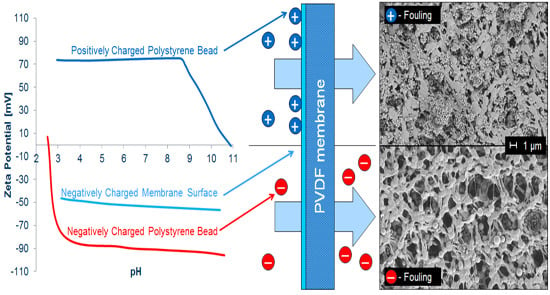
Tailoring Membrane Surface Charges: A Novel Study on Electrostatic Interactions during Membrane Fouling
Abstract
:1. Introduction
1.1. Membrane Surface Hydrophilicity and Surface Charge
1.2. Description of Fouling Tendency
1.3. Polystyrene Beads as Model Fouling Reagent
2. Experimental Section
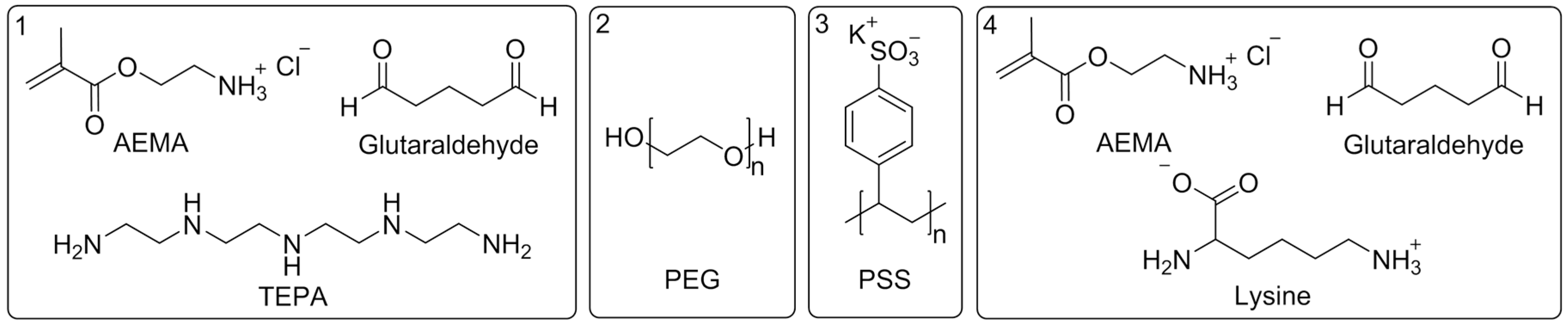
2.1. Reagents and Materials
2.2. Synthesis and Characterization of Differently Charged PS Beads
| Particle charge | Initiator | Impeller speed (rpm) | Time (h) |
|---|---|---|---|
| positive | AIBA | 200 | 5 |
| negative | KPS | 400 | 2 |
| uncharged | AIBN | 200 | 2 |
2.3. Membrane Modification and Characterization
2.4. Membrane Fouling with PS Beads
