Specificity of Thermal Destruction of Nonwoven Mixture Systems Based on Bast and Viscose Fibers
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties, and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Fengel, D.; Wegener, G. Wood—Chemistry, Ultrastructure; Fengel, D., Wegener, G., Eds.; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1984; p. 613. [Google Scholar]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Bismarck, S.; Mishra, M.; Lampke, T. Plant Fibers as Reinforcement for Green Composites. In Natural Fibres, Biopolymers and Biocomposites; Mohanty, A.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 37–108. [Google Scholar] [CrossRef]
- Kozasowski, R.M.; Mackiewicz-Talarczyk, M.; Allam, A.M. Bast fibres: Flax. In Handbook of Natural Fibres: Types, Properties and Factors Affecting Breeding and Cultivation; Kozlowski, R.M., Ed.; Woodhead Publishing Series in Textiles Sawston: Cambridge, UK, 2012; pp. 56–113. [Google Scholar] [CrossRef]
- Simon, M.; Fulchiron, R.; Gouanvé, F. Water Sorption and Mechanical Properties of Cellulosic Derivative Fibers. Polymers 2022, 14, 2836. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Bouloc, P.; Allegret, S.; Arnaud, L. Hemp: Industrial Production and Uses; Bouloc, P., Ed.; CABI: Delémont, Switzerland, 2013; p. 328. [Google Scholar]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New developments in fiber hemp (Cannabis sativa L.) breeding. Ind. Crop. Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Röhrmann, S.; Kolodziejczyk, K.; Zajączkowska, D.; Kaniewska, A.; Pawłowska, A. Hemp Fibers—Structure and Properties. Materials 2020, 13, 644. [Google Scholar] [CrossRef]
- Placet, V.; François, C.; Day, A.; Beaugrand, J.; Ouagne, P. Industrial Hemp Transformation for Composite Applications: Influence of Processing Parameters on the Fibre Properties. In Advances in Natural Fibre Composites; Fangueiro, R., Rana, S., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Angulu, M.; Gusovius, H.-J. Retting of Bast Fiber Crops Like Hemp and Flax—A Review for Classification of Procedures. Fibers 2024, 12, 28. [Google Scholar] [CrossRef]
- Mazian, B.; Bergeret, A.; Benezet, J.-C.; Malhautier, L. A Comparative Study of the Effect of Field Retting Time on the Properties of Hemp Fibres Harvested at Different Growth Stages. Fibers 2019, 7, 108. [Google Scholar] [CrossRef]
- Law, A.D.; McNees, C.R.; Moe, L.A. The Microbiology of Hemp Retting in a Controlled Environment: Steering the Hemp Microbiome towards More Consistent Fiber Production. Agronomy 2020, 10, 492. [Google Scholar] [CrossRef]
- van der Werf, H.M.G.; Turunen, L. The environmental impacts of the production of hemp and flax textile yarn. Ind. Crop. Prod. 2008, 27, 1–10. [Google Scholar] [CrossRef]
- Baley, C. Analysis of the flax fibres tensile behaviour and analysis of the tensile stiffness increase. Compos. Part A Appl. Sci. Manuf. 2002, 33, 939–948. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J. (Ed.) Handbook of Nonwovens; Woodhead Publishing: Cambridge, UK, 2006; p. 650. [Google Scholar]
- Horrocks, A.R.; Anand, S.C. Handbook of Technical Textiles: Technical Textile Processes, 2nd ed.; Horrocks, A.R., Anand, S.C., Eds.; Woodhead Publishing: Cambridge, UK, 2015; p. 394. [Google Scholar]
- Shen, L.; Worrell, E.; Patel, M.K. Environmental impact assessment of man-made cellulose fibres. Resour. Conserv. Recycl. 2010, 55, 260–274. [Google Scholar] [CrossRef]
