The Effect of Different pH and Temperature Values on Ca2+, F−, PO43−, OH−, Si, and Sr2+ Release from Different Bioactive Restorative Dental Materials: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Type
2.2. Testing Conditions of the Materials
2.2.1. pH Measurements
2.2.2. Fluoride and Phosphate Ion Release
2.2.3. Assessment of Calcium, Silicon, and Strontium Concentrations
2.3. Statistical Analysis
3. Results
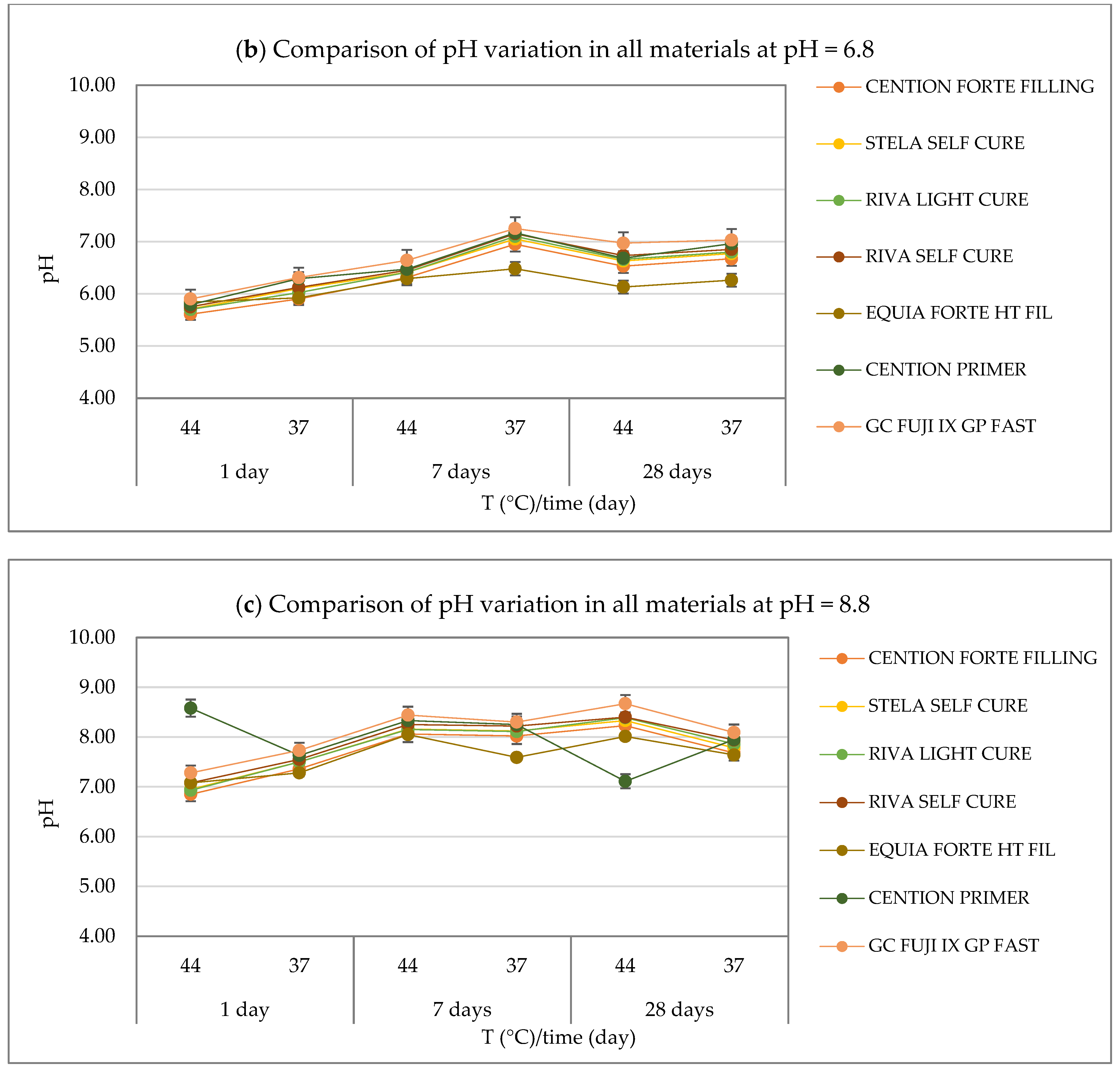
3.1. pH Measurements Results
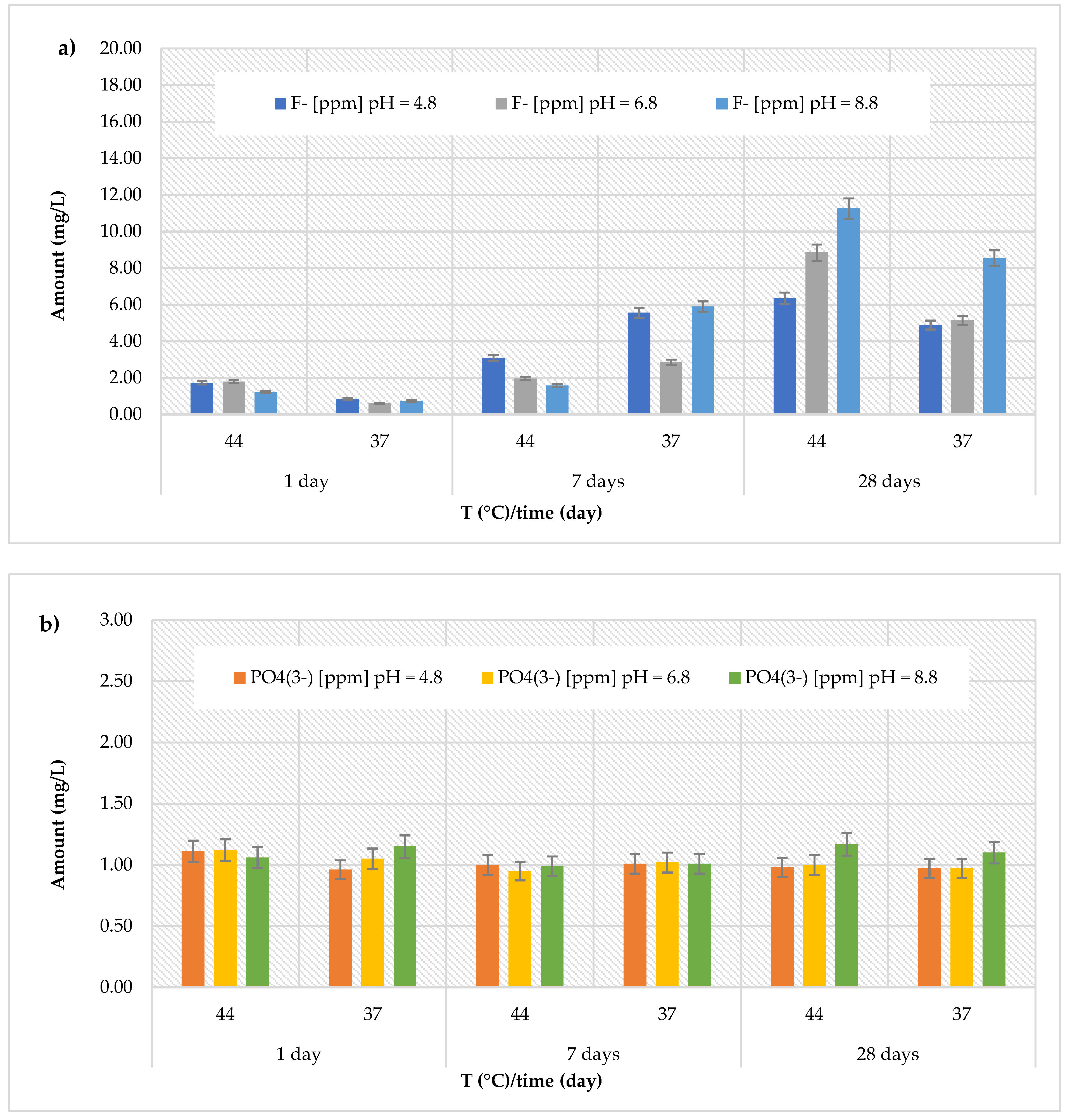
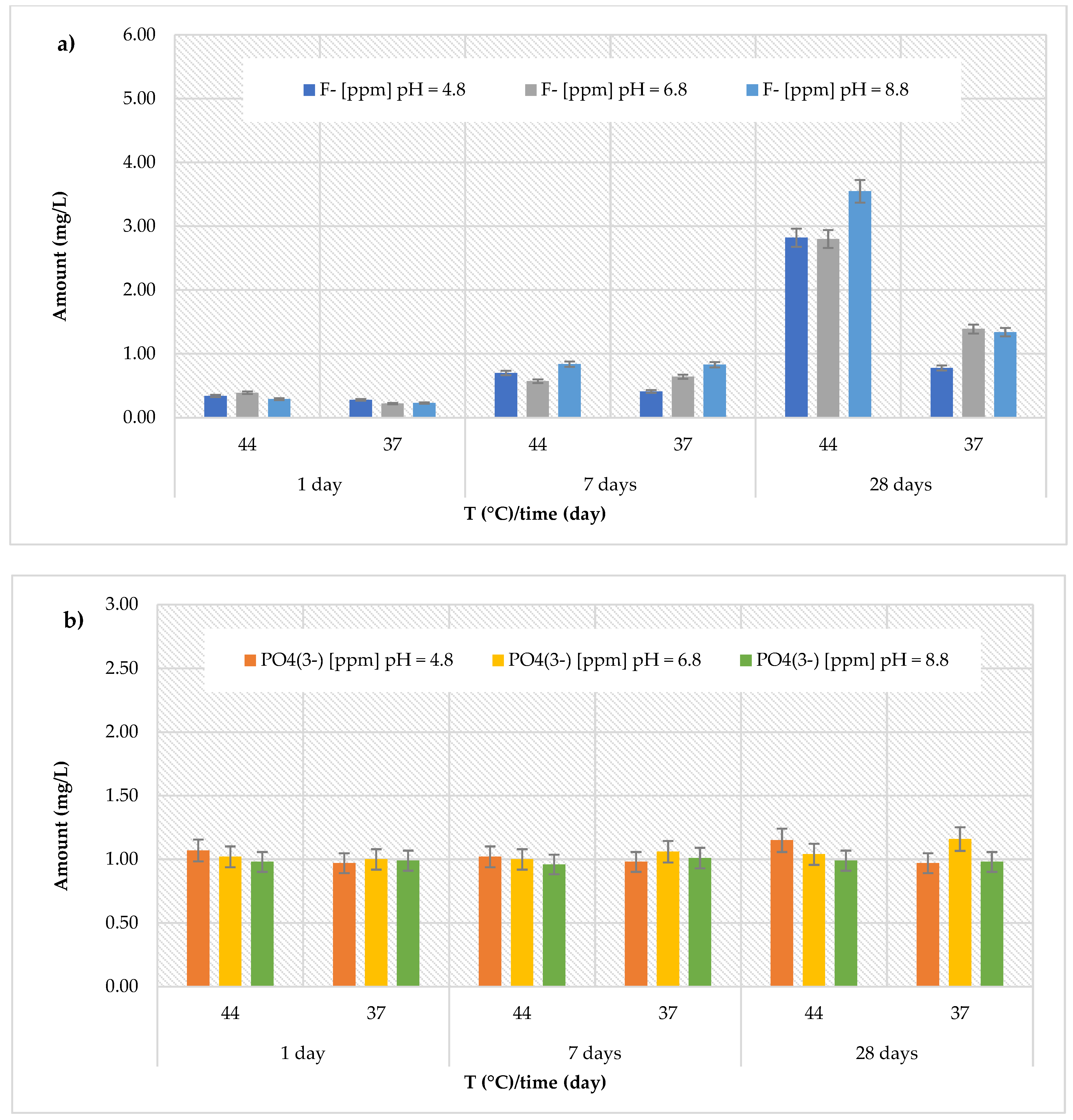
3.2. Release of Fluoride and Phosphate Ions
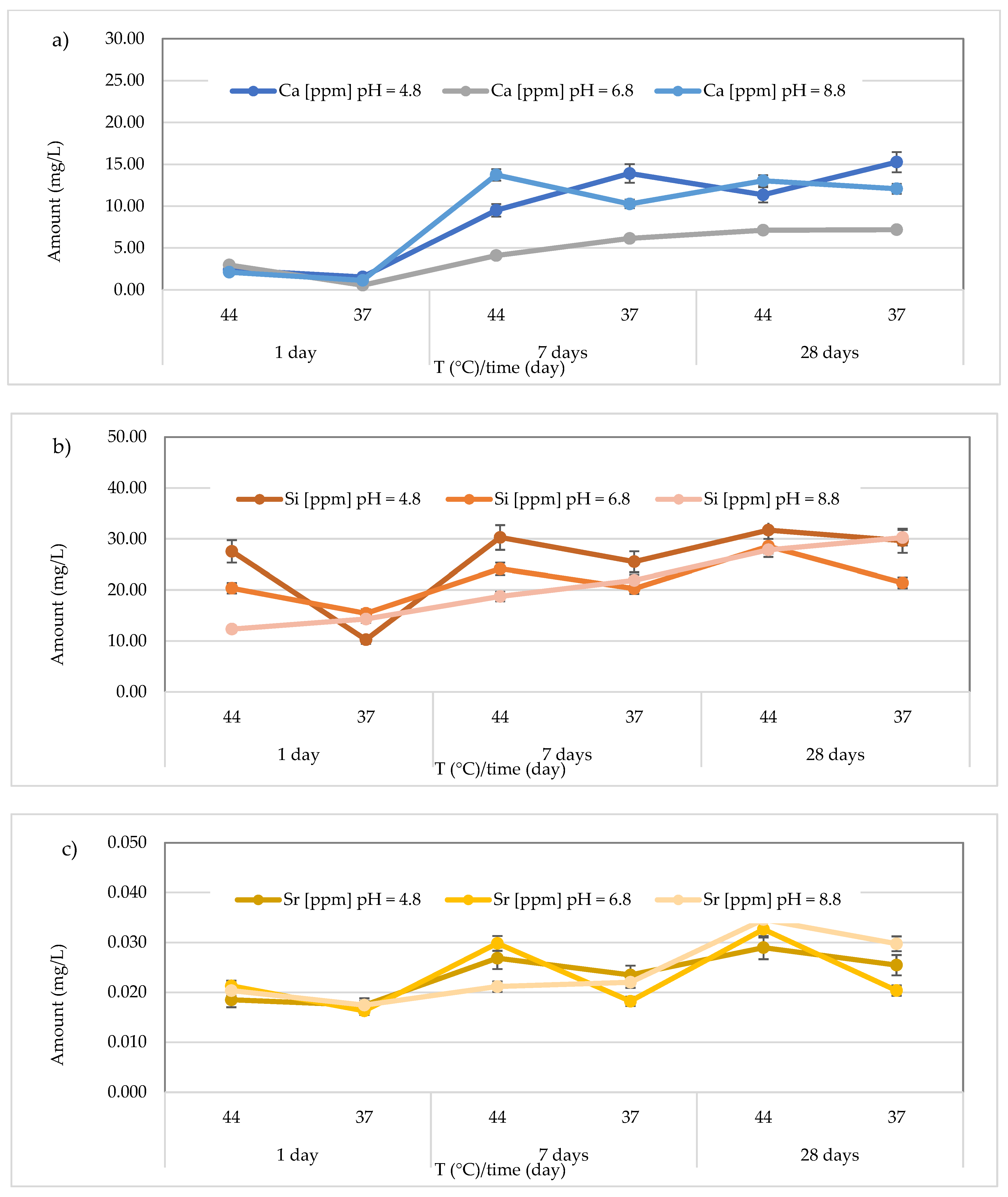
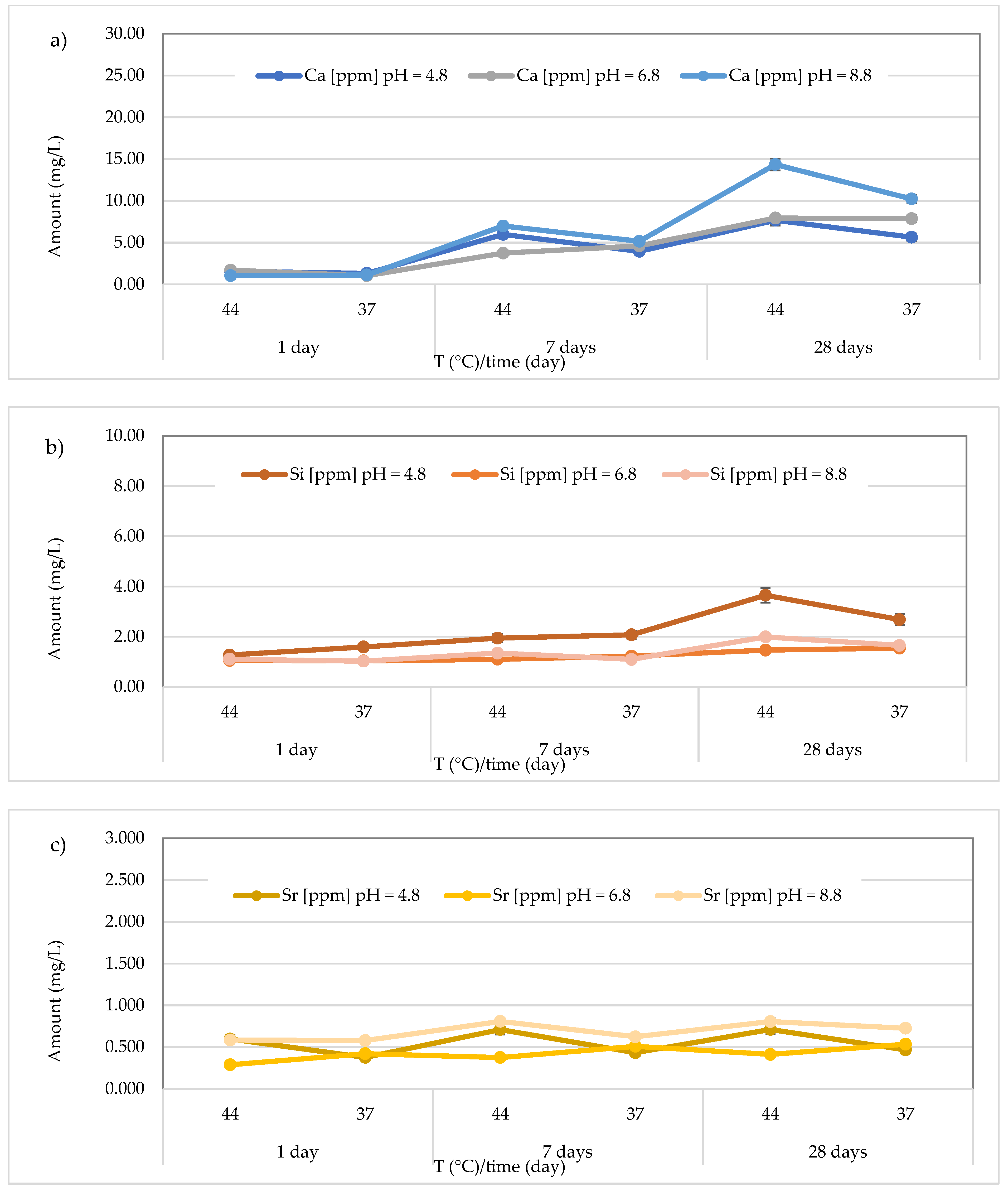
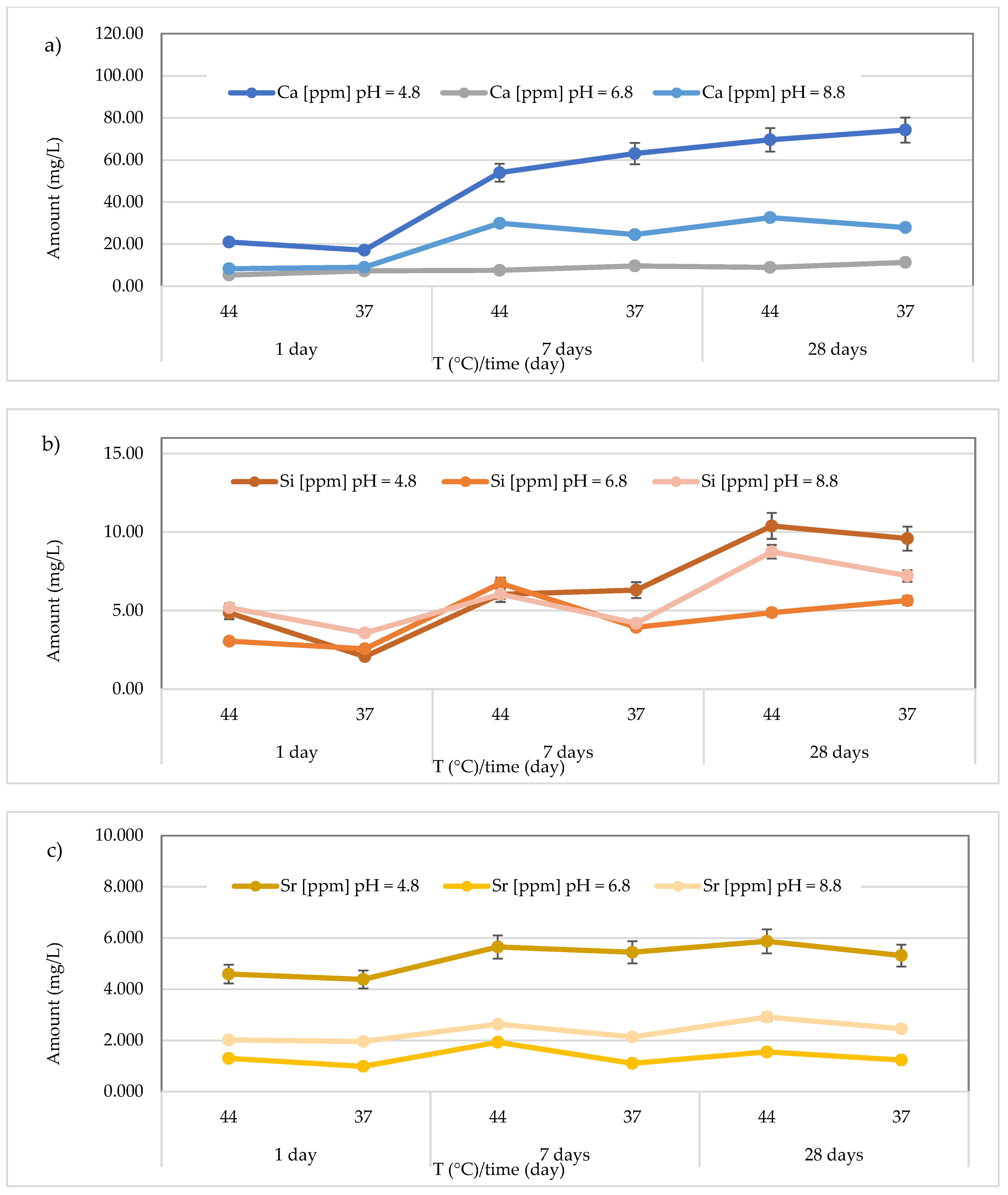
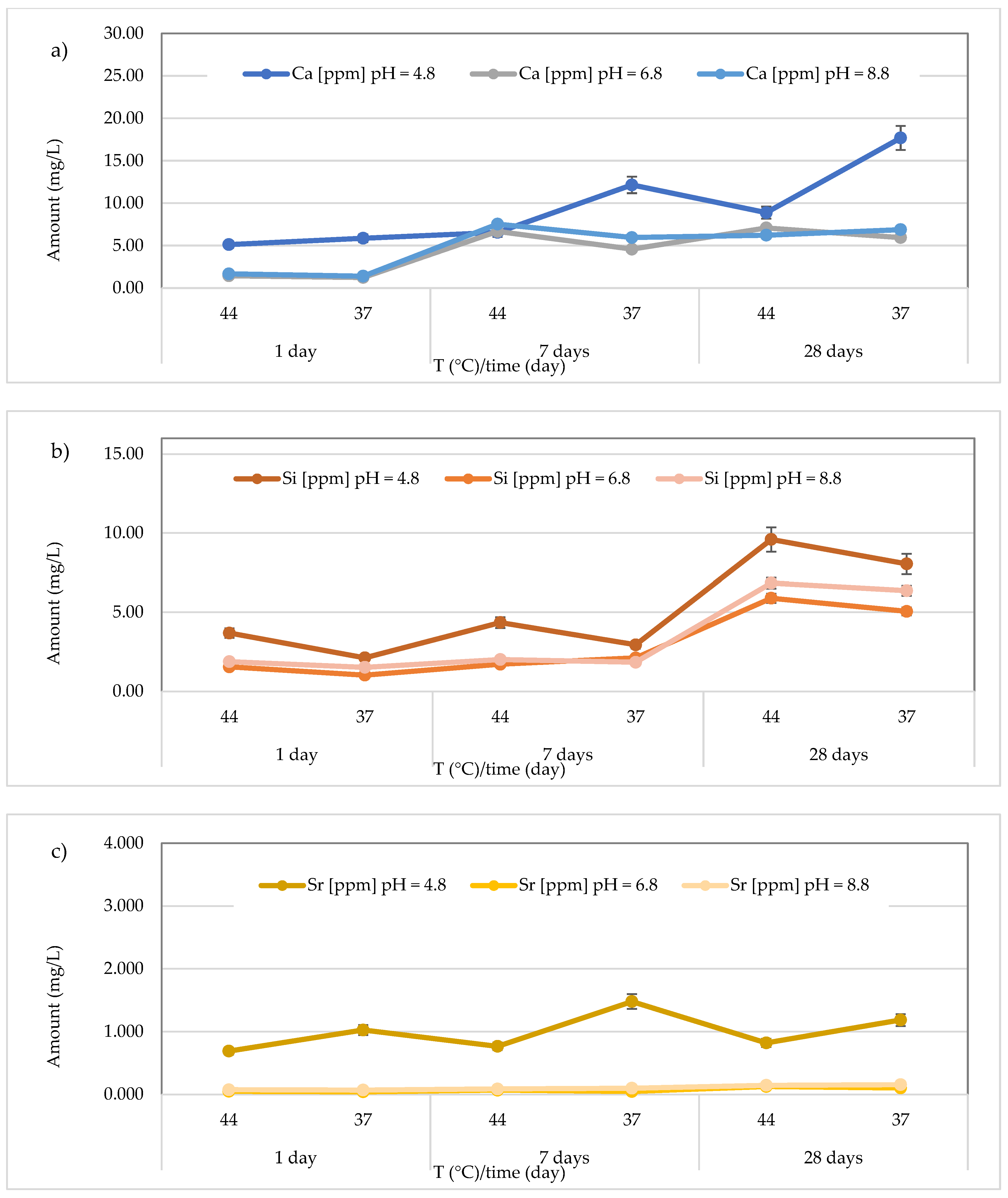
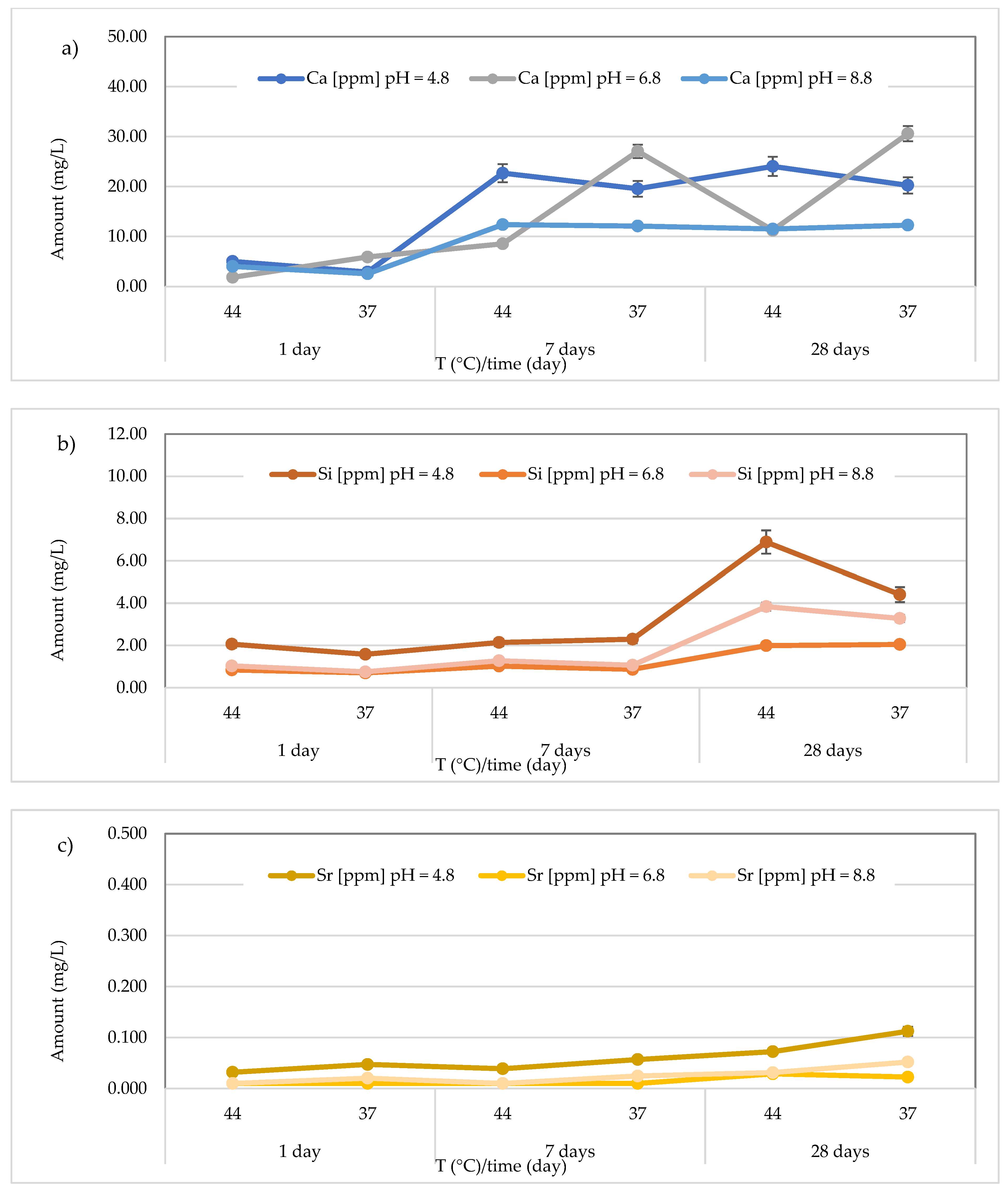
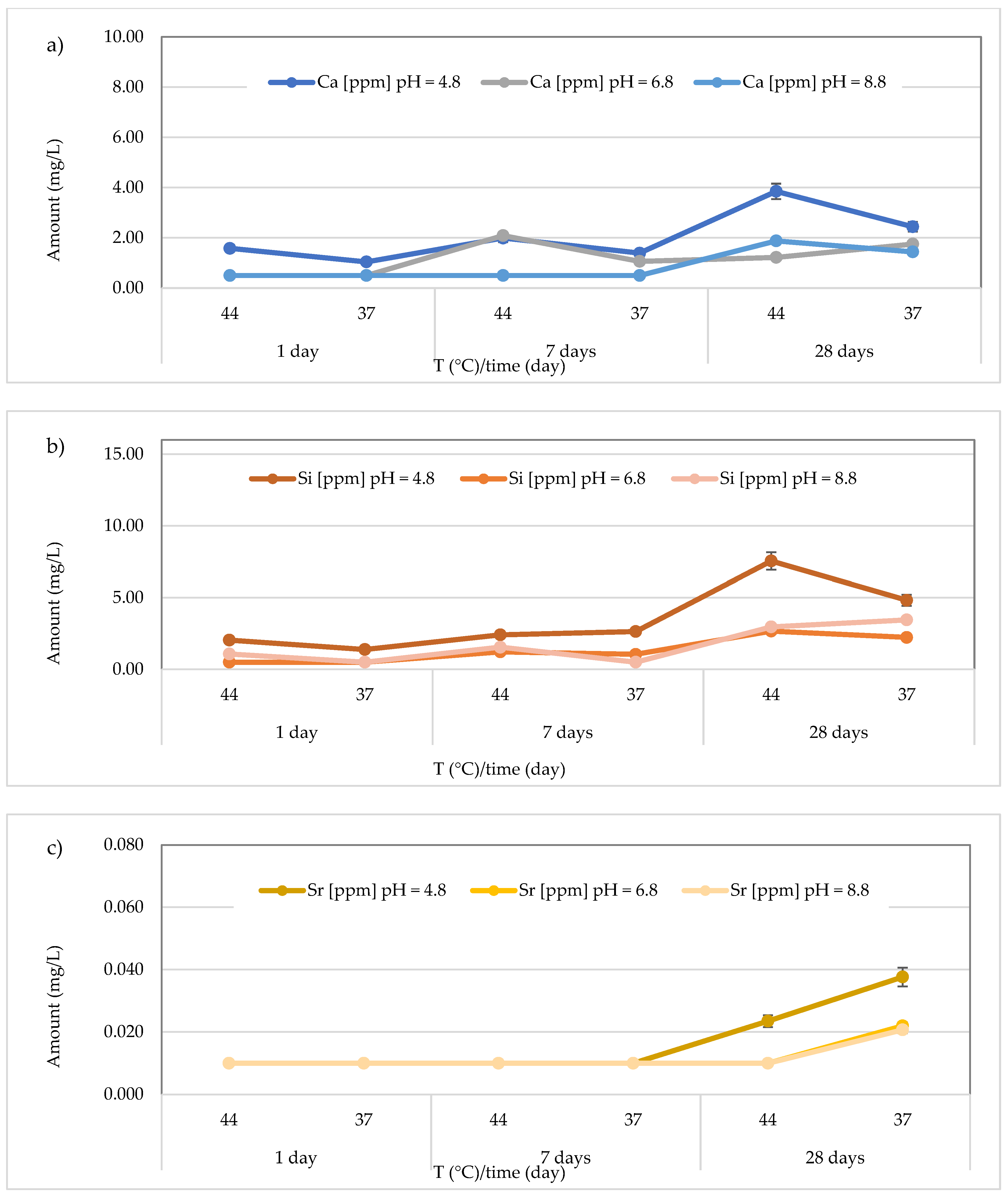
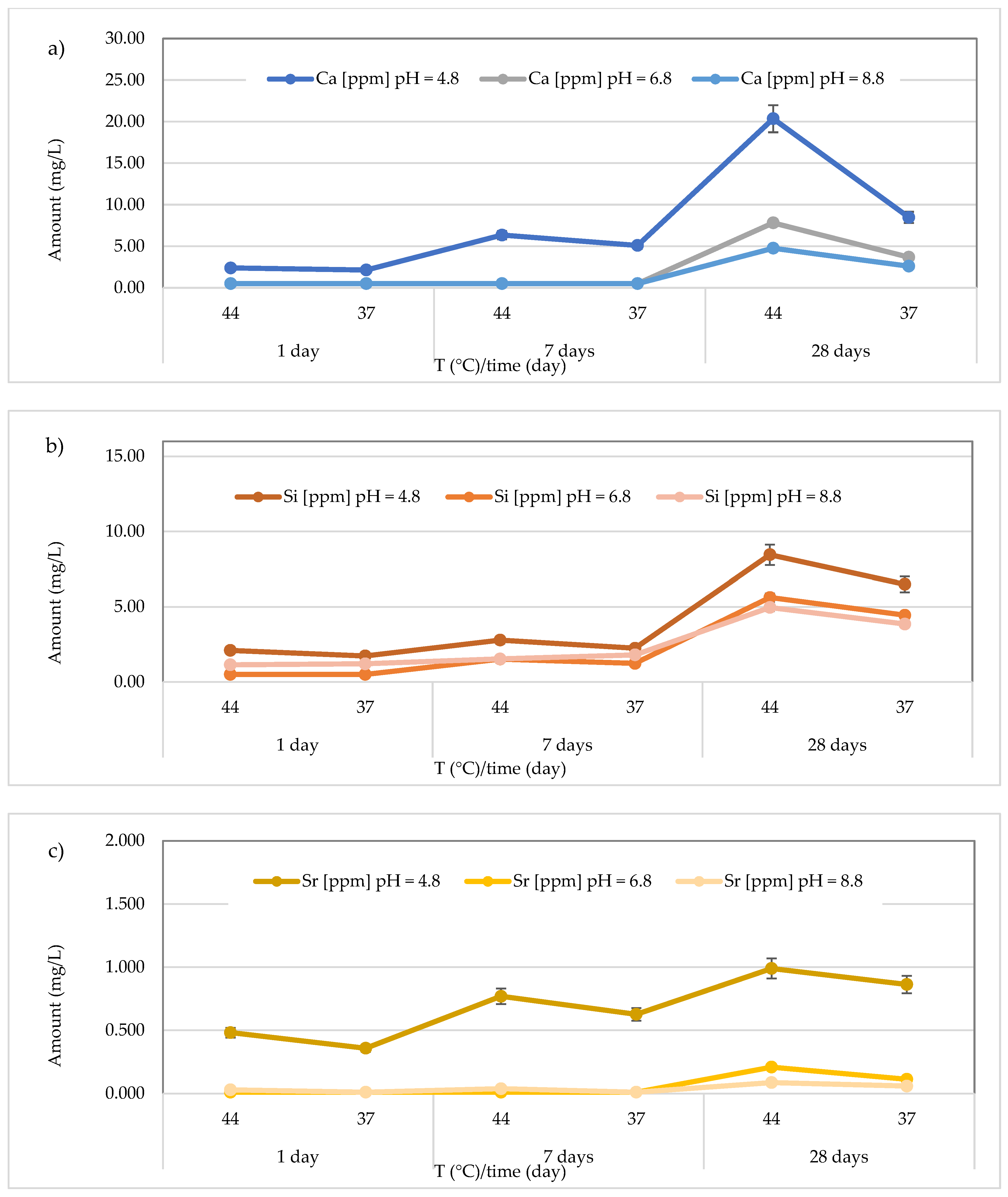
3.3. Release of Calcium, Silicon, and Strontium
3.4. Results of the Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ausiello, P.; Ciaramella, S.; De Benedictis, A.; Lanzotti, A.; Tribst, J.P.M.; Watts, D.C. The use of different adhesive filling material and mass combinations to restore class II cavities under loading and shrinkage effects: A 3D-FEA. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, H.; Weir, M.D.; Oates, T.W.; Zhou, X.; Wang, S.; Cheng, L.; Xu, H.H.K. Novel bioactive dental restorations to inhibit secondary caries in enamel and dentin under oral biofilms. J. Dent. 2023, 133, 104497. [Google Scholar] [CrossRef] [PubMed]
- Abouelleil, H.; Attik, N.; Chiriac, R.; Toche, F.; Ory, A.; Zayakh, A.; Grosgogeat, B.; Pradelle-Plasse, N. Comparative study of two bioactive dental materials. Dent. Mater. 2024, 40, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.C.; Quesada, F.J. Biomechanical and Chemical Behavior of Various Bioactive Materials in Class II Restorations. ODOVTOS Int. J. Dent. Sci. 2023, 25, 44–57. [Google Scholar]
