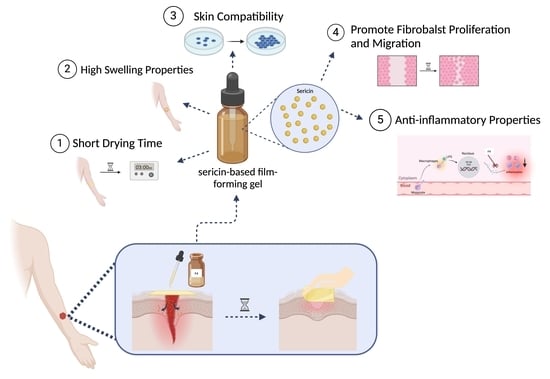
Innovative Sericin-Based Film-Forming Gel for Wound Healing: Development and Performance Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sericin Extract
2.2. Preparation of Film-Forming Gel Formulations
2.3. Determination of Film Evaporation Times on the Skin and the Slide
2.4. Determination of pH
2.5. Determination of Spreadability
2.6. Assessment of Stability
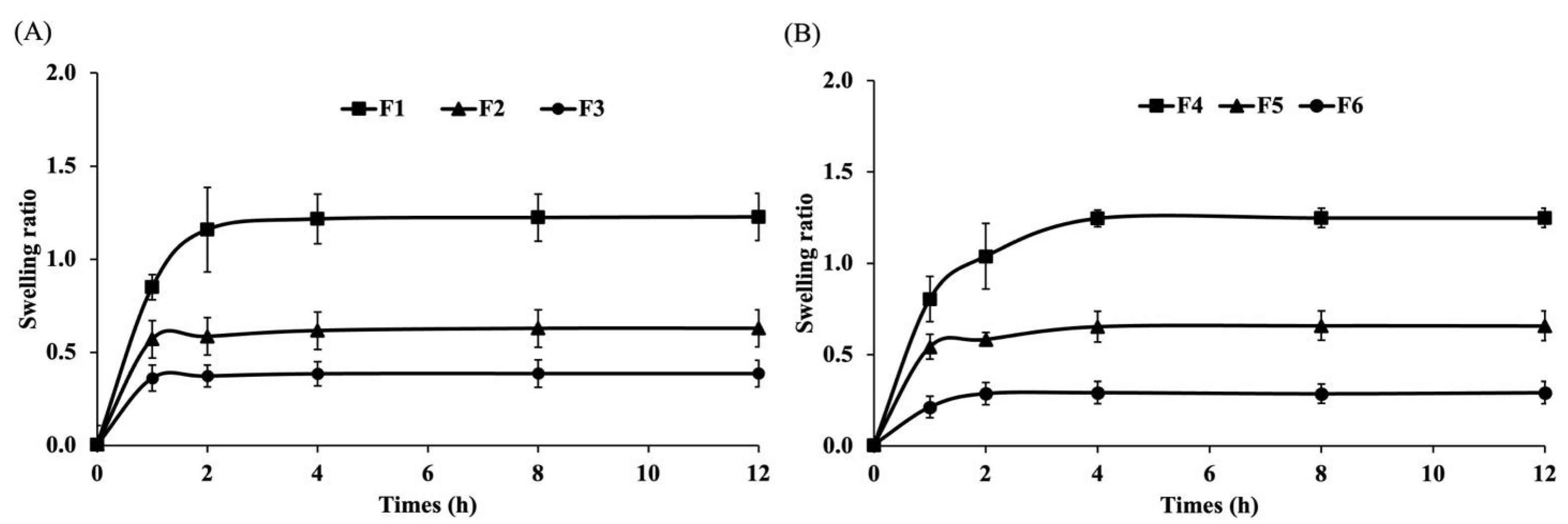
2.7. Evaluation of Swelling Ratio
2.8. Collection of Film-Forming Gel Supernatant
2.9. Fibroblast Cell and Macrophage Cell Culture
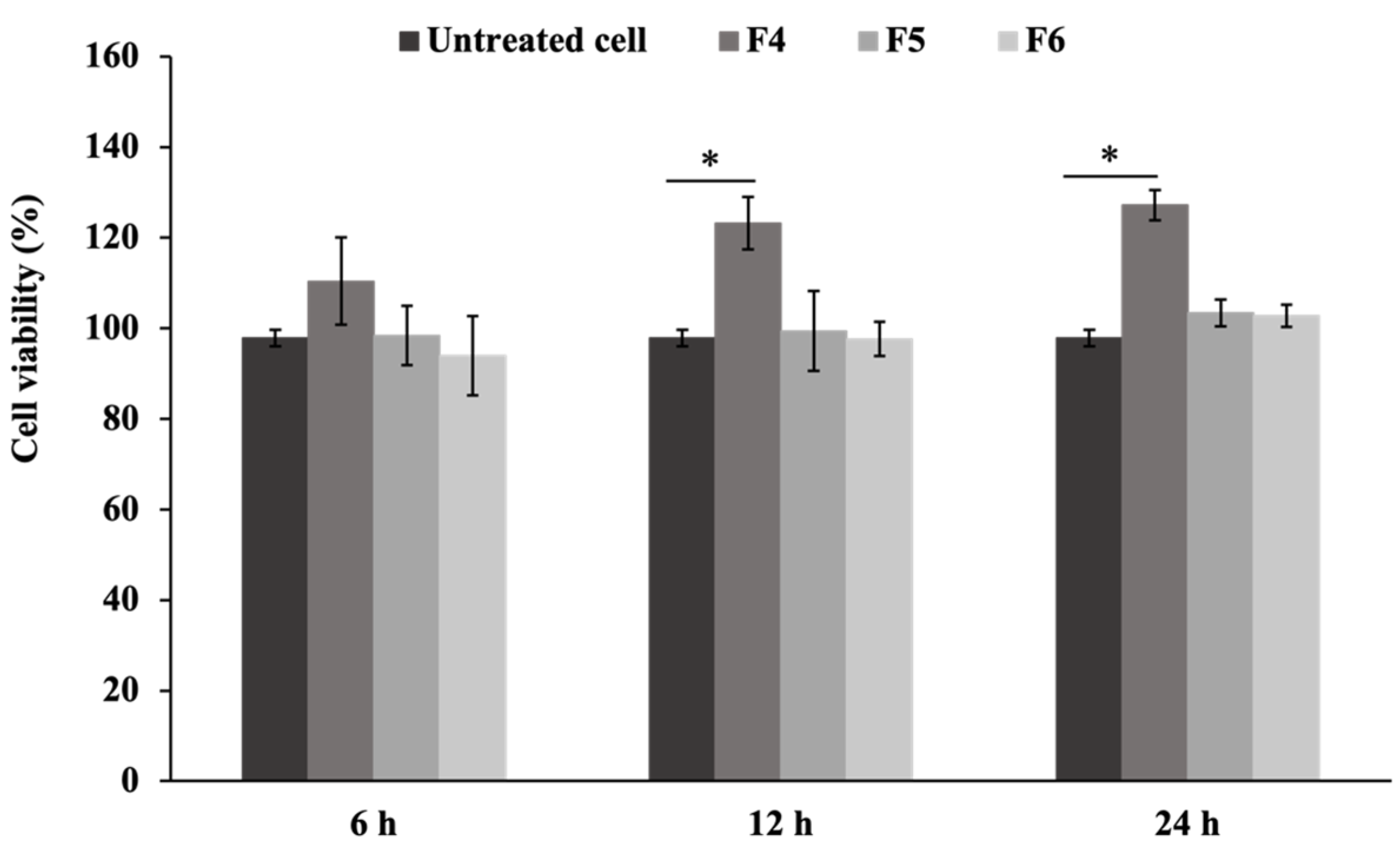
2.10. Evaluation of Cell Viability
2.11. Scratch Wound Assay
2.12. Determination of Nitric Oxide Concentration
2.13. Real-Time Quantitative PCR Analysis
2.14. Western Blot Assay
2.15. Statistical Analysis
3. Results and Discussion
3.1. Characteristics and Physical Properties of the Film-Forming Gels
3.1.1. Characteristics of the Film-Forming Gel
3.1.2. Swelling Capacity of the Film-Forming Gels
3.2. Biological Activities of the Film-Forming Gels
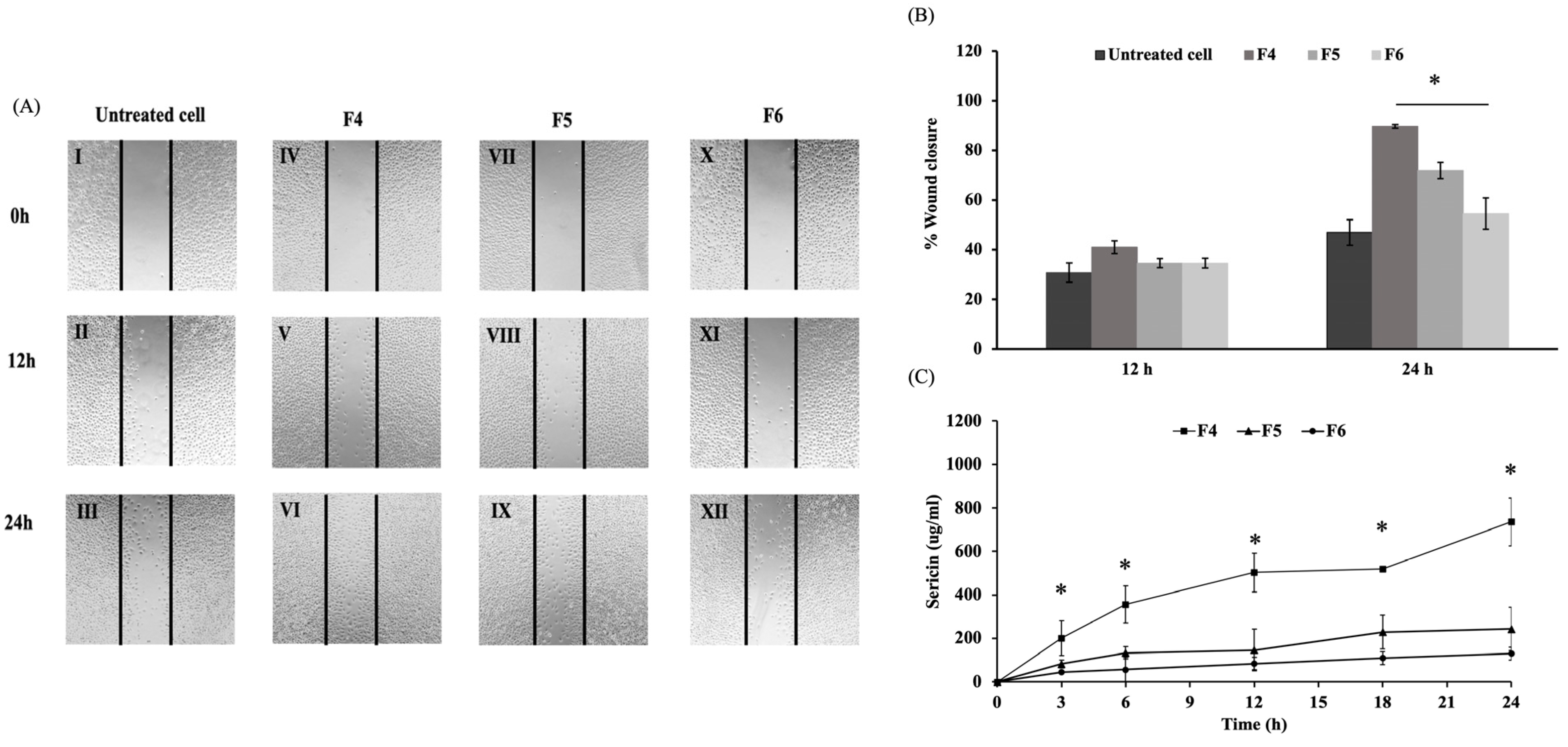
3.2.1. Effects on Wound Healing
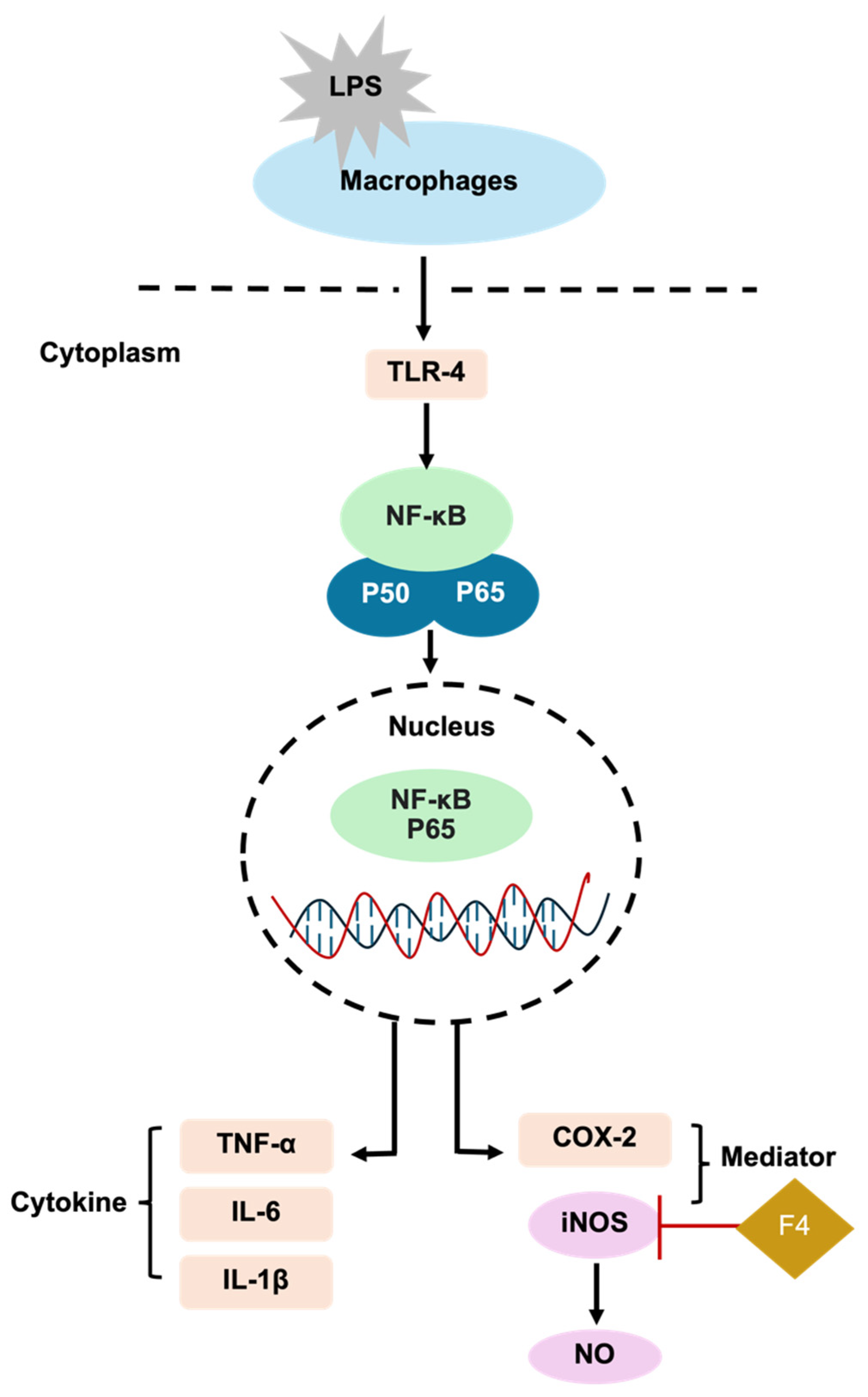
3.2.2. Effects on Inflammatory Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. Commun. 2021, 174, 105841. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human wounds and its burden: An updated compendium of estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Cai, R.; Wang, Y.; Liu, L.; Zuo, H.; Zhao, P.; Umar, A.; Mao, C.; Xia, Q.; He, H. Bioinspired design of AgNPs embedded silk sericin-based sponges for efficiently combating bacteria and promoting wound healing. Mater. Des. 2019, 180, 107940. [Google Scholar] [CrossRef]
