Design, Characterization, and Preparation of New Smart Photoactive Polymers and Their Capacity for Photodynamic Antimicrobial Action in Organic Film
Abstract
1. Introduction
2. Experimental Design
2.1. Reagents
2.2. Characterization
2.3. Procedure for Photochromic Agent Synthesis
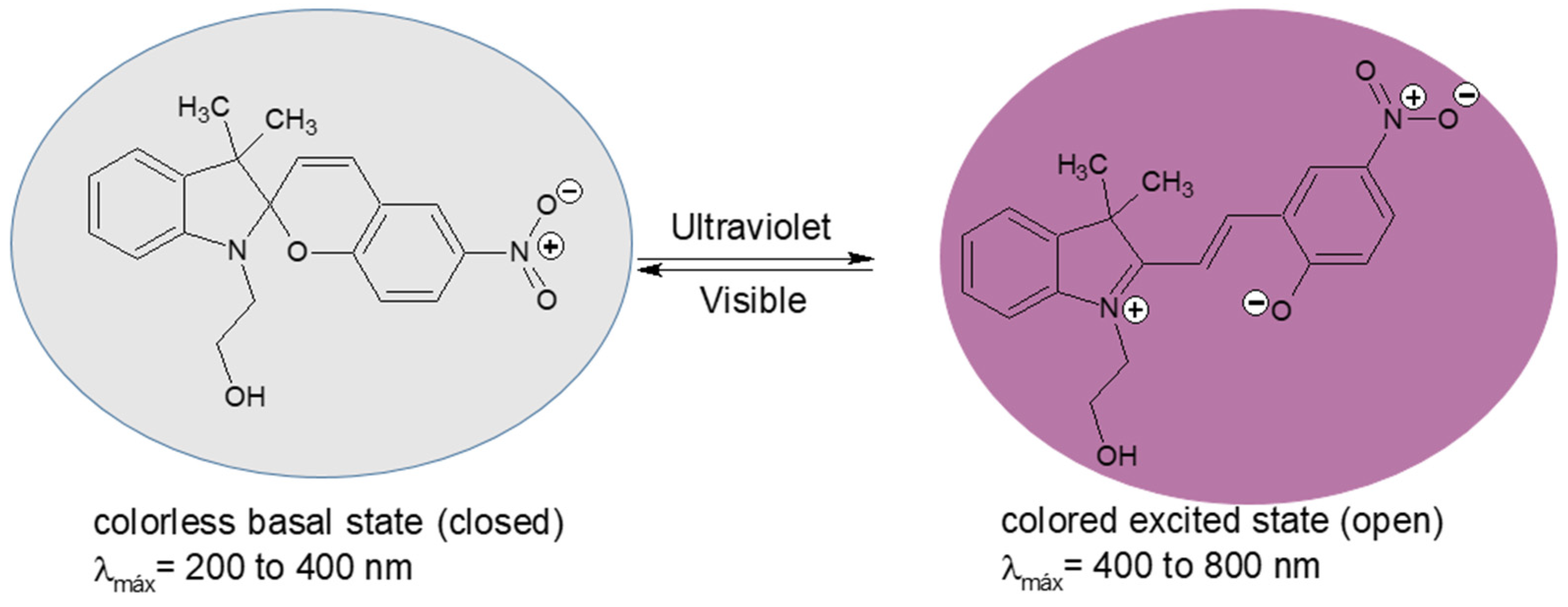
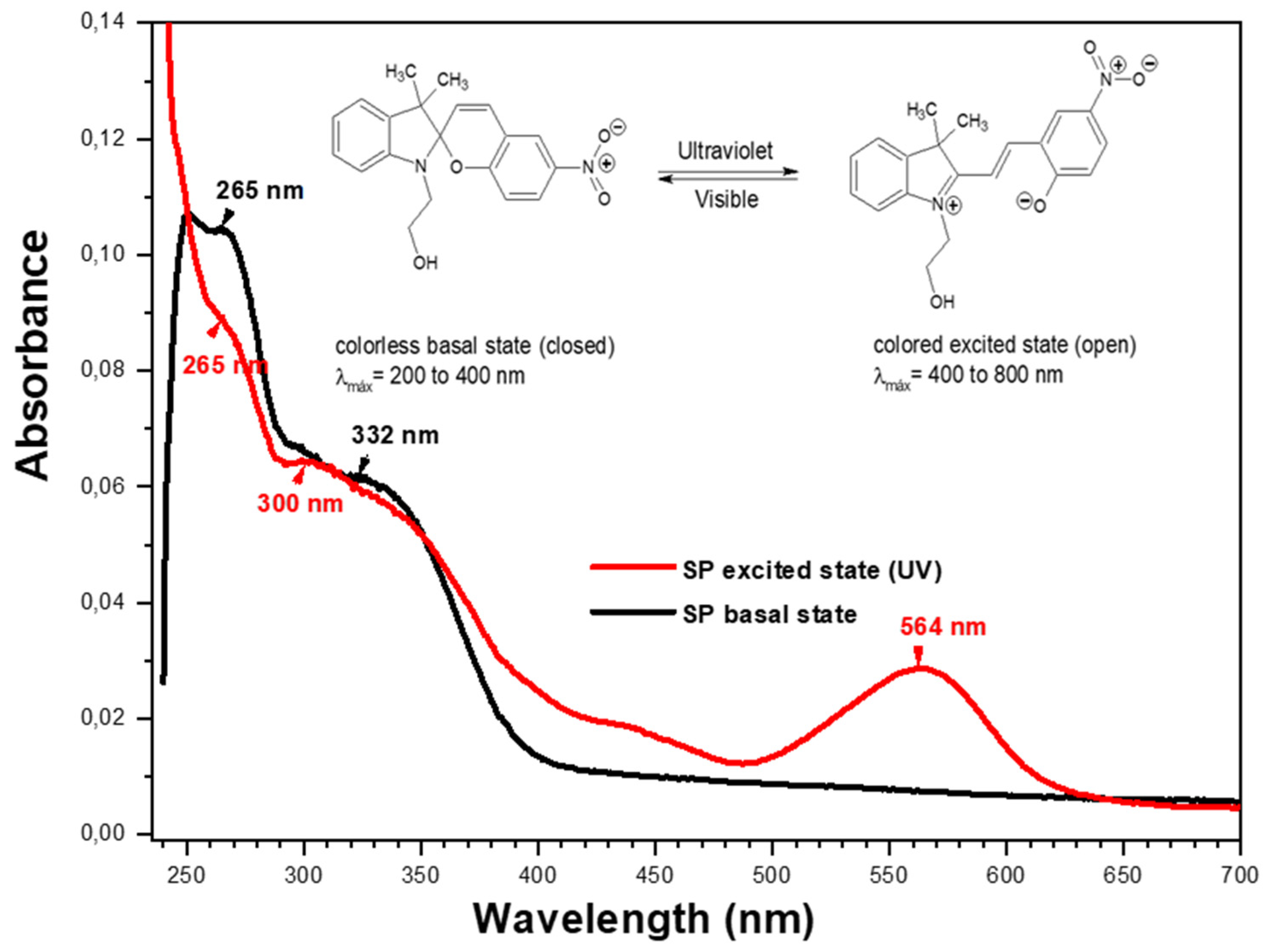
2.4. Characterization of SP by UV-Visible Spectrophotometry
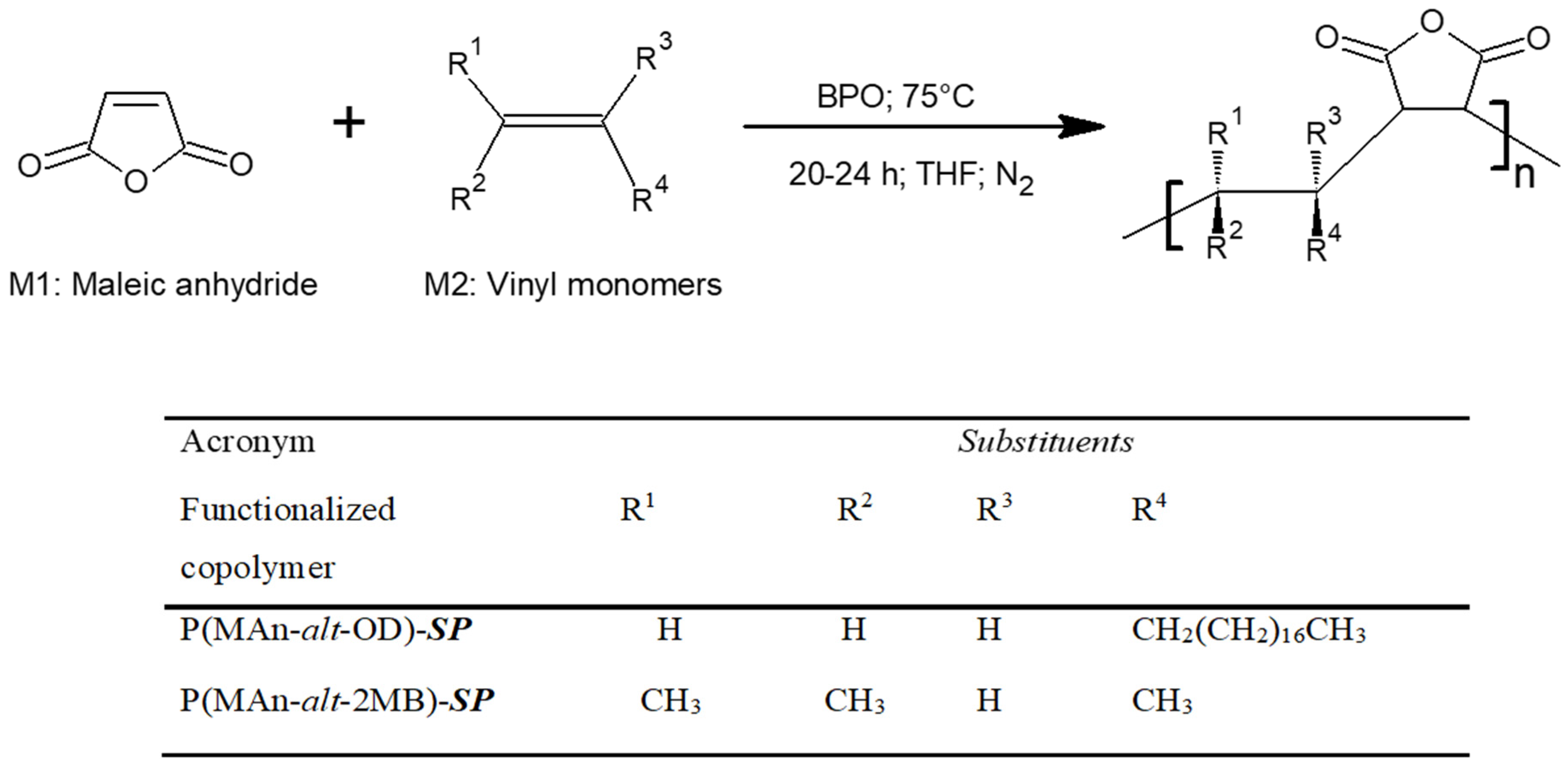