- Ghilotti, J.; Fernández, I.; Sanz, J.L.; Melo, M.; Llena, C. Remineralization Potential of Three Restorative Glass Ionomer Ce-ments: An In Vitro Study. J. Clin. Med. 2023, 12, 2434. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Can glass polyalkenoate (glass-ionomer) dental cements be considered bioactive? A review. Heliyon 2024, 10, e25239. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, M.J.; Kim, K.M.; Yang, S.Y.; Seo, J.Y.; Choi, S.H.; Kwon, J.S. Enamel Demineralization Resistance and Remineralization by Various Fluoride-Releasing Dental Restorative Materials. Materials 2021, 14, 4554. [Google Scholar] [CrossRef]
- Yokoyama, A.; Hashimoto, M.; Nezu, T.; Kaga, N.; Tanaka, Y.; Sato, M.; Endo, K.; Toshima, H. Inhibition of enamel demineralization by an ion-releasing tooth-coating material. Am. J. Dent. 2019, 32, 27–30. [Google Scholar]
- Zhao, J.; Platt, J.A.; Xie, D. Characterization of a novel light-cured star-shape poly (acrylic acid)-composed glass-ionomer cement: Fluoride release, water sorption, shrinkage, and hygroscopic expansion. Eur. J. Oral Sci. 2009, 117, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Gunay, A.; Celenk, S.; Adiguzel, O.; Cangul, S.; Ozcan, N.; Eroglu Cakmakoglu, E. Comparison of antibacterial activity, cyto-toxicity, and fluoride release of glass ionomer restorative dental cements in dentistry. Med. Sci. Monit. 2023, 29, e939065-1–e939065-8. [Google Scholar]
- Cury, J.A.; Tenuta, L.M. Enamel remineralization: Controlling the caries disease or treating early caries lesions? Braz. Oral Res. 2009, 23, 23–30. [Google Scholar] [CrossRef]
