Hydrogel-Based Systems as Smart Food Packaging: A Review
Abstract
1. Introduction
2. Current Requirements for Food Packaging
3. Hydrogel-Based Films for Food Packaging
3.1. Synthetic Hydrogel-Based Food Packaging
3.2. Natural Hydrogel-Based Food Packaging
3.3. Hybrid Hydrogel-Based Food Packaging
4. Hydrogel-Based AP
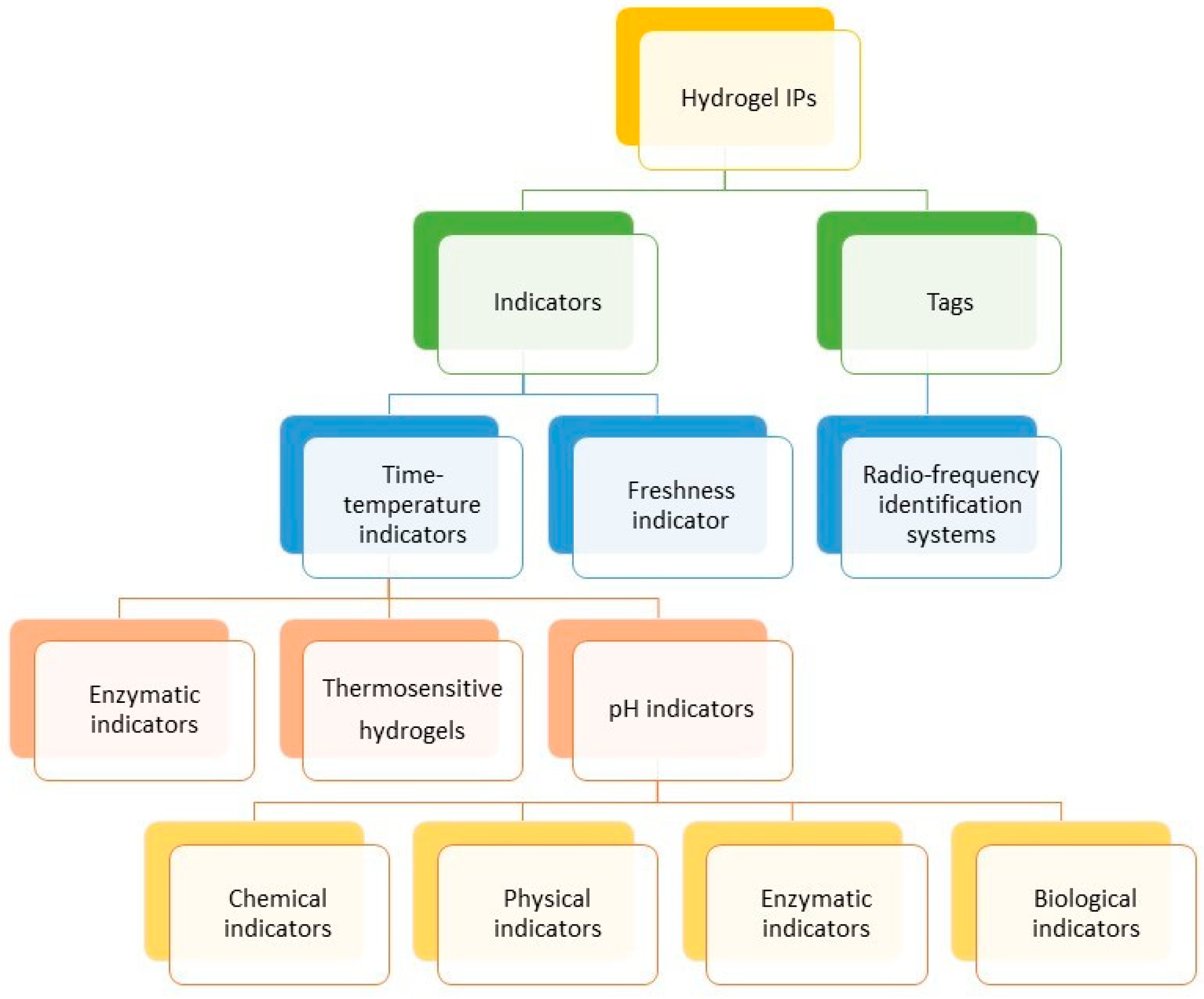
5. Hydrogel-Based IP
5.1. Time–Temperature Indicators (TTIs)
5.2. Radio-Frequency Identification Systems (RFID)
5.3. Freshness Indicators
6. Combination of Active and Intelligent Hydrogel-Based Packaging
7. Comparison and Discussion of the Mechanical Properties of Conventional Non-Biodegradable and Degradable Food Packaging, and Currently Studied Approaches That Are Essential for Food Packaging
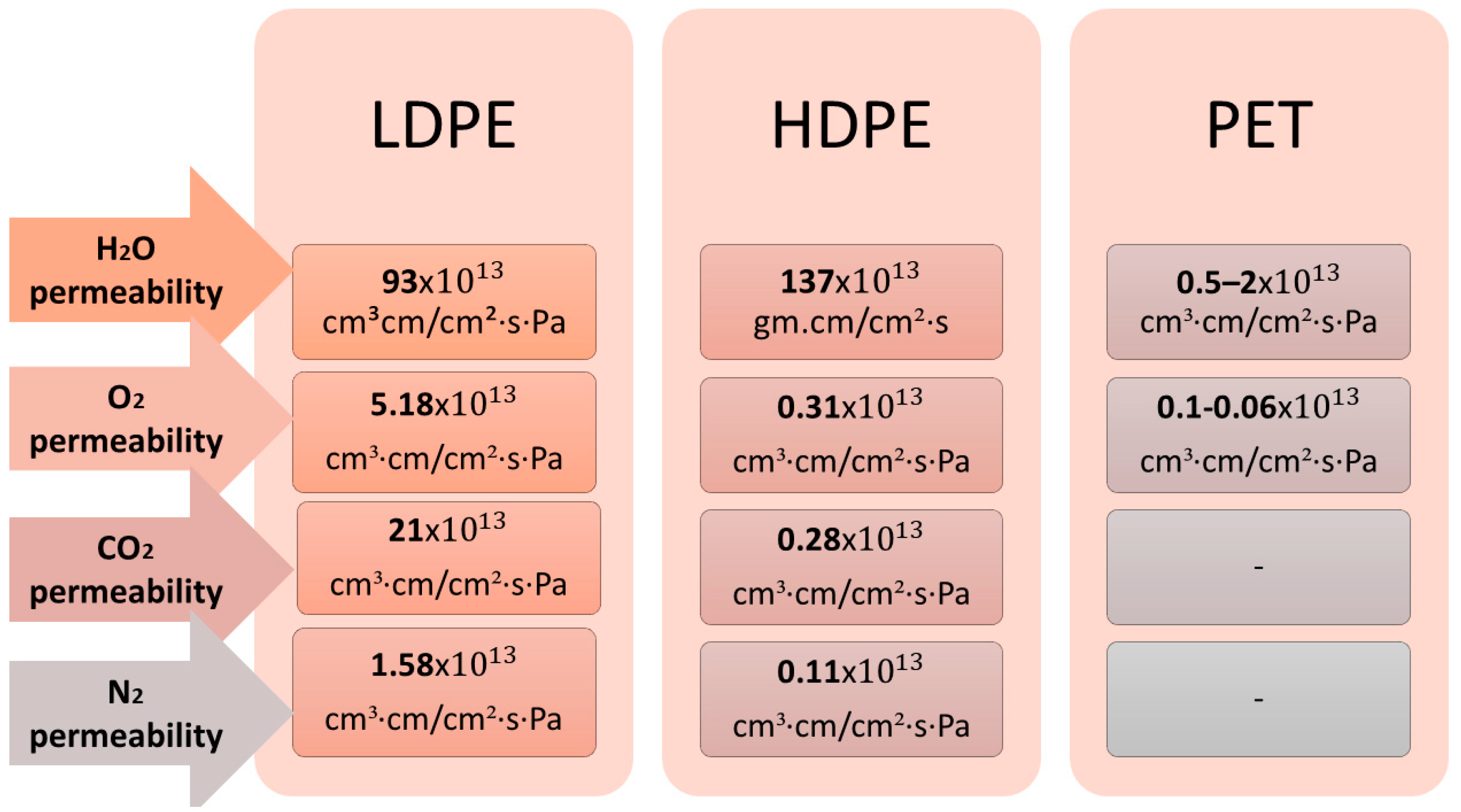
| Material | Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation (%) | Type of Food Packaging | Refs. |
|---|---|---|---|---|---|
| Low-Density Polyethylene (LDPE) | 0.11–0.45 | 2.7–200 | 100–956 |
| [103,144,145] |
| High-Density Polyethylene (HDPE) | 0.6–1.1 | 17–45 | 10–1200 |
| [144,145,146] |
| PP | 1.1–1.5 | 31–43 | 500–650 |
| [144,145] |
| PET | 2.8–4.1 | 48–270 | 45–100 |
| [144,145] |
| PC | 2.4 | 65.5 | 110 |
| [142,147] |
| PVA | - | 37.5 | 126 |
| [119,148] |
| Polyvinylidene Chloride (PVDC) | 0.3–1.1 | 48–148 | 40–100 |
| [142,144] |
| EVOH | 2.1–2.6 | 59–77 | 230–380 |
| [142,144] |
| PA | 0.69–1.7 | 41–165 | 300–400 |
| [144] |
| PS | - | 30–60 | - |
| [142,145] |
| PCV | up to 4.1 | 10–55 | 14–450 |
| [144,145] |
| PTFE | - | 7–28 | - | - | [142] |
| Material | Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation (%) | Type of Food Packaging | Refs. |
|---|---|---|---|---|---|
| PLA | 2.3 | 32 | 5 |
| [142] |
| PCL | 0.21–0.44 | 20–42 | 2.5–6 |
| [142,149] |
| PHB | 3.5–4 | 43 | 5–8 |
| [142,149] |
| PHBV | 0.2 | 25–40 | 13–20 |
| [142,150,151] |
| Material | Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation (%) | Extending Shelf Life | Refs. |
|---|---|---|---|---|---|
| TOCNF3/CPNIPAM-AM | 5.4 × 10−6 | - | 20.1 | 5 days | [76] |
| CS/PU/EBs | - | 0.77–4.90 | 135–196 | - | [69] |
| HPMC/SKEO | 0.003–0.013 | 19–44 | 10–17 | 30 days | [82] |
| CPTE | 0.003 | 0.09 | c.a. 210 | 7 days | [83] |
| CMC/PVA/PEI/TA | - | 0.38 | 400 | 7 days | [71] |
| CMC/MMT/ ε-PL | 0.317 | 9.2 | 22.5 | 2 days | [152] |
| CMC/SA /Thymus vulgaris purified leaves extract (TVE). | - | 62.2 | 61.1 | 25 days | [153] |
| CMC/PVA/Aloe vera | - | 12.8 | 30 | 13 days | [154] |
| PU/CS/ZnO NPs | - | 8.1 | 2 | 9 days | [90] |
| MC/GA/ZnO NPs | 2.42 | 60.3 | 8 | 27 days | [93] |
| CMC/GEL/ZnO NPs | 0.8 | 45 | 10 | - | [94] |
| CMC/PVA/CuS NPs | 0.03 | 0.034 | 180 | 6 days | [84] |
| GEL/CMC, PVA, and SiO2NPs | - | 6.81 | 118 | - | [95] |
| CMC/NFC/KMnO4 | 0.1 | 34 | 180 | 30 days | [96] |
| Material | Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation (%) | Extending Shelf Life | Refs. |
|---|---|---|---|---|---|
| PVP-CMC-BC-GG | 0.94 | 25.9 | 21 | 15 days | [140] |
| PVP-CMC-BC-GG-EOs | 1.4–1.87 GPa | c.a. 39 MPa | c.a. 6 | 12 days | [138] |
| CS/BSSCE | 20–23 MPa | 65–74 | - | [137] | |
| PVA/CS/PTA/CaCl2 | - | 39 MPa | 56.5 | - | [139] |
| PVA-CS/nano-ZnO/SA/cyanidin chloride | - | 23–31 MPa | 20–34 | - | [139] |
| CG-ELE | - | 13.2 MPa | 5 | - | [120] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef] [PubMed]
- Sudheer, S.; Bandyopadhyay, S.; Bhat, R. Sustainable polysaccharide and protein hydrogel-based packaging materials for food products: A review. Int. J. Biol. Macromol. 2023, 248, 125845. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.K.; Shakya, S.; Kumar, P.; Madhavi, J.; Murugaiyan, J.; Rao, M.V.R. Trends in packaging material for food products: Historical background, current scenario, and future prospects. J. Food Sci. Technol. 2021, 58, 4069–4082. [Google Scholar] [CrossRef]
- Romão, S.; Bettencourt, A.; Ribeiro, I.A.C. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968. [Google Scholar] [CrossRef] [PubMed]
- PlasticsEurope. Circular Economy for Plastics—An European Analysis. 2024. Available online: https://plasticseurope.org/knowledge-hub/the-circular-economy-for-plastics-a-european-analysis-2024/ (accessed on 19 March 2024).
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef]
