Rapid Assembly of Block Copolymer Thin Films via Accelerating the Swelling Process During Solvent Annealing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
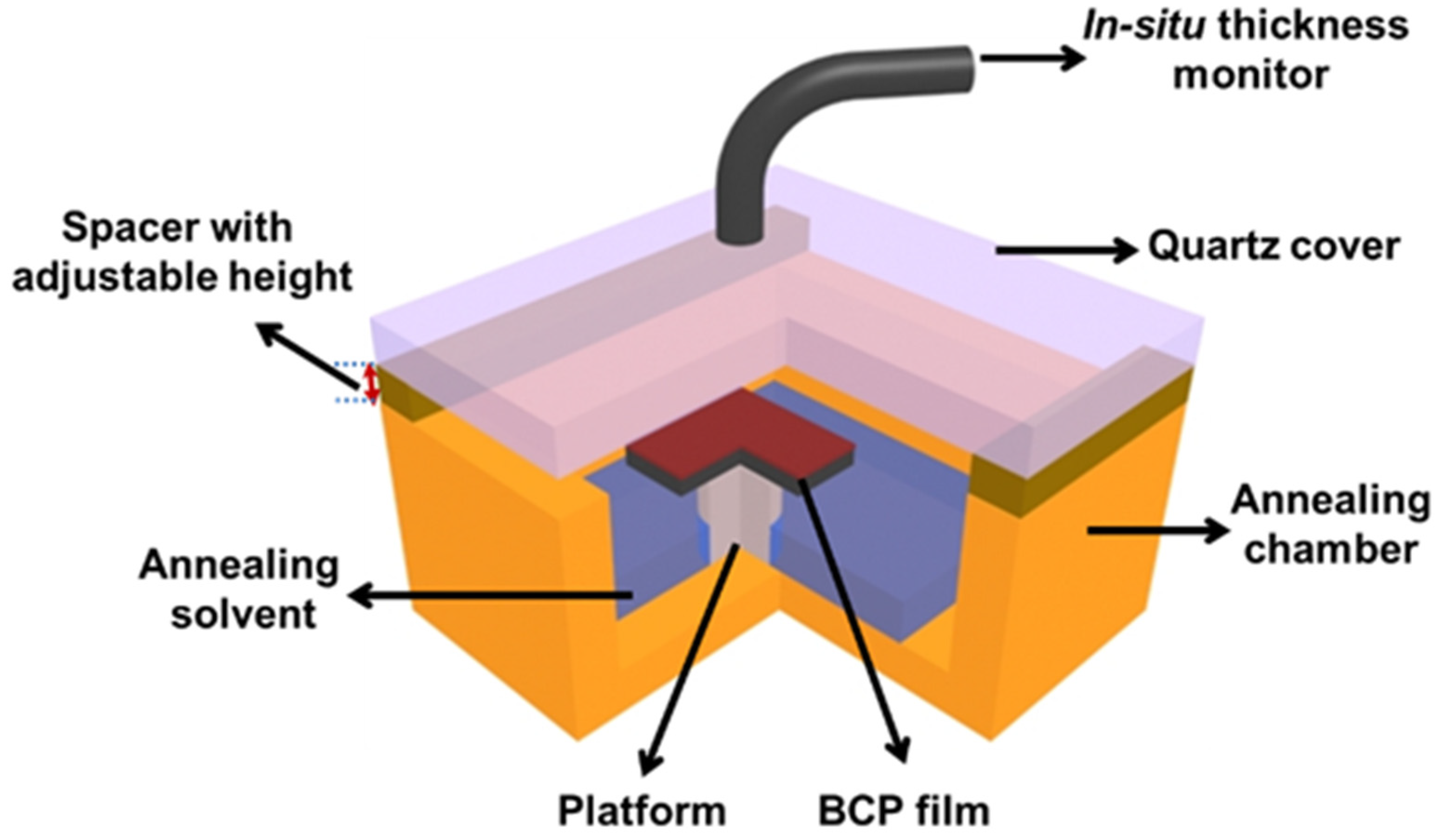
2.2. Solvent Annealing of BCP Thin Films
2.3. Fabrication of the Patterned Substrate
2.4. Fabrication of Pt Nanoarrays on the Annealing BCP Thin Films
2.5. Characterizations
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bates, C.M.; Bates, F.S. 50th Anniversary Perspective: Block Polymers—Pure Potential. Macromolecules 2017, 50, 3–22. [Google Scholar] [CrossRef]
- Fontelo, R.; Reis, R.L.; Novoa-Carballal, R.; Pashkuleva, I. Preparation, Properties, and Bioapplications of Block Copolymer Nanopatterns (Adv. Healthcare Mater. 1/2024). Adv. Healthc. Mater. 2024, 13, 2470001. [Google Scholar] [CrossRef]
- Cummins, C.; Lundy, R.; Walsh, J.J.; Ponsinet, V.; Fleury, G.; Morris, M.A. Enabling future nanomanufacturing through block copolymer self-assembly: A review. Nano Today 2020, 35, 100936. [Google Scholar] [CrossRef]
- Lo, T.-Y.; Krishnan, M.R.; Lu, K.-Y.; Ho, R.-M. Silicon-containing block copolymers for lithographic applications. Prog. Polym. Sci. 2018, 77, 19–68. [Google Scholar] [CrossRef]
- Hughes, R.A.; Menumerov, E.; Neretina, S. When lithography meets self-assembly: A review of recent advances in the directed assembly of complex metal nanostructures on planar and textured surfaces. Nanotechnology 2017, 28, 282002. [Google Scholar] [CrossRef]
- Li, W.; Müller, M. Directed self-assembly of block copolymers by chemical or topographical guiding patterns: Optimizing molecular architecture, thin-film properties, and kinetics. Prog. Polym. Sci. 2016, 54–55, 47–75. [Google Scholar] [CrossRef]