- De Caluwé, T.; Vercruysse, C.W.J.; Declercq, H.A.; Martens, L.; Verbeeck, R.M.H. Bioactivity and biocompatibility of two flu-oride-containing bioactive glasses for dental applications. Dent. Mater. 2016, 32, 1414–1428. [Google Scholar] [CrossRef]
- Sasaki, J.; Kiba, W.; Abe, G.L.; Katata, C.; Hashimoto, M.; Kitagawa, H.; Imazato, S. Fabrication of strontium-releasable in-organic cement by incorporation of bioactive glass. Dent. Mater. 2019, 35, 780–788. [Google Scholar] [CrossRef]
- Imazato, S.; Nakatsuka, T.; Kitagawa, H.; Sasaki, J.-I.; Yamaguchi, S.; Ito, S.; Takeuchi, H.; Nomura, R.; Nakano, K. Multiple-Ion Releasing Bioactive Surface Pre-Reacted Glass-Ionomer (S-PRG) Filler: Innovative Technology for Dental Treatment and Care. J. Funct. Biomater. 2023, 14, 236. [Google Scholar] [CrossRef]
- Abdalla, M.M.; Sayed, O.; Lung, C.Y.K.; Rajasekar, V. Applications of bioactive strontium compounds in dentistry. J. Funct. Biomater. 2024, 15, 216. [Google Scholar] [CrossRef] [PubMed]
- Go, H.B.; Lee, M.J.; Seo, J.Y.; Byun, S.Y.; Kwon, J.S. Mechanical properties and sustainable bacterial resistance effect of strontium-modified phosphate-based glass microfiller in dental composite resins. Sci. Rep. 2023, 13, 17763. [Google Scholar] [CrossRef]
- de Oliveira Roma, F.R.V.; de Oliveira, T.J.L.; Bauer, J.; Firoozmand, L.M. Resin-modified glass ionomer enriched with BIO-GLASS: Ion-release, bioactivity and antibacterial effect. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.Y.; Mei, M.L.; Li, Q.L.; Lo, E.C.; Chu, C.H. Methods for biomimetic remineralization of human dentine: A systematic review. Int. J. Mol. Sci. 2015, 16, 4615–4627. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.L.; Vela, B.F.; Pires Silva Borges, L.; Trinca, R.B.; Pfeifer, C.S.; Braga, R.R. Compositional boundaries for functional dental composites containing calcium orthophosphate particles. J. Mech. Behav. Biomed. Mater. 