- Perepelkin, K.E. Ways of developing chemical fibres based on cellulose: Viscose fibres and their prospects. Part 1. Development of viscose fibre technology. Alternative hydrated cellulose fibre technology. Fibre Chem. 2008, 40, 10–23. [Google Scholar] [CrossRef]
- Strunk, P.; Lindgren, A.; Eliasson, B.; Agnemo, R. Chemical changes of cellulose pulps in the processing to viscose dope. Cellul. Chem. Technol. 2012, 46, 559–569. [Google Scholar]
- Hernberg, S.; Tolonen, M.; Nurminen, M. Eight-year follow-up of viscose rayon workers exposed to carbon disulfide. Scand. J. Work. Environ. Health. 1976, 2, 27–30. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Razumeev, K.E.; Kalyamina, E.Y.; Aniskova, V.A.; Fedorova, N.E.; Egorova, M.A.; Kozlov, A.A. Production of Technical Nonwoven Materials. Fibre Chem. 2023, 55, 125–128. [Google Scholar] [CrossRef]
- Pividal, P.; Rocha, A. Development and mechanical characterization of needle-punched kapok-flax nonwovens for technical uses. J. Nat. Fibers 2019, 18, 705–716. [Google Scholar] [CrossRef]
- Makarov, I.S.; Smyslov, A.G.; Palchikova, E.E.; Vinogradov, M.I.; Shandryuk, G.A.; Levin, I.S.; Arkharova, N.A.; Kulichikhin, V.G. Nonwoven materials based on natural and artificial fibers. Cellulose 2024, 31, 1927–1940. [Google Scholar] [CrossRef]
- Shahzad, K. Hemp fiber and its composites—A review. J. Compos. Mater. 2012, 46, 973–986. [Google Scholar] [CrossRef]
- Makarov, I.S.; Smyslov, A.G.; Chernenko, D.N.; Vinogradov, M.I.; Legkov, S.A.; Levin, I.S.; Arkharova, N.A.; Kulichikhin, V.G. Preparation of Nonwoven Carbon Materials from Fabrics Based on Flax Cellulose and Viscose Fibers. Polym. Sci.-A. 2023, 65, 175–185. [Google Scholar] [CrossRef]
- Smyslov, A.G.; Vinogradov, M.I.; Kulichikhin, V.G.; Makarov, I.S.; Palchikova, E.E.; Chernenko, D.N. Non-Woven Fibrous Material. Patent RU2793403C1, 3 April 2023. [Google Scholar]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Fangueiro, R. Physical Modification of Natural Fibers and Thermoplastic Films for Composites—A Review. J. Thermoplast. Compos. Mater. 2009, 22, 135–162. [Google Scholar] [CrossRef]
- Ornaghi, H.L., Jr.; Poletto, M.; Zattera, A.J.; Amico, S.C. Correlation of the thermal stability and the decomposition kinetics of six different vegetal fibers. Cellulose 2014, 21, 177–188. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zeni, M.; Zattera, A.J. Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes. Polym. Degrad. Stab. 2011, 96, 679–685. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Badı’a, J.D.; Santonja-Blasco, L.; Moriana, R.; Ribes-Greus, A. Thermal analysis applied to the characterization of degradation in soil of polyactide: II. On the thermal stability and thermal decomposition kinetics. Polym. Degrad. Stab. 2010, 95, 2192–2199. [Google Scholar] [CrossRef]
- Demirbas, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Manya, J.J.; Velo, E.; Puigjaner, L. Kinetics of biomass pyrolysis: A reformulated three-parallel-reactions model. Ind. Eng. Chem. Res 2003, 42, 434–441. [Google Scholar] [CrossRef]
- Varhegyi, G.; Antal, J.J.M.; Jakab, E.; Szabo, P. Kinetic modeling of biomass pyrolysis. J. Anal. Appl. Pyrolysis 1997, 42, 73–87. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.K.; Khilar, C. Influence of mineral matter on biomass pyrolysis characteristics. Fuel 1995, 74, 1812–1822. [Google Scholar] [CrossRef]
