A Preliminary Stability Assessment of Three State-of-the-Art CAD/CAM Materials Under Human Gingival Cell Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Raman Spectroscopy
2.3. Energy Dispersive X-Ray Spectroscopy (EDS) and Scanning Electron Microscopy (SEM)
2.4. Atomic Force Microscopy
2.5. Hardness Test
3. Results
3.1. Raman Spectra
3.2. EDS (Energy-Dispersive X-Ray Spectroscopy)
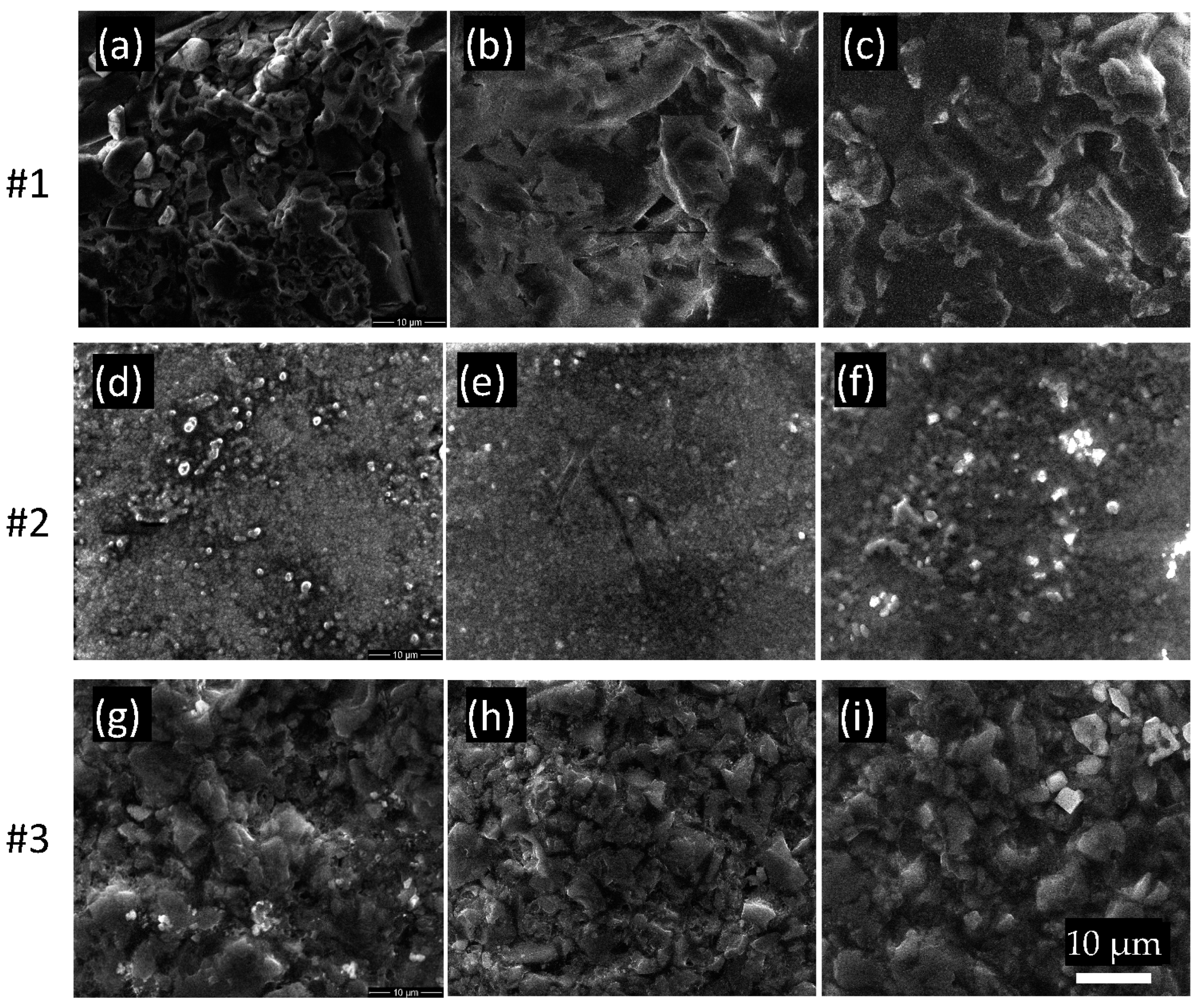
3.3. Scanning Electron Microscopy (SEM)
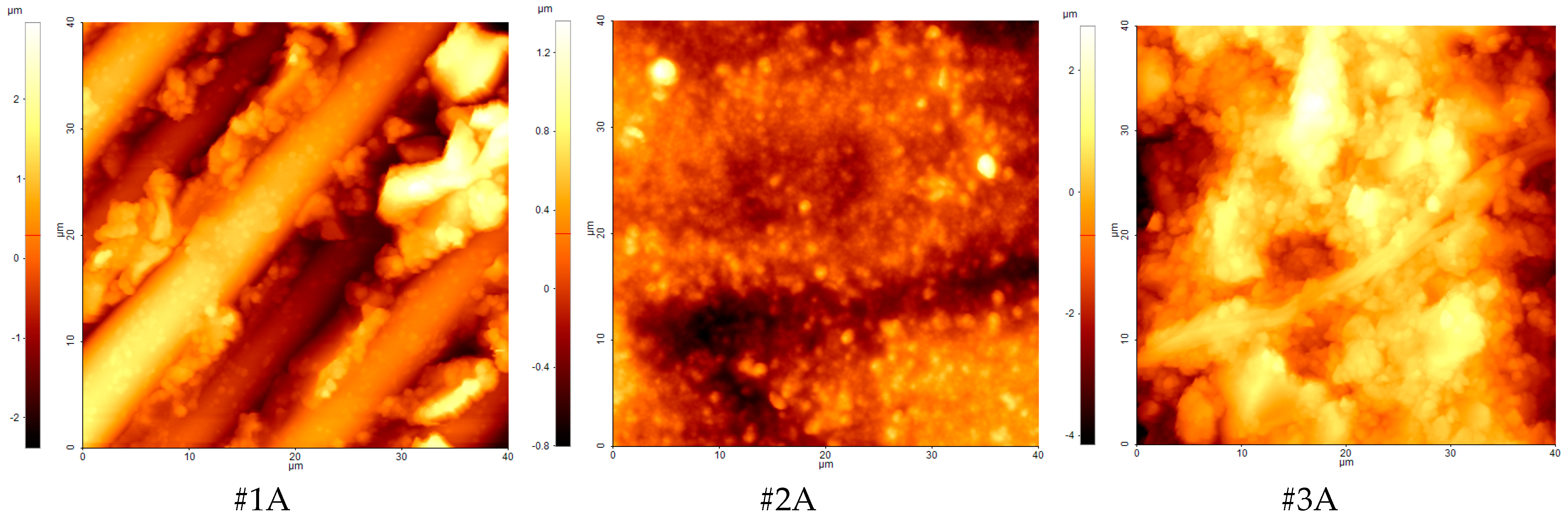
3.4. AFM
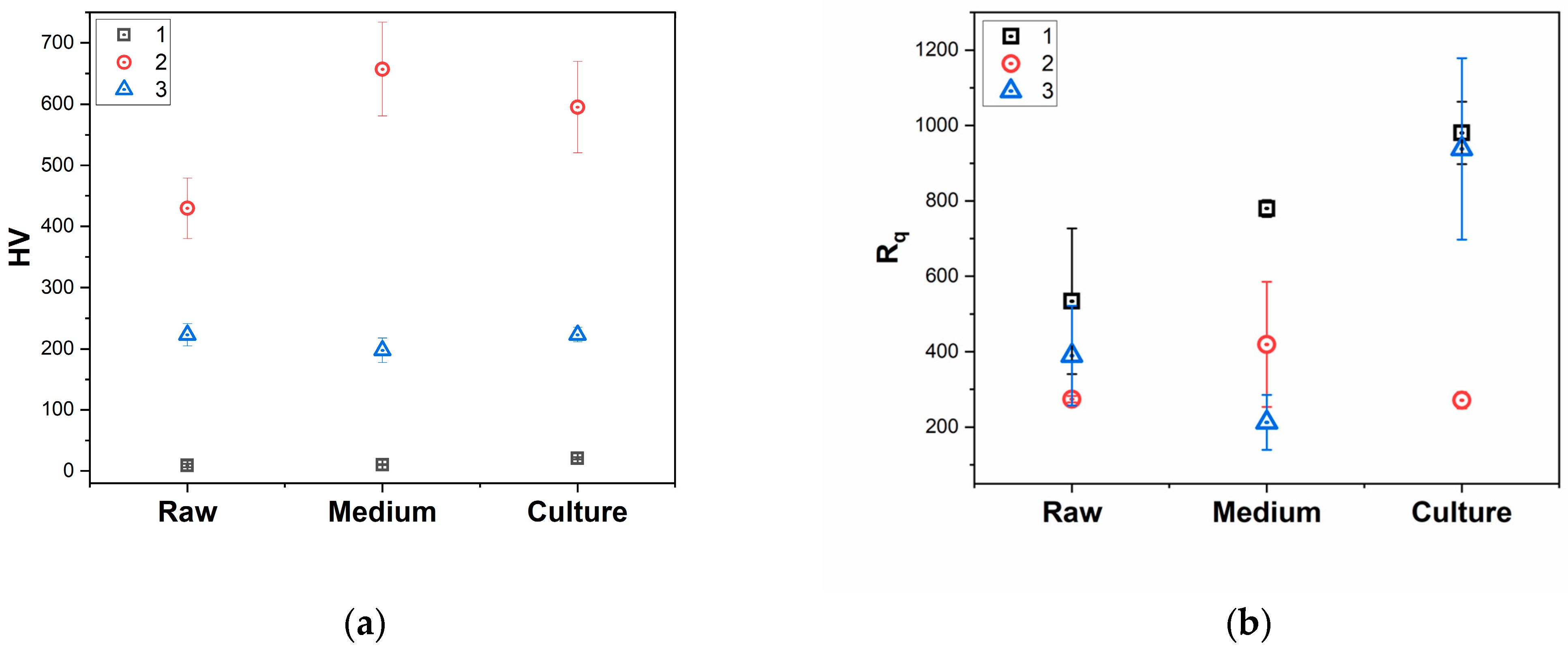
3.5. Microhardness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanno, K.; Abdul-Monem, M. Effect of denture cleansers on the physical and mechanical properties of CAD-CAM milled and 3D printed denture base materials: An in vitro study. J. Prosthet. Dent. 2023, 130, 798.e1–798.e8. [Google Scholar] [CrossRef]
- Pereira, A.C.; Troconis, C.C.M.; Curinga, M.S.; Curinga, M.S.E.; Barão, V.; da Fonte Porto Carreiro, A. Bond strength between denture lining material and CAD-CAM denture base resin: A systematic review and meta-analysis. J. Prosthet. Dent. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wei, D.; Tian, J.; Zhang, Y.; Lin, Y.; Di, P. Quantitative analysis of the color in six CAD-CAM dental materials of varied thickness and surface roughness: An in vitro study. J. Prosthet. Dent. 2024, 131, 292.e1–292.e9. [Google Scholar] [CrossRef] [PubMed]
- Abuhammoud, S.; Emtier, B.; Fu, C.C.; Rojas-Rueda, S.; Jurado, C.; Afrashtehfar, K. Fracture resistance of CAD/CAM milled versus direct hand-made interim laminate veneers. Saudi Dent. J. 2024, 36, 920–925. [Google Scholar] [CrossRef]
- Sahin, M.; Ünalan, F.; Mutlu, I. Corrosion, ion release, and surface hardness of Ti-6Al-4V and cobalt-chromium alloys produced by CAD-CAM milling and laser sintering. J. Prosthet. Dent. 2022, 128, 529.e1–529.e10. [Google Scholar] [CrossRef] [PubMed]
