Sulfur and Peroxide Cross-Linking of Lignosulfonate-Filled Compounds Based on Acrylonitrile–Butadiene Rubber and Styrene–Butadiene Rubber
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Fabrication and Curing
2.2.2. Determination of Curing Characteristics
2.2.3. Determination of Cross-Link Density
2.2.4. Investigation of Physical–Mechanical Characteristics
2.2.5. Microscopic Analysis
3. Results and Discussion
3.1. Curing Process and Cross-Link Density
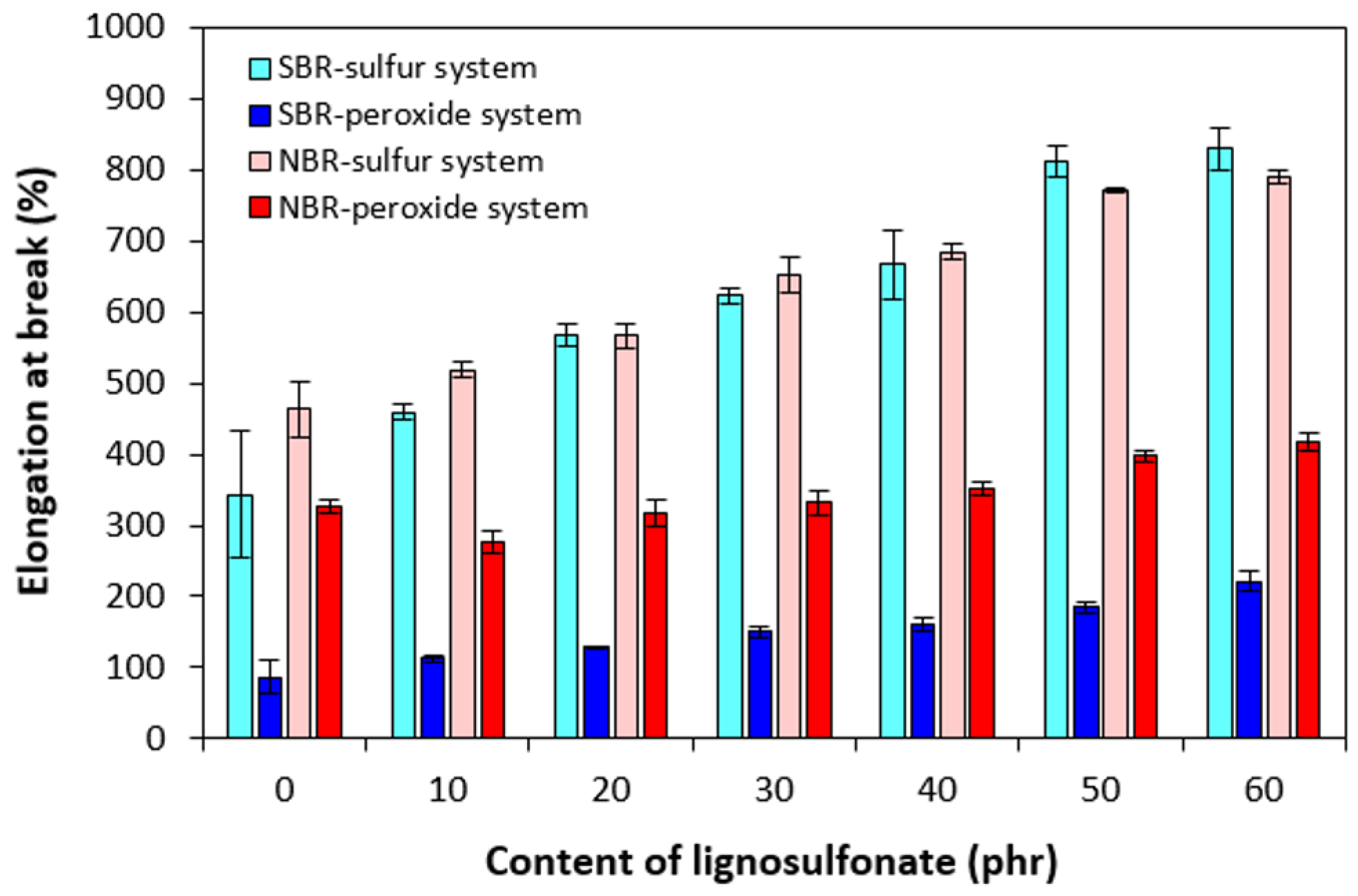
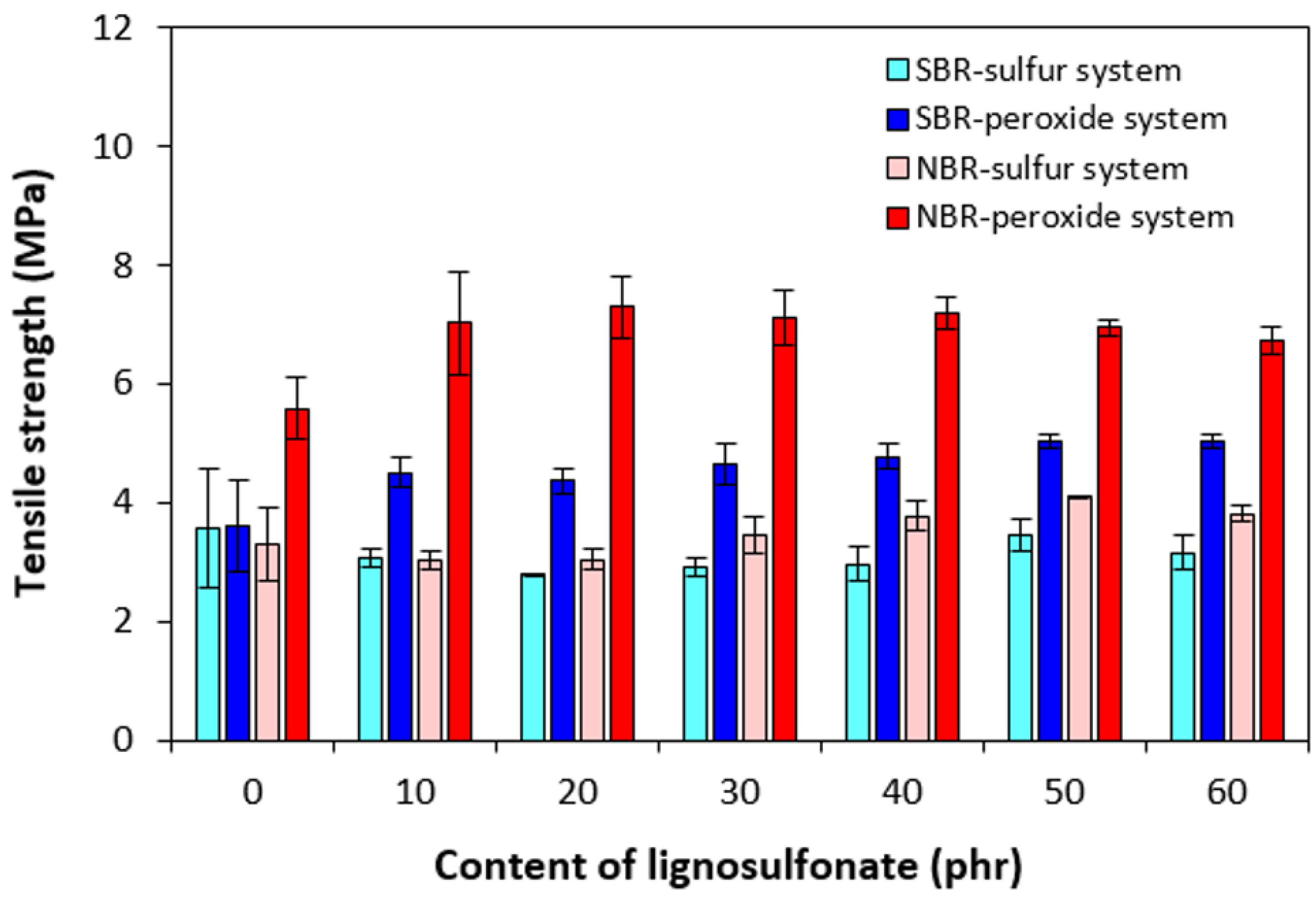
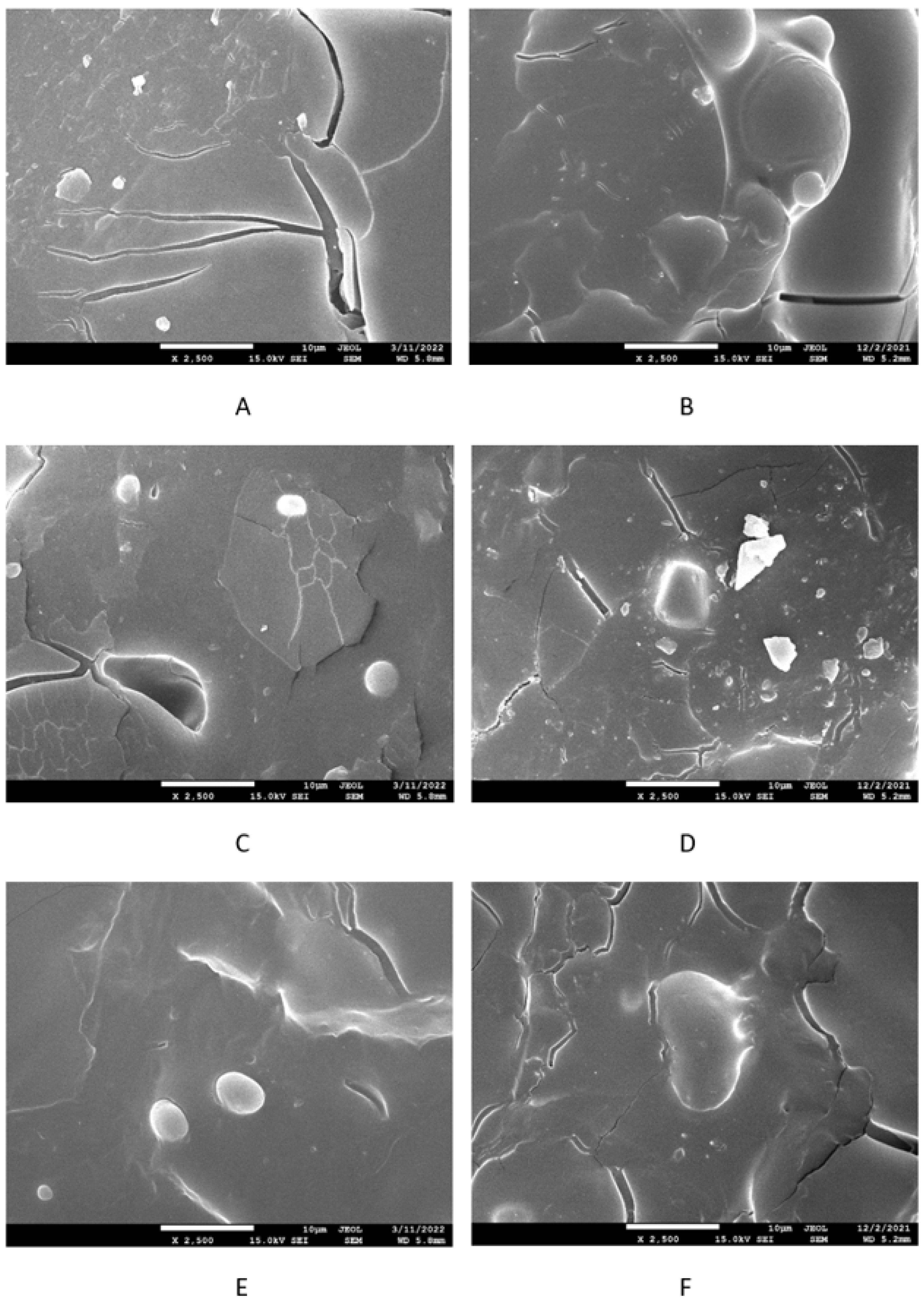
3.2. Physical–Mechanical Properties and Morphology
3.3. Dynamic–Mechanical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ruwoldt, J. A critical review of the physicochemical properties of lignosulfonates: Chemical structure and behavior in aqueous solution, at surfaces and interfaces. Surfaces 2020, 3, 622–648. [Google Scholar] [CrossRef]
- Hemmilä, V.; Hosseinpourpia, R.; Adamopoulos, S.; Eceiza, A. Characterization of wood-based industrial biorefinery lignosulfonates and supercritical water hydrolysis lignin. Waste Biomass Valori 2020, 11, 5835–5845. [Google Scholar]
- Lu, X.; Gu, X.; Shi, Y. A review on lignin antioxidants: Their sources, isolations, antioxidant activities and various applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [PubMed]
- Morena, A.G.; Bassegoda, A.; Natan, M.; Jacobi, G.; Banin, E.; Tzanov, T. Antibacterial properties and mechanisms of action of sonoenzymatically synthesized lignin-based nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 37270–37279. [Google Scholar]
- Wang, H.; Qiu, X.; Liu, W.; Fu, F.; Yang, D. A novel lignin/ZnO hybrid nanocomposite with excellent UV absorption ability and its application in transparent polyurethane coating. Ind. Eng. Chem. Res. 2017, 56, 11133–11141. [Google Scholar]
- Younesi-Kordkheili, H.; Pizzi, A. A comparison among lignin modification methods on the properties of lignin–phenol–formaldehyde resin as wood adhesive. Polymers 2021, 13, 3502. [Google Scholar] [CrossRef]
