Substance-Based Medical Device in Wound Care: Bridging Regulatory Clarity and Therapeutic Innovation
Abstract
1. Introduction
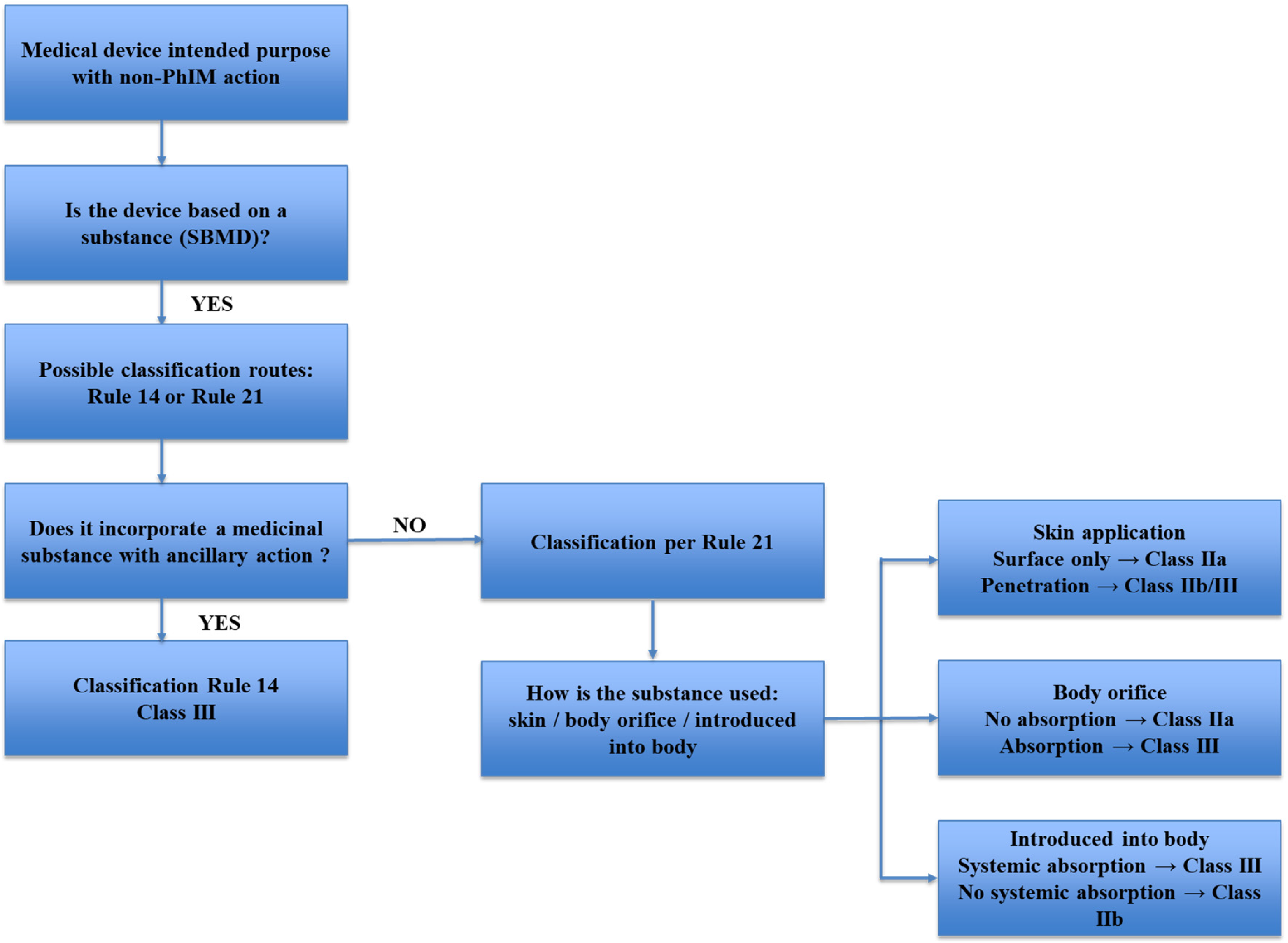
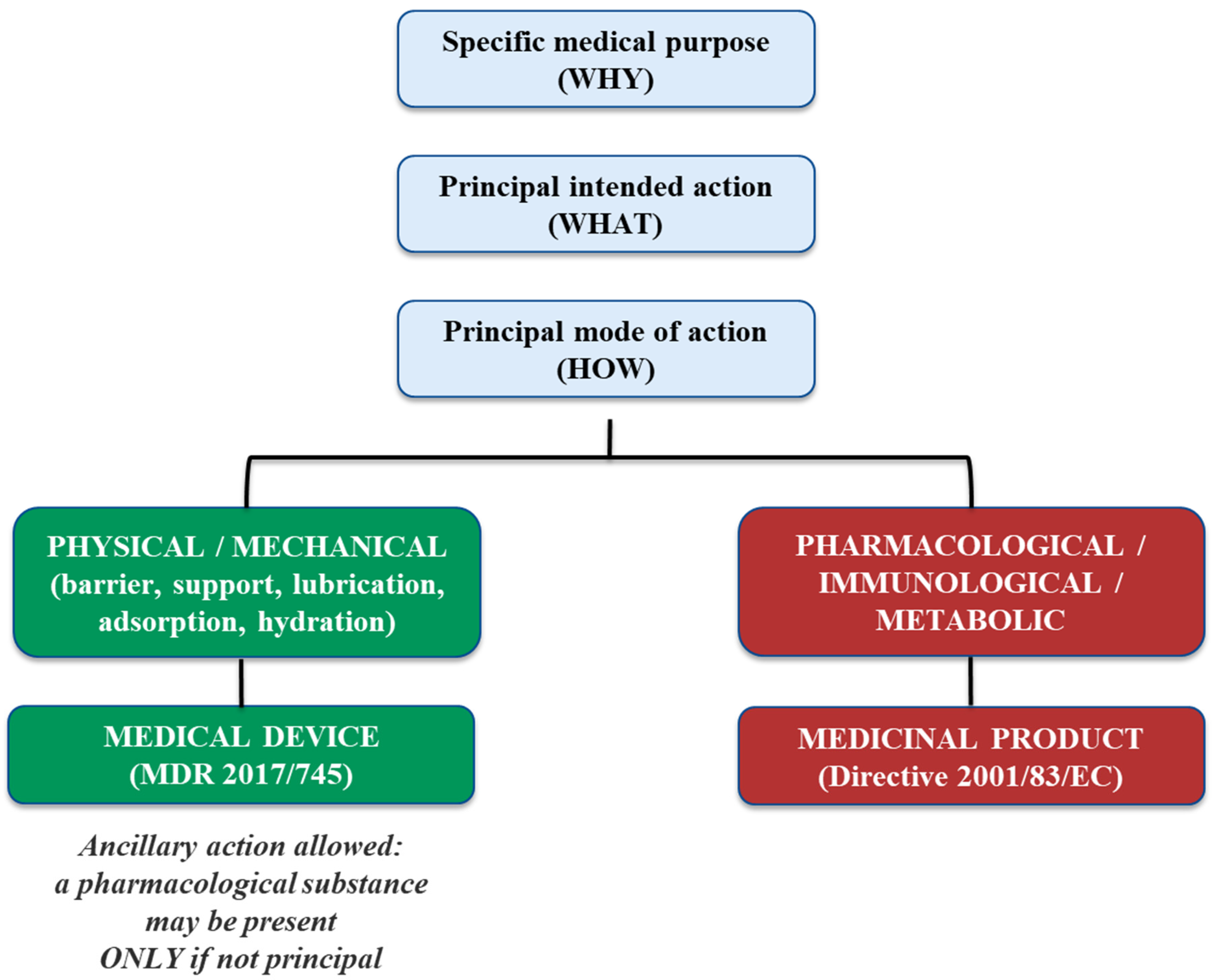
2. Substance-Based Medical Devices (SBMDs)
- -
- class III if they, or their products of metabolism, are systemically absorbed by the human body in order to achieve the intended purpose;
- -
- class III if they achieve their intended purpose in the stomach or lower gastrointestinal tract and they, or their products of metabolism, are systemically absorbed by the human body;
- -
- class IIa if they are applied to the skin or if they are applied in the nasal or oral cavity as far as the pharynx, and achieve their intended purpose on those cavities; and
- -
- class IIb in all other cases.”