- Yang, H.P.; Yan, R.; Chin, T.; Liang, D.T.; Chen, H.P.; Zheng, C.G. TGAFTIR analysis of palm oil wastes pyrolysis. Energy Fuel 2004, 18, 1814–1821. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Pyrolysis characteristics of biomass and biomass components. Fuel 1996, 75, 987–998. [Google Scholar] [CrossRef]
- Orfao, J.; Antunes, F.; Figueiredo, J. Pyrolysis kinetics of lignocellulosic materials-three independent reaction model. Fuel 1999, 78, 349–358. [Google Scholar] [CrossRef]
- Rao, T.R.; Sharma, A. Pyrolysis rates of biomass materials. Energy 1998, 23, 973–978. [Google Scholar] [CrossRef]
- Yang, H.P.; Yan, R.; Chen, H.P.; Zheng, C.G.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuel 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Liang, Y.; Ries, M.E.; Hine, P.J. Pyrolysis activation energy of cellulosic fibres investigated by a method derived from the first order global model. Carbohydr. Polym. 2023, 305, 120518. [Google Scholar] [CrossRef]
- Milosavljevic, I.; Suuberg, E.M. Cellulose Thermal Decomposition Kinetics: Global Mass Loss Kinetics. Ind. Eng. Chem. Res. 1995, 34, 1081–1091. [Google Scholar] [CrossRef]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B. Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Martí-Rossell’o, T.; Zhang, X. Experimental study on the ignition characteristics of cellulose, hemicellulose, lignin and their mixtures. J Energy Inst. 2019, 92, 1303–1312. [Google Scholar] [CrossRef]
- Fan, Y.; Cai, Y.; Li, X.; Jiao, L.; Xia, J.; Deng, X. Effects of the cellulose, xylan and lignin constituents on biomass pyrolysis characteristics and bio-oil composition using the simplex lattice mixture design method. Energy Convers. Manag. 2017, 138, 106–118. [Google Scholar] [CrossRef]
- Mamleev, V.; Bourbigot, S.; Yvon, J. Kinetic analysis of the thermal decomposition of cellulose: The main step of mass loss. J. Anal. Appl. Pyrolysis 2007, 80, 151–165. [Google Scholar] [CrossRef]
- Smyslov, A.G. Method for Producing Cellulose. Patent RU2779000C1, 9 September 2022. [Google Scholar]
- GOST 6840-78; Method for Determination of Alpha-Cellulose Content. Russian State Standard: Moscow, Russia, 1984. Available online: https://meganorm.ru/Data2/1/4294822/4294822889.pdf (accessed on 19 March 2025).
- GOST 6841-77; Cellulose. Method for Determination of Resins and Fats. Russian State Standard: Moscow, Russia, 2023. Available online: https://files.stroyinf.ru/Data2/1/4294822/4294822888.pdf (accessed on 19 March 2025).
- GOST 16932-93; Cellulose. Determination of Dry Matter Content. Russian State Standard: Moscow, Russia, 2023. Available online: https://meganorm.ru/Data2/1/4294835/4294835684.pdf (accessed on 19 March 2025).
- GOST 25438-82; Methods for Determining Intrinsic Viscosity. Russian State Standard: Moscow, Russia, 2015. Available online: https://meganorm.ru/Data2/1/4294828/4294828997.pdf (accessed on 19 March 2025).
- French, A.D.; Santiago Cintron, M. Cellulose polymorphy, crystallite size, and the segal crystallinity index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Fink, H.P.; Hofmann, D.; Philipp, B. Some aspects of lateral chain order in cellulosics from X-ray scattering. Cellulose 1995, 2, 51–70. [Google Scholar] [CrossRef]
- GOST 10213.4-2002; Textile Fibers. Determination of Fiber Length and Their Distribution by Length (by Measuring Individual Fibers). Russian State Standard: Moscow, Russia, 2002. Available online: https://meganorm.ru/Data2/1/4294816/4294816682.pdf (accessed on 19 March 2025).