2023, 144, 105928. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Joiner, A.; Chang, J. The remineralization of enamel: A review of the literature. J. Dent. 2014, 42, S12–S20. [Google Scholar] [CrossRef] [PubMed]
- Sáez, M.D.M.; López, G.L.; Atlas, D.; de la Casa, M.L. Evaluation of pH and calcium ion diffusion from calcium hydroxide pastes and MTA. Acta Odontol. Latinoam. 2017, 30, 26–32. [Google Scholar]
- Gandolfi, M.G.; Chersoni, S.; Acquaviva, G.L.; Piana, G.; Prati, C.; Mongiorgi, R. Fluoride release and absorption at different pH from glass-ionomer cements. Dent. Mater. 2006, 22, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is secondary caries with composites a material-based problem? Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.A.; Martins, C.S.; de Oliveira Cardoso Demarchi, A.C.; de Godoy, L.F.; Kuga, M.C.; Yamashita, J.C. Calcium and hydroxide release from different pulp-capping materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, e66–e69. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Cintra, L.T.A.; Duarte, M.A.H.; Rossi-Fedele, G.; Gavini, G.; Sousa-Neto, M.D. Mechanism of action of Bioactive Endodontic Materials. Braz. Dent. J. 2023, 34, 1–11. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; Ciapetti, G.; Prati, C. Biomimetic remineralization of human dentin using promising innovative calcium-silicate hybrid “smart” materials. Dent. Mater. 2011, 27, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tarle, A.; Tauböck, T.T. Experimental Bioactive Glass-Containing Composites and Commercial Restorative Materials: Anti-Demineralizing Protection of Dentin. Biomedicines 2021, 9, 1616. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, L.; Ionescu, A.C.; Carella, F.; Adamiano, A.; Brambilla, E.; Iafisco, M. Antimicrobial activity of remineralizing ion-doped amorphous calcium phosphates for preventive dentistry. Front. Mater. 2022, 9, 846130. [Google Scholar] [CrossRef]
- Di Lauro, A.; Di Duca, F.; Montuori, P.; Dal Piva, A.M.O.; Tribst, J.P.M.; Borges, A.L.S.; Ausiello, P. Fluoride and Calcium Release from Alkasite and Glass Ionomer Restorative Dental Materials: In Vitro Study. J. Funct. Biomater. 2023, 14, 109. [Google Scholar] [CrossRef]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Effect of strontium-doped bioactive glass-ceramic containing toothpaste on prevention of artificial dentine caries formation: An in vitro study. BMC Oral Health 2022, 22, 288. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.R.G.; Balsamo, M.; Imazato, S. Composite restoration on posterior teeth. In Modern Operative Dentistry; Springer: Cham, Switzerland, 2020; pp. 577–630. [Google Scholar]
- Ausiello, P.; Ciaramella, S.; Lanzotti, A.; Ventre, M.; Borges, A.L.; Tribst, J.P.; Dal Piva, A.; Garcia-Godoy, F. Mechanical behavior of Class I cavities restored by different material combinations under loading and polymerization shrinkage stress. A 3D-FEA study. Am. J. Dent. 2019, 32, 55–60. [Google Scholar] [PubMed]
- di Lauro, A.E.; Ciaramella, S.; Tribst, J.P.M.; Aliberti, A.; Ausiello, P. Comparison of Bulk Polymeric Resin Composite and Hybrid Glass Ionomer Cement in Adhesive Class I Dental Restorations: A 3D Finite Element Analysis. Polymers 2024, 16, 2525. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Suh, B.I.; Yang, J. Antibacterial dental restorative materials: A review. Am. J. Dent. 2018, 31, 6B–12B. [Google Scholar]