Topical Substance-Based Medical Devices
3. Market Dynamics
4. Topical Medicinal Products
5. Innovations in Wound Dressings: From Traditional Approaches to SBMDs
| Polymer | Advantages | Disadvantages | References |
|---|---|---|---|
| Chitosan | Antimicrobial, hemostatic, biocompatible and biodegradable | Solubility limitations, mechanical constraints, heterogeneity and batch variability | [48,49,50,51,52] |
| Polyvinylalcohol | Biocompatible, water solubility, hemostatic, high-water uptake, low production costs | Non-antimicrobial, limited mechanical properties | [53,54,55,56,57,58,59] |
| Cellulose | Biocompatible, hemostatic, water absorption capacity | Non-antimicrobial, limited mechanical properties | [60,61,62,63] |
| Polycaprolactone | Biocompatible, mechanical strength, controllable degradation rate, cost-effectiveness and scalability | Non-antimicrobial, hydrophobicity, poor absorption capacity | [64,65,66,67] |
| Gelatin | Biocompatible, hemostatic, versatile formulation capability, water absorption capacity | Non-antimicrobial, limited mechanical properties, thermosensitive, batch variability, high production costs | [68,69,70,71] |
| Starch | Biocompatible, water absorption capacity, cost-effectiveness, versatile formulation capability, excellent water vapor transmission rate | Non-antimicrobial, limited mechanical properties, environmental sensitivity, batch variability, high production costs and scalability challenges | [72,73,74,75,76] |
| Collagen | Biocompatible, hemostatic, angiogenic, versatile formulation capability, biodegradable, promote fibroblast proliferation and collagen deposition | Non-antimicrobial, high production costs, manufacturing complexity, weak mechanical properties, immunogenicity and allergic sensitization, ethical/religious limitations | [77,78,79,80,81,82,83] |
| Hyaluronic acid | Biocompatible, anti-inflammatory, antioxidant, optimal moist wound management, angiogenic, versatile formulation capability | Weak mechanical properties, high production costs, batch variability, maceration risk for high swelling ratio, non-antimicrobial | [34,84,85,86,87,88,89,90,91] |
| Alginate | Biocompatible, exudate absorption, moisture management, hemostatic, anti-inflammatory, versatile formulation capability, cost-effectiveness | Weak mechanical properties, non-antimicrobial, rapid enzymatic degradation, complex production, high variability | [92,93,94,95,96,97,98,99] |
| Polylactic acid | Biocompatible, superior mechanical properties, promotes angiogenesis and collagen deposition, optimal moisture management, versatile formulation capability | Slow degradation rate, acid degradation by-products, inherent hydrophobicity, low cell affinity, non-antimicrobial, high production costs, | [100,101,102,103,104,105,106] |
| Carrageenan | Biocompatible, superior exudate absorption and moisture management, hemostatic, versatile formulation capability, antioxidant, anti-inflammatory | Weak mechanical properties, non-antimicrobial, sourcing and batch variability, high production costs, batch | [107,108,109,110,111,112,113] |
| Antimicrobial Agents | Advantages | Disadvantages | References |
|---|---|---|---|
| Silver | Broad-spectrum antimicrobial, bactericidal, anti-inflammatory | Cytotoxicity, argyria and systemic silver accumulation | [114,115,116,117,118,119,120] |
| Metals and metal oxides nanoparticles (silver, zinc oxide, iron oxide, cerium dioxide, titanium dioxide) | Broad-spectrum antimicrobial, anti-inflammatory, antioxidant | Dispersion and accumulation in different organs of the body, leading to toxicity | [121,122,123,124,125] |
| Non-metal nanoparticles (dendrimers, ferritins, micelles, liposomes) | Broad-spectrum antimicrobial, low immunogenicity, and encapsulation capacity | High production costs | [38,126,127,128,129] |
| Iodine | Broad-spectrum antimicrobial, bactericidal, fungicidal, rapid efficacy, anti-inflammatory, cost-effectiveness, | Local tissue toxicity and irritation, thyroid dysfunction risk | [130,131,132,133,134,135,136] |
| Biguanides: polyhexamethylene biguanide (PHMB), chlorhexidine | Cationic emulsifier and broad-spectrum antimicrobial, bactericidal, virucidal, cysticidal, promotes tissue granulation and wound healing | Possibly cytotoxic, repeated prolonged exposure may cause sensitization | [38,137,138,139,140,141] |
| Plant-derived natural compounds (oregano, tea tree oil, lavender) | Broad-spectrum antimicrobial, bactericidal, insecticidal, analgesic, antioxidant, and anti-inflammatory effects | Frequent application and/or the use of high concentrations may be necessary, batch variability | [142,143,144,145,146,147,148] |
| Antimicrobial peptides (AMPs) | Broad-spectrum antimicrobial, biofilm penetration and disruption, minimal resistance development | Some AMPs might be sensitive to light, heat, and moisture; proper storage conditions are crucial to maintain their efficacy and cytotoxicity at higher concentrations. High production costs | [149,150,151,152,153,154] |
6. Examples of Registered Topical Substance-Based Medical Devices
| Trade Name | Manufacturer | Risk Class | Applicable Legislation | Reference |
|---|---|---|---|---|
| Bioepithelia base crema | Kethema farmaceutici srl | Class I | Reg. UE 2017/745 | [155] |
| Alovex ferite spray | Nirial pharma s.r.l. | Class IIa | Reg. UE 2017/745 | [156] |
| Ialuset | Ibsa farmaceutici italia srl | Class IIb | Reg. UE 2017/745 | [157] |
| Iodosorb ointment | Smith and nephew medical limited | Class III | Reg. UE 2017/745 | [158] |
| Sofargen repair | Sofar s.p.a. | Class IIa | Directive 93/42/EEC on Medical Devices | [159] |
| Fitostimoline plus crema | Farmaceutici damor s.p.a. | Class IIb | Directive 93/42/EEC on Medical Devices | [160] |
| Connettivinabio plus crema | Fidia farmaceutici s.p.a. | Class III | Directive 93/42/EEC on Medical Devices | [161] |
| ialuset plus | Ibsa farmaceutici italia srl | Class III | Directive 93/42/EEC on Medical Devices | [162] |
7. Conclusions
8. Key Takeaways
- -
- Topical substance-based medical devices offer significant therapeutic potential in wound care, particularly when formulated with hydrating biopolymers (e.g., hyaluronic acid) and antimicrobial agents (e.g., silver sulfadiazine, AgNPs).