3. Results and Discussion
3.1. Characteristics of PS Beads with Different Surface Charge

3.2. Characteristics of Modified PVDF Membranes
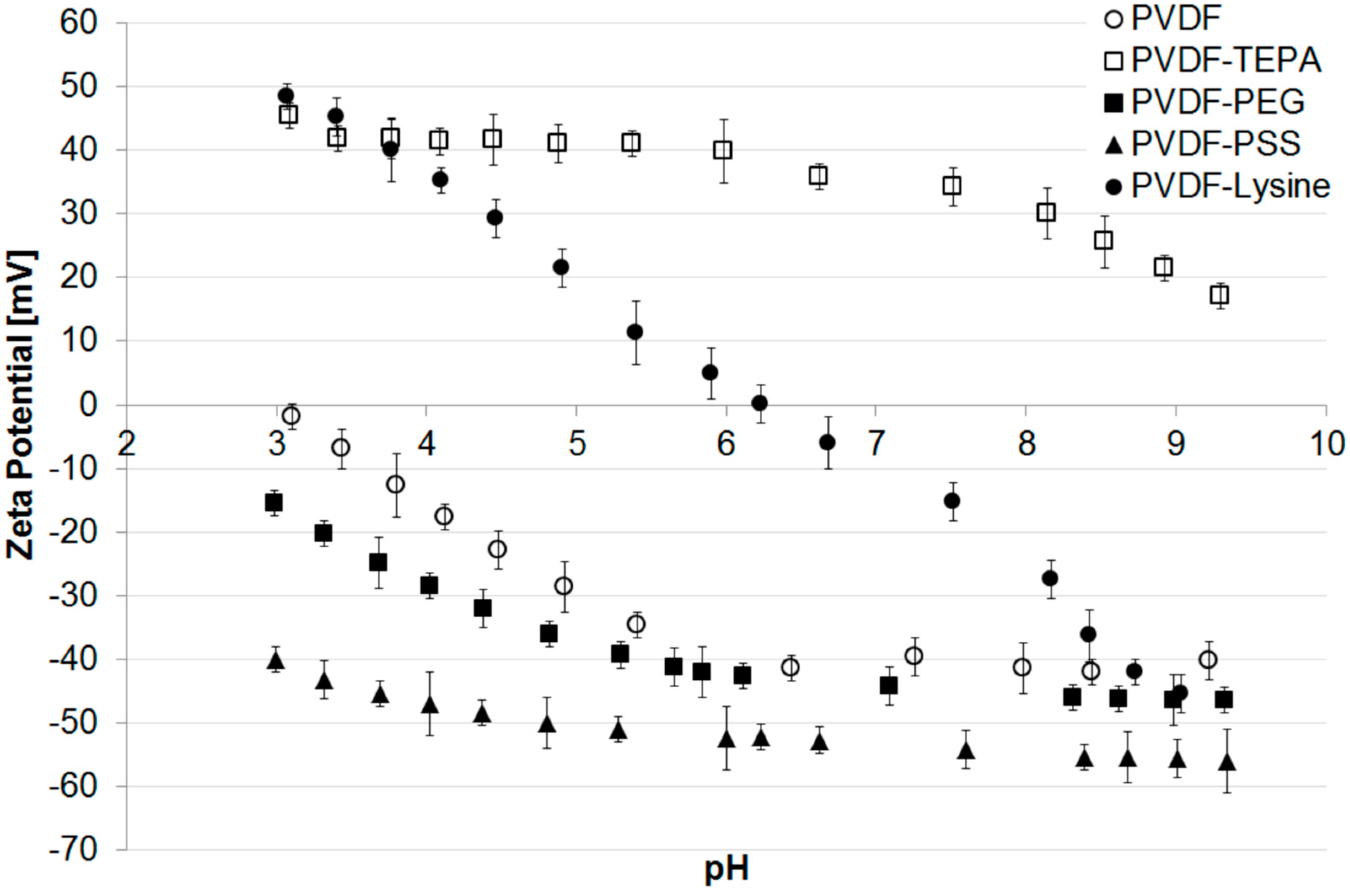
3.3. Membrane Fouling with PS Beads

| Determined parameter | PVDF | PVDF-TEPA | PVDF-PEG | PVDF-PSS | PVDF-Lysine |
|---|---|---|---|---|---|
| Initial water contact angle (°) | 132 ± 1 | 25 ± 1 | 92 ± 6 | 121 ± 2 | 114 ± 5 |
| Drop absorption rate * (°/min) | 1.6 ± 0.1 | ** | 3.5 ± 0.1 | 1.6 ± 0.3 | 1.8 ± 0.3 |
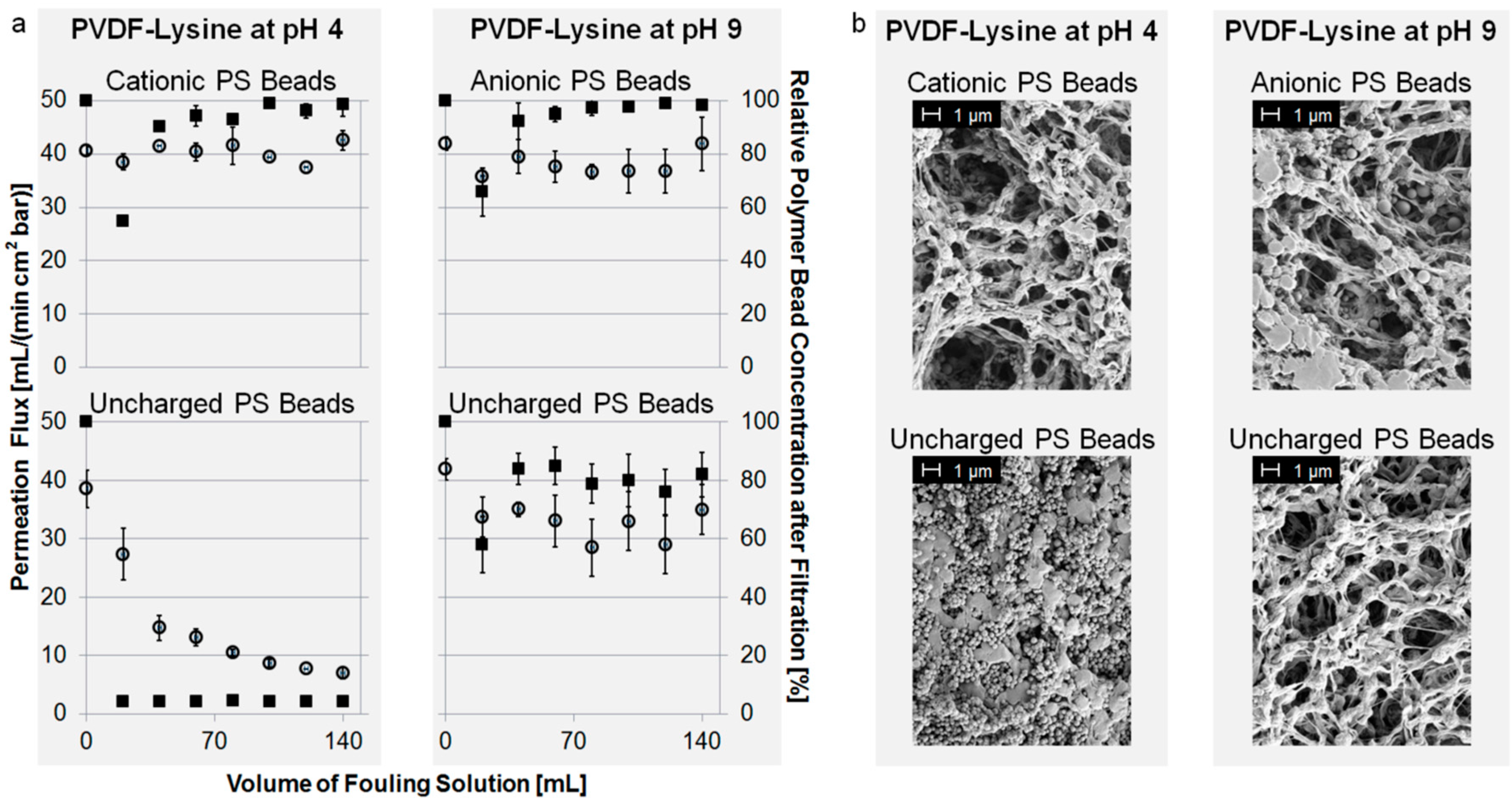
4. Conclusions
- Highly hydrophobic systems like the pristine PVDF membrane are mainly fouled due to hydrophobic interactions while electrostatic interactions are less important.
- Electrostatic attractive interactions are the driving forces of fouling when the membrane surface and the fouling reagent are oppositely charged (e.g., PVDF-PSS + cationic PS beads or PVDF-TEPA + anionic PS beads).
- Electrostatic repulsive interactions are the dominating forces for obtaining fouling resistance when the membrane surface and the fouling reagent are evenly charged (e.g., PVDF-PSS + anionic PS beads, PVDF-TEPA + cationic PS beads, PVDF-Lysine + anionic PS beads at pH 9 or PVDF-Lysine + cationic PS beads at pH 4).
- Hydrophilized, non-charged membranes like the PVDF-PEG membrane show fouling characteristics that can only be explained when both hydrophilicity and electrostatic interactions are considered.