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Mclean, D.S.; Stack, K.R.; Lu, X.; Strand, A.; Sundberg, A. Self-assembly of alkyl chains of fatty acids in papermaking systems: A review of related pitch issues, hydrophobic sizing, and pH effects. BioResources 2020, 15, 4591–4635. [Google Scholar] [CrossRef]
- Makarov, I.S.; Bondarenko, G.N.; Kuznetsova, L.K. Hemicellulose in the process of obtaining cellulose fibers and membranes. Phys. Fibrous Mater. Struct. Prop. High Technol. Mater. Smartex 2016, 1, 142–149. [Google Scholar]
- Ramiah, M. Thermogravimetric and differential thermal analysis of cellulose, hemicellulose, and lignin. J. Appl. Polym. Sci. 1970, 14, 1323–1337. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Z. Effects of cellulose, hemicellulose and lignin on biomass pyrolysis kinetics. Korean J. Chem. Eng. 2020, 37, 1660–1668. [Google Scholar] [CrossRef]
- Kim, U.-J.; Eom, S.H.; Wada, M. Thermal decomposition of native cellulose: Influence on crystallite size. Polym. Degrad. Stab. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Forte, M.M.C.; Santana, R.M.C. Thermal decomposition of wood: Influence of wood components and cellulose crystallite size. Bioresour. Technol. 2012, 109, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Capart, R.; Khezami, L.; Burnhamb, A.K. Assessment of Various Kinetic Models for the Pyrolysis of a Microgranular Cellulose. Thermochim. Acta 2004, 417, 79–89. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, Y.; Chen, H.; Yang, H. Pyrolysis Chemistry and Mechanisms: Interactions of Primary Components. In Production of Biofuels and Chemicals with Pyrolysis. Biofuels and Biorefineries; Fang, Z., Smith, R.L., Jr., Xu, L., Eds.; Springer: Singapore, 2020; Volume 10, pp. 113–137. [Google Scholar] [CrossRef]