- -
- The principal mode of action is the defining criterion for classification under MDR, impacting whether a product is regulated as a medical device (Rule 21), a drug-device combination (Rule 14), or a medicinal product.
- -
- Products containing silver or SSD exemplify borderline challenges, as their antimicrobial activity may be ancillary (Rule 14) or primary (MPs), depending on concentration, presentation, and intended purpose.
- -
- The interpretation of Rule 14 has been clarified by MDCG 2022-5 Rev.1 and the AESGP Position Paper, which stress the need for a case-by-case assessment and scientific justification of the mode of action.
- -
- Inconsistent classification practices across EU Member States delay market access, increase regulatory burden, and hinder innovation, particularly for complex, multifunctional, or natural-origin substances.
- -
- A more harmonized, flexible, and evidence-based regulatory approach is urgently needed to support the development of effective, safe, and innovative wound care solutions.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical drug delivery: History, percutaneous absorption, and product development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef] [PubMed]
- Jeary, T.; Sutch, J. An Overview of the Impact of the MDR (EU) 2017/745 for Combination Products and Substance-Based Devices. BSI Group. 2025. Available online: https://www.bsigroup.com (accessed on 15 October 2025).
- Marletta, M. Regulation 2017/745 on medical devices, two major innovations: 1) the physiological action of devices consisting of natural materials such as vegetal matrices; 2) the chemical-physical-mechanical action of devices made of “substances”, which as such are artificial derivatives. Front. Drug Saf. Regul. Front. Media SA 2024, 4, 1389406. [Google Scholar] [CrossRef]
- Leone, M.G. Medical devices made of substances: Regulatory perspectives and market challenges. Front. Drug Saf. Regul. 2022, 2, 952013. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Aronson, J.K.; Ferner, R.E. Clarification of Terminology in Drug Safety. Drug Saf. 2005, 28, 851–870. [Google Scholar] [CrossRef]
- MDCG MDCGD. MDCG 2022-5 Rev. 1 Guidance on Borderline Between Medical Devices and Medicinal Products Under Regulation (EU) 2017/745 on Medical Devices; European Commission: Brussels, Belgium, 2024. [Google Scholar]
- Racchi, M.; Govoni, S.; Lucchelli, A.; Capone, L.; Giovagnoni, E. Insights into the definition of terms in European medical device regulation. Expert Rev. Med. Devices 2016, 13, 907–917. [Google Scholar] [CrossRef]
- MDCG MMDCGD. Guidance on Transitional Provisions for Consultations of Authorities on Devices Incorporating a Substance Which May be Considered a Medicinal Product and Which Has Action Ancillary to that of the Device, as Well as on Devices Manufactured Using TSE Susceptible Animal Tissues; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- AESGP. Association of the European Self-Care Industry AESGP Position Paper on Classification Rule 21. 2018. Available online: www.aesgp.eu (accessed on 24 July 2025).
- European Commission. MEDDEV 2.1/3 Rev. 3—Guidelines on Borderline Products, Drug-Delivery Products and Medical Devices Incorporating an Ancillary Medicinal Substance or an Ancillary Human Blood Derivative. 2009. Available online: https://ec.europa.eu/docsroom/documents/10328/attachments/1/translations (accessed on 30 October 2025).
- European Commission. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/ 385/EEC and 93/42/EEC; Official Journal of the European Union; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Giovagnoni, E. Substance-based medical devices made of natural substances: An opportunity for therapeutic innovation. Front. Drug Saf. Regul. 2022, 2, 998114. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M. Mucoadhesive polymers in substance-based medical devices: Functional ingredients or what else? Front. Drug Saf. Regul. 2023, 3, 1227763. [Google Scholar] [CrossRef]
- Marletta, M. The new regulation 2017/745: An opportunity for innovation. Pharmadvances 2020, 1. [Google Scholar] [CrossRef]
- Racchi, M.; Govoni, S. The concept of non-pharmacological mechanism of action in medical devices made of substances in practice: What pharmacology can do to promote the scientific implementation of the European medical device regulation. Pharmadvances 2020, 1, 4–12. [Google Scholar] [CrossRef]
- MedTech Europe. European Medical Technology Market Statistics. 2023. Available online: https://www.medtecheurope.org/datahub/market (accessed on 30 October 2025).
- European Commission. Directive 2001/83/EC of the European Parliament and of the Council on the Community Code Relating to Medicinal Products for Human Use. 2001. Available online: https://eur-lex.europa.eu/eli/dir/2001/83/oj (accessed on 30 October 2025).
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of Action of Topical Corticosteroids in Psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef]
- Axon, E.; Chalmers, J.R.; Santer, M.; Ridd, M.J.; Lawton, S.; Langan, S.M.; Grindlay, D.J.C.; Muller, I.; Roberts, A.; Ahmed, A.; et al. Safety of topical corticosteroids in atopic eczema: An umbrella review. BMJ Open 2021, 11, 46476. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Guttman-Yassky, E.; Thaçi, D.; Irvine, A.D.; Stein Gold, L.; Blauvelt, A.; Simpson, E.L.; Chu, C.-Y.; Liu, Z.; Lima, R.G.; et al. Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2023, 388, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Life Sciences Law Review—European Union Chapter. 2017. Available online: https://www.cov.com/-/media/files/corporate/publications/2017/03/life_sciences_law_review_european_union_2017.pdf (accessed on 15 October 2025).