- Bauer, A.-S.; Tacker, M.; Uysal-Unalan, I.; Cruz, R.M.S.; Varzakas, T.; Krauter, V. Recyclability and Redesign Challenges in Multilayer Flexible Food Packaging—A Review. Foods 2021, 10, 2702. [Google Scholar] [CrossRef]
- Pivnenko, K.; Eriksen, M.K.; Martín-Fernández, J.A.; Eriksson, E.; Astrup, T.F. Recycling of plastic waste: Presence of phthalates in plastics from households and industry. Waste Manag. 2016, 54, 44–52. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, P.J.P.; Quintans, J.D.S.S.; Freitas, M.M.; Cerqueira, M.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef]
- Badía, J.D.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Thermal analysis as a quality tool for assessing the influence of thermo-mechanical degradation on recycled poly (ethylene terephthalate). Polym. Test. 2009, 28, 169–175. [Google Scholar] [CrossRef]
- Müller, A.J.; Feijoo, J.L.; Alvarez, M.E.; Febles, A.C. The calorimetric and mechanical properties of virgin and recycled poly (ethylene terephthalate) from beverage bottles. Polym. Eng. Sci. 1987, 27, 796–803. [Google Scholar] [CrossRef]
- Yadav, P.; Silvenius, F.; Katajajuuri, J.M.; Leinonen, I. Life cycle assessment of reusable plastic food packaging. J. Clean. Prod. 2024, 448, 141529. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Barrow, C.J.; Adhikari, B. The future of bioplastics in food packaging: An industrial perspective. Food Packag. Shelf Life 2024, 43, 101279. [Google Scholar] [CrossRef]
- Bucci, D.Z.; Tavares LB, B.; Sell, I. Biodegradation and physical evaluation of PHB packaging. Polym. Test. 2007, 26, 908–915. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Kolbuk, D.; Mikulowski, G.; Ciechomska, I.A.; Sajkiewicz, P. Methylcellulose/agarose hydrogel loaded with short electrospun PLLA/laminin fibers as an injectable scaffold for tissue engineering/3D cell culture model for tumour therapies. RSC Adv. 2023, 13, 11889–11902. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Carvalho, A.; Gil, M.H.; Mendes, A.; Bártolo, P.J. Influence of Aloe vera on water absorption and enzymatic in vitro degradation of alginate hydrogel films. Carbohydr. Polym. 2013, 98, 311–320. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Sáha, P. Biodegradable hydrogel film for food packaging. In Recent Researches in Geography, Geology, Energy, Environment and Biomedicine, Proceedings of the 4th WSEAS International Conference on EMESEG’11, Corfu Island, Greece, 14–16 July 2011; World Scientific and Engineering Academy and Society (WSEAS): Attica, Greece, 2011; pp. 329–334. [Google Scholar]
- Platt, D. The future of biodegradable films for flexible packaging. In The Future of Biodegradable Films for Flexible Packaging; Pira International Ltd.: Letherhead, UK, 2006. [Google Scholar]
- Robertson, G. State-of-the-art biobased food packaging materials. In Environmentally Compatible Food Packaging; Woodhead Publishing: Sawston, CA, USA, 2008. [Google Scholar]
- Thapliyal, D.; Karale, M.; Diwan, V.; Kumra, S.; Arya, R.K.; Verros, G.D. Current status of sustainable food packaging regulations: Global perspective. J. Sustain. 2024, 16, 5554. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- de Carvalho, A.P.A.; Junior, C.A.C. Green strategies for active food packagings: A systematic review on active properties of graphene-based nanomaterials and biodegradable polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of polysaccharide-based materials as advanced food packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Int. Food Res. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Al Mahmud, M.Z.; Mobarak, M.H.; Hossain, N. Emerging trends in biomaterials for sustainable food packaging: A comprehensive review. Heliyon 2024, 10, e24122. [Google Scholar] [CrossRef]
- Enríquez-Martínez, V.; Niembro-García, I.J.; Marmolejo-Saucedo, J.A. A Life Cycle Assessment (LCA) of Antibacterial Gel Production. In Computer Science and Engineering in Health Services, Proceedings of the 4th EAI International Conference, COMPSE 2020, Online, 26 November 2020; Marmolejo-Saucedo, J.A., Vasant, P., Litvinchev, I., Rodríguez-Aguilar, R., Saucedo-Martínez, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 12–27. [Google Scholar]