- Schwärzler, A.; Ludwig, B.; Chitan, P.; Lettner, S.; Sagl, B.; Jonke, E. Transfer accuracy of 3D printed versus CAD/CAM milled surgical guides for temporary orthodontic implants: A preclinical micro CT study. J. Dent. 2024, 146, 105060. [Google Scholar] [CrossRef] [PubMed]
- Ausiello, P.; Di Lauro, A.E.; Tribst, J.P.M.; Watts, D. Stress distribution in resin-based CAD-CAM implant-supported crowns. Dent. Mater. 2023, 39, 114–122. [Google Scholar] [CrossRef]
- Ling, L.; Lai, T.; Malyala, R. Fracture toughness and brittleness of novel CAD/CAM resin composite block. Dent. Mater. 2022, 38, e308–e317. [Google Scholar] [CrossRef] [PubMed]
- Almejrad, L.; Almansour, A.; Bartlett, D.; Austin, R. CAD/CAM leucite-reinforced glass-ceramic for simulation of attrition in human enamel in vitro. Dent. Mater. 2024, 40, 173–178. [Google Scholar] [CrossRef]
- Oudkerk, J.; Herman, R.; Eldafrawy, M.; Wulfman, C.; Ernst, M.; Vanheusden, A.; Mainjot, A. Intraoral wear of PICN CAD-CAM composite restorations used in severe tooth wear treatment: 5-year results of a prospective clinical study using 3D profilometry. Dent. Mater. 2024, 40, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Brambilla, E.; Pires, P.; López-Castellano, A.; Alambiaga-Caravaca, A.; Lenardi, C.; Sauro, S. Physical-chemical and microbiological performances of graphene-doped PMMA for CAD/CAM applications before and after accelerated aging protocols. Dent. Mater. 2022, 38, 1470–1481. [Google Scholar] [CrossRef]
- Elraggal, A.; Abdelraheem, I.; Watts, D.; Roy, S.; Dommeti, V.; Alshabib, A.; Althaqafi, K.; Afifi, R. Biomechanical reinforcement by CAD-CAM materials affects stress distributions of posterior composite bridges: 3D finite element analysis. Dent. Mater. 2024, 40, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, M.Y.; Al Humayyani, N.; Alwthinani, F.K.; Alzahrani, A.H.; Alotaibi, A.O.; Yousef, M.; Ahmed, A.S.; Ali, A. In vitro evaluation of the mechanical and optical properties of 3D printed vs CAD/CAM milled denture teeth materials. Saudi Dent. J. 2024, 61. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, G.; Mahn, E.; Sanchez, J.; Berrera, S.; Prado, M.; Bernasconi, V. Hybrid Ceramics in Dentistry: A Literature Review. J. Clin. Res. Dent. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, J.; Lee, H.; Scherrer, S.; Lee, H. Strength-limiting damage and defects of dental CAD/CAM full-contour zirconia ceramics. Dent. Mater. 2024, 40, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.M.; de Oliveira Dal Piva, A.; Pereira, G.K.R.; Valandro, L.; Rippe, M.P.; Feilzer, A.; Kleverlaan, C.; Tribst, J.M. Effect of brushing simulation on the wear behavior of repaired CAD-CAM restorations. Int. Dent. J. 2024, 74, 999–1005. [Google Scholar] [CrossRef]
- Didangelou, P.; Dionysopoulos, D.; Papadopoulos, C.; Strakas, D.; Mourouzis, P.; Tolidis, K. Evaluation of repair bond strength of a dental CAD/CAM resin composite after surface treatment with two Er, Cr: YSGG laser protocols following artificial aging. J. Mech. Behav. Biomed. Mater. 2023, 146, 106101. [Google Scholar] [CrossRef]
- Yiğit, E.; Erdoğan, H.G.; Eyüboğlu, T.; Özcan, M. Effect of Various Beverages on Adhesion of Repaired CAD/CAM Restorative Materials. J. Funct. Biomater. 2023, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, R.; Marocho, S.S.; Griggs, J.; Borba, M. CAD/CAM versus 3D-printing/pressed lithium disilicate monolithic crowns: Adaptation and fatigue behavior. J. Dent. 2022, 123, 104181. [Google Scholar] [CrossRef]
- Sola, D.; Chueca, E.; Wang, S.; Peña, J. Surface Activation of Calcium Zirconate-Calcium Stabilized Zirconia Eutectic Ceramics with Bioactive Wollastonite-Tricalcium Phosphate Coatings. J. Funct. Biomater. 2023, 14, 510. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, F.; Sánchez-González, E.; Borrero-López, Ó.; Hoffman, M. In-vitro study of the sliding-wear of CAD/CAM dental composite materials. Tribol. Int. 2024, 202, 110314. [Google Scholar] [CrossRef]