- Chang, T.X.; Huang, H.Y.; He, T.B. Directed Self-Assembly of Diblock Copolymer Thin Films on Prepatterned Metal Nanoarrays. Macromol. Rapid Commun. 2016, 37, 161–167. [Google Scholar] [CrossRef]
- Bates, C.M.; Maher, M.J.; Janes, D.W.; Ellison, C.J.; Willson, C.G. Block Copolymer Lithography. Macromolecules 2014, 47, 2–12. [Google Scholar] [CrossRef]
- Ross, C.A.; Berggren, K.K.; Cheng, J.Y.; Jung, Y.S.; Chang, J.-B. Three-Dimensional Nanofabrication by Block Copolymer Self-Assembly. Adv. Mater. 2014, 26, 4386–4396. [Google Scholar] [CrossRef]
- Luo, M.; Epps, T.H., III. Directed Block Copolymer Thin Film Self-Assembly: Emerging Trends in Nanopattern Fabrication. Macromolecules 2013, 46, 7567–7579. [Google Scholar] [CrossRef]
- Koo, K.; Ahn, H.; Kim, S.-W.; Ryu, D.Y.; Russell, T.P. Directed self-assembly of block copolymers in the extreme: Guiding microdomains from the small to the large. Soft Matter 2013, 9, 9059–9071. [Google Scholar] [CrossRef]
- Chang, T.; Du, B.; Huang, H.; He, T. Highly Tunable Complementary Micro/Submicro-Nanopatterned Surfaces Combining Block Copolymer Self-Assembly and Colloidal Lithography. ACS Appl. Mater. Interfaces 2016, 8, 22705–22713. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Wan, L.; Liu, C.-C.; Nealey, P.F. Directed self-assembly of block copolymers on chemical patterns: A platform for nanofabrication. Prog. Polym. Sci. 2016, 54–55, 76–127. [Google Scholar] [CrossRef]
- Hu, H.; Gopinadhan, M.; Osuji, C.O. Directed self-assembly of block copolymers: A tutorial review of strategies for enabling nanotechnology with soft matter. Soft Matter 2014, 10, 3867–3889. [Google Scholar] [CrossRef]
- Ji, S.; Nagpal, U.; Liao, W.; Liu, C.-C.; de Pablo, J.J.; Nealey, P.F. Three-dimensional Directed Assembly of Block Copolymers together with Two-dimensional Square and Rectangular Nanolithography. Adv. Mater. 2011, 23, 3692–3697. [Google Scholar] [CrossRef] [PubMed]