- Yates, M.R.; Barlow, C.Y. Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Banerjee, R.; Ray, S.S. Sustainability and life cycle assessment of thermoplastic polymers for packaging: A review on fundamental principles and applications. Macromol. Mater. Eng. 2022, 307, 2100794. [Google Scholar] [CrossRef]
- Piringer, O.G.; Baner, A.L. Permeation of gases, water vapor and volatile organic compounds. In Plastic Packaging Materials for Food: Barrier Function, Mass Transport, Quality Assurance and Legislation; John Wiley & Sons: Hoboken, NJ, USA, 2000; pp. 239–243. [Google Scholar]
- Michaels, A.S.; Bixler, H.J. Solubility of gases in polyethylene. J. Polym. Sci. 1961, 50, 393–412. [Google Scholar] [CrossRef]
- Lange, J.; Wyser, Y. Recent innovations in barrier technologies for plastic packaging—A review. Packag. Technol. Sci. 2003, 16, 149–158. [Google Scholar] [CrossRef]
- Kamal, M.R.; Jinnah, I.A.; Utracki, L.A. Permeability of oxygen and water vapor through polyethylene/polyamide films. Polym. Eng. Sci. 1984, 24, 1337–1347. [Google Scholar] [CrossRef]
- Ghosal, K.; Ghosh, S. Biodegradable polymers from lignocellulosic biomass and synthetic plastic waste: An emerging alternative for biomedical applications. Mater. Sci. Eng. R Rep. 2023, 156, 100761. [Google Scholar] [CrossRef]
- de Carvalho, A.P.A.; Conte-Junior, C.A. Food-derived biopolymer kefiran composites, nanocomposites and nanofibers: Emerging alternatives to food packaging and potentials in nanomedicine. Trends Food Sci. Technol. 2021, 116, 370–386. [Google Scholar] [CrossRef]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Nanotechnologies in the food industry—Recent developments, risks and regulation. Trends Food Sci. 2012, 24, 30–46. [Google Scholar] [CrossRef]
- Khalesi, H.; Lu, W.; Nishinari, K.; Fang, Y. New insights into food hydrogels with reinforced mechanical properties: A review on innovative strategies. Adv. Colloid Interface Sci. 2020, 285, 102278. [Google Scholar] [CrossRef]
- Niemczyk, B.; Sajkiewicz, P.; Kolbuk, D. Injectable hydrogels as novel materials for central nervous system regeneration. J. Neural Eng. 2018, 15, 051002. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk-Soczynska, B.; Gradys, A.; Kolbuk, D.; Krzton-Maziopa, A.; Sajkiewicz, P. Crosslinking kinetics of methylcellulose aqueous solution and its potential as a scaffold for tissue engineering. Polymers 2019, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Espitia, P.J.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y. Recent applications of regenerated cellulose films and hydrogels in food packaging. Curr. Opin. Food Sci. 2022, 43, 7–17. [Google Scholar] [CrossRef]
- Hussain, S.; Akhter, R.; Maktedar, S.S. Advancements in sustainable food packaging: From eco-friendly materials to innovative technologies. Sustain. Food Technol. 2024, 2, 1297–1364. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Zaszczyńska, A.; Zabielski, K.; Sajkiewicz, P. Hydrogel, electrospun and composite materials for bone/cartilage and neural tissue engineering. Materials 2021, 14, 6899. [Google Scholar] [CrossRef]
- Singh, A.K.; Itkor, P.; Lee, Y.S. State-of-the-Art Insights and Potential Applications of Cellulose-Based Hydrogels in Food Packaging: Advances towards Sustainable Trends. Gels 2023, 9, 433. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Benlikaya, R.; Slobodian, P.; Saha, P. Breathable and polyol based hydrogel food packaging. J. Biobased Mater. Bioenergy 2015, 9, 136–144. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Biodegradation of PVP–CMC hydrogel film: A useful food packaging material. Carbohydr. Polym. 2012, 89, 346–353. [Google Scholar] [CrossRef]
- Campanella, G.; Ghaani, M.; Quetti, G.; Farris, S. On the origin of primary aromatic amines in food packaging materials. Trends Food Sci. Technol. 2015, 46, 137–143. [Google Scholar] [CrossRef]
- Szabó, B.S.; Petrovics, N.; Kirchkeszner, C.; Nyiri, Z.; Bodai, Z.; Eke, Z. Stability study of primary aromatic amines in aqueous food simulants under storage conditions of food contact material migration studies. Food Packag. Shelf Life 2022, 33, 100909. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Şen, F.; Uzunsoy, İ.; Başturk, E.; Kahraman, M.V. Antimicrobial agent-free hybrid cationic starch/sodium alginate polyelectrolyte films for food packaging materials. Carbohydr. Polym. 2015, 170, 264–270. [Google Scholar] [CrossRef]