2.5. Preparation of Photoactive Alternating Copolymers
2.6. Determination of Antimicrobial Photodynamic Properties
3. Results and Discussion
3.1. Characterization of Alternating Copolymers
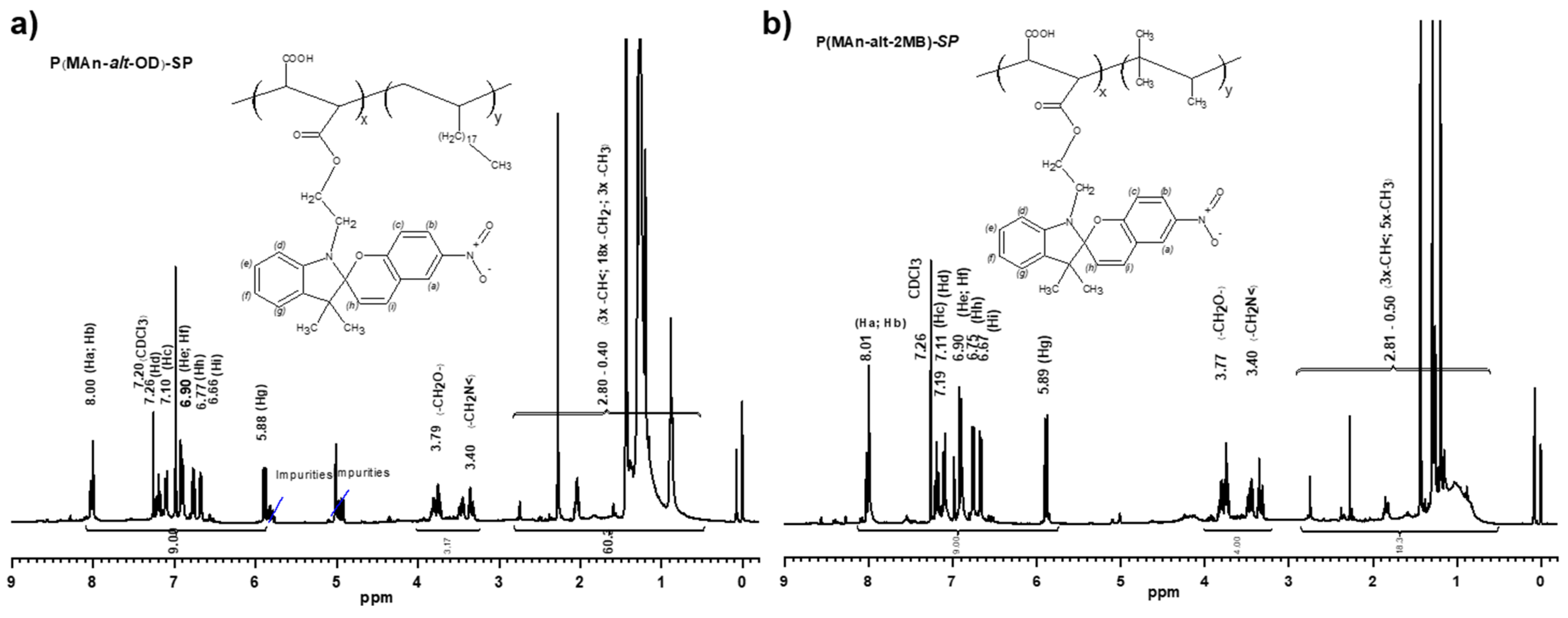
3.1.1. Characterization by FT-IR and 1H-NMR
3.1.2. Characterization by 1H-NMR of Copolymers P(MAn-alt-OD)-SP and P(MAn-alt-OD)-SP
3.1.3. Degrees of Functionalization
- = the number of aliphatic protons in fraction X;
- = the number of aliphatic protons in fraction Y;
- = the number of methylene protons;
- = the number of aromatic protons;
- = integral aliphatic protons;
- = integral aromatic protons;
- = integral methylene protons;
- = a numerical value, the mathematical operation of η1, η2, η4, , ;
- = a numerical value, the mathematical operation of η1, η2, η3, , ;
- = the comonomer fraction in X;
- = the comonomer fraction in Y.