- Zaichenko, V.M.; Lavrenov, V.A.; Faleeva, Y.M. Study of the Slow Pyrolysis of Lignin, Hemicellulose, and Cellulose and the Effect of Their Interaction in Plant Biomas. Solid Fuel Chem. 2023, 6, 66–74. [Google Scholar] [CrossRef]
- Johansen, J.M.; Jensen, P.A.; Glarborg, P.; De Martini, N.; Ek, P.; Mitchell, R.E. High Heating Rate Devolatilization Kinetics of Pulverized Biomass Fuels. Energy Fuels 2018, 32, 12955–12961. [Google Scholar] [CrossRef]
- Tabakaev, R.B.; Astafev, A.V.; Ivashutenko, A.S.; Yazykov, N.A.; Zavorin, A.S. Bulletin of the Tomsk Polytechnic University. Geo Assets Eng. 2021, 332, 74–84. [Google Scholar]
- Prusov, A.N.; Prusova, S.M.; Zakharov, A.G.; Bazanov, A.V.; Smirnov, P.R.; Radugin, M.V. Chemical transformation of technical fiber of flax, hemp and jute to cellulose and their pyrolysis. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 2016, 59, 97–104. [Google Scholar]
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-Reinforced Polymer Composites: Manufacturing, Properties, and Applications. Polymers 2019, 11, 1667. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic Bionanocomposites: A Review of Preparation, Properties and Applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Luo, L.; Guo, X.; Zhang, Z.; Chai, M.; Rahman, M.M.; Zhang, X.; Cai, J. Insight into pyrolysis kinetics of lignocellulosic biomass: Isoconversional kinetic analysis by the modified friedman method. Energy Fuels 2020, 34, 4874–4881. [Google Scholar] [CrossRef]
- Özsin, G. Assessing thermal behaviours of cellulose and poly (methyl methacrylate) during co-pyrolysis based on an unified thermoanalytical study. Bioresour. Technol. 2020, 300, 122700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Feng, Q.; Hu, J.; Sun, G.; Evrendilek, F.; Liu, H.; Liu, J. Performance and mechanism of bamboo residues pyrolysis: Gas emissions, by-products, and reaction kinetics. Sci. Total Environ. 2022, 838, 156560. [Google Scholar] [CrossRef] [PubMed]