- A zeta potential of nearly zero (PVDF-Lysine membrane at pH 7) results in membrane fouling that can be prevented by, e.g., changes in pH.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haynes, C.A.; Norde, W. Structures and stabilities of adsorbed proteins. J. Colloid Interface Sci. 1995, 169, 313–328. [Google Scholar] [CrossRef]
- Wahlgren, M.; Arnebrant, T. Protein adsorption to solid surfaces. Trends Biotechnol. 1991, 9, 201–208. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Feng, C.; Matsuura, T. The art of surface modification of synthetic polymeric membranes. J. Appl. Polym. Sci. 2010, 115, 855–895. [Google Scholar] [CrossRef]
- Liu, S.X.; Kim, J.T.; Kim, S. Effect of polymer surface modification on polymer–protein interaction via hydrophilic polymer grafting. J. Food Sci. 2008, 73, E143–E150. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Saxena, N.; Prabhavathy, C.; de, S.; DasGupta, S. Flux enhancement by argon–oxygen plasma treatment of polyethersulfone membranes. Sep. Purif. Technol. 2009, 70, 160–165. [Google Scholar] [CrossRef]
- Chang, Y.; Ko, C.-Y.; Shih, Y.-J.; Quémener, D.; Deratani, A.; Wei, T.-C.; Wang, D.-M.; Lai, J.-Y. Surface grafting control of pegylated poly(vinylidene fluoride) antifouling membrane via surface-initiated radical graft copolymerization. J. Membr. Sci. 2009, 345, 160–169. [Google Scholar] [CrossRef]
- Du, J.R.; Peldszus, S.; Huck, P.M.; Feng, X. Modification of poly(vinylidene fluoride) ultrafiltration membranes with poly(vinyl alcohol) for fouling control in drinking water treatment. Water Res. 2009, 43, 4559–4568. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Belfort, G. Surface modification of poly(ether sulfone) ultrafiltration membranes by low-temperature plasma-induced graft polymerization. J. Appl. Polym. Sci. 1999, 72, 1699–1711. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Shi, Q.; Su, Y.; Chen, W.; Peng, J.; Nie, L.; Zhang, L.; Jiang, Z. Grafting short-chain amino acids onto membrane surfaces to resist protein fouling. J. Membr. Sci. 2011, 366, 398–404. [Google Scholar] [CrossRef]
- Schulze, A.; Marquardt, B.; Kaczmarek, S.; Schubert, R.; Prager, A.; Buchmeiser, M.R. Electron beam-based functionalization of poly(ethersulfone) membranes. Macromol. Rapid Commun. 2010, 31, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Marquardt, B.; Went, M.; Prager, A.; Buchmeiser, M.R. Electron beam-based functionalization of polymer membranes. Water Sci. Technol. 2012, 65, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Boulares-Pender, A.; Thomas, I.; Prager, A.; Schulze, A. Surface modification of polyamide and poly(vinylidene fluoride) membranes. J. Appl. Polym. Sci. 2013, 128, 322–331. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Characterization of PPO membranes by oxygen plasma etching, gas separation and atomic force microscopy. J. Membr. Sci. 2000, 171, 273–284. [Google Scholar] [CrossRef]
- Asfardjani, K.; Segui, Y.; Aurelle, Y.; Abidine, N. Effect of plasma treatments on wettability of polysulfone and polyetherimide. J. Appl. Polym. Sci. 1991, 43, 271–281. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Lopez, L.C.; Favia, P.; d’Agostino, R.; Gordano, A.; Drioli, E. New pvdf membranes: The effect of plasma surface modification on retention in nanofiltration of aqueous solution containing organic compounds. Water Res. 2007, 41, 4309–4316. [Google Scholar] [CrossRef] [PubMed]
- Vandencasteele, N.; Reniers, F. Plasma-modified polymer surfaces: Characterization using XPS. J. Electron Spectrosc. Relat. Phenom. 2010, 178–179, 394–408. [Google Scholar] [CrossRef]
- Kull, K.R.; Steen, M.L.; Fisher, E.R. Surface modification with nitrogen-containing plasmas to produce hydrophilic, low-fouling membranes. J. Membr. Sci. 2005, 246, 203–215. [Google Scholar] [CrossRef]
- Ingemar, L. Surface physics and biological phenomena. Phys. Scr. 1983, T4, 5–13. [Google Scholar]