3.1.4. Functionalization of P(MAn-alt-OD)-SP and P(MAn-alt-2MB)-SP
3.1.5. Characterization of Copolymers by UV-Visible Spectrophotometry
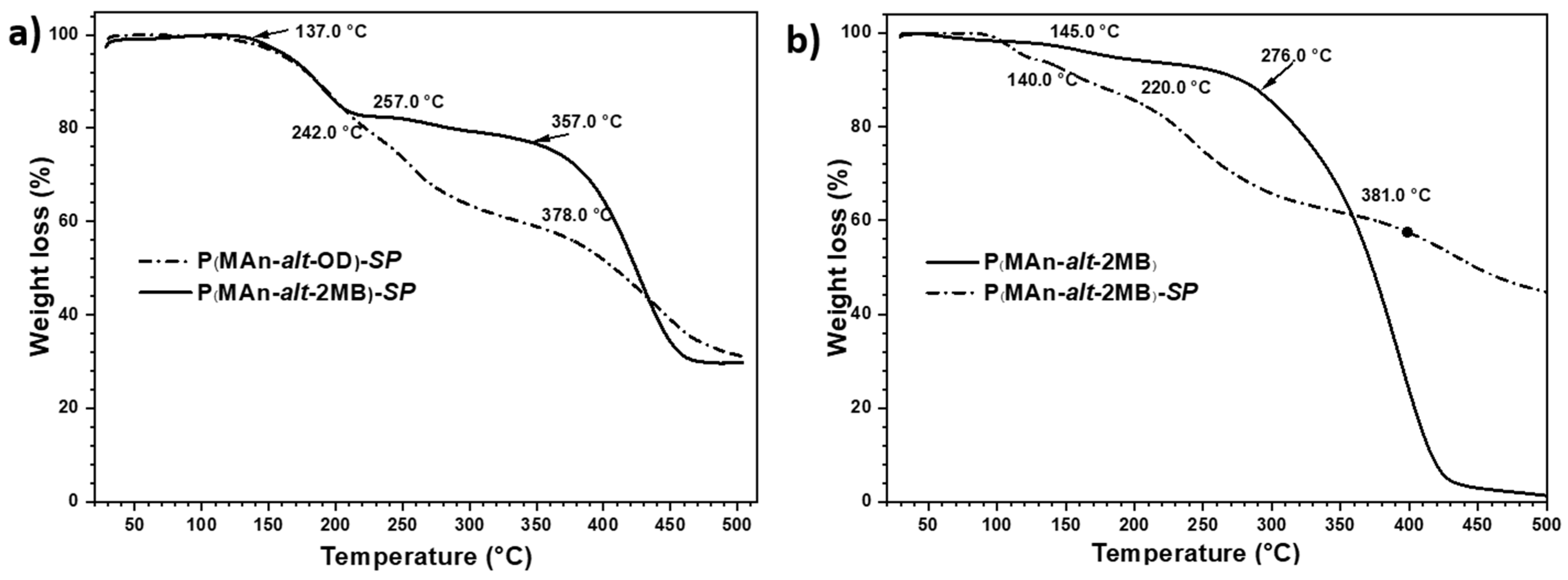
3.2. Thermal Decomposition Analysis by TGA
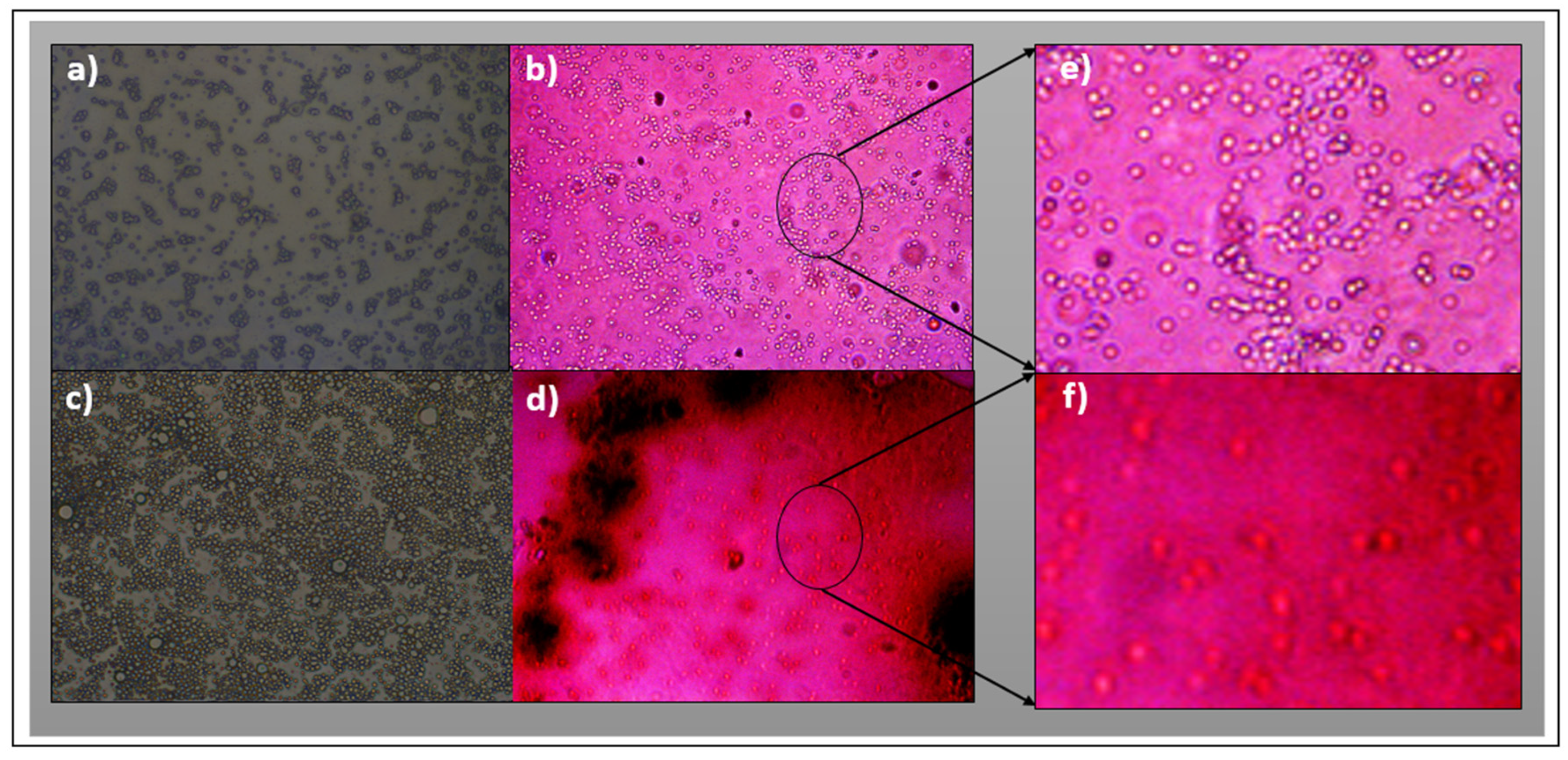
3.3. Characterization by Fluorescence Optical Microscopy
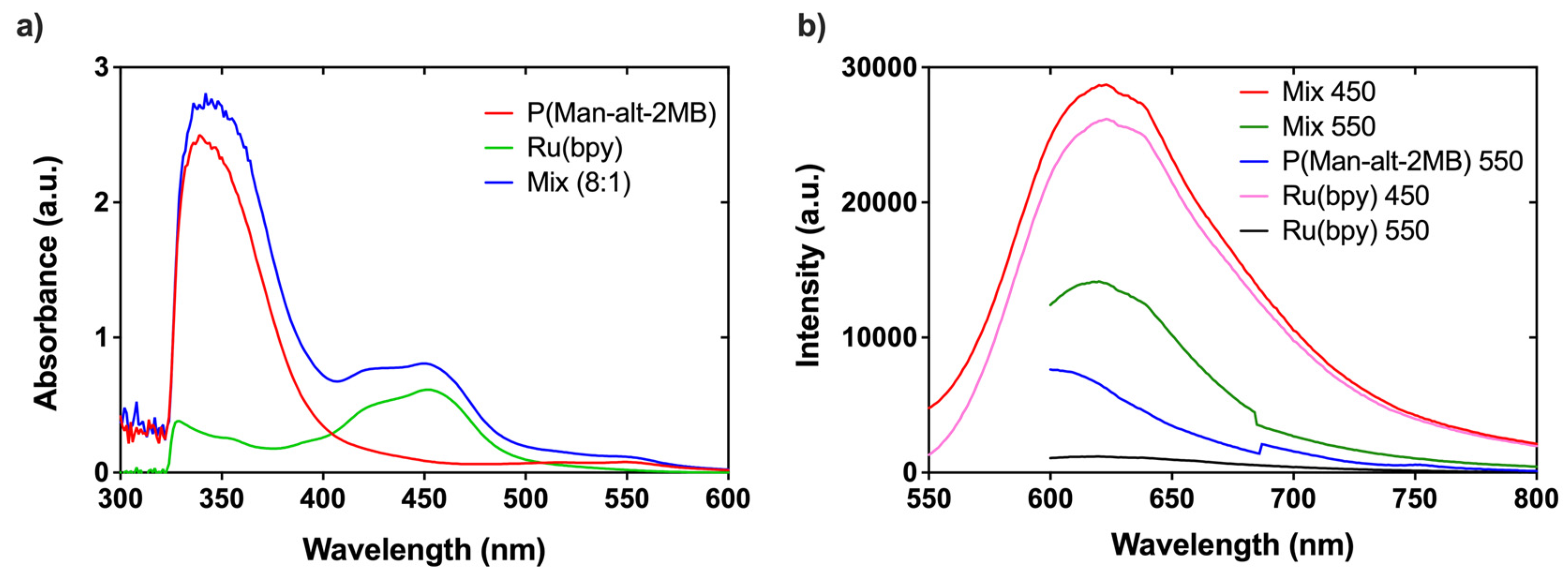
3.4. Determination of Antimicrobial Properties Based on Photodynamic Therapy (PDT)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, P.N.; Williams, D.J. Introduction to Nonlinear Optical Effect in Molecules and Polymers; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- García, A.E.; Elizalde, L.E.; Guillén, L.; de los Santos, G.; Medellín, D.I. Síntesis y evaluación fotocromática de compuestos espirobenzopiránicos. J. Mex. Chem. Soc. 2004, 48, 269–274. [Google Scholar]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Raymo, F.M. Chiroptical Switching Based On Photoinduced Proton Transfer Between Homopolymers Bearing Side-Chain Spiropyran and Azopyridine Moieties. Macromol. Chem. Phys. 2008, 209, 2049–2060. [Google Scholar] [CrossRef]
- de los Santos, G.; Elizalde, L.E.; Castro, B.; García, A.E.; Medellín, D.I. Sociedad Química de México: [Revista]. Rev. De La Soc. Química De México 1996, 48, 332–337. [Google Scholar]
- Nunes, S.P.; Behzad, A.R.; Hooghan, B.; Sougrat, R.; Karunakaran, M.; Pradeep, N.; Vainio, U.; Peinemann, K.-V. Switchable PH-Responsive Polymeric Membranes Prepared via Block Copolymer Micelle Assembly. ACS Nano 2011, 5, 3516–3522. [Google Scholar] [CrossRef]
- Hilke, R.; Pradeep, N.; Madhavan, P.; Vainio, U.; Behzad, A.R.; Sougrat, R.; Nunes, S.P.; Peinemann, K.-V. Block Copolymer Hollow Fiber Membranes with Catalytic Activity and PH-Response. ACS Appl. Mater. Interfaces 2013, 5, 7001–7006. [Google Scholar] [CrossRef]
- Amado, F.D.R.; Gondran, E.; Ferreira, J.Z.; Rodrigues, M.A.S.; Ferreira, C.A. Synthesis and Characterisation of High Impact Polystyrene/Polyaniline Composite Membranes for Electrodialysis. J. Memb. Sci. 2004, 234, 139–145. [Google Scholar] [CrossRef]
- Wijnhoven, J.E.G.J.; Vos, W.L. Preparation of Photonic Crystals Made of Air Spheres in Titania. Science (1979) 1998, 281, 802–804. [Google Scholar] [CrossRef]