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N. In Vitro and In Vivo Characterization Methods for Evaluation of Modern Wound Dressings. Pharmaceutics 2023, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.M.; Leite, J.M.d.S.; Medeiros, K.d.A.; Assis, K.M.A.d.; Borges, J.C.; Santana, L.M.B.; de Carvalho Moreira, L.M.C.; Alves, L.P.; de Oliveira, T.K.B.; de Souza da Silveira, J.W.; et al. Pentoxifylline/Chitosan Films on Wound Healing: In Vitro/In Vivo Evaluation. Pharmaceutics. Pharmaceutics 2023, 15, 1122. [Google Scholar] [CrossRef]
- Torres, A.; Rego, L.; Martins, M.S.; Ferreira, M.S.; Cruz, M.T.; Sousa, E.; Almeida, I.F. How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies. Pharmaceuticals 2023, 16, 573. [Google Scholar] [CrossRef]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Vazifehdoost, M.; Alhashemi, S.H.; Davoudi, H.; Zarrabi, A.; Dehshahri, A.; Fekri, H.S.; Mohammadinejad, R.; Thakur, V.K. Next-Generation Hydrogels as Biomaterials for Biomedical Applications: Exploring the Role of Curcumin. ACS Omega 2023, 8, 8960. [Google Scholar] [CrossRef]
- Khalil, A.M.; Abdel-Monem, R.A.; Darwesh, O.M.; Hashim, A.I.; Nada, A.A.; Rabie, S.T. Synthesis, Characterization, and Evaluation of Antimicrobial Activities of Chitosan and Carboxymethyl Chitosan Schiff-Base/Silver Nanoparticles. J. Chem. 2017, 2017, 1434320. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Hu, X.H.; Chu, L.Q.; Jia, S.R.; Xie, Y.Y.; Zhong, C. Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int. J. Biol. Macromol. 2019, 122, 380–387. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Hyaluronic Acid-Based Scaffolds as Potential Bioactive Wound Dressings. Polymers 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef]
- Sudhakar, K.; Ji, S.M.; Kummara, M.R.; Han, S.S. Recent Progress on Hyaluronan-Based Products for Wound Healing Applications. Pharmaceutics 2022, 14, 2235. [Google Scholar] [CrossRef]
- Brouillard, C.; Bursztejn, A.C.; Latarche, C.; Cuny, J.F.; Truchetet, F.; Goullé, J.P.; Schmutz, J.L. Silver absorption and toxicity evaluation of silver wound dressings in 40 patients with chronic wounds. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2295–2299. [Google Scholar] [CrossRef]
- Yousefian, F.; Hesari, R.; Jensen, T.; Obagi, S.; Rgeai, A.; Damiani, G.; Bunick, C.G.; Grada, A. Antimicrobial Wound Dressings: A Concise Review for Clinicians. Antibiotics 2023, 12, 1434. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Elliott, J.A.; Verma, L.; Brandon, A.; Persaud, R.; Ayello, E.A. Update: Topical Antimicrobial Agents for Chronic Wounds. Adv. Ski. Wound Care 2017, 30, 438–450. [Google Scholar] [CrossRef]
- Pangli, H.; Vatanpour, S.; Hortamani, S.; Jalili, R.; Ghahary, A. Incorporation of Silver Nanoparticles in Hydrogel Matrices for Controlling Wound Infection. J. Burn Care Res. 2020, 42, 785. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Constantin, M.; Solcan, G.; Ichim, D.L.; Rata, D.M.; Horodincu, L.; Solcan, C. Composite Hydrogels with Embedded Silver Nanoparticles and Ibuprofen as Wound Dressing. Gels 2023, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gálvez, J.; Martínez-Isasi, S.; Gómez-Salgado, J.; Rumbo-Prieto, J.M.; Sobrido-Prieto, M.; Sánchez-Hernández, M.; García-Martínez, M.; Fernández-García, D. Cytotoxicity and concentration of silver ions released from dressings in the treatment of infected wounds: A systematic review. Front. Public Health 2024, 12, 1331753. [Google Scholar] [CrossRef]
- Jodar, K.S.P.; Balcão, V.M.; Chaud, M.V.; Tubino, M.; Yoshida, V.M.H.; Oliveira, J.M.; Vila, M.M. Development and Characterization of a Hydrogel Containing Silver Sulfadiazine for Antimicrobial Topical Applications. J. Pharm. Sci. 2015, 104, 2241–2254. [Google Scholar] [CrossRef]
- Liu, X.; Gan, H.; Hu, C.; Sun, W.; Zhu, X.; Meng, Z.; Gu, R.; Wu, Z.; Dou, G. Silver sulfadiazine nanosuspension-loaded thermosensitive hydrogel as a topical antibacterial agent. Int. J. Nanomed. 2018, 14, 289–300. [Google Scholar] [CrossRef]
- Dutt, Y.; Pandey, R.P.; Dutt, M.; Gupta, A.; Vibhuti, A.; Raj, V.S.; Chang, C.-M.; Priyadarshini, A. Silver Nanoparticles Phytofabricated through Azadirachta indica: Anticancer, Apoptotic, and Wound-Healing Properties. Antibiotics 2023, 12, 121. [Google Scholar] [CrossRef]
- AESGP. AESGP Position Paper on Classification Rule 14. 2018. Available online: https://aesgp.eu/content/uploads/2020/07/AESGP-position-paper-on-Rule-14_October-2018.pdf (accessed on 15 September 2025).
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533. [Google Scholar] [CrossRef]
- Rajinikanth, S.B.; Rajkumar, D.S.R.; Keerthika, K.; Vijayaragavan, V. Chitosan-Based Biomaterial in Wound Healing: A Review. Cureus 2024, 16, e55193. [Google Scholar] [CrossRef]
- Sheokand, B.; Vats, M.; Kumar, A.; Srivastava, C.M.; Bahadur, I.; Pathak, S.R. Natural polymers used in the dressing materials for wound healing: Past, present and future. J. Polym. Sci. 2023, 61, 1389–1414. [Google Scholar] [CrossRef]
- Ianev, D.; Vigani, B.; Valentino, C.; Sorrenti, M.; Catenacci, L.; Bonferoni, M.C.; Ruggeri, M.; Sandri, G.; Mori, M.; Rossi, S. Whey protein-chitosans complexes as sustainable and value-added biomaterials for wound healing. Carbohydr. Polym. Technol. Appl. 2025, 9, 100643. [Google Scholar] [CrossRef]
- Pino, P.; Vigani, B.; Valentino, C.; Ianev, D.; Ruggeri, M.; Boselli, C.; Cornaglia, A.I.; Grisoli, P.; Onida, B.; Bosco, F.; et al. Sustainable whey proteins-nanostructured zinc oxide-based films for the treatment of chronic wounds: New insights from biopharmaceutical studies. Int. J. Biol. Macromol. 2024, 263, 130655. [Google Scholar] [CrossRef] [PubMed]
- Dorkhani, E.; Faryabi, A.; Noorafkan, Y.; Heirani, A.; Behboudi, B.; Fazeli, M.S.; Kazemeini, A.; Keramati, M.R.; Keshvari, A.; Tafti, S.M.A. Biomedical properties and hemostatic efficacy of polyvinyl alcohol (PVA) based hydrogel in experimental rat liver injury model. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000231198803. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Arisawa, E.A.L.S.; Filho, A.L.M.M.; Figueredo-Silva, J.; Alves, N.; da Silveira, C.H.; Vieira, L. Bioabsorbable Poly(vinyl alcohol)–Citric Acid Dressings: Wound Healing Studies in an Experimental In Vivo Model. Eur. Burn. J. 2025, 6, 18. [Google Scholar] [CrossRef]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl alcohol (PVA) as Wound Dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- Yudaev, P.; Butorova, I.; Chuev, V.; Posokhova, V.; Klyukin, B.; Chistyakov, E. Wound Gel with Antimicrobial Effects Based on Polyvinyl Alcohol and Functional Aryloxycyclotriphosphazene. Polymers 2023, 15, 2831. [Google Scholar] [CrossRef]