- Lisý, A.; Ház, A.; Nadányi, R.; Jablonský, M.; Šurina, I. About hydrophobicity of lignin: A review of selected chemical methods for lignin valorisation in biopolymer production. Energies 2022, 15, 6213. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Li Tan, C.; Wang, G.; Kai, D. How far is Lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar]
- Fabbri, F.; Bischof, S.; Mayr, S.; Gritsch, S.; Bartolome, M.J.; Schwaiger, N.; Guebitz, G.M.; Weiss, R. The biomodified lignin patform: A review. Polymers 2023, 15, 1694. [Google Scholar]
- Saadan, R.; Alaoui, C.H.; Ihammi, A.; Chigr, M.; Fatimi, A. A brief overview of lignin extraction and isolation processes: From lignocellulosic biomass to added-value biomaterials. Environ. Earth Sci. Proc. 2024, 31, 3. [Google Scholar] [CrossRef]
- Alam, M.M.; Greco, A.; Rajabimashhadi, Z.; Corcione, C.E. Efficient and environmentally friendly techniques for extracting lignin from lignocellulose biomass and subsequent uses: A review. Clean. Mater. 2024, 13, 100253. [Google Scholar] [CrossRef]
- Shorey, R.; Salaghi, A.; Fatehi, P.; Mekonnen, T.H. Valorization of lignin for advanced material applications: A review. RSC Sustain. 2024, 2, 804. [Google Scholar] [CrossRef]
- Gonçalves, S.; Ferra, J.; Paiva, N.; Martins, J.; Carvalho, L.H.; Magalhães, F.D. Lignosulphonates as an alternative to non-renewable binders in wood-based materials. Polymers 2021, 13, 4196. [Google Scholar] [CrossRef]
- Madyaratri, E.W.; Ridho, M.R.; Aristri, M.A.; Lubis, M.A.R.; Iswanto, A.H.; Nawawi, D.S.; Antov, P.; Kristak, L.; Majlingová, A.; Fatriasari, W. Recent advances in the development of fire-resistant biocomposites—A review. Polymers 2022, 14, 362. [Google Scholar] [CrossRef]
- Guterman, R.; Molinari, V.; Josef, E. Ionic liquid lignosulfonate: Dispersant and binder for preparation of biocomposite materials. Angew. Chem. Int. Ed. 2019, 58, 13044–13050. [Google Scholar] [CrossRef]
- Breilly, D.; Fadlallah, S.; Froidevaux, V.; Colas, A.; Allais, F. Origin and industrial applications of lignosulfonates with a focus on their use as superplasticizers in concrete. Constr. Build. Mater. 2021, 301, 124065. [Google Scholar] [CrossRef]