- Sinturel, C.; Vayer, M.; Morris, M.; Hillmyer, M.A. Solvent Vapor Annealing of Block Polymer Thin Films. Macromolecules 2013, 46, 5399–5415. [Google Scholar] [CrossRef]
- Campbell, I.P.; Hirokawa, S.; Stoykovich, M.P. Processing Approaches for the Defect Engineering of Lamellar-Forming Block Copolymers in Thin Films. Macromolecules 2013, 46, 9599–9608. [Google Scholar] [CrossRef]
- Jin, C.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Nanopatterning via Solvent Vapor Annealing of Block Copolymer Thin Films. Chem. Mater. 2017, 29, 176–188. [Google Scholar] [CrossRef]
- Sinturel, C.; Bates, F.S.; Hillmyer, M.A. High χ–Low N Block Polymers: How Far Can We Go? ACS Macro Lett. 2015, 4, 1044–1050. [Google Scholar] [CrossRef]
- Aissou, K.; Mumtaz, M.; Fleury, G.; Portale, G.; Navarro, C.; Cloutet, E.; Brochon, C.; Ross, C.A.; Hadziioannou, G. Sub-10 nm Features Obtained from Directed Self-Assembly of Semicrystalline Polycarbosilane-Based Block Copolymer Thin Films. Adv. Mater. 2015, 27, 261–265. [Google Scholar] [CrossRef]
- Yokoyama, H.; Kramer, E.J. Self-Diffusion of Asymmetric Diblock Copolymers with a Spherical Domain Structure. Macromolecules 1998, 31, 7871–7876. [Google Scholar] [CrossRef]
- Lodge, T.P.; Dalvi, M.C. Mechanisms of Chain Diffusion in Lamellar Block Copolymers. Phys. Rev. Lett. 1995, 75, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese Lupi, F.; Giammaria, T.J.; Ceresoli, M.; Seguini, G.; Sparnacci, K.; Antonioli, D.; Gianotti, V.; Laus, M.; Perego, M. Rapid thermal processing of self-assembling block copolymer thin films. Nanotechnology 2013, 24, 315601. [Google Scholar] [CrossRef] [PubMed]