- Kumar, M.; Ulbricht, M. Novel ultrafiltration membranes with adjustable charge density based on sulfonated poly(arylene ether sulfone) block copolymers and their tunable protein separation performance. Polymer 2014, 55, 354–365. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, X.; Huang, X.; Waite, T.D.; Wen, X. Combined effect of membrane and foulant hydrophobicity and surface charge on adsorptive fouling during microfiltration. J. Membr. Sci. 2011, 373, 140–151. [Google Scholar] [CrossRef]
- Hadidi, M.; Zydney, A.L. Fouling behavior of zwitterionic membranes: Impact of electrostatic and hydrophobic interactions. J. Membr. Sci. 2014, 452, 97–103. [Google Scholar] [CrossRef]
- Zhang, Q.; Vecitis, C.D. Conductive CNT-PVDF membrane for capacitive organic fouling reduction. J. Membr. Sci. 2014, 459, 143–156. [Google Scholar] [CrossRef]
- Dudchenko, A.V.; Rolf, J.; Russell, K.; Duan, W.; Jassby, D. Organic fouling inhibition on electrically conducting carbon nanotube–polyvinyl alcohol composite ultrafiltration membranes. J. Membr. Sci. 2014, 468, 1–10. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Relating nanofiltration membrane performance to membrane charge (electrokinetic) characteristics. Environ. Sci. Technol. 2000, 34, 3710–3716. [Google Scholar] [CrossRef]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Levitsky, I.; Gitis, V. Electrostatic repulsion as a mechanism in fouling of ultrafiltration membranes. Water Sci. Technol. 2008, 58, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Han, M.J.; Baroña, G.N.B.; Jung, B. Effect of surface charge on hydrophilically modified poly(vinylidene fluoride) membrane for microfiltration. Desalination 2011, 270, 76–83. [Google Scholar] [CrossRef]
- Lamminen, M.O.; Walker, H.W.; Weavers, L.K. Effect of fouling conditions and cake layer structure on the ultrasonic cleaning of ceramic membranes. Sep. Sci. Technol. 2006, 41, 3569–3584. [Google Scholar] [CrossRef]
- Wang, Y.; Wicaksana, F.; Tang, C.Y.; Fane, A.G. Direct microscopic observation of forward osmosis membrane fouling. Environ. Sci. Technol. 2010, 44, 7102–7109. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hakim, L.F.; Bowman, C.N.; Davis, R.H. Factors affecting membrane fouling reduction by surface modification and backpulsing. J. Membr. Sci. 2001, 189, 255–270. [Google Scholar] [CrossRef]
- Bondy, C.M.; Santeufemio, C. Analysis of fouling within microporous membranes in biopharmaceutical applications using latex microsphere suspensions. J. Membr. Sci. 2010, 349, 12–24. [Google Scholar] [CrossRef]
- Teychene, B.; Loulergue, P.; Guigui, C.; Cabassud, C. Development and use of a novel method for in line characterisation of fouling layers electrokinetic properties and for fouling monitoring. J. Membr. Sci. 2011, 370, 45–57. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Doan, H.; Lohi, A.; Cheng, C.-H. Modeling of fouling and foulant attachments on heterogeneous membranes in ultrafiltration of latex solution. Sep. Purif. Technol. 2014, 135, 199–210. [Google Scholar] [CrossRef]
- Gordano, A.; Arcella, V.; Drioli, E. New HYFLON AD composite membranes and AFM characterization. Desalination 2004, 163, 127–136. [Google Scholar] [CrossRef]
- Lamminen, M.O.; Walker, H.W.; Weavers, L.K. Mechanisms and factors influencing the ultrasonic cleaning of particle-fouled ceramic membranes. J. Membr. Sci. 2004, 237, 213–223. [Google Scholar] [CrossRef]
- Lamminen, M.O.; Walker, H.W.; Weavers, L.K. Cleaning of particle-fouled membranes during cross-flow filtration using an embedded ultrasonic transducer system. J. Membr. Sci. 2006, 283, 225–232. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Warszyhski, P. Role of electrostatic interactions in particle adsorption. Adv. Colloid Interface Sci. 1996, 63, 41–149. [Google Scholar] [CrossRef]
- Adamczyk, Z. Particle adsorption and deposition: Role of electrostatic interactions. Adv. Colloid Interface Sci. 2003, 100, 267–347. [Google Scholar] [CrossRef]