- Munir, F.; Tahir, H.M.; Ali, S.; Ali, A.; Tehreem, A.; Zaidi, S.; Adnan, M.; Ijaz, F. Characterization and evaluation of silk sericin-based hydrogel: A promising biomaterial for efficient healing of acute wounds. ACS Omega 2023, 8, 32090–32098. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- Mondal, M.I.H.; Islam, M.M.; Haque, M.I.; Ahmed, F. Natural, biodegradable, biocompatible and bioresorbable medical textile materials. In Medical Textiles from Natural Resources; Elsevier: Amsterdam, The Netherlands, 2022; pp. 87–116. [Google Scholar]
- Ruszymah, B.H.I.; Chowdhury, S.R.; Manan, N.A.B.A.; Fong, O.S.; Adenan, M.I.; Saim, A.B. Aqueous extract of Centella asiatica promotes corneal epithelium wound healing in vitro. J. Ethnopharmacol. 2012, 140, 333–338. [Google Scholar] [CrossRef]
- Radenahmad, N.; Saleh, F.; Sayoh, I.; Sawangjaroen, K.; Subhadhirasakul, P.; Boonyoung, P.; Rundorn, W.; Mitranun, W. Young coconut juice can accelerate the healing process of cutaneous wounds. BMC Complement. Altern. Med. 2012, 12, 252. [Google Scholar] [CrossRef]
- Arango, M.C.; Montoya, Y.; Peresin, M.S.; Bustamante, J.; Álvarez-López, C. Silk sericin as a biomaterial for tissue engineering: A review. Int. J. Polym. Mater. 2021, 70, 1115–1129. [Google Scholar] [CrossRef]
- Silva, A.S.; Costa, E.C.; Reis, S.; Spencer, C.; Calhelha, R.C.; Miguel, S.P.; Ribeiro, M.P.; Barros, L.; Vaz, J.A.; Coutinho, P. Silk Sericin: A promising sustainable biomaterial for biomedical and pharmaceutical applications. Polymers 2022, 14, 4931. [Google Scholar] [CrossRef]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, S.; Jabbari, H.; Yousofi, H.; Fathi, A.; Mahmoodi, S.; Jafarian, M.J.; Shomali, N.; Shotorbani, S.S. Regulatory effect of sericin protein in inflammatory pathways; A comprehensive review. Pathol. Res. Pract. 2023, 243, 154369. [Google Scholar] [CrossRef] [PubMed]
- Manesa, K.C.; Kebede, T.G.; Dube, S.; Nindi, M.M. Fabrication and characterization of sericin-PVA composite films from Gonometa postica, Gonometa rufobrunnea, and Argema mimosae: Potentially applicable in biomaterials. ACS Omega 2022, 7, 19328–19336. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Guo, H. Research progress of polyvinyl alcohol water-resistant film materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef]