- Chiari, M.D.; Rodrigues, M.C.; Xavier, T.A.; de Souza, E.M.; Arana-Chavez, V.E.; Braga, R.R. Mechanical properties and ion release from bioactive restorative composites containing glass fillers and calcium phosphate nano-structured particles. Dent. Mater. 2015, 31, 726–733. [Google Scholar] [CrossRef]
- Pires, P.M.; Rosa, T.d.C.; Ribeiro-Lages, M.B.; Duarte, M.L.; Cople Maia, L.; Neves, A.d.A.; Sauro, S. Bioactive Restorative Materials Applied over Coronal Dentine—A Bibliometric and Critical Review. Bioengineering 2023, 10, 731. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Garcia, I.M.; Mokeem, L.; Weir, M.D.; Xu, H.H.K.; Montoya, C.; Orrego, S. Developing Bioactive Dental Resins for Restorative Dentistry. J. Dent. Res. 2023, 102, 1180–1190. [Google Scholar] [CrossRef]
- Madi, F.; Sidhu, S.K.; Nicholson, J.W. The effect of temperature and ionic solutes on the fluoride release and recharge of glass-ionomer cements. Dent. Mater. 2020, 36, e9–e14. [Google Scholar] [CrossRef]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Remineralizing effect of a new strontium-doped bioactive glass and fluoride on demineralized enamel and dentine. J. Dent. 2021, 108, 103633. [Google Scholar] [CrossRef]
- Li, J.; Han, Q.; Zhang, L.; Zhang, J.; Zhong, Y. Efficacy of a toothpaste containing paeonol, potassium nitrate, and strontium chloride on dentine hypersensitivity: A double-blind randomized controlled trial in Chinese adults. Heliyon 2023, 9, e14634. [Google Scholar] [CrossRef] [PubMed]
- Elshahawy, W.; Ajlouni, R.; James, W.; Abdellatif, H.; Watanabe, I. Elemental ion release from fixed restorative materials into patient saliva. J. Oral Rehabil. 2013, 40, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tarle, A.; Prskalo, K.; Tauböck, T.T. Effect of adhesive coating on calcium, phosphate, and fluoride release from experimental and commercial remineralizing dental restorative materials. Sci. Rep. 2022, 12, 10272. [Google Scholar] [CrossRef] [PubMed]