- Heydari, R.; Bavandi, S.; Javadian, S.R. Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4 °C. Food Sci. Nutr. 2015, 3, 188–194. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Gong, W.; Huang, H.B.; Wang, X.C.; He, W.Y.; Hou, Y.Y.; Hu, J.N. Construction of a sustained-release hydrogel using gallic acid and lysozyme with antimicrobial properties for wound treatment. Biomater. Sci. 2022, 10, 6836–6849. [Google Scholar] [CrossRef]
- Farris, S.; Schaich, K.M.; Liu, L.; Piergiovanni, L.; Yam, K.L. Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: A review. Trends Food Sci. Technol. 2009, 20, 316–332. [Google Scholar] [CrossRef]
- Li, Y.; Shan, P.; Yu, F.; Li, H.; Peng, L. Fabrication and characterization of waste fish scale-derived gelatin/sodium alginate/carvacrol loaded ZIF-8 nanoparticles composite films with sustained antibacterial activity for active food packaging. Int. J. Biol. Macromol. 2023, 230, 123192. [Google Scholar] [CrossRef] [PubMed]
- Boccia, A.C.; Pulvirenti, A.; Cerruti, P.; Silvetti, T.; Brasca, M. Antimicrobial starch-based cryogels and hydrogels for dual-active food packaging applications. Carbohydr. Polym. 2024, 342, 122340. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; Fini, E.; Christiansen, J.D.C. The effect of pH on the viscoelastic response of alginate–montmorillonite nanocomposite hydrogels. Molecules 2024, 29, 244. [Google Scholar] [CrossRef]
- Galante, R.; Pinto, T.J.; Colaco, R.; Serro, A.P. Sterilization of hydrogels for biomedical applications: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2472–2492. [Google Scholar] [CrossRef]
- Galante, R.; Rediguieri, C.F.; Kikuchi, I.S.; Vasquez, P.A.S.; Colaco, R.; Serro, A.P.; Pinto, T.J.A.; Santos, H.A. About the sterilization of chitosan hydrogel nanoparticles. PLoS ONE 2016, 11, e0168862. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Benyathiar, P.; Mishra, D.K. Aseptic processing and packaging. In Food Safety Engineering. Food Engineering Series; Springer: Cham, Switzerland, 2020; pp. 661–692. [Google Scholar]
- Labay, C.; Hamouda, I.; Tampieri, F.; Ginebra, M.P.; Canal, C. Production of reactive species in alginate hydrogels for cold atmospheric plasma-based therapies. Sci. Rep. 2019, 9, 16160. [Google Scholar] [CrossRef]
- Kamlow, M.A.; Vadodaria, S.; Gholamipour-Shirazi, A.; Spyropoulos, F.; Mills, T. 3D printing of edible hydrogels containing thiamine and their comparison to cast gels. Food Hydrocoll. 2021, 116, 106550. [Google Scholar] [CrossRef]
- Kim, Y.H.; Priyadarshi, R.; Kim, J.W.; Kim, J.; Alekseev, D.G.; Rhim, J.W. 3D-printed pectin/carboxymethyl cellulose/ZnO bio-inks: Comparative analysis with the solution casting method. Polymers 2022, 14, 4711. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent advances in 3D printing of tough hydrogels: A review. Compos. B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Ghosal, A.; Bala, J.; Nikkhah-Moshaie, R.; Wani, W.A.; Manickam, P.; Nair, M. Nanocomposite hydrogels: Advances in nanofillers used for nanomedicine. Gels 2018, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, J.; Sun, N.; Jiang, Y.; Yu, Y.; Lai, G.; Yang, X. UV−Curable antibacterial and pH−sensitive eugenol functionalized chitosan−polyurethane hydrogels for shelf−life extension of chicken. Food Control. 2025, 168, 110918. [Google Scholar] [CrossRef]
- Wahid, F.; Bai, H.; Wang, F.P.; Xie, Y.Y.; Zhang, Y.W.; Chu, L.Q.; Zhong, C. Facile synthesis of bacterial cellulose and polyethyleneimine based hybrid hydrogels for antibacterial applications. Cellulose 2020, 27, 369–383. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Zhang, Y.; Xu, E.; Yan, S.; Xu, H.; Li, M. Recent advances in the fabrication, characterization and application of starch-based materials for active food packaging: Hydrogels and aerogels. Sustain. Food Technol. 2024, 2, 615–634. [Google Scholar] [CrossRef]
- Bodbodak, S.; Rafiee, Z. Recent trends in active packaging in fruits and vegetables. In Eco-Friendly Technology for Postharvest Produce Quality; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–125. [Google Scholar]