- Majewski, P.W.; Yager, K.G. Latent Alignment in Pathway-Dependent Ordering of Block Copolymer Thin Films. Nano Lett. 2015, 15, 5221–5228. [Google Scholar] [CrossRef]
- Majewski, P.W.; Yager, K.G. Millisecond Ordering of Block Copolymer Films via Photothermal Gradients. ACS Nano 2015, 9, 3896–3906. [Google Scholar] [CrossRef]
- Jin, H.M.; Park, D.Y.; Jeong, S.-J.; Lee, G.Y.; Kim, J.Y.; Mun, J.H.; Cha, S.K.; Lim, J.; Kim, J.S.; Kim, K.H.; et al. Flash Light Millisecond Self-Assembly of High χ Block Copolymers for Wafer-Scale Sub-10 nm Nanopatterning. Adv. Mater. 2017, 29, 1700595. [Google Scholar] [CrossRef]
- Campbell, I.P.; He, C.; Stoykovich, M.P. Topologically Distinct Lamellar Block Copolymer Morphologies Formed by Solvent and Thermal Annealing. ACS Macro Lett. 2013, 2, 918–923. [Google Scholar] [CrossRef]
- Zhang, X.; Harris, K.D.; Wu, N.L.Y.; Murphy, J.N.; Buriak, J.M. Fast Assembly of Ordered Block Copolymer Nanostructures through Microwave Annealing. ACS Nano 2010, 4, 7021–7029. [Google Scholar] [CrossRef]
- Jin, C.; Murphy, J.N.; Harris, K.D.; Buriak, J.M. Deconvoluting the Mechanism of Microwave Annealing of Block Copolymer Thin Films. ACS Nano 2014, 8, 3979–3991. [Google Scholar] [CrossRef]
- Borah, D.; Senthamaraikannan, R.; Rasappa, S.; Kosmala, B.; Holmes, J.D.; Morris, M.A. Swift Nanopattern Formation of PS-b-PMMA and PS-b-PDMS Block Copolymer Films Using a Microwave Assisted Technique. ACS Nano 2013, 7, 6583–6596. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, W.-C.; Borsali, R. Carbohydrate-Based Block Copolymer Thin Films: Ultrafast Nano-Organization with 7 nm Resolution Using Microwave Energy. Adv. Mater. 2017, 29, 1701645. [Google Scholar] [CrossRef] [PubMed]