- Mascaro, B.A.; Tejada-Casado, M.; Fonseca, R.G.; Reis, J.M.D.S.N.; Pérez, M.M. Exploring the optical behavior and relative translucency parameter of CAD-CAM resin-based composites, polymer-infiltrated ceramic network, and feldspar porcelain. Dent. Mater. 2024, 40, 1954–1961. [Google Scholar] [CrossRef]
- Redwan, H.; Fan, Y.; Giordano, R. Effect of machining damage on the surface roughness and flexural strength of CAD-CAM materials. J. Prosthet. Dent. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Şenol, A.A.; Gençer, B.K.; Kaya, B.D.; Kahramanoğlu, E.; Atalı, P.Y.; Tarçın, B. Evaluation of the shear bond strength between CAD/CAM blocks and sonic/thermoviscous bulk-fill composites with different surface treatments. Int. J. Adhes. Adhes. 2024, 134, 103805. [Google Scholar] [CrossRef]
- Wierichs, R.J.; Kramer, E.J.; Reiss, B.; Roccuzzo, A.; Raabe, C.; Yilmaz, B.; Abou-Ayash, S. Longevity and risk factors of CAD-CAM manufactured implant-supported all-ceramic crowns—A prospective, multi-center, practice-based cohort study. Dent. Mater. 2024, 40, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Gatin, E.; Ciucu, C.; Ciobanu, G.; Berlic, C. Investigation and comparative survey of some dental restorative materials. Optoelectron. Adv. Mater. Rapid Commun. 2008, 2, 284–290. [Google Scholar]
- Gatin, E.; Iordache, S.-M.; Matei, E.; Luculescu, C.-R.; Iordache, A.-M.; Grigorescu, C.; Ilici, R. Raman Spectroscopy as Spectral Tool for Assessing the Degree of Conversion after Curing of Two Resin-Based Materials Used in Restorative Dentistry. Diagnostics 2022, 12, 1993. [Google Scholar] [CrossRef]
- Jennes, M.-E.; Tomakidi, P.; Husari, A.; Hellwig, E.; Polydorou, O.; Schulz, S.D. Response of human gingival keratinocytes to hybrid CAD/CAM material eluates. Dent. Mater. 2022, 38, 1532–1546. [Google Scholar] [CrossRef]