| Material | Manufacturer | Type | Curing Mechanism | Composition | Bioactive Properties |
|---|---|---|---|---|---|
| Cention Forte Filling Material | Ivoclar (Schaan, Liechtenstein) | Alkasite | Self curing with light curing option | Ca-fluorosilicate glass, Ba-Al silicate glass, copolymer, Ca-Ba-Al fluorosilicate glass, 25–50% UDMA, 2.5–10% ytterbium trifluoride, 10–25% aromatic aliphatic UDMA, DCP and PEG-400-DMA | Releases hydroxide, calcium and fluoride ions |
| Cention Primer | Ivoclar (Schaan, Liechtenstein) | Self etching primer | Self curing | HEMA, MDP, Bis-GMA, D3MA, ethanol, methacrylate-modified polyacrylic acid, silicon dioxide, potassium hydroxide and campherquinone | |
| Stela Self Cure | SDI (Victoria, Australia) | Resin-based restorative material | Self curing | 10–25% UDMA, 5–15% GDMA, 1–10% silica amorphous, 3–7% ytterbium fluoride, 1–5% MDP | Releases fluoride, calcium and strontium ions |
| Fuji IX GP Fast | GC Corp (Tokyo, Japan) | Glass ionomer | Self setting | Alumino-silicate glass, 25–<50% Polyacrylic acid, water, 5–<10% Tartaric acid | Stimulates remineralization through ion exchange |
| Riva Light Cure HV | SDI (Victoria, Australia) | High viscosity glass ionomer | Light curing | 10–20% HEMA, 10–20% HEMA-phosphate derivative, 1–10% GDMA, 1–7% DMAEMA, 1–5% tartaric acid, 0–1% EDMAB, 0–1% camphorquinone, 0–1% BHT, >90% glass, oxide, 1–10% silica amorphous | Releases fluoride and strontium ions |
| Riva Self Cure | SDI (Victoria, Australia) | Glass ionomer | Self curing | 20–30% acrylic acid homopolymer, 10–15% tartaric acid, 90–95% fluoro aluminosilicate glass | Releases fluoride and strontium ions |