- Imada, M.; Noda, S.; Chutinan, A.; Tokuda, T.; Murata, M.; Sasaki, G. Coherent Two-Dimensional Lasing Action in Surface-Emitting Laser with Triangular-Lattice Photonic Crystal Structure. Appl. Phys. Lett. 1999, 75, 316–318. [Google Scholar] [CrossRef]
- Katsonis, N.; Lubomska, M.; Pollard, M.; Feringa, B.; Rudolf, P. Synthetic Light-Activated Molecular Switches and Motors on Surfaces. Prog. Surf. Sci. 2007, 82, 407–434. [Google Scholar] [CrossRef]
- Hugel, T.; Holland, N.B.; Cattani, A.; Moroder, L.; Seitz, M.; Gaub, H.E. Single-Molecule Optomechanical Cycle. Science (1979) 2002, 296, 1103–1106. [Google Scholar] [CrossRef]
- Byrne, R.J.; Stitzel, S.E.; Diamond, D. Photo-Regenerable Surface with Potential for Optical Sensing. J. Mater. Chem. 2006, 16, 1332. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, T.; Kim, E. Electropolymerization of an EDOT-Modified Diarylethene. Tetrahedron Lett. 2007, 48, 249–254. [Google Scholar] [CrossRef]
- Raymo, F.M.; Tomasulo, M. Optical Processing with Photochromic Switches. Chem.—A Eur. J. 2006, 12, 3186–3193. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, I.; Deniz, E.; Raymo, F.M. Fluorescence Modulation with Photochromic Switches in Nanostructured Constructs. Chem. Soc. Rev. 2009, 38, 1859–1867. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and Spirooxazines for Memories and Switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Samat, A.; Lokshin, V. Thermochromism of Organic Compounds. In Organic Photochromic and Thermochromic Compounds; Kluwer Academic Publishers: Boston, MA, USA, 2002; pp. 415–466. [Google Scholar] [CrossRef]
- Rätzsch, M. Alternating Maleic Anhydride Copolymers. Prog. Polym. Sci. 1988, 13, 277–337. [Google Scholar] [CrossRef]
- Huang, J.; Turner, S.R. Recent Advances in Alternating Copolymers: The Synthesis, Modification, and Applications of Precision Polymers. Polymer 2017, 116, 572–586. [Google Scholar] [CrossRef]
- Heiligman-Rim, R.; Hirshberg, Y.; Fischer, E. 29. Photochromism in Some Spiropyrans. Part III. The Extent of Phototransformation. J. Chem. Soc. (Resumed) 1961, 156–157. [Google Scholar] [CrossRef]
- Malik, M.Z.A.; Nazri, S.A.A.A.; Zainuddin, M.T.; Islam, N.Z.M.; Aziz, N.M.A.N.A.; Isha, K.M. Effects of Papain Incorporation on the Photo-Transformation Stability of 5-Bromo-8-Methoxy-6-Nitro Bips. Adv. Mater. Res. 2014, 879, 73–78. [Google Scholar] [CrossRef]