- He, H.; Cai, R.; Wang, Y.; Tao, G.; Guo, P.; Zuo, H.; Chen, L.; Liu, X.; Zhao, P.; Xia, Q. Preparation and characterization of silk sericin/PVA blend film with silver nanoparticles for potential antimicrobial application. Int. J. Biol. Macromol. 2017, 104, 457–464. [Google Scholar] [CrossRef]
- Khasraghi, A.H.; Thomas, L.M. Preparation and evaluation of lornoxicam film-forming gel. Drug Inven. Today 2019, 11, 1906–1913. [Google Scholar]
- Noosak, C.; Jantorn, P.; Meesane, J.; Voravuthikunchai, S.; Saeloh, D. Dual-functional bioactive silk sericin for osteoblast responses and osteomyelitis treatment. PLoS ONE 2022, 17, e0264795. [Google Scholar] [CrossRef]
- Pichayakorn, W.; Suksaeree, J.; Boonme, P.; Amnuaikit, T.; Taweepreda, W.; Ritthidej, G.C. Deproteinized natural rubber film forming polymeric solutions for nicotine transdermal delivery. Pharm. Dev. Technol. 2013, 18, 1111–1121. [Google Scholar] [CrossRef]
- Sumayya, A.S.; Muraleedhara Kurup, G. In vitro anti-inflammatory potential of marine macromolecules cross-linked bio-composite scaffold on LPS stimulated RAW 264.7 macrophage cells for cartilage tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2021, 32, 1040–1056. [Google Scholar] [CrossRef]
- Pugar, D.; Haramina, T.; Leskovac, M.; Ćurković, L. Preparation and characterization of poly(vinyl-alcohol)/chitosan polymer blend films chemically crosslinked with glutaraldehyde: Mechanical and thermal investigations. Molecules 2024, 29, 5914. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Li, J.; Brennan, M.; Brennan, C.; Chen, H.; Qin, Y.; Yuan, M. Smart indicator film based on sodium alginate/polyvinyl alcohol/TiO2 containing purple garlic peel extract for visual monitoring of beef freshness. Polymers 2023, 15, 4308. [Google Scholar] [CrossRef] [PubMed]