- Mao, S.; Ren, Y.; Wei, C.; Chen, S.; Ye, X.; Jinhu, T. Development of novel EGCG/Fe loaded sodium alginate-based packaging films with antibacterial and slow-release properties. Food Hydrocol. 2023, 145, 109032. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, L.; McClements, D.J.; Qiu, C.; Li, C.; Zhang, Z.; Miao, M.; Tian, Y.; Zhu, K.; Jin, Z. Stimulus-responsive hydrogels in food science: A review. Food Hydrocoll. 2022, 124, 107218. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Hamoud, Y.A.; Xu, X.; Liu, H.; Wang, S.; Sheteiwy, M.; Dong, F.; Guo, L.; Qian, Y.; Li, P. Thermo-/pH-responsive preservative delivery based on TEMPO cellulose nanofiber/cationic copolymer hydrogel film in fruit packaging. Int. J. Biol. Macromol. 2021, 183, 1911–1924. [Google Scholar] [CrossRef]
- Dagnas, S.; Membré, J.-M. Predicting and preventing mold spoilage of food products. J. Food Prot. 2013, 76, 538–551. [Google Scholar] [CrossRef]
- Snyder, A.B.; Martin, N.; Wiedmann, M. Microbial Food Spoilage: Impact, Causative Agents and Control Strategies. Nat. Rev. Microbiol. 2024, 22, 528–542. [Google Scholar] [CrossRef]
- Vegad, U.; Patel, M.; Khunt, D.; Zupančič, O.; Chauhan, S.; Paudel, A. pH stimuli-responsive hydrogels from non-cellulosic biopolymers for drug delivery. Front. Bioeng. Biotechnol. 2023, 11, 1270364. [Google Scholar] [CrossRef] [PubMed]
- Madivoli, E.S.; Schwarte, J.V.; Kareru, P.G.; Gachanja, A.N.; Fromm, K.M. Stimuli-Responsive and Antibacterial Cellulose-Chitosan Hydrogels Containing Polydiacetylene Nanosheets. Polymers 2023, 15, 1062. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kim, J.T.; Roy, S.; Jayakumar, A. Recent advances in carboxymethyl cellulose-based active and intelligent packaging materials: A comprehensive review. Int. J. Biol. Macromol. 2024, 259, 129194. [Google Scholar] [CrossRef] [PubMed]
- Aghajani, F.; Rafati, H.; Aliahmadi, A.; Moghimi, R. Novel nanoemulsion-loaded hydroxyl propyl methyl cellulose films as bioactive food packaging materials containing Satureja Khuzestanica essential oil. Carbohydr. Polym. Technol. Appl. 2024, 8, 100544. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Yang, Y.; Li, Y. Self-healing cellulose-based hydrogels: From molecular design to multifarious applications. Carbohydr. Polym. 2025, 347, 122738. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Guo, M.; Jin, T.Z.; Arabi, S.A.; He, Q.; Ismail, B.B.; Hu, Y.; Liu, D. Antimicrobial and UV Blocking Properties of Composite Chitosan Films with Curcumin Grafted Cellulose Nanofiber. Food Hydrocoll. 2021, 112, 106337. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Huang, L.; Chen, G.; Wei, Z.; Mo, Q.; Chen, Q. Development of chlorine dioxide sustained-release device using carboxymethyl cellulose-polyvinyl alcohol-β-cyclodextrin ternary hydrogel and a new sustained-release kinetic model. Cellulose 2023, 30, 3073–3082. [Google Scholar] [CrossRef]
- Gang, F.; Xu, M.; Zhang, S.; Zhang, C.; He, J.; Xiao, Y.; Zhang, J. Biodegradable active composite hydrogel packaging for postharvest climacteric bananas preservation. Food Chem. 2024, 442, 138494. [Google Scholar] [CrossRef]
- Yang, H.; Li, L.; Li, C.; Xu, Z.; Tao, Y.; Lu, J.; Wang, H. Multifunctional and antimicrobial carboxymethyl cellulose-based active hydrogel film for fruits packaging and preservation. Food Biosci. 2024, 59, 104005. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, S.; Xia, X.; Tan, M.; Lv, Y.; Cheng, Y.; Wang, H. High-performance carboxymethyl cellulose-based hydrogel film for food packaging and preservation system. Int. J. Biol. Macromol. 2022, 223, 1126–1137. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, G.; Jiang, Q.; Fan, J.; Wang, S.; Chen, J. Carboxymethyl cellulose-based photothermal film: A sustainable packaging with high barrier and tensile strength for food long-term antibacterial protection. Int. J. Biol. Macromol. 2024, 276, 133910. [Google Scholar] [CrossRef] [PubMed]
- Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan–zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Kurabetta, L.K.; Masti, S.P.; Gunaki, M.N.; Hunashyal, A.A.; Eelager, M.P.; Chougale, R.B.; Kumar, S.P. A synergistic influence of gallic acid/ZnO NPs to strengthen the multifunctional properties of methylcellulose: A conservative approach for tomato preservation. Int. J. Biol. Macromol. 2024, 277, 134191. [Google Scholar] [CrossRef]