- Wagner, K.; Byrne, R.; Zanoni, M.; Gambhir, S.; Dennany, L.; Breukers, R.; Higgins, M.; Wagner, P.; Diamond, D.; Wallace, G.G.; et al. A Multiswitchable Poly(Terthiophene) Bearing a Spiropyran Functionality: Understanding Photo- and Electrochemical Control. J. Am. Chem. Soc. 2011, 133, 5453–5462. [Google Scholar] [CrossRef]
- Willner, I.; Willner, B. Layered Molecular Optoelectronic Assemblies. J. Mater. Chem. 1998, 8, 2543–2556. [Google Scholar] [CrossRef]
- Willner, I.; Willner, B. Photoswitchable Biomaterials as Grounds for Optobioelectronic Devices. Bioelectrochem. Bioenerg. 1997, 42, 43–57. [Google Scholar] [CrossRef]
- Scarmagnani, S.; Walsh, Z.; Slater, C.; Alhashimy, N.; Paull, B.; Macka, M.; Diamond, D. Polystyrene Bead-Based System for Optical Sensing Using Spiropyran Photoswitches. J. Mater. Chem. 2008, 18, 5063. [Google Scholar] [CrossRef]
- Lahsoune, M.; Boutayeb, H.; Zerouali, K.; Belabbes, H.; El Mdaghri, N. Prévalence et État de Sensibilité Aux Antibiotiques d’Acinetobacter Baumannii Dans Un CHU Marocain. Med. Mal. Infect. 2007, 37, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella Pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Ko, W.-C. Community-Acquired Klebsiella Pneumoniae Bacteremia: Global Differences in Clinical Patterns. Emerg. Infect. Dis. 2002, 8, 160–166. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Diekema, D.J.; Pfaller, M.A.; Schmitz, F.J.; Smayevsky, J.; Bell, J.; Jones, R.N.; Beach, M. Survey of Infections Due to Staphylococcus Species: Frequency of Occurrence and Antimicrobial Susceptibility of Isolates Collected in the United States, Canada, Latin America, Europe, and the Western Pacific Region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 32 (Suppl. 2), S114–S132. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella spp. as Nosocomial Pathogens. Clin. Microbiol Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Nadasy, K.A.; Domiati-Saad, R.; Tribble, M.A. Invasive Klebsiella Pneumoniae Syndrome in North America. Clin. Infect. Dis. 2007, 45, e25–e28. [Google Scholar] [CrossRef] [PubMed]