- Alamoush, R.A.; Kushnerev, E.; Yates, J.M.; Satterthwaite, J.D.; Silikas, N. Response of two gingival cell lines to CAD/CAM composite blocks. Dent. Mater. 2020, 36, 1214–1225. [Google Scholar] [CrossRef]
- Pecho, O.E.; Alvarez-Lloret, P.; Ionescu, A.M.; Cardona, J.C.; Ghinea, R.; Sánchez-Sánchez, P.; Perez, M.M.; Della Bona, A. Influence of microstructure on optical properties of CAD-CAM lithium disilicate glass-ceramics. Dent. Mater. 2024, 40, 1927–1936. [Google Scholar] [CrossRef]
- Muehlemann, E.; Chantler, J.G.M.; Smajovic, M.; Strauss, F.J. A custom CAD-CAM resilient attachment: A dental technique. J. Prosthet. Dent. 2024, in press. [Google Scholar] [CrossRef]
- Picolo, M.Z.D.; Kury, M.; Romário-Silva, D.; Rosalen, P.L.; Pecorari, V.G.A.; Gianinni, M.; Cavalli, V. Effects of gastric acid and mechanical toothbrushing in CAD-CAM restorative materials: Mechanical properties, surface topography, and biofilm adhesion. J. Mech. Behav. Biomed. Mater. 2023, 138, 105606. [Google Scholar] [CrossRef] [PubMed]
- Gil-Pozo, A.; Astudillo-Rubio, D.; Cascales, A.; Inchingolo, F.; Hirata, R.; Sauro, S.; Delgado-Gaete, A. Effect of gastric acids on the mechanical properties of conventional and CAD/CAM resin composites—An in-vitro study. J. Mech. Behav. Biomed. Mater. 2024, 155, 106565. [Google Scholar] [CrossRef]
- Ellakany, P.; Aly, N.; Alameer, S.; Alshehri, T.; Fouda, S. Assessment of color stability and translucency of various CAD/CAM ceramics of different compositions and Thicknesses: An in vitro study. Saudi Dent. J. 2024, 36, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Innoprot. Cell-Based Assay Kits Collagen I-Cell Culture Surface Coating Kit. Available online: https://innoprot.com/product/collagen-i-cell-culture-surface-coating-kit/ (accessed on 23 July 2024).
- Innoprot. Human Gingival Epithelial Cells. Available online: https://innoprot.com/product/human-gingival-epithelial-cells/ (accessed on 23 July 2024).