- Entekhabi, E.; Haghbin Nazarpak, M.; Sedighi, M.; Kazemzadeh, A. Predicting degradation rate of genipin cross-linked gelatin scaffolds with machine learning. Mater. Sci. Eng. C 2020, 107, 110362. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.G. Production and application of biomaterials based on polyvinyl alcohol (PVA) as wound dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- Noosak, C.; Iamthanaporn, K.; Meesane, J.; Voravuthikunchai, S.P.; Sotthibandhu, D.S. Bioactive functional sericin/polyvinyl alcohol hydrogel: Biomaterials for supporting orthopedic surgery in osteomyelitis. J. Mater. Sci. 2023, 58, 5477–5488. [Google Scholar] [CrossRef]
- Dinescu, S.; Galateanu, B.; Albu, M.; Cimpean, A.; Dinischiotu, A.; Costache, M. Sericin enhances the bioperformance of collagen-based matrices preseeded with human-adipose derived stem cells (hADSCs). Int. J. Mol. Sci. 2013, 14, 1870–1889. [Google Scholar] [CrossRef]
- Nursal, F.K.; Nining; Rahmani, A. Effect of glycerin as plasticizer in formulation of grape seed oil (Vitis vinifera L.) emulgel peel-off mask. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2021. [Google Scholar]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk nanospheres and microspheres from silk/PVA blend films for drug delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef]
- Parhi, R.; Goli, V.V.N. Design and optimization of film-forming gel of etoricoxib using research surface methodology. Drug Deliv. Transl. Res. 2020, 10, 498–514. [Google Scholar] [CrossRef]
- Neha, V.; Saudagar, R. Formulation, development and evaluation of film-forming gel for prolonged dermal delivery of terbinafine hydrochloride. Int. J. Pharma Sci. Res. 2014, 5, 537–554. [Google Scholar]