- Giulieri, S.G.; Tong, S.Y.C.; Williamson, D.A. Using Genomics to Understand Meticillin- and Vancomycin-Resistant Staphylococcus Aureus Infections. Microb. Genom. 2020, 6, e000324. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, F.; Kang, J.; Wang, X.; Yin, D.; Dang, W.; Duan, J. Prevalence of Multidrug Resistant Gram-Positive Cocci in a Chinese Hospital over an 8-Year Period. Int. J. Clin. Exp. Med. 2015, 8, 9462–9469. [Google Scholar]
- Pizarro, G.D.C.; Alavia, W.; González, K.; Díaz, H.; Marambio, O.G.; Martin-Trasanco, R.; Sánchez, J.; Oyarzún, D.P.; Neira-Carrillo, A. Design and Study of a Photo-Switchable Polymeric System in the Presence of ZnS Nanoparticles under the Influence of UV Light Irradiation. Polymers 2022, 14, 945. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium Complexes as Antimicrobial Agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Raymo, F.M.; Tomasulo, M. Electron and Energy Transfer Modulation with Photochromic Switches. Chem. Soc. Rev. 2005, 34, 327–336. [Google Scholar] [CrossRef]
- Raymo, F.M.; Yildiz, I. Luminescent Chemosensors Based on Semiconductor Quantum Dots. Phys. Chem. Chem. Phys. 2007, 9, 2036. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Nayak, D.; Choudhary, R.B. Augmented Optical and Electrical Properties of PMMA-ZnS Nanocomposites as Emissive Layer for OLED Applications. Opt. Mater. 2019, 91, 470–481. [Google Scholar] [CrossRef]
- Singh, R.; Choudhary, R.B.; Kandulna, R. Optical Band Gap Tuning and Thermal Properties of PMMA-ZnO Sensitized Polymers for Efficient Exciton Generation in Solar Cell Application. Mater. Sci. Semicond. Process 2019, 103, 104623. [Google Scholar] [CrossRef]
- Kole, A.K.; Gupta, S.; Kumbhakar, P.; Ramamurthy, P.C. Nonlinear Optical Second Harmonic Generation in ZnS Quantum Dots and Observation on Optical Properties of ZnS/PMMA Nanocomposites. Opt. Commun. 2014, 313, 231–237. [Google Scholar] [CrossRef]




| Maleic Anhydride (MAn) | COOH | Ester -COOR | -NO2 | ||
|---|---|---|---|---|---|
| Copolymer | Asymmetric (C=O) | Symmetrical (C=O) | Tension (C=O) | Tension (C=O) | Tension (N-O) |
| P(MAn-alt-OD) | --- | 1780.0 | 1707.5 | --- | --- |
| P(MAn-alt-OD)-SP | --- | 1780.0 | --- | 1720.0 | 1460.0 |
| P(MAn-alt-2MB) | 1857.0 | 1780.0 | 1719.0 | --- | --- |
| P(MAn-alt-2MB)-SP | 1857.0 | 1780.0 | --- | 1728.0 | 1460.0 |