- Afzal, A.; Jalalah, M.; Noor, A.; Khaliq, Z.; Qadir, M.B.; Masood, R.; Nazir, A.; Ahmad, S.; Ahmad, F.; Irfan, M.; et al. Development and Characterization of Drug Loaded PVA/PCL Fibres for Wound Dressing Applications. Polymers 2023, 15, 1355. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, H.J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.-M.; He, J. Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef]
- Ji, W.; Mizanur, M.; Khan, R.; Mahamudul, M.; Rumon, H. Synthesis of PVA-Based Hydrogels for Biomedical Applications: Recent Trends and Advances. Gels 2025, 11, 88. [Google Scholar] [CrossRef]
- Bukatuka, C.F.; Mbituyimana, B.; Xiao, L.; Qaed Ahmed, A.A.; Qi, F.; Adhikari, M.; Shi, Z.; Yang, G. Recent Trends in the Application of Cellulose-Based Hemostatic and Wound Healing Dressings. J. Funct. Biomater. 2025, 16, 151. [Google Scholar] [CrossRef]
- Tudoroiu, E.E.; Dinu-Pîrvu, C.E.; Kaya, M.G.A.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/Biocompatible Hydrogels Dual-Reinforced by Cellulose as Ultrastretchable and Rapid Self-Healing Wound Dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Islam, M.S.; Christopher, L.P. Sustainable Production of Cellulose-Based Hydrogels with Superb Absorbing Potential in Physiological Saline. ACS Omega 2019, 4, 9419–9426. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, M.O.; Altun, E.; Crabbe-Mann, M.; Brako, F.; Koc, F.; Ozen, G.; Kuruca, S.E.; Edirisinghe, U.; Luo, C.J.; Gunduz, O.; et al. Cellular interactions with bacterial cellulose: Polycaprolactone nanofibrous scaffolds produced by a portable electrohydrodynamic gun for point-of-need wound dressing. Int. Wound J. 2018, 15, 789. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Golchin, A.; Maymand, M.M.; Mansouri, F.; Ardeshirylajimi, A. Electrospun Polycaprolactone Nanofibers: Current Research and Applications in Biomedical Application. Adv. Pharm. Bull. 2021, 12, 658. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Zeng, W.; Cheng, N.M.; Liang, X.; Hu, H.; Luo, F.; Jin, J.; Li, Y.-W. Electrospun polycaprolactone nanofibrous membranes loaded with baicalin for antibacterial wound dressing. Sci. Rep. 2022, 12, 10900. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef]
- Cao, H.; Wang, J.; Hao, Z.; Zhao, D. Gelatin-based biomaterials and gelatin as an additive for chronic wound repair. Front. Pharmacol. 2024, 15, 1398939. [Google Scholar] [CrossRef]
- Pei, Y.; Ye, D.; Zhao, Q.; Wang, X.; Zhang, C.; Huang, W.; Zhang, N.; Liu, S.; Zhang, L. Effectively promoting wound healing with cellulose/gelatin sponges constructed directly from a cellulose solution. J. Mater. Chem. B 2015, 3, 7518–7528. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef]
- Garavito, J.; Peña-Venegas, C.P.; Castellanos, D.A. Production of Starch-Based Flexible Food Packaging in Developing Countries: Analysis of the Processes, Challenges, and Requirements. Foods 2024, 13, 4096. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Singhal, N.; Vashistha, P.; Jain, R.; Bist, Y.; Gaur, A.; Wagri, N.K. Starch–biomacromolecule complexes: A comprehensive review of interactions, functional materials, and applications in food, pharma, and packaging. Carbohydr. Polym. Technol. Appl. 2025, 11, 101001. [Google Scholar] [CrossRef]
- Sringam, J.; Pankongadisak, P.; Trongsatitkul, T.; Suppakarn, N. Improving Mechanical Properties of Starch-Based Hydrogels Using Double Network Strategy. Polymers 2022, 14, 3552. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Agheb, M.; Navid, S.; Ebrahimpour, K. Cornstarch-based wound dressing incorporated with hyaluronic acid and propolis: In vitro and in vivo studies. Carbohydr. Polym. 2019, 216, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yin, J.; Shi, Y.; Cheng, J.; Fang, Y.; Huang, C.; Yu, W.; Liu, M.; Yang, Z.; Zhou, H.; et al. Starch and chitosan-based antibacterial dressing for infected wound treatment via self-activated NO release strategy. Int. J. Biol. Macromol. 2022, 220, 1177–1187. [Google Scholar] [CrossRef]
- Pasaribu, K.M.; Ilyas, S.; Tamrin, T.; Radecka, I.; Swingler, S.; Gupta, A.; Stamboulis, A.G.; Gea, S. Bioactive bacterial cellulose wound dressings for burns with collagen in-situ and chitosan ex-situ impregnation. Int. J. Biol. Macromol. 2023, 230, 123118. [Google Scholar] [CrossRef]
- Salthouse, D.; Goulding, P.D.; Reay, S.L.; Jackson, E.L.; Xu, C.; Ahmed, R.; Mearns-Spragg, A.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Amine-reactive crosslinking enhances type 0 collagen hydrogel properties for regenerative medicine. Front. Bioeng. Biotechnol. 2024, 12, 1391728. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Ding, X.; Yu, Y.; Yang, C.; Wu, D.; Zhao, Y. Multifunctional GO Hybrid Hydrogel Scaffolds for Wound Healing. Research 2022, 2022, 9850743. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of Collagen-Based Hydrogel in Skin Wound Healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Xia, Z.; Qin, X.; Wang, X.; Lu, W.; Luo, Q.; Zhang, Z.; Xiong, X. The clinical efficacy of collagen dressing on chronic wounds: A meta-analysis of 11 randomized controlled trials. Front. Surg. 2022, 9, 978407. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Bratu, A.G.; Niculescu, A.G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. [Google Scholar] [CrossRef]
- Grabowski, M.; Gmyrek, D.; Żurawska, M.; Trusek, A. Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems. Gels 2025, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Cerminati, S.; Leroux, M.; Anselmi, P.; Peirú, S.; Alonso, J.C.; Priem, B.; Menzella, H.G. Low cost and sustainable hyaluronic acid production in a manufacturing platform based on Bacillus subtilis 3NA strain. Appl. Microbiol. Biotechnol. 2021, 105, 3075–3086. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, L.; Shen, X. Development of hyaluronic acid-based hydrogels for chronic diabetic wound healing: A review. Int. J. Biol. Macromol. 2025, 308, 142273. [Google Scholar] [CrossRef]
- Romanò, C.L.; Vecchi, E.D.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic Acid and Its Composites as a Local Antimicrobial/Antiadhesive Barrier. J. Bone Jt. Infect. 2017, 2, 63. [Google Scholar] [CrossRef]