- Antov, P.; Mantanis, G.I.; Savov, V. Development of wood composites from recycled fibres bonded with magnesium lignosulfonate. Forests 2020, 11, 613. [Google Scholar] [CrossRef]
- Mohamad Aini, N.A.; Othman, N.; Hussin, M.H.; Sahakaro, K.; Hayeemasae, N. Lignin as alternative reinforcing filler in the rubber industry: A review. Front. Mater. 2020, 6, 329. [Google Scholar] [CrossRef]
- Thungphotrakul, N.; Dittanet, P.; Loykulnunt, S.; Tanpichai, S.; Parpainainar, P. Synthesis of sodium lignosulfonate from lignin extracted from oil palm empty fruit bunches by acid/ alkaline treatment for reinforcement in natural rubber composites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 526, 012022. [Google Scholar] [CrossRef]
- Kruželák, J.; Džuganová, M.; Kvasničáková, A.; Preťo, J.; Hronkovič, J.; Hudec, I. Influence of plasticizers on cross-linking process, morphology, and properties of lignosulfonate-filled rubber compounds. Polymers 2025, 17, 393. [Google Scholar] [CrossRef]
- An, D.; Cheng, S.; Jiang, C.; Duan, X.; Yang, B.; Zhang, Z.; Li, J.; Liu, Y.; Wong, C.P. A novel environmentally friendly boron nitride/lignosulfonate/natural rubber composite with improved thermal conductivity. J. Mater. Chem. C 2020, 8, 4801–4809. [Google Scholar]
- Džuganová, M.; Stoček, R.; Pöschl, M.; Kruželák, J.; Kvasničáková, A.; Hronkovič, J.; Preťo, J. Strategy for reducing rubber wear emissions: The prospect of using calcium lignosulfonate. Express Polym. Lett. 2024, 18, 1277–1290. [Google Scholar]
- Nardelli, F.; Calucci, L.; Carignani, E.; Borsacchi, S.; Cettolin, M.; Arimondi, M.; Giannini, L.; Geppi, M.; Martini, F. Influence of sulfur-curing conditions on the dynamics and crosslinking of rubber networks: A time-domain NMR study. Polymers 2022, 14, 767. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Fefar, M.M.; Griggs, T.; Akutagawa, K.; Chen, B.; Busfield, J.J.C. Recyclable sulfur cured natural rubber with controlled disulfide metathesis. Commun. Mater. 2024, 5, 212. [Google Scholar]