- Thanh, N.Q.; Mai, D.H.; Le, T.P.A.; Do, N.H.; Le, P.K. Novel chitosan/polyvinyl alcohol gel encapsulating ethanolic Centella asiatica extract for cosmeceutical applications. Polym. Bull. 2025, 82, 523–541. [Google Scholar] [CrossRef]
- Monton, C.; Luprasong, C.; Suksaeree, J.; Songsak, T. Preparation and evaluation of film forming polymeric dispersion containing Centella asiatica extract for skin application. Int. J. Pharma Sci. Res. 2021, 21, 73–81. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal pH of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag. Res. 2011, 30, 217–224. [Google Scholar] [CrossRef]
- Djiobie Tchienou, G.E.; Tsatsop Tsague, R.K.; Mbam Pega, T.F.; Bama, V.; Bamseck, A.; Dongmo Sokeng, S.; Ngassoum, M.B. Multi-response optimization in the formulation of a topical cream from natural ingredients. Cosmetics 2018, 5, 7. [Google Scholar] [CrossRef]
- Elkhenany, H.; Abou-Shanab, A.M.; Magdy, S.; Kamar, S.S.; Salah, R.A.; El Badri, N. Comprehensive evaluation of ethanol-preserved amniotic extracts: Exploring antioxidant properties, proliferation enhancement, protective efficacy and regeneration potential in wound healing. J. Drug Deliv. Sci. Technol. 2024, 100, 106062. [Google Scholar] [CrossRef]
- Marto, J.; Baltazar, D.; Duarte, A.; Fernandes, A.; Gouveia, L.; Militão, M.; Salgado, A.; Simões, S.; Oliveira, E.; Ribeiro, H.M. Topical gels of etofenamate: In vitro and in vivo evaluation. Pharm. Dev. Technol. 2015, 20, 710–715. [Google Scholar] [CrossRef]
- Gennari, C.G.M.; Selmin, F.; Ortenzi, M.A.; Franzé, S.; Musazzi, U.M.; Casiraghi, A.; Minghetti, P.; Cilurzo, F. In situ film forming fibroin gel intended for cutaneous administration. Int. J. Pharm. 2016, 511, 296–302. [Google Scholar] [CrossRef]
- Dubey, V.; Daschakraborty, S. Influence of glycerol on the cooling effect of pair hydrophobicity in water: Relevance to proteins’ stabilization at low temperature. Phys. Chem. Chem. Phys. 2019, 21, 800–812. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Um, J.-Y.; Chung, B.-Y.; Lee, S.-Y.; Park, J.-S.; Kim, J.-C.; Park, C.-W.; Kim, H.-O. Moisturizer in patients with inflammatory skin diseases. Medicina 2022, 58, 888. [Google Scholar] [CrossRef]