- Kim, D.S.; Seong, K.Y.; Lee, H.; Kim, M.J.; An, S.M.; Jeong, J.S.; Kim, S.Y.; Kang, H.-G.; Jang, S.; Hwang, D.-Y.; et al. Antiadhesive Hyaluronic Acid-Based Wound Dressings Promote Wound Healing by Preventing Re-Injury: An In Vivo Investigation. Biomedicines 2024, 12, 510. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, W.; Liu, C.; An, M.; Li, S.; Guo, L.; Fan, Y.; Zhang, X. Gelatin/hyaluronic acid-based in situ forming hydrogel promotes wound regeneration by the synergy of ROS-scavenging and pro-healing activity. Regen. Biomater. 2025, 12, rbaf052. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Ning, F.; Du, C.; Chen, M.; Feng, C.; Dong, C.-M. Dynamic Hyaluronic Acid Hydrogels for Comprehensively Regulating Inflammation, Angiogenesis, and Metabolism to Effectively Proheal Diabetic Wounds. ACS Appl. Mater. Interfaces 2024, 16, 70256–70273. [Google Scholar] [CrossRef]
- Lee, H.M.; Jang, E.J.; Choi, K.H.; Na, Y.C. Comparative evaluation of hyaluronic acid-based dressing versus hydrocolloid dressing in rat dermal wound healing. Arch. Craniofac. Surg. 2024, 25, 224. [Google Scholar] [CrossRef]
- Pastrana-Alta, R.Y.; Huarote-Garcia, E.; Egusquiza-Huamani, M.A.; Baena-Moncada, A.M. Antimicrobial activity of chitosan, alginate, pectin, and cellulose-based biopolymer composites with silver, copper oxide, and zinc oxide nanoparticles. RSC Adv. 2025, 15, 35807–35843. [Google Scholar] [CrossRef]
- Ramírez-Partida, A.E.; García-Cayuela, T.; Amador-Castro, L.F.; Alper, H.S.; Carrillo-Nieves, D. Towards a biorefinery processing Sargassum seaweed: Techno-economic assessment of alginate and fucoidan production through SuperPro Designer® process simulation. Environ. Technol. Innov. 2024, 34, 103587. [Google Scholar] [CrossRef]
- Babić Radić, M.M.; Vukomanović, M.; Nikodinović-Runić, J.; Tomić, S. Gelatin-/Alginate-Based Hydrogel Scaffolds Reinforced with TiO2 Nanoparticles for Simultaneous Release of Allantoin, Caffeic Acid, and Quercetin as Multi-Target Wound Therapy Platform. Pharmaceutics 2024, 16, 372. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Bao, D.; Ta, F.; Liu, D.; Zhang, D.; Zhang, Z.; Fan, Z. Multifunctional Alginate Hydrogel Protects and Heals Skin Defects in Complex Clinical Situations. ACS Omega 2020, 5, 17152–17159. [Google Scholar] [CrossRef] [PubMed]
- Mujawar, S.S.; Arbade, G.K.; Rukwal, S.; Tripathi, V.; Mane, M.; Sharma, R.K.; Kashte, S.B. 3D printed sodium Alginate-Gelatin hydrogel loaded with Santalum album oil as an antibacterial Full-Thickness wound healing and scar reduction Scaffold: In vitro and in vivo study. Int. J. Pharm. 2025, 670, 125164. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Kuś, M.; Jurak, J.; Rybka, M.; Kuczeriszka, M.; Stradczuk-Mazurek, M.; Konop, M. Biomedical potential of alginate wound dressings—From preclinical studies to clinical applications: A review. Int. J. Biol. Macromol. 2025, 309, 142908. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Froelich, A.; Jakubowska, E.; Wojtyłko, M.; Jadach, B.; Gackowski, M.; Gadziński, P.; Napierała, O.; Ravliv, Y.; Osmałek, T. Alginate-Based Materials Loaded with Nanoparticles in Wound Healing. Pharmaceutics 2023, 15, 1142. [Google Scholar] [CrossRef]
- Mou, L.; Li, J.; Lu, Y.; Li, G.; Li, J. Polylactic acid: A future universal biobased polymer with multifunctional performance—From monomer synthesis, and processing to applications: A review. J. Hazard. Mater. Adv. 2025, 18, 100757. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Weng, Q.H.; Hu, M.H.; Wang, J.F.; Hu, J.J. Enhancing the Flexibility and Hydrophilicity of PLA via Polymer Blends: Electrospinning vs. Solvent Casting. Polymers 2025, 17, 800. [Google Scholar] [CrossRef] [PubMed]
- Kayadurmus, H.M.; Rezaei, A.; Ilhan, E.; Cesur, S.; Sahin, A.; Gunduz, O.; Kalaskar, D.M.; Ekren, N. Whey protein-loaded 3D-printed poly (lactic) acid scaffolds for wound dressing applications. Biomed. Mater. 2024, 19, 045045. [Google Scholar] [CrossRef] [PubMed]
- Heydari, P.; Zargar Kharazi, A.; Shariati, L. Enhanced wound regeneration by PGS/PLA fiber dressing containing platelet-rich plasma: An in vitro study. Sci. Rep. 2024, 14, 12019. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Chen, H.L.; Chung, J.W.Y.; Yan, V.C.M.; Wong, T.K.S. Polylactic Acid-Based Biomaterials in Wound Healing: A Systematic Review. Adv. Ski. Wound Care 2023, 36, 1–8. [Google Scholar] [CrossRef]
- Ditta, L.A.; Rao, E.; Provenzano, F.; Sánchez, J.L.; Santonocito, R.; Passantino, R.; Costa, M.A.; Sabatino, M.A.; Dispenza, C.; Giacomazza, D.; et al. Agarose/κ-carrageenan-based hydrogel film enriched with natural plant extracts for the treatment of cutaneous wounds. Int. J. Biol. Macromol. 2020, 164, 2818–2830. [Google Scholar] [CrossRef]
- Khajavi, M.; Raoufi, Z.; Abdollahi, S. Investigating the potential of collagen/carrageenan trilayer sponges with optimal therapeutic and physical properties for the treatment of pressure ulcers. Int. J. Biol. Macromol. 2025, 306, 141743. [Google Scholar] [CrossRef]
- Biranje, S.S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Bangde, P.; Dandekar, P.; Adivarekar, R.V. Cytotoxicity and hemostatic activity of chitosan/carrageenan composite wound healing dressing for traumatic hemorrhage. Carbohydr. Polym. 2020, 239, 116106. [Google Scholar] [CrossRef]
- P, A.; Joy, J.M.; Visnuvinayagam, S.; Remya, S.; Mathew, S. Development of κ-carrageenan-based transparent and absorbent biodegradable films for wound dressing applications. Int. J. Biol. Macromol. 2024, 282, 137084. [Google Scholar] [CrossRef]
- Rode, M.P.; Batti Angulski, A.B.; Gomes, F.A.; da Silva, M.M.; Jeremias, T.d.S.; de Carvalho, R.G.; Vieira, D.C.I.; Oliveira, L.F.C.; Maia, L.F.; Trentin, A.G.; et al. Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Ianev, D.; Adami, M.; Valentino, C.; Ruggeri, M.; Boselli, C.; Cornaglia, A.I.; Sandri, G.; Rossi, S. Porous Functionally Graded Scaffold prepared by a single-step freeze-drying process. A bioinspired approach for wound care. Int. J. Pharm. 2024, 656, 124119. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, V.; Lyczak, J.; Turner, K.; Martinuzzi, R.J. Impact of Silver-Containing Wound Dressings on Bacterial Biofilm Viability and Susceptibility to Antibiotics during Prolonged Treatment. Antimicrob. Agents Chemother. 2010, 54, 5120. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, T.; Nigusse, T.; Dhanaraju, M.D. Silver Nanoparticles as Real Topical Bullets for Wound Healing. J. Am. Coll. Clin. Wound Spec. 2012, 3, 82. [Google Scholar] [CrossRef]
- Liang, K.; Liu, Y.; Jiang, F. Analysis of therapeutic effect of silver-based dressings on chronic wound healing. Int. Wound J. 2024, 21, e70006. [Google Scholar] [CrossRef]
- Isak, V.; Beerli, T.; Cozzio, A.; Flatz, L. A Rare Case of Localized Argyria on the Face. Case Rep. Dermatol. 2019, 11, 23. [Google Scholar] [CrossRef]
- Steck, M.B.; Murray, B.P. Silver Toxicity; StatPearls Publishing: Orlando, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK604211/ (accessed on 17 December 2025).