- Naebpetch, W.; Junhasavasdikul, B.; Saetung, A.; Tulyapitak, T.; Nithi-Uthai, N. Influence of accelerator/sulphur and co-agent/peroxide ratios in mixed vulcanisation systems on cure characteristics, mechanical properties and heat aging resistance of vulcanised SBR. Plast. Rubber Compos. 2016, 45, 436–444. [Google Scholar]
- Shahrampour, H.; Motavalizadehkakhky, A. The Effects of sulfur curing systems (insoluble-rhombic) on physical and thermal properties of the matrix polymeric of styrene butadiene rubber. Pet. Chem. 2017, 57, 700–704. [Google Scholar]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulphur and peroxide vulcanisation of rubber compounds—Overview. Chem. Pap. 2016, 70, 1533–1555. [Google Scholar] [CrossRef]
- Rodríguez Garraza, A.L.; Mansilla, M.A.; Depaoli, E.L.; Macchi, C.; Cerveny, S.; Marzocca, A.J.; Somoza, A. Comparative study of thermal, mechanical and structural properties of polybutadiene rubber isomers vulcanized using peroxide. Polym. Test. 2016, 52, 117–123. [Google Scholar]
- Peidayesh, H.; Nógellová, Z.; Chodák, I. Effects of peroxide and sulfur curing systems on physical and mechanical properties of nitrile rubber composites: A comparative study. Materials 2024, 17, 71. [Google Scholar]
- Wei, B.X.; Yi, X.T.; Xiong, Y.J.; Wei, X.J.; Wu, Y.D.; Huang, Y.D.; He, J.M.; Bai, Y.P. The preparation and characterization of a carbon fiber-reinforced epoxy resin and EPDM composite using the co-curing method. RSC Adv. 2020, 10, 20588. [Google Scholar]
- Bhattacharya, A.B.; Gopalan, A.M.; Chatterjee, T.; Vennemann, N.; Naskar, K. Exploring the thermomechanical properties of peroxide/co-agent assisted thermoplastic vulcanizates through temperature scanning stress relaxation measurements. Polym. Eng. Sci. 2021, 61, 2466–2476. [Google Scholar]
- Laing, B.; De Keyzer, J.; Seveno, D.; Van Bael, A. Effect of co-agents on adhesion between peroxide cured ethylene–propylene–diene monomer and thermoplastics in two-component injection molding. J. Appl. Polym. Sci. 2020, 137, 48414. [Google Scholar]
- Hayichelaeh, C.; Boonkerd, K. Enhancement of the properties of carbon-black-filled natural rubber compounds containing soybean oil cured with peroxide through the addition of coagents. Ind. Crop Prod. 2022, 187, 115306. [Google Scholar]