- Park, W.I.; Kim, K.; Jang, H.-I.; Jeong, J.W.; Kim, J.M.; Choi, J.; Park, J.H.; Jung, Y.S. Directed Self-Assembly with Sub-100 Degrees Celsius Processing Temperature, Sub-10 Nanometer Resolution, and Sub-1 Minute Assembly Time. Small 2012, 8, 3762–3768. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tempeler, J.; Danylyuk, S.; Böker, A.; Tsarkova, L. Disclosing Topographical and Chemical Patterns in Confined Films of High-Molecular-Weight Block Copolymers under Controlled Solvothermal Annealing. Polymers 2024, 16, 1943. [Google Scholar] [CrossRef]
- Cheng, X.; Böker, A.; Tsarkova, L. Temperature-Controlled Solvent Vapor Annealing of Thin Block Copolymer Films. Polymers 2019, 11, 1312. [Google Scholar] [CrossRef]
- Gotrik, K.W.; Ross, C.A. Solvothermal Annealing of Block Copolymer Thin Films. Nano Lett. 2013, 13, 5117–5122. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, Y.; Park, W.I.; Hur, Y.H.; Jeong, J.W.; Sim, D.M.; Baek, K.M.; Lee, J.H.; Kim, M.-J.; Jung, Y.S. Eliminating the Trade-Off between the Throughput and Pattern Quality of Sub-15 nm Directed Self-Assembly via Warm Solvent Annealing. Adv. Funct. Mater. 2015, 25, 306–315. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, J.M.; Jeong, J.W.; Jung, Y.S. Deep-Nanoscale Pattern Engineering by Immersion-Induced Self-Assembly. ACS Nano 2014, 8, 10009–10018. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Bhaway, S.M.; Vogt, B.D.; Douglas, J.F.; Al-Enizi, A.; Elzatahry, A.; Sharma, A.; Karim, A. Direct Immersion Annealing of Thin Block Copolymer Films. ACS Appl. Mater. Interfaces 2015, 7, 21639–21645. [Google Scholar] [CrossRef]
- Longanecker, M.; Modi, A.; Dobrynin, A.; Kim, S.; Yuan, G.; Jones, R.; Satija, S.; Bang, J.; Karim, A. Reduced Domain Size and Interfacial Width in Fast Ordering Nanofilled Block Copolymer Films by Direct Immersion Annealing. Macromolecules 2016, 49, 8563–8571. [Google Scholar] [CrossRef]