- Manesa, K.C.; Kebede, T.G.; Dube, S.; Nindi, M.M. Profiling of silk sericin from cocoons of Three Southern African wild silk moths with a focus on their antimicrobial and antioxidant properties. Materials 2020, 13, 5706. [Google Scholar] [CrossRef] [PubMed]
- Kunz, R.; Brancalhão, R.M.C.; Ribeiro, L.; Natali, M. Silkworm sericin: Properties and biomedical applications. Biomed. Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, Ó.; Alves, P.; Granja, P.L.; Soares, R.; et al. In situ forming silk sericin-based hydrogel: A novel wound healing biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-L.; Kang, E.-B.; Yun, S.-G.; Park, D.-b.; Lim, J.-O.; Suh, J.-S. Effect of a silk sericin and Methylsulfonylmethane (MSM) blends on inflammatory response and wound healing. Appl. Sci. 2023, 13, 288. [Google Scholar] [CrossRef]
- Prakash, M.; Mathikere Naganna, C.; Radhakrishnan, V.; Somayaji, P.; Sabu, L. Therapeutic potential of silkworm sericin in wound healing applications. Wound Repair Regen. 2024, 32, 916–940. [Google Scholar] [CrossRef]
- Cam, M.; Yildiz, S.; Alenezi, H.; Cesur, S.; Ozcan, G.; Erdemir Cilasun, G.; Edirisinghe, U.; Akakin, D.; Kuruca, D.; Kabasakal, L.; et al. Evaluation of burst release and sustained release of pioglitazone-loaded fibrous mats on diabetic wound healing: An in vitro and in vivo comparison study. J. R. Soc. Interface. 2020, 17, 20190712. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric oxide: Physiological functions, delivery, and biomedical applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef]