- Dumanlı, A.G.; Windle, A.H.J. Mater. Sci. 2012, 47, 4236; Dumanlı, A.G.; Windle, A.H. Carbon fibres from cellulosic precursors: A review. J. Mater. Sci. 2012, 47, 4236–4250. [Google Scholar] [CrossRef]
- Karacan, I.; Soy, T. Enhancement of Oxidative Stabilization of Viscose Rayon Fibers Impregnated with Ammonium Sulfate Prior to Carbonization and Activation Steps. J. Appl. Polym. Sci. 2013, 128, 1239–1249. [Google Scholar] [CrossRef]
- Biranje, S.S.; Kaushik, S.; Marewad, D.; Yadav, A.; Vankundre, V.; Panse, M.; Joshi, I.; Goli, A.; Shahid, M.; Kulkarni, K.; et al. Applications of nanocellulose and its derivatives in developing sustainable textiles. Cellulose 2024, 31, 5343–5379. [Google Scholar] [CrossRef]
- Zhang, J.; Fleury, E.; Chen, Y.; Brook, M. Flame retardant lignin-based silicone composites. RSC Adv. 2015, 5, 103907–103914. [Google Scholar] [CrossRef]
- Chen, H.Q.; Xu, Y.J.; Jiang, Z.M.; Jin, X.; Liu, Y.; Zhang, L.; Zhang, C.J.; Yan, C. The thermal degradation property and flame-retardant mechanism of coated knitted cotton fabric with chitosan and APP by LBL assembly. J. Therm. Anal. Calorim. 2020, 140, 591–602. [Google Scholar] [CrossRef]

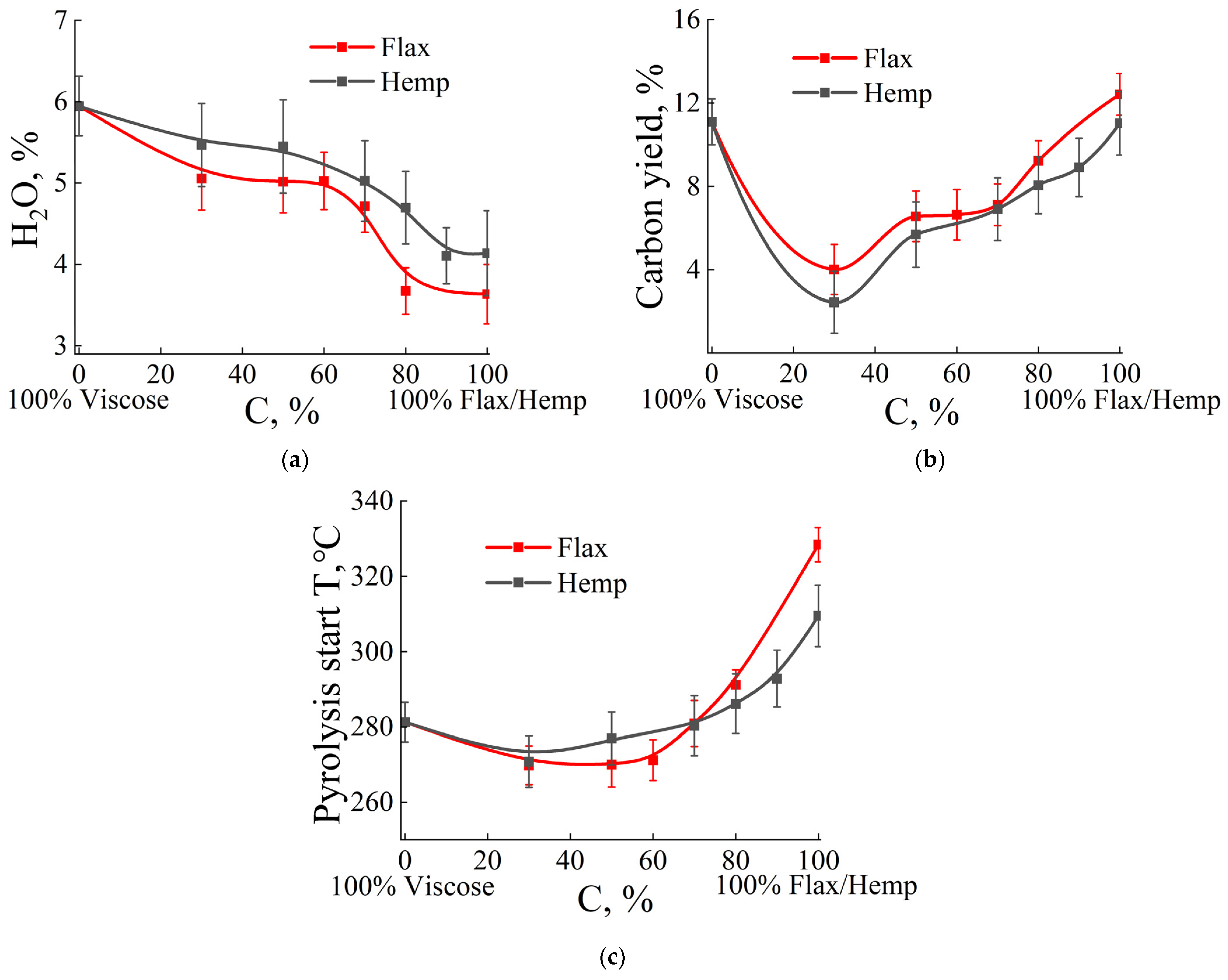


| Fiber Type | α—Fraction, % | H2O, % | Degree of Polymerization | Fats and Resins, % |
|---|---|---|---|---|
| Flax | 97.4 | 5 | >1000 | 0.48 |
| Hemp | 98 | 4.2 | >1000 | 0.43 |
| Viscose | 91 | 5.9 | 256 | 0.29 |
| Element | Content in Fibers, ppm | ||
|---|---|---|---|
| Flax | Viscose | Hemp | |
| Al | 228 | 44 | 116 |
| Ca | 899 | 105 | 2905 |
| Fe | 130 | 69 | 177 |
| K | 102 | 132 | 77 |
| Mg | 260 | 12 | 640 |
| Na | 614 | 2785 | 1637 |
| Zn | 13 | 38 | 23 |
| Sample | Water, % | Pyrolysis Start Temperature, °C | Carbon Yield, % | T of Maximum Rate of Water Loss, °C | T Maximum Rate of the Mass Loss, °C | Maximum Rate of the Mass Loss, 1/°C |