- Jeong, J.W.; Hur, Y.H.; Kim, H.-j.; Kim, J.M.; Park, W.I.; Kim, M.J.; Kim, B.J.; Jung, Y.S. Proximity Injection of Plasticizing Molecules to Self-Assembling Polymers for Large-Area, Ultrafast Nanopatterning in the Sub-10-nm Regime. ACS Nano 2013, 7, 6747–6757. [Google Scholar] [CrossRef]
- Arias-Zapata, J.; Böhme, S.; Garnier, J.; Girardot, C.; Legrain, A.; Zelsmann, M. Ultrafast Assembly of PS-PDMS Block Copolymers on 300 mm Wafers by Blending with Plasticizers. Adv. Funct. Mater. 2016, 26, 5690–5700. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.; Cho, J.-Y.; Yang, S.R.; Kim, J.M.; Yim, S.; Lee, H.; Jung, Y.S. In Situ Nanolithography with Sub-10 nm Resolution Realized by Thermally Assisted Spin-Casting of a Self-Assembling Polymer. Adv. Mater. 2015, 27, 4814–4822. [Google Scholar] [CrossRef]
- Jung, H.; Woo, S.; Choe, Y.; Ryu, D.Y.; Huh, J.; Bang, J. Single Step Process for Self-Assembled Block Copolymer Patterns via in Situ Annealing during Spin-Casting. ACS Macro Lett. 2015, 4, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Thorkelsson, K.; Bai, P.; Zhang, Z.; Sun, C.; Xu, T. Rapid fabrication of hierarchically structured supramolecular nanocomposite thin films in one minute. Nat. Commun. 2014, 5, 4053. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, Y.; He, Y.; Wang, L.; Huang, H.; He, T. Solvent-Vapor-Induced Rapid Assembly of Block-Copolymer Film via Prevacuumizing. Macromol. Chem. Phys. 2014, 215, 1092–1097. [Google Scholar] [CrossRef]
- Selkirk, A.; Prochukhan, N.; Lundy, R.; Cummins, C.; Gatensby, R.; Kilbride, R.; Parnell, A.; Vasquez, J.B.; Morris, M.; Mokarian-Tabari, P. Optimization and Control of Large Block Copolymer Self-Assembly via Precision Solvent Vapor Annealing. Macromolecules 2021, 54, 1203–1215. [Google Scholar] [CrossRef]