- Cai, X.; Sha, F.; Zhao, C.; Zheng, Z.; Zhao, S.; Zhu, Z.; Zhu, H.; Chen, J.; Chen, Y. Synthesis and anti-inflammatory activity of novel steroidal chalcones with 3β-pregnenolone ester derivatives in RAW 264.7 cells in vitro. Steroids 2021, 171, 108830. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Zhu, J.; Wu, J.; Geng, S.; Zhong, C. Magnesium isoglycyrrhizinate suppresses LPS-induced inflammation and oxidative stress through inhibiting NF-κB and MAPK pathways in RAW264.7 cells. Bioorg. Med. Chem. 2019, 27, 516–524. [Google Scholar]
- Mu, K.; Yu, S.; Kitts, D.D. The role of Nitric Oxide in regulating intestinal redox status and intestinal epithelial cell functionality. Int. J. Mol. Sci. 2019, 20, 1755. [Google Scholar] [CrossRef]
- Anavi, S.; Tirosh, O. iNOS as a metabolic enzyme under stress conditions. Free Radic. Biol. Med. 2020, 146, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dutta, S.; Pati, S.; Sarkar, T.; Paul, S.; Chakraborty, S.; Basak, U.; Dhar, S.; Das, T.; Sa, G. Nuclear factor kappa-B: The El Dorado of inflammatory immune response. Cell. Mol. Immunol. 2024, 3, 6–19. [Google Scholar] [CrossRef]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001, 480–481, 243–268. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Towiwat, P.; Srichana, T. Anti-inflammatory potential of silk sericin. Nat. Prod. Commun. 2013, 8, 501–504. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, W.; Zhang, Q.; Guo, H.; Dong, Z.; Zhao, P.; Xia, Q. Multi-omics integration to reveal the mechanism of sericin inhibiting LPS-induced inflammation. Int. J. Mol. Sci. 2022, 24, 259. [Google Scholar] [CrossRef]


| Formulations | Component Concentrations (%w/v) | |||||
|---|---|---|---|---|---|---|
| 10% PVA | 0.25% Sericin | 0.5% Sericin | 1% Sericin | 95% Ethanol | Glycerol | |
| F1 | 6.5 | - | - | 0.2 | 7.9 | 6.3 |
| F2 | 6.5 | - | 0.1 | - | 7.9 | 6.3 |
| F3 | 6.5 | 0.05 | - | - | 7.9 | 6.3 |
| F4 | 6.5 | - | - | 0.15 | 11.85 | 6.3 |
| F5 | 6.5 | - | 0.075 | - | 11.85 | 6.3 |
| F6 | 6.5 | 0.0375 | - | - | 11.85 | 6.3 |
| Primer | Sequence (5′-3′) | Reference |