- Zafar, A.; Khosa, M.K.; Noor, A.; Qayyum, S.; Saif, M.J. Carboxymethyl cellulose/gelatin hydrogel films loaded with zinc oxide nanoparticles for sustainable food packaging applications. Polymers 2022, 14, 5201. [Google Scholar] [CrossRef]
- Sagar, S.; Khalid, U.; Azim, W.; Kanwal, M.; Hossain, N. Reinforcement of biodegradable SiO2NPs-modified cellulose-gelatin hydrogel films with antioxidant and antibacterial properties as potential food packaging composite. J. Clust. Sci. 2024, 35, 1613–1624. [Google Scholar] [CrossRef]
- Pirsa, S. Nanocomposite base on carboxymethylcellulose hydrogel: Simultaneous absorbent of ethylene and humidity to increase the shelf life of banana fruit. Int. J. Biol. Macromol. 2021, 193, 300–310. [Google Scholar] [CrossRef]
- Bhasney, S.; Dhar, P.; Katiyar, V. Sustainable Polymers for Food Packaging: An Introduction; Katiyar, V., Ed.; De Gruyter: Berlin, Germany, 2020; p. 104. [Google Scholar]
- Auras, R.A.; Singh, S.P.; Singh, J.J. Evaluation of oriented poly (lactide) polymers vs. existing PET and oriented PS for fresh food service containers. Packag. Technol. Sci. 2005, 18, 207–216. [Google Scholar] [CrossRef]
- Kathuria, A.; Abiad, M.G.; Auras, R. Toughening of poly(L-lactic acid) with Cu3BTC2 metal organicframework crystals. Polymers 2013, 54, 6979–6986. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Investigating the toxicological effects of nanomaterials in food packaging associated with human health and the environment. JHM Lett. 2024, 5, 100125. [Google Scholar] [CrossRef]
- Ahari, H.; Lahijani, L.K. Migration of silver and copper nanoparticles from food coating. Coatings 2021, 11, 380. [Google Scholar] [CrossRef]
- Râpă, M.; Stefan, M.; Popa, P.A.; Toloman, D.; Leostean, C.; Borodi, G.; Matei, E. Electrospun nanosystems based on PHBV and ZnO for ecological food packaging. Polymers 2021, 13, 2123. [Google Scholar] [CrossRef] [PubMed]
- Athauda, T.; Banerjee, P.C.; Karmakar, N.C. Microwave characterization of chitosan hydrogel and its use as a wireless pH sensor in smart packaging applications. IEEE Sens. J. 2020, 20, 8990–8996. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Luo, Y.; Wu, T.; Chen, X.; Wang, Y.; Xie, J. Advanced applications of chitosan-based hydrogels: From biosensors to intelligent food packaging system. Trends Food Sci. Technol. 2021, 110, 822–832. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Q.; Li, T.T.; Lou, C.W.; Hung, C.Y.; Lin, J.H. Exploration and application prospect of the advanced technology of time–temperature indicators. Text. Res. J. 2025, 95, 404–428. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Zhang, M.; Xu, T.; Du, H.; Pang, B.; Si, C. Sustainable polysaccharide-based materials for intelligent packaging. Carbohydr. Polym. 2023, 313, 120851. [Google Scholar] [CrossRef]
- Du, H.; Sun, X.; Chong, X.; Yang, M.; Zhu, Z.; Wen, Y. A review on smart active packaging systems for food preservation: Applications and future trends. Trends Food Sci. Technol. 2023, 141, 104200. [Google Scholar] [CrossRef]
- Abekoon, T.; Buthpitiya, B.L.S.K.; Sajindra, H.; Samarakoon, E.R.J.; Jayakody, J.A.D.C.A.; Kantamaneni, K.; Rathnayake, U. A comprehensive review to evaluate the synergy of intelligent food packaging with modern food technology and artificial intelligence field. Discov. Sustain. 2024, 5, 160. [Google Scholar] [CrossRef]
- Girardeau, A.; Biscola, V.; Keravec, S.; Corrieu, G.; Fonseca, F. Application of lactic acid bacteria in time-temperature integrators. In Lactic Acid Bact: A Funct Approach; Taylor & Francis: Boca Raton, FL, USA, 2020. [Google Scholar]
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of Time Temperature Indicators as Quality Monitors in Food Packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Srinivasa Gopal, T.K. Smart packaging systems for food applications: A review. J Food Sci Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Roberts, S.; Xia, Y. Cover Picture: Nanocrystal-Based Time—Temperature Indicators. Chem. Eur. J. 2010, 16, 12493. [Google Scholar] [CrossRef]
- Kerry, J.P.; O’Grady, M.N.; Hogan, S.A. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef]
- Kim, J.U.; Ghafoor, K.; Ahn, J.; Shin, S.; Lee, S.H.; Shahbaz, H.M.; Shin, H.H.; Kim, S.; Park, J. Kinetic modeling and characterization of a diffusion-based time-temperature indicator (TTI) for monitoring microbial quality of non-pasteurized angelica juice. LWT Food Sci.Technol. 2016, 67, 143–150. [Google Scholar] [CrossRef]