- Kruželák, J.; Kvasničáková, A.; Hložeková, K.; Hudec, I. Influence of dicumyl peroxide and Type I and II co-agents on cross-linking and physical–mechanical properties of rubber compounds based on NBR. Plast. Rubber Compos. 2020, 49, 307–320. [Google Scholar]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Synergistic mechanisms underlie the peroxide and coagent improvement of natural-rubber-toughened poly(3-hydroxybutyrate-co-3-hydroxyvalerate) mechanical performance. Polymers 2019, 11, 565. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Vulcanization of rubber compounds with peroxide curing systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar]
- Kraus, G. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar]
- Hosseini, S.M.; Razzaghi-Kashani, M. On the role of nano-silica in the kinetics of peroxide vulcanization of ethylene propylene diene rubber. Polymer 2017, 133, 8–19. [Google Scholar]
- Nikolova, S.; Mihaylov, M.; Dishovsky, N. Mixed peroxide/sulfur vulcanization of ethylene-propylene terpolymer based on composites. Curing characteristics, curing kinetics and mechanical properties. J. Chem. Technol. Metall. 2022, 57, 881–894. [Google Scholar]
- Wang, H.; Zhuang, T.; Shi, X.; Van Duin, M.; Zhao, S. Peroxide cross-linking of EPDM using moving die rheometer measurements. II. Effects of the process oils. Rubber Chem. Technol. 2018, 91, 561–576. [Google Scholar]
- George, B.; Alex, R. Stable free radical assisted scorch control in peroxide vulcanization of EPDM. Rubber Sci. 2013, 27, 135–145. [Google Scholar]
- Choi, S.S.; Kim, J.C. Lifetime prediction and thermal aging behaviors of SBR and NBR composites using crosslink density changes. J. Ind. Eng. Chem. 2012, 18, 1166–1170. [Google Scholar] [CrossRef]
- Valentín, J.L.; Posadas, P.; Fernández-Torres, A.; Malmierca, M.A.; González, L.; Chassé, W.; Saalwächter, K. Inhomogeneities and chain dynamics in diene rubbers vulcanized with different cure systems. Macromolecules 2010, 43, 4210–4222. [Google Scholar] [CrossRef]