- Horvat, A.; Sevink, G.J.A.; Zvelindovsky, A.V.; Krekhov, A.; Tsarkova, L. Specific Features of Defect Structure and Dynamics in the Cylinder Phase of Block Copolymers. ACS Nano 2008, 2, 1143–1152. [Google Scholar] [CrossRef]
- Harrison, C.; Cheng, Z.; Sethuraman, S.; Huse, D.A.; Chaikin, P.M.; Vega, D.A.; Sebastian, J.M.; Register, R.A.; Adamson, D.H. Dynamics of pattern coarsening in a two-dimensional smectic system. Phys. Rev. E 2002, 66, 011706. [Google Scholar] [CrossRef]
- Gu, X.; Gunkel, I.; Hexemer, A.; Gu, W.; Russell, T.P. An In Situ Grazing Incidence X-Ray Scattering Study of Block Copolymer Thin Films During Solvent Vapor Annealing. Adv. Mater. 2014, 26, 273–281. [Google Scholar] [CrossRef]
- Takahashi, H.; Laachi, N.; Delaney, K.T.; Hur, S.-M.; Weinheimer, C.J.; Shykind, D.; Fredrickson, G.H. Defectivity in Laterally Confined Lamella-Forming Diblock Copolymers: Thermodynamic and Kinetic Aspects. Macromolecules 2012, 45, 6253–6265. [Google Scholar] [CrossRef]
- Mishra, V.; Fredrickson, G.H.; Kramer, E.J. Effect of Film Thickness and Domain Spacing on Defect Densities in Directed Self-Assembly of Cylindrical Morphology Block Copolymers. ACS Nano 2012, 6, 2629–2641. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.R.; Cochran, E.; Fredrickson, G.H.; Kramer, E.J. Temperature Dependence of Order, Disorder, and Defects in Laterally Confined Diblock Copolymer Cylinder Monolayers. Macromolecules 2005, 38, 6575–6585. [Google Scholar] [CrossRef]