|---|---|---|---|---|---|---|
| 100% Flax | 3.63 ± 0.365 | 328.41 ± 4.56 | 12.415 ± 1 | 79.87 ± 1.98 | 365.55 ± 4 | −0.018 ± 0.0011 |
| 80% Flax/20% Viscose | 3.673 ± 0.287 | 291.24 ± 3.94 | 9.27 ± 0.97 | 79.6 ± 2.05 | 359.32 ± 4.31 | −0.014 ± 0.001 |
| 70% Flax/30% Viscose | 4.716 ± 0.321 | 280.96 ± 6.1 | 7.12 ± 1 | 84.7 ± 4.72 | 358.13 ± 3.96 | −0.014 ± 0.0011 |
| 60% Flax/40% Viscose | 5.027 ± 0.353 | 271.19 ± 5.46 | 6.64 ± 1.21 | 82.64 ± 3.84 | 345.29 ± 3.84 | −0.0145 ± 0.0012 |
| 50% Flax/50% Viscose | 5.015 ± 0.379 | 270.03 ± 6 | 6.56 ± 1.21 | 79.99 ± 3.46 | 337.04 ± 4.5 | −0.0149 ± 0.0012 |
| 30% Flax/70% Viscose | 5.055 ± 0.385 | 269.78 ± 5.17 | 4.02 ± 1.2 | 79.85 ± 1.57 | 336.6 ± 4.71 | −0.0151 ± 0.0012 |
| 100% Viscose | 5.946 ± 0.368 | 281.3 ± 5.3 | 11.1 ± 1.1 | 83.75 ± 2.74 | 336.3 ± 4.23 | 0.015 ± 0.001 |
| Sample | Water, % | Pyrolysis Start Temperature, °C | Carbon Yield, % | T of Maximum Rate of Water Loss, °C | T Maximum Rate of the Mass Loss, °C | Maximum Rate of the Mass Loss, 1/°C |
|---|---|---|---|---|---|---|
| 100% Hemp | 4.139 ± 0.522 | 309.54 ± 8.17 | 11.03 ± 1.53 | 82.68 ± 1.49 | 360.01 ± 5.98 | −0.0182 ± 0.001 |
| 90% Hemp/10% Viscose | 4.107 ± 0.345 | 292.87 ± 7.55 | 8.91 ± 1.4 | 84 ± 1.18 | 357.95 ± 5.27 | −0.0175 ± 0.001 |
| 80% Hemp/20% Viscose | 4.698 ± 0.447 | 286.17 ± 7.93 | 8.06 ± 1.37 | 84.27 ± 2.09 | 352.04 ± 4.98 | −0.0138 ± 0.001 |
| 70% Hemp/30% Viscose | 5.027 ± 0.496 | 280.37 ± 8.01 | 6.91 ± 1.5 | 82.57 ± 2.14 | 353.06 ± 6.09 | −0.0131 ± 0.001 |
| 50% Hemp/50% Viscose | 5.452 ± 0.572 | 277 ± 7.03 | 5.69 ± 1.57 | 81.68 ± 2.04 | 340.73 ± 6.01 | −0.0138 ± 0.001 |
| 30% Hemp/70% Viscose | 5.469 ± 0.512 | 270.8 ± 6.87 | 2.44 ± 1.49 | 80.25 ± 1.52 | 340.43 ± 5.78 | −0.0141 ± 0.001 |