- González, L.; Rodríguez, A.; Marcos-Fernández, A.; Valentín, J.L.; Fernández-Torres, A. Effect of network heterogeneities on the physical properties of nitrile rubbers cured with dicumyl peroxide. J. Appl. Polym. Sci. 2007, 103, 3377–3382. [Google Scholar] [CrossRef]
- Kruželák, J.; Kvasničáková, A.; Hudec, I. Peroxide curing systems applied for cross-linking of rubber compounds based on SBR. Adv. Ind. Eng. Polym. Res. 2020, 3, 120–128. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, T.; Liu, Y.; Zhang, A.; Yuan, C.; Zhang, W. Cross-linking process of cis-polybutadiene rubber with peroxides studied by two-dimensional infrared correlation spectroscopy: A detailed tracking. RSC Adv. 2015, 5, 10231–10242. [Google Scholar] [CrossRef]
- González, L.; Rodríguez, A.; Del Campo, A.; Marcos-Fernández, A. Effect of heterogeneities on the physical properties of elastomers derived from butadiene cured with dicumyl peroxide. Polym. Int. 2004, 53, 1426–1430. [Google Scholar] [CrossRef]
- Valentín, J.L.; Rodríguez, A.; Marcos-Fernández, A.; Gonzáles, L. Dicumyl peroxide cross-linking of nitrile rubbers with different content in acrylonitrile. J. Appl. Polym. Sci. 2005, 96, 1–5. [Google Scholar] [CrossRef]
- Charoeythornkhajhornchai, P.; Samthong Ch Somwangthanaroj, A. Influence of sulfenamide accelerators on cure kinetics and properties of natural rubber foam. J. Appl. Polym. Sci. 2017, 134, 44822. [Google Scholar] [CrossRef]
- Ghosh, J.; Ghorai, S.; Jalan, A.K.; Roy, M.; De, D. Manifestation of accelerator type and vulcanization system on the properties of silica-reinforced SBR/devulcanize SBR blend vulcanizates. Adv. Polym. Technol. 2018, 37, 2636–2650. [Google Scholar] [CrossRef]
- Lian, Q.; Li, Y.; Li, K.; Cheng, J.; Zhang, J. Insights into the vulcanization mechanism through a simple and facile approach to the sulfur cleavage behavior. Macromolecules 2017, 50, 803–810. [Google Scholar] [CrossRef]
- Sheng, C.; Hu, Z.; Martin, H.; Duan, Y.; Zhang, J. Effect of a small amount of sulfur on the physical and mechanical properties of peroxide-cured fully saturated HNBR compounds. J. Appl. Polym. Sci. 2015, 132, 41612. [Google Scholar] [CrossRef]