- Henry, C.; Minier, J.-P.; Lefèvre, G. Towards a description of particulate fouling: From single particle deposition to clogging. Adv. Colloid Interface Sci. 2012, 185, 34–76. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, Y.; Fievet, P.; Magnenet, C.; Déon, S.; Szymczyk, A. Electrokinetic characterisation of particle deposits from streaming potential coupled with permeate flux measurements during dead-end filtration. J. Membr. Sci. 2011, 378, 224–232. [Google Scholar] [CrossRef]
- Inukai, S.; Tanma, T.; Orihara, S.; Konno, M. A simple method for producing micron-sized, highly monodisperse polystyrene particles in aqueous media: Effects of impeller speed on particle size distribution. Chem. Eng. Res. Des. 2001, 79, 901–905. [Google Scholar] [CrossRef]
- Duracher, D.; Sauzedde, F.; Elaissari, A.; Perrin, A.; Pichot, C. Cationic amino-containing N-isopropyl-acrylamide–styrene copolymer latex particles: 1-Particle size and morphology vs. polymerization process. Colloid Polym. Sci. 1998, 276, 219–231. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Park, Y.-S.; Lee, J.S. Controlled synthesis of spherical polystyrene beads and their template-assisted manual assembly. Bull. Korean Chem. Soc. 2014, 35, 2281–2284. [Google Scholar] [CrossRef]
- Schulze, A.; Maitz, M.F.; Zimmermann, R.; Marquardt, B.; Fischer, M.; Werner, C.; Went, M.; Thomas, I. Permanent surface modification by electron-beam-induced grafting of hydrophilic polymers to PVDF membranes. RSC Adv. 2013, 3, 22518–22526. [Google Scholar] [CrossRef]
- Boulares-Pender, A.; Prager, A.; Elsner, C.; Buchmeiser, M.R. Surface-functionalization of plasma-treated polystyrene by hyperbranched polymers and use in biological applications. J. Appl. Polym. Sci. 2009, 112, 2701–2709. [Google Scholar] [CrossRef]
- Zimmermann, R.; Dukhin, S.; Werner, C. Electrokinetic measurements reveal interfacial charge at polymer films caused by simple electrolyte ions. J. Phys. Chem. B 2001, 105, 8544–8549. [Google Scholar] [CrossRef]
- Zimmermann, R.; Freudenberg, U.; Schweiß, R.; Küttner, D.; Werner, C. Hydroxide and hydronium ion adsorption—A survey. Curr. Opin. Colloid Interface Sci. 2010, 15, 196–202. [Google Scholar] [CrossRef]
- Ray, W.J., Jr.; Puvathingal, J.M. A simple procedure for removing contaminating aldehydes and peroxides from aqueous solutions of polyethylene glycols and of nonionic detergents that are based on the polyoxyethylene linkage. Anal. Biochem. 1985, 146, 307–312. [Google Scholar] [CrossRef]
- Jurnak, F. Effect of chemical impurities in polyethylene glycol on macromolecular crystallization. J. Cryst. Growth 1986, 76, 577–582. [Google Scholar] [CrossRef]
- Burke, S.E.; Barrett, C.J. Acid-base equilibria of weak polyelectrolytes in multilayer thin films. Langmuir 2003, 19, 3297–3303. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2008, 11, 77–89. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breite, D.; Went, M.; Prager, A.; Schulze, A. Tailoring Membrane Surface Charges: A Novel Study on Electrostatic Interactions during Membrane Fouling. Polymers 2015, 7, 2017-2030. https://doi.org/10.3390/polym7101497
Breite D, Went M, Prager A, Schulze A. Tailoring Membrane Surface Charges: A Novel Study on Electrostatic Interactions during Membrane Fouling. Polymers. 2015; 7(10):2017-2030. https://doi.org/10.3390/polym7101497
Chicago/Turabian StyleBreite, Daniel, Marco Went, Andrea Prager, and Agnes Schulze. 2015. "Tailoring Membrane Surface Charges: A Novel Study on Electrostatic Interactions during Membrane Fouling" Polymers 7, no. 10: 2017-2030. https://doi.org/10.3390/polym7101497
APA StyleBreite, D., Went, M., Prager, A., & Schulze, A. (2015). Tailoring Membrane Surface Charges: A Novel Study on Electrostatic Interactions during Membrane Fouling. Polymers, 7(10), 2017-2030. https://doi.org/10.3390/polym7101497