| Name | n1 | n2 | n3 | n4 | Ialiph | Imeth | Iar | X | Y | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P(MAn-alt-OD)-SP | 8.00 | 10.0 | 4.00 | 9.00 | 48.0 | 6.06 | --- | 0.40 | 0.60 | ||
| P(MAn-alt-OD)-SP | 8.00 | 10.0 | 4.00 | 9.00 | 48.0 | --- | 2.53 | 0.40 | 0.60 | ||
| P(MAn-alt-2MB)-SP | 8.00 | 10.0 | 4.00 | 9.00 | 18.3 | 4.00 | --- | 0.49 | 0.51 | ||
| P(MAn-alt-2MB)-SP | 8.00 | 10.0 | 4.00 | 9.00 | 18.3 | --- | 9.00 | 0.49 | 0.51 | ||
| Compounds | λmax in Basal State (nm) | λmax in Excited State (nm) | |||||
|---|---|---|---|---|---|---|---|
| Photochromic agent | 265 | 332 | -- | 265 | 300 | -- | 564 |
| P(MAn-alt-OD)-SP | 320 | -- | -- | 318 | -- | -- | 560 |
| P(MAn-alt-2MB)-SP | 268 | 300 | 337 | 268 | 300 | 337 | 566 |
| Case | Eg (eV) | |
|---|---|---|
| Before Irradiation | After Irradiation | |
| Photochromic agent (SP) | 3.00 | 2.00 |
| P(MAn-alt-OD)-SP | 3.73 | 3.02 |
| P(MAn-alt-2MB)-SP | 4.53 | 3.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marambio, O.G.; Barrera, F.I.; Martin-Trasancos, R.; Sánchez, J.; Palavecino, C.E.; Pizarro, G.d.C. Design, Characterization, and Preparation of New Smart Photoactive Polymers and Their Capacity for Photodynamic Antimicrobial Action in Organic Film. Polymers 2025, 17, 1247. https://doi.org/10.3390/polym17091247
Marambio OG, Barrera FI, Martin-Trasancos R, Sánchez J, Palavecino CE, Pizarro GdC. Design, Characterization, and Preparation of New Smart Photoactive Polymers and Their Capacity for Photodynamic Antimicrobial Action in Organic Film. Polymers. 2025; 17(9):1247. https://doi.org/10.3390/polym17091247
Chicago/Turabian StyleMarambio, Oscar G., Franco I. Barrera, Rudy Martin-Trasancos, Julio Sánchez, Christian Erick Palavecino, and Guadalupe del C. Pizarro. 2025. "Design, Characterization, and Preparation of New Smart Photoactive Polymers and Their Capacity for Photodynamic Antimicrobial Action in Organic Film" Polymers 17, no. 9: 1247. https://doi.org/10.3390/polym17091247
APA StyleMarambio, O. G., Barrera, F. I., Martin-Trasancos, R., Sánchez, J., Palavecino, C. E., & Pizarro, G. d. C. (2025). Design, Characterization, and Preparation of New Smart Photoactive Polymers and Their Capacity for Photodynamic Antimicrobial Action in Organic Film. Polymers, 17(9), 1247. https://doi.org/10.3390/polym17091247