- Zou, S.B.; Yoon, W.Y.; Han, S.K.; Jeong, S.H.; Cui, Z.J.; Kim, W.K. Cytotoxicity of silver dressings on diabetic fibroblasts. Int. Wound J. 2012, 10, 306. [Google Scholar] [CrossRef]
- You, C.; Li, Q.; Wang, X.; Wu, P.; Ho, J.K.; Jin, R.; Zhang, L.; Shao, H.; Han, C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7, 10489. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, D.; Nie, F.; Wang, Y.; Chong, Y. Iron Nanoparticles Open Up New Directions for Promoting Healing in Chronic Wounds in the Context of Bacterial Infection. Pharmaceutics 2023, 15, 2327. [Google Scholar] [CrossRef]
- Nosrati, H.; Heydari, M. Titanium dioxide nanoparticles: A promising candidate for wound healing applications. Burn. Trauma 2025, 13, tkae069. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Y.; Bao, S.; Yao, L.; Fu, X.; Yu, Y.; Lyu, H.; Pang, H.; Guo, S.; Zhang, H.; et al. Cerium oxide nanoparticles in wound care: A review of mechanisms and therapeutic applications. Front. Bioeng. Biotechnol. 2024, 12, 1404651. [Google Scholar] [CrossRef] [PubMed]
- Dam, P.; Celik, M.; Ustun, M.; Saha, S.; Saha, C.; Kacar, E.A.; Kugu, S.; Karagulle, E.N.; Tasoglu, S.; Buyukserin, F.; et al. Wound healing strategies based on nanoparticles incorporated in hydrogel wound patches. RSC Adv. 2023, 13, 21345–21364. [Google Scholar] [CrossRef] [PubMed]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.Ș.; Dițu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO Nanoparticles-Modified Dressings to Inhibit Wound Pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef]
- Tetteh-Quarshie, S.; Blough, E.R.; Jones, C.B. Exploring Dendrimer Nanoparticles for Chronic Wound Healing. Front. Med. Technol. 2021, 3, 661421. [Google Scholar] [CrossRef]
- Roy, M.; Roy, A.; Rustagi, S.; Pandey, N. An Overview of Nanomaterial Applications in Pharmacology. Biomed. Res. Int. 2023, 2023, 4838043. [Google Scholar] [CrossRef]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef]
- Ding, C.; Liu, X.; Zhang, S.; Sun, S.; Yang, J.; Chai, G.; Wang, N.; Ma, S.; Ding, Q.; Liu, W. Multifunctional hydrogel bioscaffolds based on polysaccharide to promote wound healing: A review. Int. J. Biol. Macromol. 2024, 259, 129356. [Google Scholar] [CrossRef]
- Grada, A.; Obagi, Z.; Phillips, T. Management of chronic wounds in patients with pemphigus. Chronic Wound Care Manag. Res. 2019, 6, 89–98. [Google Scholar] [CrossRef]
- Vercammen, Y.; Dauwe, D.; De Vlieger, G.; Houthoofd, S.; Desmet, L.; Casaer, M.P. Povidone Iodine Disinfection Associated with Hypothyroidism and Potentially Contributing to Prolonged Kidney Failure. Case Rep. Crit. Care 2021, 2021, 5528210. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, J.; Liu, Y.; Ben, C.; Li, H.; Cheng, D.; Sun, Y.; Guang-Yi, W.; Zhu, S. Analysis of povidone iodine, chlorhexidine acetate and polyhexamethylene biguanide as wound disinfectants: In vitro cytotoxicity and antibacterial activity. BMJ Nutr. Prev. Health 2023, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Naseri, E.; Ahmadi, A. A review on wound dressings: Antimicrobial agents, biomaterials, fabrication techniques, and stimuli-responsive drug release. Eur. Polym. J. 2022, 173, 111293. [Google Scholar] [CrossRef]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on Wound Antisepsis: Update 2018. Ski. Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef]
- Kanagalingam, J.; Feliciano, R.; Hah, J.H.; Labib, H.; Le, T.A.; Lin, J.C. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. Int. J. Clin. Pract. 2015, 69, 1247–1256. [Google Scholar] [CrossRef]
- Hübner, N.O.; Kramer, A. Review on the Efficacy, Safety and Clinical Applications of Polihexanide, a Modern Wound Antiseptic. Ski. Pharmacol. Physiol. 2010, 23, 17–27. [Google Scholar] [CrossRef]
- Guiomar, A.J.; Urbano, A.M. Polyhexanide-Releasing Membranes for Antimicrobial Wound Dressings: A Critical Review. Membranes 2022, 12, 1281. [Google Scholar] [CrossRef]
- Günther, F.; Blessing, B.; Dapunt, U.; Mischnik, A.; Mutters, N.T. Ability of chlorhexidine, octenidine, polyhexanide and chloroxylenol to inhibit metabolism of biofilm-forming clinical multidrug-resistant organisms. J. Infect. Prev. 2020, 22, 12. [Google Scholar] [CrossRef]
- Sukakul, T.; Dahlin, J.; Pontén, A.; Antelmi, A.; Bruze, M.; Hamnerius, N.; Hauksson, I.; Isaksson, M.; Lejding, T.; Svedman, C. Contact allergy to polyhexamethylene biguanide (polyaminopropyl biguanide). Contact Dermat. 2021, 84, 326–331. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Isopencu, G.O.; Covaliu-Mierlă, C.I.; Deleanu, I.M. From Plants to Wound Dressing and Transdermal Delivery of Bioactive Compounds. Plants 2023, 12, 2661. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.d.S.S.; Guimarães, A.G. Effects of Carvacrol, Thymol and essential oils containing such monoterpenes on wound healing: A systematic review. J. Pharm. Pharmacol. 2019, 71, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, S.; Tsvetkova, A.; Stamova, S.; Ermenlieva, N.; Tsankova, G.; Georgieva, E.; Peycheva, K.; Panayotova, V.; Voynikov, Y. Antibacterial Effects of Bulgarian Oregano and Thyme Essential Oils Alone and in Combination with Antibiotics Against Klebsiella pneumoniae and Pseudomonas aeruginosa. Microorganisms 2025, 13, 843. [Google Scholar] [CrossRef] [PubMed]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Cuttle, L.; Kempf, M.; Kravchuk, O.; George, N.; Liu, P.Y.; Chang, H.E.; Mill, J.; Wang, X.-Q.; Kimble, R.M. The efficacy of Aloe vera, tea tree oil and saliva as first aid treatment for partial thickness burn injuries. Burns 2008, 34, 1176–1182. [Google Scholar] [CrossRef]