- Meng, J.; Qian, J.; Tang, Y. A solid-state time-temperature indicator used in chilled fresh pork monitoring. Packag. Technol. Sci. 2018, 31, 353–360. [Google Scholar] [CrossRef]
- Dove, S.A. Improving the Robustness and Reliability of Population-Based Global Biodiversity Indicators. Ph.D. Dissertation, University College London (UCL), London, UK, 2023. [Google Scholar]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Mirzaei, A.; Jorshari, Y.B.; Jananshir, S.; Noori, M.; Mahdavi, M. Colorimetric pH-sensitive hydrogel film based on kappa-carrageenan containing quercetin or eucalyptus leaf extract for freshness monitoring of chicken meat. Food Chem. X 2024, 22, 101307. [Google Scholar] [CrossRef]
- Kurek, M.; Hlupić, L.; Ščetar, M.; Bosiljkov, T.; Galić, K. Comparison of Two pH Responsive Color Changing Bio-Based Films Containing Wasted Fruit Pomace as a Source of Colorants. J. Food Sci. 2019, 84, 2490–2498. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Skinner, G.A. Water-based colourimetric optical indicators for the detection of carbon dioxide. Analyst 2010, 135, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, S.; Lee, K.; Baek, S.; Seo, J. Development of a pH indicator composed of high moisture-absorbing materials for real-time monitoring of chicken breast freshness. Food Sci. Biotechnol. 2017, 26, 37–42. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Ye, K.; He, W.; Wei, H.; Dang, L. A novel pH-sensitive antibacterial bilayer film for intelligent packaging. Biomass Conv. Bioref. 2024, 14, 14303–14316. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of cellulose nanofiber-based functional color indicator film incorporated with shikonin extracted from Lithospermum erythrorhizon root. Food Hydrocoll. 2021, 114, 106566. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Jensen, I.J.; Kurek, M.; Lerfall, J. Novel colorimetric indicators based on alginate hydrogel beads containing anthocyanin for visual freshness monitoring of shrimp and minced chicken. LWT 2024, 199, 116127. [Google Scholar] [CrossRef]
- Silva-Corrêa, K.M.; Stefani, R. Intelligent Films Based on Lobeira Fruit Starch for Fresh Chicken Meat Quality Monitoring. Eur. J. Nutr. Food Saf. 2024, 16, 222–232. [Google Scholar] [CrossRef]
- Jiang, R.; Yang, F.; Kang, X.; Li, X.; Jia, W.; Pan, L.; Yang, L. Background-Free Imaging of Food Freshness Using Curcumin-Functionalized Upconversion Reversible Hydrogel Patch. Small 2024, 21, 2405812. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, J.; Liu, F.; Gui, X.; Tu, Y. Preparation of a colorimetric hydrogel indicator reinforced with modified aramid nanofiber employing natural anthocyanin to monitor shrimp freshness. J. Food Sci. 2024, 89, 5461–5472. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Long, T.; Yuan, S.; Qi, P.; Han, L.; Hao, J. A pH-indicating smart tag based on porous hydrogel as food freshness sensors. J. Colloid Interface Sci. 2023, 647, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, W.; Yan, K.; Ding, F.; Shi, X.; Deng, H.; Du, Y. Electrochemical writing on edible polysaccharide films for intelligent food packaging. Carbohydr. Polym. 2018, 186, 236–242. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Alighourchi, H.; Barzegar, M. Some physicochemical characteristics and degradation kinetic of anthocyanin of reconstituted pomegranate juice during storage. J. Food Eng. 2009, 90, 179–185. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Zandraa, O.; Pummerová, M.; Sáha, P. Essential Oil Based PVP-CMC-BC-GG Functional Hydrogel Sachet for ‘Cheese’: Its Shelf Life Confirmed with Anthocyanin (Isolated from Red Cabbage) Bio Stickers. Foods 2020, 9, 307. [Google Scholar] [CrossRef]
- Qi, Y.; Li, Y. Comparison of nano-zinc oxide or calcium chloride incorporated polyvinyl alcohol/chitosan/anthocyanin films for active and intelligent packaging. Colloid Polym. Sci. 2024, 302, 1711–1723. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U.V.; Sáha, P. Bacterial cellulose and guar gum based modified PVP-CMC hydrogel films: Characterized for packaging fresh berries. Food Packag. Shelf Life 2019, 22, 100402. [Google Scholar] [CrossRef]