- Strohmeier, L.; Balasooriya, W.; Schrittesser, B.; van Duin, M.; Schlögl, S. Hybrid in situ reinforcement of EPDM rubber compounds based on phenolic novolac resin and ionic coagent. Appl. Sci. 2022, 12, 2432. [Google Scholar] [CrossRef]
- Siaw, C.; Baharulrazi, N.; Che Man, S.H.; Othman, N. Effect of zinc dimethacrylate concentrations on properties of emulsion styrene butadiene rubber/butadiene rubber blends. Plast. Rubber Compos. 2023, 52, 315–329. [Google Scholar] [CrossRef]
- Li, C.; Yuan, Z.; Ye, L. Facile construction of enhanced multiple interfacial interactions in EPDM/zinc dimethacrylate (ZDMA) rubber composites: Highly reinforcing effect and improvement mechanism of sealing resilience. Compos. A Appl. Sci. 2019, 126, 105580. [Google Scholar] [CrossRef]
- Cao, Y.; Gu, N.; Hu, S.; Jin, R.; Zhang, J. Preparation and properties of zinc-diacrylate-modified montmorillonite/rubber nanocomposite. Appl. Mech. Mater. 2012, 182–183, 47–51. [Google Scholar] [CrossRef]
- Henning, S.K.; Costin, R. Fundamentals of curing elastomers with peroxides and coagents. Rubber World 2006, 233, 28–35. [Google Scholar]

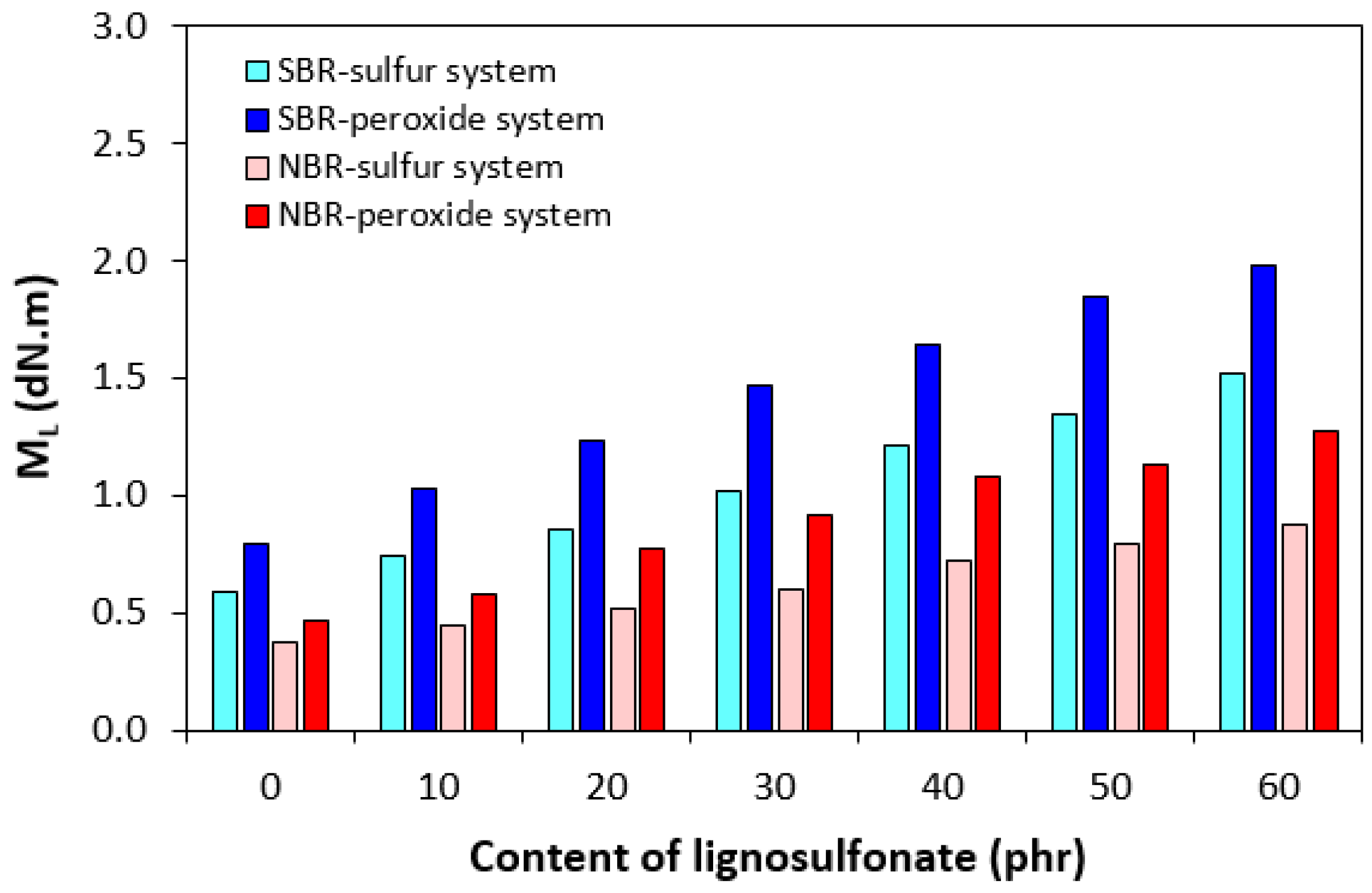

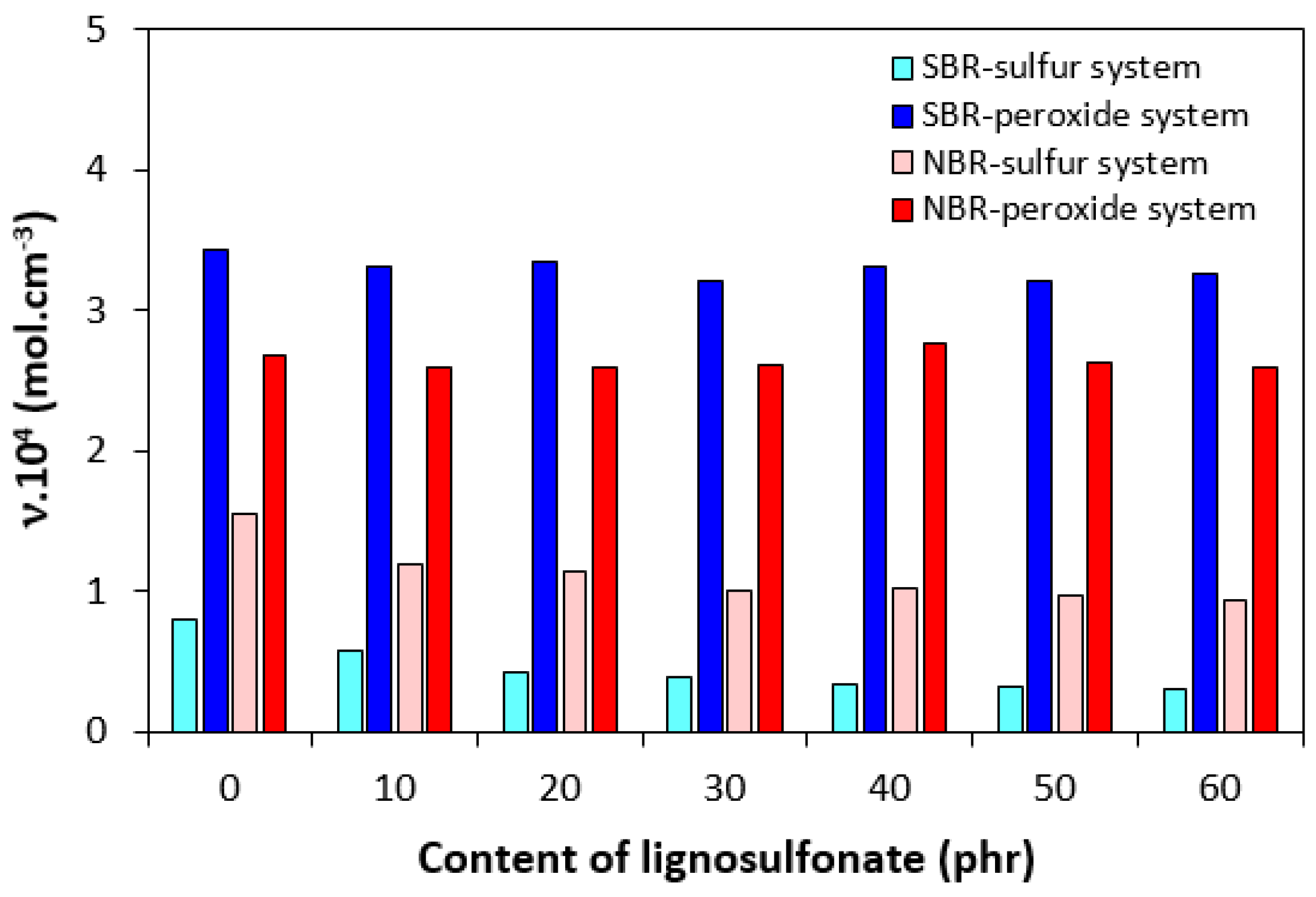
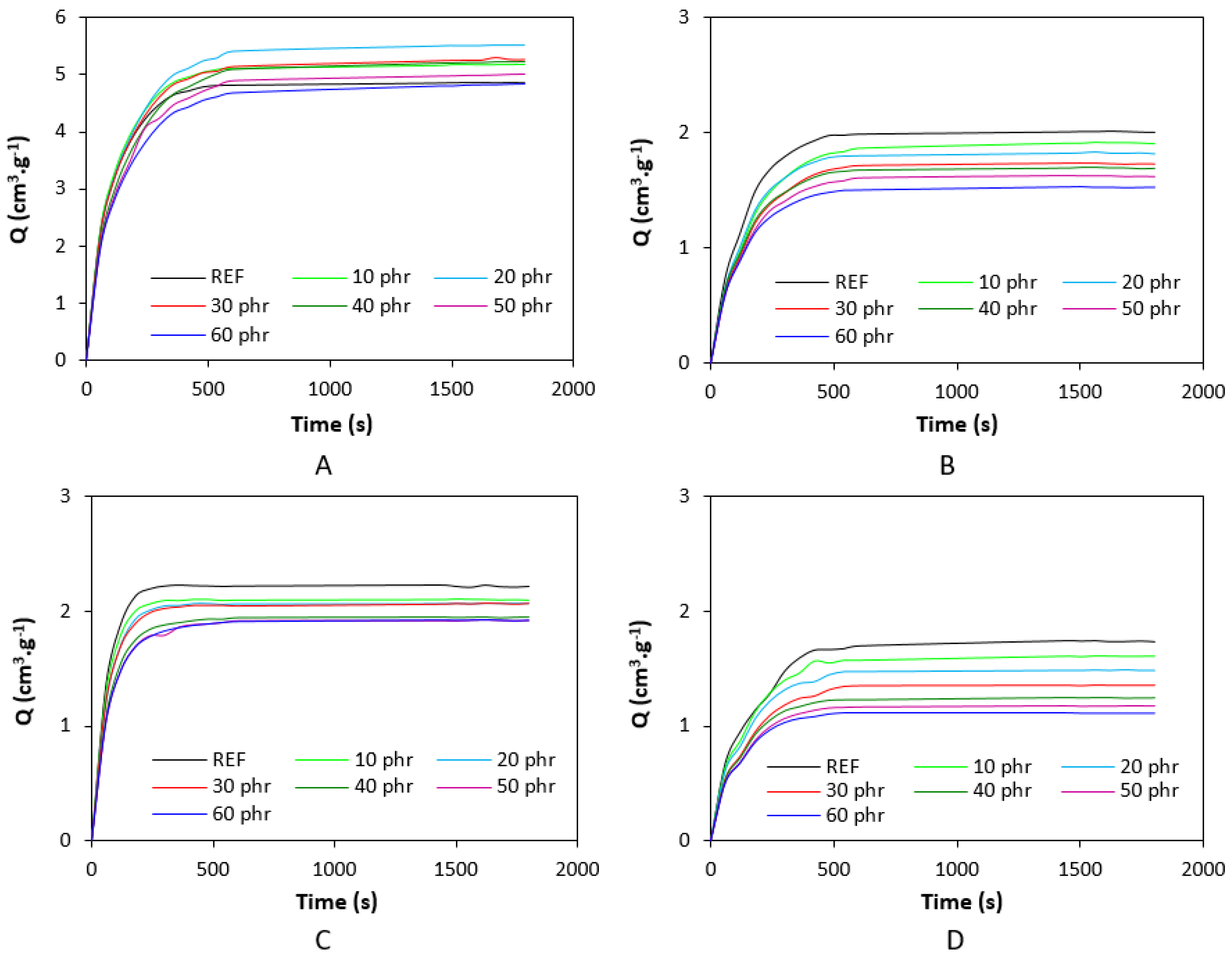
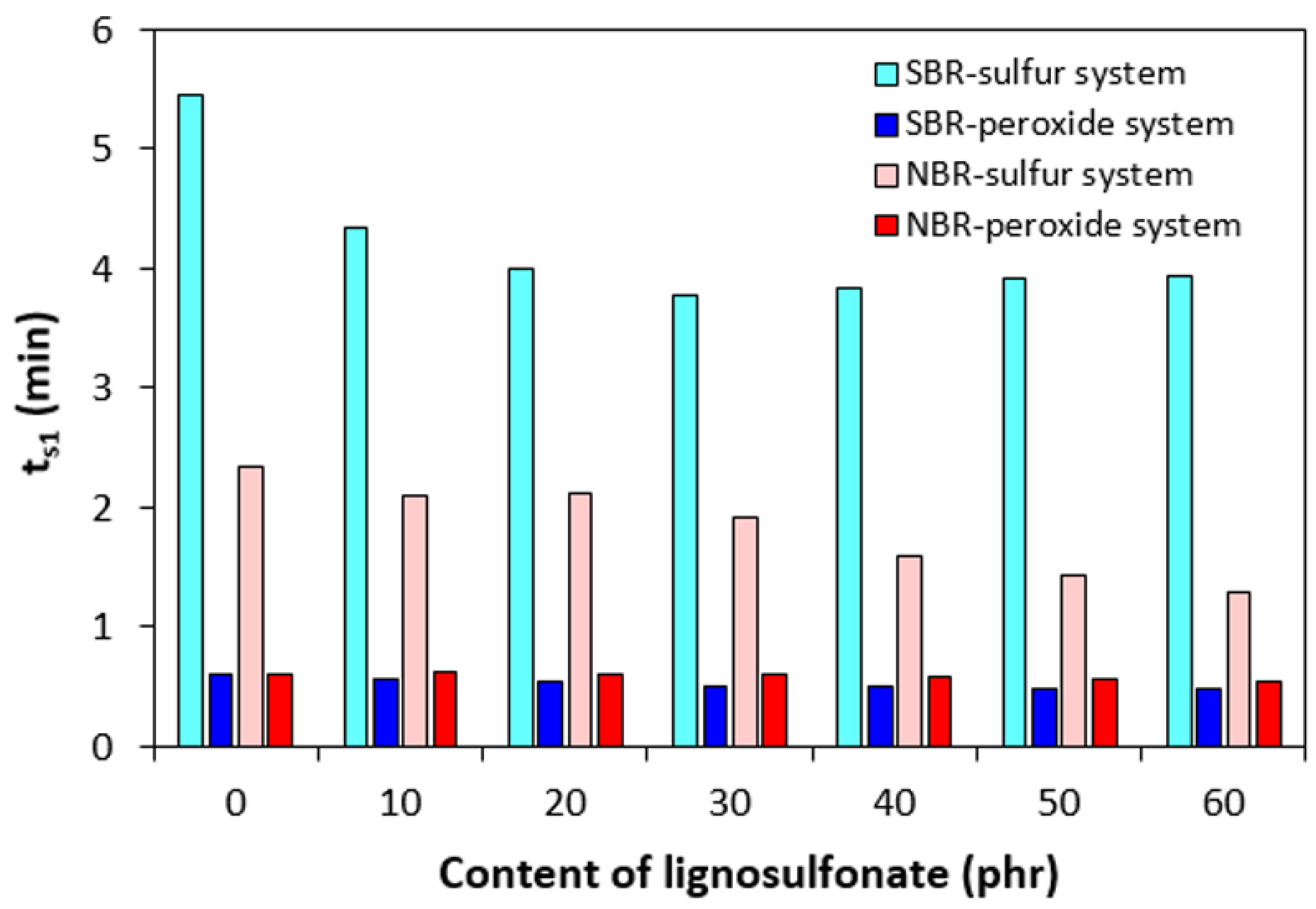




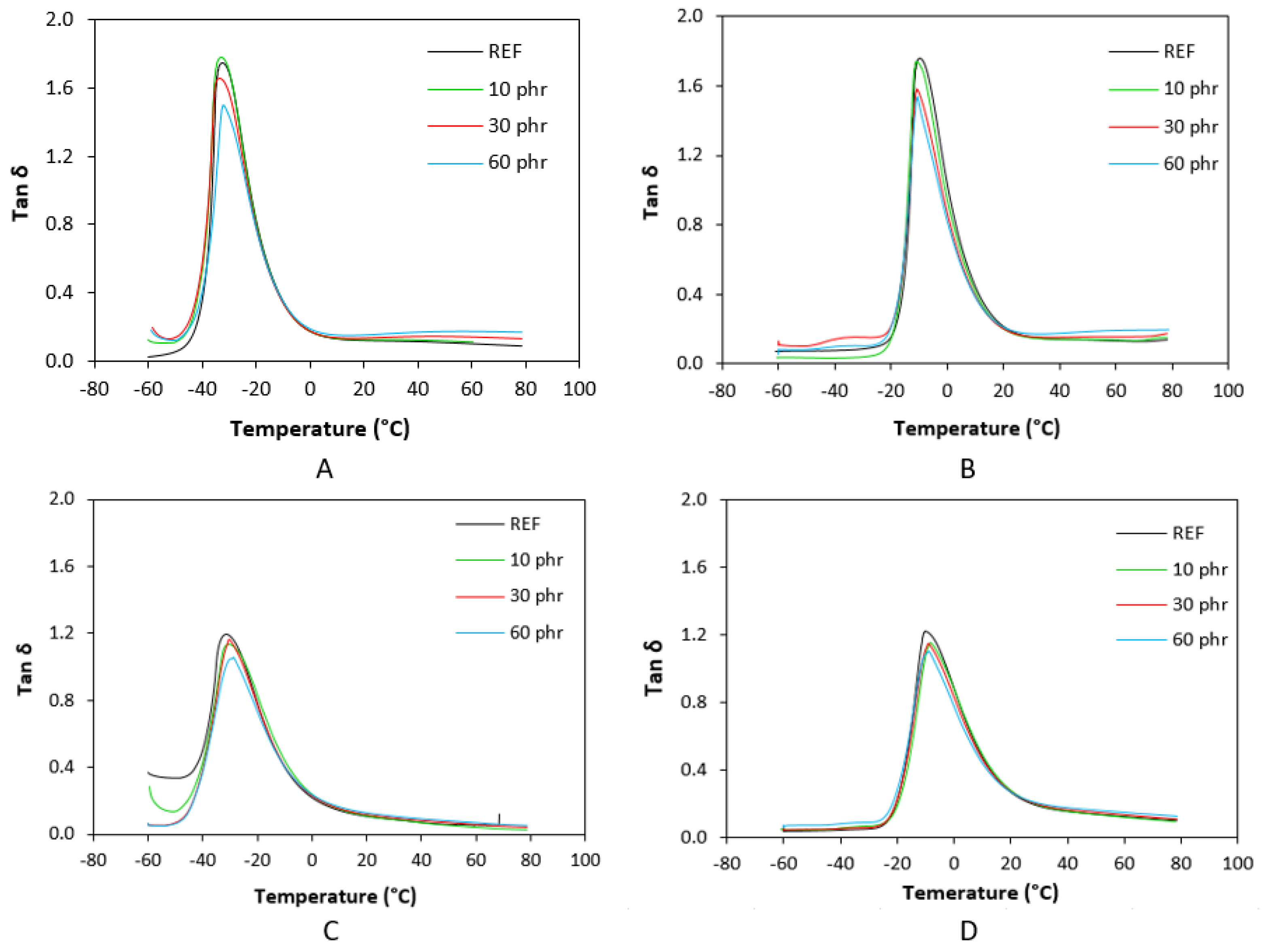
| Lignosulfonate (phr) | Tg (°C) | tan δ at Tg | tan δ (−50 °C) | tan δ (−20 °C) | tan δ (0 °C) | tan δ (20 °C) | tan δ (50 °C) |
|---|---|---|---|---|---|---|---|
| 0 | −32.4 | 1.74 | 0.06 | 0.83 | 0.18 | 0.12 | 0.11 |
| 10 | −33.0 | 1.77 | 0.11 | 0.81 | 0.17 | 0.13 | 0.12 |
| 30 | −33.6 | 1.66 | 0.14 | 0.79 | 0.18 | 0.14 | 0.15 |
| 60 | −32.4 | 1.50 | 0.12 | 0.77 | 0.18 | 0.15 | 0.17 |
| Lignosulfonate (phr) | Tg (°C) | tan δ at Tg | tan δ (−50 °C) | tan δ (−20 °C) | tan δ (0 °C) | tan δ (20 °C) | tan δ (50 °C) |
|---|---|---|---|---|---|---|---|
| 0 | −31.3 | 1.20 | 0.33 | 0.78 | 0.22 | 0.10 | 0.06 |
| 10 | −30.7 | 1.13 | 0.14 | 0.82 | 0.24 | 0.11 | 0.05 |
| 30 | −30.4 | 1.16 | 0.06 | 0.77 | 0.23 | 0.12 | 0.07 |