- Baruth, A.; Seo, M.; Lin, C.H.; Walster, K.; Shankar, A.; Hillmyer, M.A.; Leighton, C. Optimization of Long-Range Order in Solvent Vapor Annealed Poly(styrene)-block-poly(lactide) Thin Films for Nano lithography. ACS Appl. Mater. Interfaces 2014, 6, 13770–13781. [Google Scholar] [CrossRef] [PubMed]
- Krevelen, D.W.V. Properties of Polymers. Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]

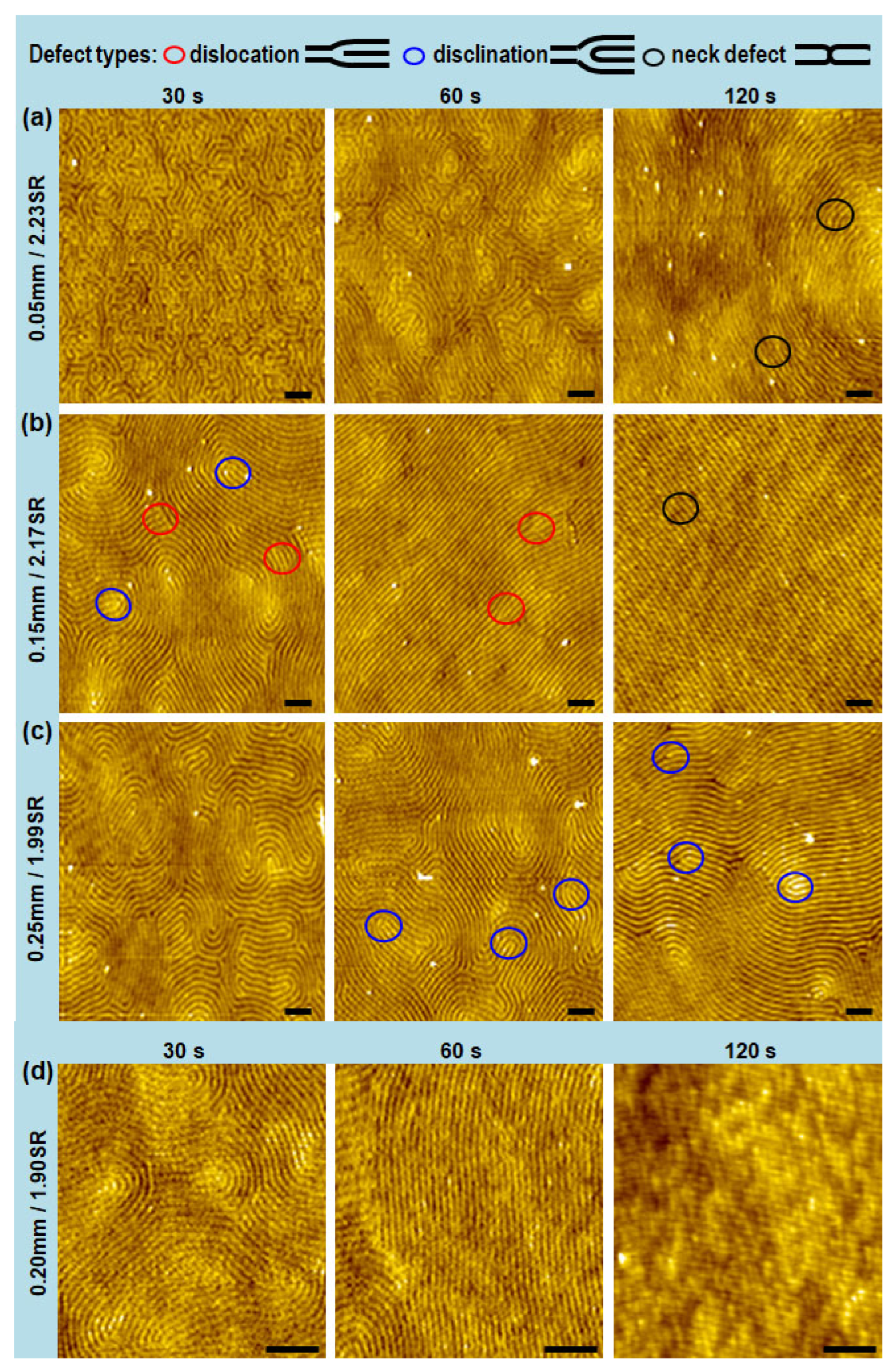

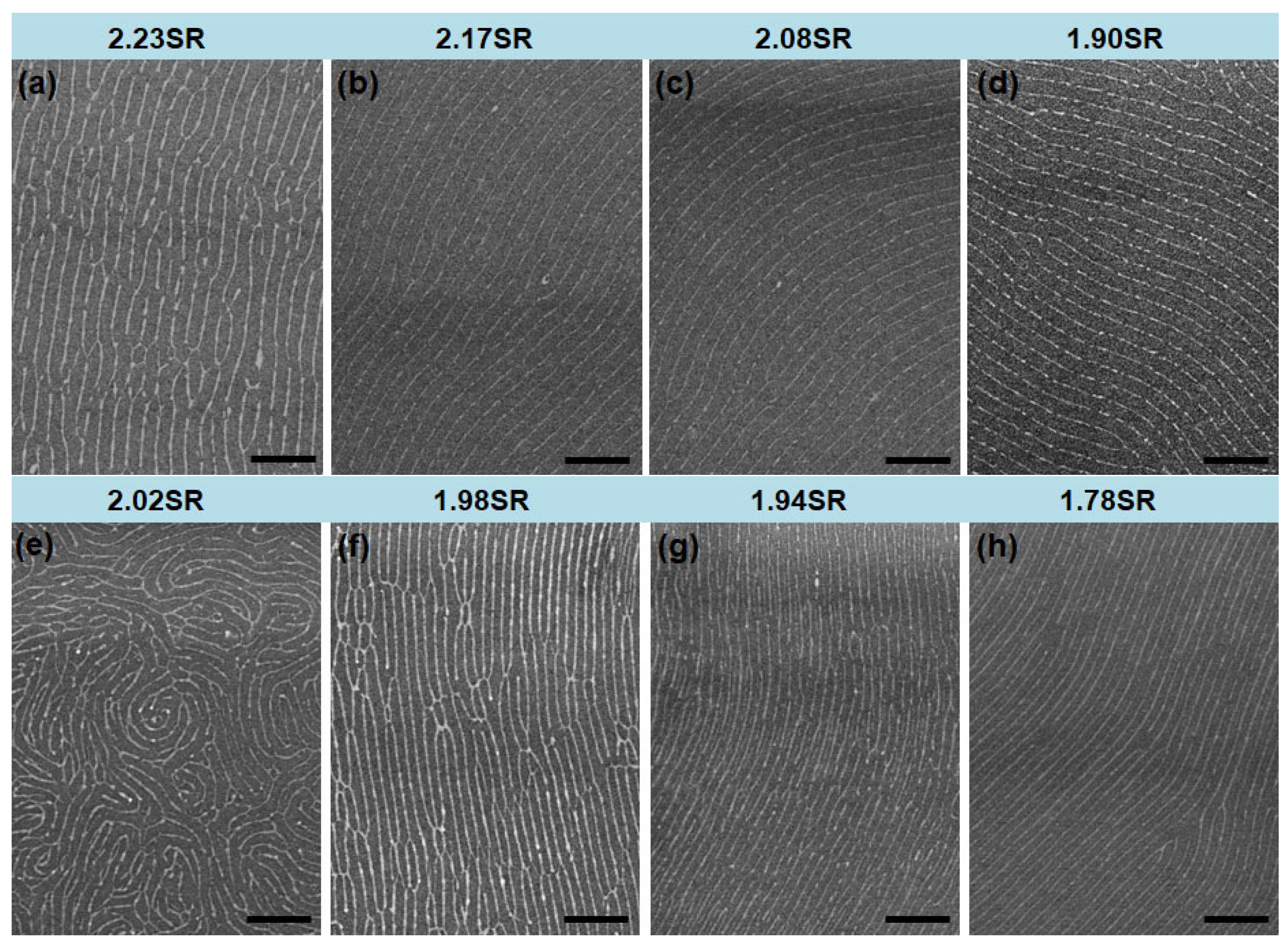
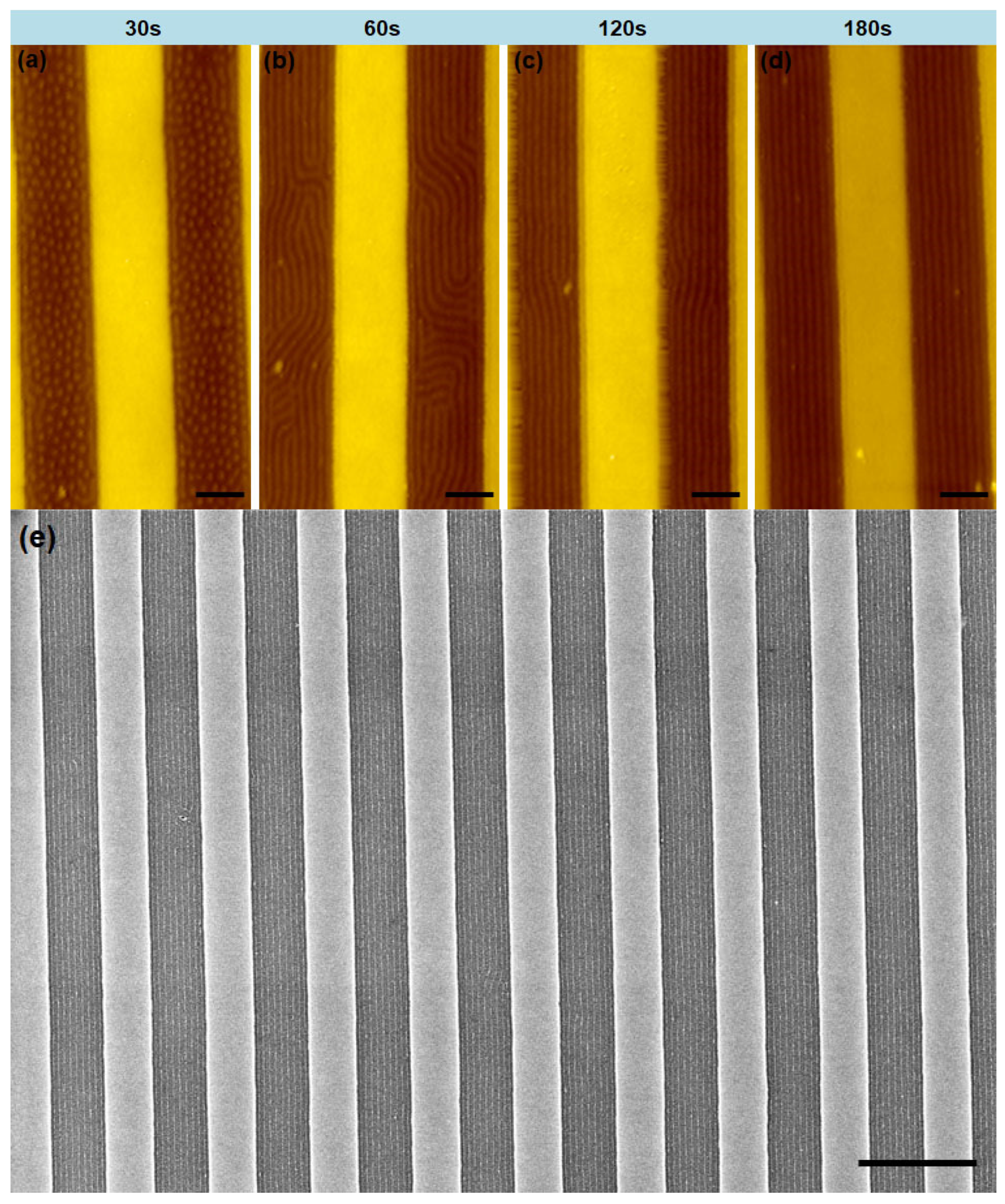
| Spacer Height (mm) | Swelling Ratio 1 | Spacer Height (mm) | Swelling Ratio 1 | ||
|---|---|---|---|---|---|
| PS62K-b-P2VP26K | 0.05 | 2.23 | PS30K-b-P2VP12.5K | 0.05 | 2.02 |
| 0.10 | 2.21 | 0.10 | 1.98 | ||
| 0.15 | 2.17 | 0.15 | 1.94 | ||
| 0.20 | 2.08 | 0.20 | 1.90 | ||
| 0.25 | 1.99 | 0.25 | 1.87 | ||
| 0.30 | 1.97 | 0.30 | – | ||
| 0.40 | 1.90 | 0.40 | 1.78 | ||
| 0.50 | 1.77 | 0.50 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shui, T.-e.; Chang, T.; Wang, Z.; Huang, H. Rapid Assembly of Block Copolymer Thin Films via Accelerating the Swelling Process During Solvent Annealing. Polymers 2025, 17, 1242. https://doi.org/10.3390/polym17091242
Shui T-e, Chang T, Wang Z, Huang H. Rapid Assembly of Block Copolymer Thin Films via Accelerating the Swelling Process During Solvent Annealing. Polymers. 2025; 17(9):1242. https://doi.org/10.3390/polym17091242
Chicago/Turabian StyleShui, Tian-en, Tongxin Chang, Zhe Wang, and Haiying Huang. 2025. "Rapid Assembly of Block Copolymer Thin Films via Accelerating the Swelling Process During Solvent Annealing" Polymers 17, no. 9: 1242. https://doi.org/10.3390/polym17091242
APA StyleShui, T.-e., Chang, T., Wang, Z., & Huang, H. (2025). Rapid Assembly of Block Copolymer Thin Films via Accelerating the Swelling Process During Solvent Annealing. Polymers, 17(9), 1242. https://doi.org/10.3390/polym17091242