- Dunn, K.; Edwards-Jones, V. The role of ActicoatTM with nanocrystalline silver in the management of burns. Burns 2004, 30, S1–S9. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Trzaskowska, M.; Przekora, A. Wound Dressing Modifications for Accelerated Healing of Infected Wounds. Int. J. Mol. Sci. 2023, 24, 2193. [Google Scholar] [CrossRef]
- Zhu, A.; Chen, B.; Ma, J.; Wang, J.; Tang, R.; Liu, L.; Sun, W.; Zheng, X.; Pan, G. Application of Antimicrobial Peptides in Wound Dressings. Drug Des. Devel. Ther. 2025, 19, 8523–8539. [Google Scholar] [CrossRef]
- Adnan, S.B.; Maarof, M.; Fauzi, M.B.; Md Fadilah, N.I. Antimicrobial Peptides in Wound Healing and Skin Regeneration: Dual Roles in Immunity and Microbial Defense. Int. J. Mol. Sci. 2025, 26, 5920. [Google Scholar] [CrossRef]
- Kanaujia, K.A.; Mishra, N.; Rajinikanth, P.S.; Saraf, S.A. Antimicrobial peptides as antimicrobials for wound care management: A comprehensive review. J. Drug Deliv. Sci. Technol. 2024, 95, 105570. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018, 9, 352601. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Ali, Z.; Mahfouz, M. Molecular farming for sustainable production of clinical-grade antimicrobial peptides. Plant Biotechnol. J. 2024, 22, 2282. [Google Scholar] [CrossRef] [PubMed]
- BIOEPITHELIA® BASE: Per il Trattamento Della Cute Infiammata. Available online: https://www.bioepithelia.it/bioepithelia-per-la-guarigione-delle-patologie-cutanee/bioepithelia-base/ (accessed on 31 July 2025).
- Ferite: Il Rimedio Alle Ferite Cutanee. Alovex Ferite. Available online: https://www.alovex.it/ferite-cutanee/rimedio (accessed on 7 August 2025).
- IALUSET—ialuset. Available online: https://ialuset.it/prodotti/ialuset/ (accessed on 7 August 2025).
- IODOSORB Global. Available online: https://www.smith-nephew.com/en/health-care-professionals/products/advanced-wound-management/iodosorb-global#overview (accessed on 7 August 2025).
- Alfasigma Medicazione Sofargen Gel Acido Ialuronico E Argento Sulfadiazina Tubetto 25 G. Available online: https://www.farmaciarossettisas.it/primo-soccorso-in-viaggio/57094-alfasigma-medicazione-sofargen-gel-acido-ialuronico-e-argento-sulfadiazina-tubetto-25-g.html?srsltid=AfmBOoqoupk_6ddzS5eqele-HLMsCVyri4qeD_Kgr2NRRj-4D0LoOqmP (accessed on 7 August 2025).
- Fitostimoline®Plus Crema—Damor Farmaceutici. Available online: https://www.damorpharma.it/product/fitostimoline-plus-crema/ (accessed on 7 August 2025).
- ConnettivinaBio Plus—Fidiaperlapelle. Available online: https://fidiaperlapelle.it/connettivinabio-plus/ (accessed on 7 August 2025).
- IALUSET PLUS—ialuset. Available online: https://ialuset.it/prodotti/ialuset-plus/ (accessed on 7 August 2025).
- Gazzabin, L.; Serantoni, S.; Palumbo, F.P.; Giordan, N. Hyaluronic acid and metallic silver treatment of chronic wounds: Healing rate and bacterial load control. J. Wound Care 2019, 28, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Dereure, O.; Czubek, M.; Combemale, P. Efficacy and safety of hyaluronic acid in treatment of leg ulcers: A double-blind RCT. J. Wound Care 2012, 21, 131–139. [Google Scholar] [CrossRef]
- Barrois, B.; Carles, M.; Rumeau, M.; Tell, L.; Toussaint, J.F.; Bonnefoy, M.; de Vathaire, F. Efficacy and tolerability of hyaluronan (ialuset®) in the treatment of pressure ulcers: A multicentre, non-randomised, pilot study. Drugs R D 2007, 8, 267–273. [Google Scholar] [CrossRef]
- De Francesco, F.; Riccio, M.; Jimi, S. Contribution of Topical Agents such as Hyaluronic Acid and Silver Sulfadiazine to Wound Healing and Management of Bacterial Biofilm. Medicina 2022, 58, 835. [Google Scholar] [CrossRef]
- Russo, R.; Carrizzo, A.; Barbato, A.; Rasile, B.R.; Pentangelo, P.; Ceccaroni, A.; Marra, C.; Alfano, C.; Losco, L. Clinical Evaluation of the Efficacy and Tolerability of Rigenase® and Polyhexanide (Fitostimoline® Plus) vs. Hyaluronic Acid and Silver Sulfadiazine (Connettivina® Bio Plus) for the Treatment of Acute Skin Wounds: A Randomized Trial. J. Clin. Med. 2022, 11, 2518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ianev, D.; Mori, M.; Vigani, B.; Valentino, C.; Ruggeri, M.; Sandri, G.; Rossi, S. Substance-Based Medical Device in Wound Care: Bridging Regulatory Clarity and Therapeutic Innovation. Polymers 2026, 18, 129. https://doi.org/10.3390/polym18010129
Ianev D, Mori M, Vigani B, Valentino C, Ruggeri M, Sandri G, Rossi S. Substance-Based Medical Device in Wound Care: Bridging Regulatory Clarity and Therapeutic Innovation. Polymers. 2026; 18(1):129. https://doi.org/10.3390/polym18010129
Chicago/Turabian StyleIanev, Daiana, Michela Mori, Barbara Vigani, Caterina Valentino, Marco Ruggeri, Giuseppina Sandri, and Silvia Rossi. 2026. "Substance-Based Medical Device in Wound Care: Bridging Regulatory Clarity and Therapeutic Innovation" Polymers 18, no. 1: 129. https://doi.org/10.3390/polym18010129
APA StyleIanev, D., Mori, M., Vigani, B., Valentino, C., Ruggeri, M., Sandri, G., & Rossi, S. (2026). Substance-Based Medical Device in Wound Care: Bridging Regulatory Clarity and Therapeutic Innovation. Polymers, 18(1), 129. https://doi.org/10.3390/polym18010129