| 60 | −28.9 | 1.05 | 0.06 | 0.73 | 0.23 | 0.13 | 0.08 |
| Lignosulfonate (phr) | Tg (°C) | tan δ at Tg | tan δ (−50 °C) | tan δ (−20 °C) | tan δ (0 °C) | tan δ (20 °C) | tan δ (50 °C) |
|---|---|---|---|---|---|---|---|
| 0 | −9.8 | 1.76 | 0.07 | 0.15 | 1.03 | 0.21 | 0.13 |
| 10 | −10.9 | 1.74 | 0.04 | 0.14 | 0.96 | 0.20 | 0.14 |
| 30 | −10.7 | 1.58 | 0.10 | 0.21 | 0.88 | 0.21 | 0.16 |
| 60 | −10.7 | 1.53 | 0.08 | 0.21 | 0.82 | 0.21 | 0.19 |
| Lignosulfonate (phr) | Tg (°C) | tan δ at Tg | tan δ (−50 °C) | tan δ (−20 °C) | tan δ (0 °C) | tan δ (20 °C) | tan δ (50 °C) |
|---|---|---|---|---|---|---|---|
| 0 | −10.1 | 1.22 | 0.04 | 0.22 | 0.89 | 0.27 | 0.14 |
| 10 | −8.2 | 1.15 | 0.05 | 0.18 | 0.88 | 0.28 | 0.14 |
| 30 | −8.9 | 1.15 | 0.05 | 0.23 | 0.83 | 0.27 | 0.15 |
| 60 | −8.9 | 1.10 | 0.07 | 0.28 | 0.77 | 0.27 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruželák, J.; Džuganová, M.; Kvasničáková, A.; Hronkovič, J.; Preťo, J.; Chodák, I.; Hudec, I. Sulfur and Peroxide Cross-Linking of Lignosulfonate-Filled Compounds Based on Acrylonitrile–Butadiene Rubber and Styrene–Butadiene Rubber. Polymers 2025, 17, 950. https://doi.org/10.3390/polym17070950
Kruželák J, Džuganová M, Kvasničáková A, Hronkovič J, Preťo J, Chodák I, Hudec I. Sulfur and Peroxide Cross-Linking of Lignosulfonate-Filled Compounds Based on Acrylonitrile–Butadiene Rubber and Styrene–Butadiene Rubber. Polymers. 2025; 17(7):950. https://doi.org/10.3390/polym17070950
Chicago/Turabian StyleKruželák, Ján, Michaela Džuganová, Andrea Kvasničáková, Ján Hronkovič, Jozef Preťo, Ivan Chodák, and Ivan Hudec. 2025. "Sulfur and Peroxide Cross-Linking of Lignosulfonate-Filled Compounds Based on Acrylonitrile–Butadiene Rubber and Styrene–Butadiene Rubber" Polymers 17, no. 7: 950. https://doi.org/10.3390/polym17070950
APA StyleKruželák, J., Džuganová, M., Kvasničáková, A., Hronkovič, J., Preťo, J., Chodák, I., & Hudec, I. (2025). Sulfur and Peroxide Cross-Linking of Lignosulfonate-Filled Compounds Based on Acrylonitrile–Butadiene Rubber and Styrene–Butadiene Rubber. Polymers, 17(7), 950. https://doi.org/10.3390/polym17070950