| Equia Forte HT Fil | GC Europe (Leuven, Belgium) | Bulk fill glass hybrid | Self setting | 95% fluoro aluminosilicate glass, 5% polyacrylic acid powder, reinforced with silicate particles, 25–<50% polyacrylic acid, 5–<10% polybasic carboxylic acid, 5–<10% tartaric acid | Promotes ion exchange; enhances remineralization and prevents demineralization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliberti, A.; Di Duca, F.; Triassi, M.; Montuori, P.; Scippa, S.; Piscopo, M.; Ausiello, P. The Effect of Different pH and Temperature Values on Ca2+, F−, PO43−, OH−, Si, and Sr2+ Release from Different Bioactive Restorative Dental Materials: An In Vitro Study. Polymers 2025, 17, 640. https://doi.org/10.3390/polym17050640
Aliberti A, Di Duca F, Triassi M, Montuori P, Scippa S, Piscopo M, Ausiello P. The Effect of Different pH and Temperature Values on Ca2+, F−, PO43−, OH−, Si, and Sr2+ Release from Different Bioactive Restorative Dental Materials: An In Vitro Study. Polymers. 2025; 17(5):640. https://doi.org/10.3390/polym17050640
Chicago/Turabian StyleAliberti, Angelo, Fabiana Di Duca, Maria Triassi, Paolo Montuori, Stefano Scippa, Mirko Piscopo, and Pietro Ausiello. 2025. "The Effect of Different pH and Temperature Values on Ca2+, F−, PO43−, OH−, Si, and Sr2+ Release from Different Bioactive Restorative Dental Materials: An In Vitro Study" Polymers 17, no. 5: 640. https://doi.org/10.3390/polym17050640
APA StyleAliberti, A., Di Duca, F., Triassi, M., Montuori, P., Scippa, S., Piscopo, M., & Ausiello, P. (2025). The Effect of Different pH and Temperature Values on Ca2+, F−, PO43−, OH−, Si, and Sr2+ Release from Different Bioactive Restorative Dental Materials: An In Vitro Study. Polymers, 17(5), 640. https://doi.org/10.3390/polym17050640