- Alpaslan, D. Use of colorimetric hydrogel as an indicator for food packaging applications. Bull. Mater. Sci. 2019, 42, 247. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Dhar, P.; Katiyar, V. 8. Polymer blends for sustainable food packaging. In Sustainable Polymers for Food Packaging; De Gruyter: Berlin, Germany, 2020; pp. 145–158. [Google Scholar]
- Gaur, S.S.; Ghosh, T.; Katiyar, V. 6 General material property requirement for food packaging applications. In Sustainable Polymers for Food Packaging; De Gruyter: Berlin, Germany, 2020; pp. 97–113. [Google Scholar]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of plastic films for modified atmosphere packaging of fruits and vegetables: A review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Raheem, D. Application of plastics and paper as food packaging materials—An overview. EJFA 2013, 25, 177–188. [Google Scholar] [CrossRef]
- Olam, M. Mechanical and Thermal Properties of HDPE/PET Microplastics, Applications, and Impact on Environment and Life; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Colina-Martínez, G. Characterization of recycled polycarbonate from electronic waste and its use in hydraulic concrete: Improvement of compressive performance. Adv. Concr Constr. 2017, 5, 563–573. [Google Scholar]
- Patil, A.S.; Waghmare, R.D.; Pawar, S.P.; Salunkhe, S.T.; Kolekar, G.B.; Sohn, D.; Gore, A.H. Photophysical Insights of Highly Transparent, Flexible and Re-Emissive PVA @ WTR-CDs Composite Thin Films: A next Generation Food Packaging Material for UV Blocking Applications. J. Photochem. Photobiol. A Chem. 2020, 400, 112647. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Li, F.; Yu, H.-Y.; Li, Y.; Abdalkarim, S.Y.H.; Zhu, J.; Zhou, Y. “Soft-rigid” synergistic reinforcement of PHBV composites with functionalized cellulose nanocrystals and amorphous recycled polycarbonate. Compos. B Eng. 2020, 206, 108542. [Google Scholar] [CrossRef]
- Dedieu, I.; Aouf, C.; Gaucel, S.; Peyron, S. Recycled Poly(hydroxybutyrate-co-valerate) as Food Packaging: Effect of Multiple Melt Processing on Packaging Performance and Food Contact Suitability. J. Polym. Environ. 2023, 31, 1019–1028. [Google Scholar] [CrossRef]
- He, Y.; Fei, X.; Li, H. Carboxymethyl cellulose-based nanocomposites reinforced with montmorillonite and ε-poly-l-lysine for antimicrobial active food packaging. J. Appl. Polym. Sci. 2020, 137, 48782. [Google Scholar] [CrossRef]
- Abdin, M.; Mabrouk, M.; El-Sebaiy, L.; Eissa, M.; El-Bana, M.; Salama, M.A.; Naeem, M.A. Composite films based on carboxy methyl cellulose and sodium alginate incorporated Thymus vulgaris purified leaves extract for food application: Assessment, antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2023, 240, 124474. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Makwana, S.H. Development of active, water-resistant carboxymethyl cellulose-poly vinyl alcohol-Aloe vera packaging film. Carbohydr. Polym. 2020, 227, 115303. [Google Scholar] [CrossRef]
- Bashir, A.; Jabeen, S.; Gull, N.; Islam, A.; Sultan, M.; Ghaffar, A.; Jamil, T. Co-concentration effect of silane with natural extract on biodegradable polymeric films for food packaging. Int. J. Biol. Macromol. 2018, 106, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Mc Millin, K.W. Advancement in meat packaging—A review. Meat Sci. 2017, 132, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, G.Q.; Zilberman, Y.; Ruan, W.; Ameri, S.; Zhang, Y.S.; Miller, E.; Sonkusale, S.R. Low cost smart phone diagnostics for food using paper-based colorimetric sensor arrays. Food Control 2017, 82, 227–232. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemczyk-Soczynska, B.; Sajkiewicz, P.Ł. Hydrogel-Based Systems as Smart Food Packaging: A Review. Polymers 2025, 17, 1005. https://doi.org/10.3390/polym17081005
Niemczyk-Soczynska B, Sajkiewicz PŁ. Hydrogel-Based Systems as Smart Food Packaging: A Review. Polymers. 2025; 17(8):1005. https://doi.org/10.3390/polym17081005
Chicago/Turabian StyleNiemczyk-Soczynska, Beata, and Paweł Łukasz Sajkiewicz. 2025. "Hydrogel-Based Systems as Smart Food Packaging: A Review" Polymers 17, no. 8: 1005. https://doi.org/10.3390/polym17081005
APA StyleNiemczyk-Soczynska, B., & Sajkiewicz, P. Ł. (2025). Hydrogel-Based Systems as Smart Food Packaging: A Review. Polymers, 17(8), 1005. https://doi.org/10.3390/polym17081005