| 100% Viscose | 5.946 ± 0.368 | 281.3 ± 5.3 | 11.1 ± 1.1 | 83.75 ± 2.74 | 336.3 ± 4.23 | −0.015 ± 0.001 |
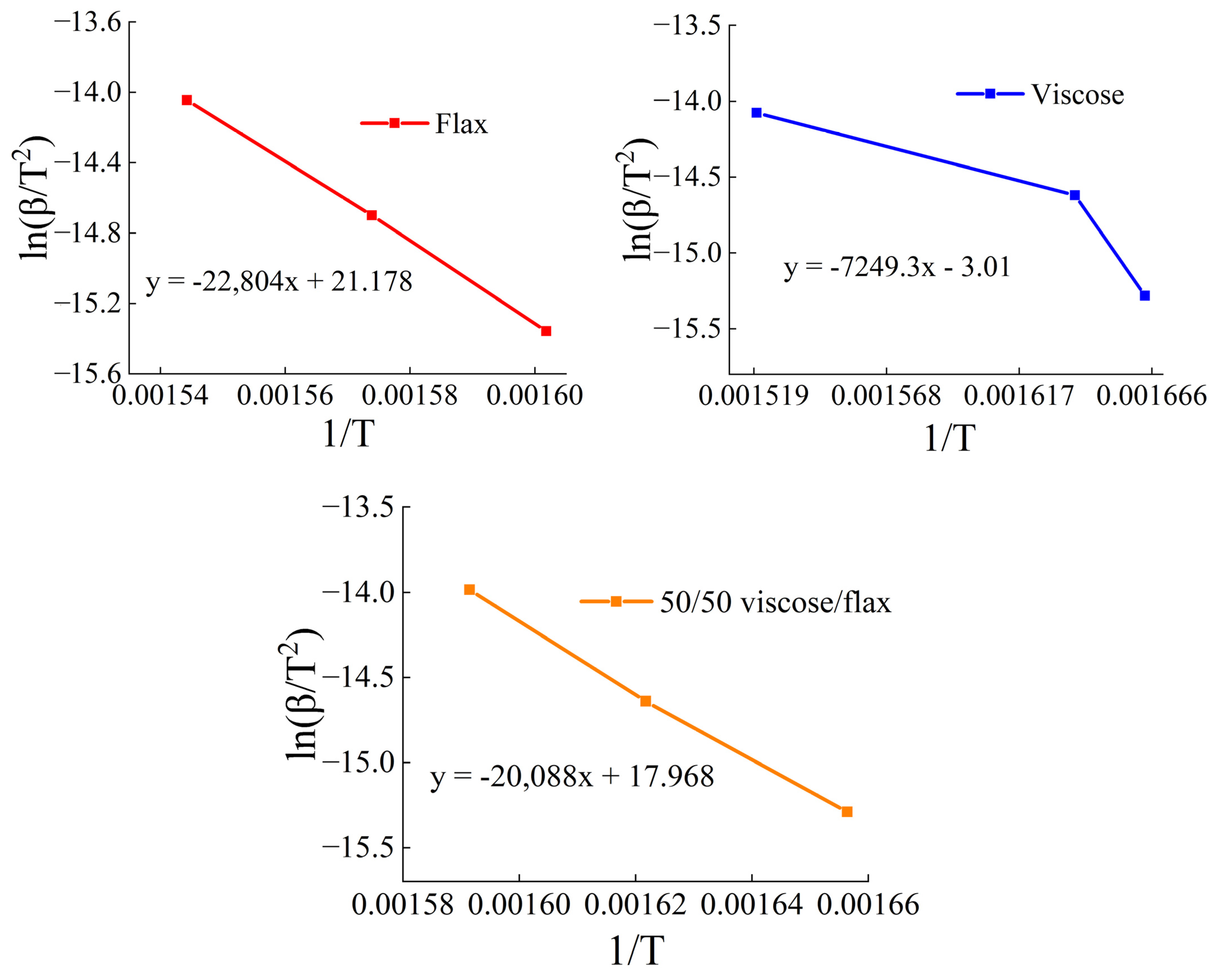
| β, K/min | Tp, °C | ||
|---|---|---|---|
| Flax | 50% Flax/50%Viscose | Viscose | |
| 5 | 351.28 | 330.72 | 328.16 |
| 10 | 362.36 | 343.62 | 337.65 |
| 20 | 374.55 | 355.35 | 384.86 |
| Sample | Ea, kJ/mol |
|---|---|
| Flax | 189.59 |
| 50% Viscose/50% Flax | 167.01 |
| Viscose | 60.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalauova, A.S.; Palchikova, E.E.; Makarov, I.S.; Shandryuk, G.A.; Abilkhairov, A.I.; Kalimanova, D.Z.; Naukenov, M.Z.; Shambilova, G.K.; Novikov, E.M.; Song, J.; et al. Specificity of Thermal Destruction of Nonwoven Mixture Systems Based on Bast and Viscose Fibers. Polymers 2025, 17, 1223. https://doi.org/10.3390/polym17091223
Kalauova AS, Palchikova EE, Makarov IS, Shandryuk GA, Abilkhairov AI, Kalimanova DZ, Naukenov MZ, Shambilova GK, Novikov EM, Song J, et al. Specificity of Thermal Destruction of Nonwoven Mixture Systems Based on Bast and Viscose Fibers. Polymers. 2025; 17(9):1223. https://doi.org/10.3390/polym17091223
Chicago/Turabian StyleKalauova, Altynay S., Ekaterina E. Palchikova, Igor S. Makarov, Georgiy A. Shandryuk, Amangeldi I. Abilkhairov, Danagul Zh. Kalimanova, Meirbek Zh. Naukenov, Gulbarshin K. Shambilova, Egor M. Novikov, Junlong Song, and et al. 2025. "Specificity of Thermal Destruction of Nonwoven Mixture Systems Based on Bast and Viscose Fibers" Polymers 17, no. 9: 1223. https://doi.org/10.3390/polym17091223
APA StyleKalauova, A. S., Palchikova, E. E., Makarov, I. S., Shandryuk, G. A., Abilkhairov, A. I., Kalimanova, D. Z., Naukenov, M. Z., Shambilova, G. K., Novikov, E. M., Song, J., & Smyslov, A. G. (2025). Specificity of Thermal Destruction of Nonwoven Mixture Systems Based on Bast and Viscose Fibers. Polymers, 17(9), 1223. https://doi.org/10.3390/polym17091223