|---|---|---|
| iNOS | Forward: CACCACCCTCCTTGTTCAAC Reverse: CAATCCACAACTCGCTCCAA | [21] |
| GAPDH | Forward: CATGGCCTTCCGTGTTCCTA Reverse: CCTGCTTCACCACCTTCTTGAT |
| Formulation | Integrity on Skin | Drying Time (min) | pH | Spreadability (g cm2/s) | |
|---|---|---|---|---|---|
| On Skin | On Glass Slide | ||||
| F1 | a | 4.29 ± 0.45 | 9.06 ± 0.22 | 5.22 ± 0.03 | 1.48 ± 0.13 |
| F2 | a | 4.54 ± 0.27 | 8.28 ± 0.20 | 5.25 ± 0.02 | 1.59 ± 0.04 |
| F3 | a | 4.43 ± 0.30 | 9.31 ± 0.05 | 5.23 ± 0.01 | 1.98 ± 0.10 |
| F4 | a | 3.54 ± 0.52 | 6.54 ± 0.18 | 5.30 ± 0.01 | 1.54 ± 0.03 |
| F5 | a | 4.18 ± 0.05 | 7.05 ± 0.15 | 5.37 ± 0.02 | 2.39 ± 0.06 |
| F6 | a | 4.18 ± 0.21 | 6.35 ± 0.26 | 5.36 ± 0.02 | 2.31 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongtechanon, S.; Noosak, C.; Jantorn, P.; Watcharanurak, P.; Swangphon, P.; Wanna, W.; Sotthibandhu, D.S. Innovative Sericin-Based Film-Forming Gel for Wound Healing: Development and Performance Evaluation. Polymers 2025, 17, 1246. https://doi.org/10.3390/polym17091246
Wongtechanon S, Noosak C, Jantorn P, Watcharanurak P, Swangphon P, Wanna W, Sotthibandhu DS. Innovative Sericin-Based Film-Forming Gel for Wound Healing: Development and Performance Evaluation. Polymers. 2025; 17(9):1246. https://doi.org/10.3390/polym17091246
Chicago/Turabian StyleWongtechanon, Suprawee, Chayanee Noosak, Pavarish Jantorn, Papitchaya Watcharanurak, Piyawut Swangphon, Warapond Wanna, and Dennapa Saeloh Sotthibandhu. 2025. "Innovative Sericin-Based Film-Forming Gel for Wound Healing: Development and Performance Evaluation" Polymers 17, no. 9: 1246. https://doi.org/10.3390/polym17091246
APA StyleWongtechanon, S., Noosak, C., Jantorn, P., Watcharanurak, P., Swangphon, P., Wanna, W., & Sotthibandhu, D. S. (2025). Innovative Sericin-Based Film-Forming Gel for Wound Healing: Development and Performance Evaluation. Polymers, 17(9), 1246. https://doi.org/10.3390/polym17091246