- Vermehren, M.; Wiesmann, N.; Deschner, J.; Brieger, J.; Al-Nawas, B.; Kämmerer, P. Comparative analysis of the impact of e-cigarette vapor and cigarette smoke on human gingival fibroblasts. Toxicol. Vitr. 2020, 69, 105005. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.A.; Kushnerev, E.; Alamoush, R.A.; Seymour, K.G.; Yates, J.M. Two Gingival Cell Lines Response to Different Dental Implant Abutment Materials: An In Vitro Study. Dent. J. 2022, 10, 192. [Google Scholar] [CrossRef]
- Gatin, E.; Nagy, P.; Paun, I.; Dubok, O.; Bucur, V.; Windisch, P. Raman Spectroscopy: Application in Periodontal and Oral Regenerative Surgery for Bone Evaluation. IRBM 2019, 40, 279–285. [Google Scholar] [CrossRef]
- Gatin, E.; Nagy, P.; Iordache, S.; Iordache, A.; Luculescu, C. Raman Spectroscopy: In Vivo Application for Bone Evaluation in Oral Reconstructive (Regenerative) Surgery. Diagnostics 2022, 12, 723. [Google Scholar] [CrossRef] [PubMed]
- Aragonez, G.; Dalla-Nora, F.; Soares, P.M.; Pereira, G.R.; Valandro, L.; Santos, S.D.; Rippe, M.P. Load-bearing capacity under fatigue of bonded-yttria tetragonal zirconia polycrystals and -yttria-stabilized zirconia: Effects of the viscosity of a dual-cured resin cement. J. Mech. Behav. Biomed. Mater. 2023, 148, 106233. [Google Scholar] [CrossRef]
- University of California. RAMAN Band Correlation Table. Available online: https://www.chem.uci.edu/~dmitryf/manuals/Raman%20correlations.pdf (accessed on 30 July 2024).
- Durand, J.; Jacquot, B.; Salehi, H.; Fages, M.; Margerit, J.; Cuisinier, F. Confocal Raman microscopic analysis of the zirconia/feldspathic ceramic interface. Dent. Mater. 2012, 28, 661–671. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.; Fateley, W.; Grasselli, J. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: USA, 1991. [Google Scholar]
- Saravia-Rojas, M.; Huanambal-Tiravanti, V.; Geng-Vivanco, R.; de Carvalho Panzeri Pires-de-Souza, F.; Balarezo-Razzeto, J. Different surface treatments on recently introduced CAD-CAM resin-modified ceramics: Implications on bond strength. J. Prosthet. Dent. 2024, in press. [Google Scholar] [CrossRef]
- Lührs, A.; Pongprueksa, P.; De Munck, J.; Geurtsen, W.; Van Meerbeek, B. Curing mode affects bond strength of adhesively luted composite CAD/CAM restorations to dentin. Dent. Mater. 2014, 30, 281–291. [Google Scholar] [CrossRef]
- Aragonez, G.; Pilecco, R.; Dapieve, K.; Burgo, T.; Guilardi, L.; Prochnow, C.; Valandro, L.; Rippe, M. Simulation of CAD/CAM milling on lithium disilicate: Mechanical and topographic analyses of surface grinding different protocols. J. Mech. Behav. Biomed. Mater. 2022, 132, 105278. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, L.; Kontonasaki, E.; Zorba, T.; Chatzistavrou, X.; Pavlidou, E.; Paraskevopoulos, K.; Sklavounos, S.; Koidis, P. Dental ceramics coated with bioactive glass: Surface changes after exposure in a simulated body fluid under static and dynamic conditions. Phys. Status Solidi (a) 2003, 198, 65–75. [Google Scholar] [CrossRef]
- Kontonasaki, E.; Zorba, T.; Papadopoulou, L.; Pavlidou, E.; Chatzistavrou, X.; Paraskevopoulos, K.; Koidis, P. Hydroxy Carbonate Apatite Formation on Particulate Bioglass In Vitro as a Function of Time. Cryst. Res. Technol. 2002, 37, 1165–1171. [Google Scholar] [CrossRef]
- Colombo, G.; Dalle-Donne, I.; Orioli, M.; Giustarini, D.; Rossi, R.; Clerici, M.; Regazzoni, L.; Aldini, G.; Milzani, A.; Butterfield, D.A.; et al. Oxidative damage in human gingival fibroblasts exposed to cigarette smoke. Free Radic. Biol. Med. 2012, 52, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Adil, N.; Ali, H.; Siddiqui, A.; Ali, A.; Ahmed, A.; El-Seedi, H.; Musharraf, S. Evaluation of cytotoxicity of areca nut and its commercial products on normal human gingival fibroblast and oral squamous cell carcinoma cell lines. J. Hazard. Mater. 2021, 403, 123872. [Google Scholar] [CrossRef]
- Gatin, E.; Luculescu, C.; Iordache, S.; Pătraşcu, I. Morphological investigation by AFM of dental ceramics under thermal processing. J. Optoelectron. Adv. Mater. 2013, 15, 1136–1141. [Google Scholar]
- Sfeatcu, R.; Luculescu, C.; Ciobanu, L.; Balan, A.; Gatin, E.; Patrascu, I. Dental Enamel Quality and Black Tooth Stain: A New Approach and Explanation by using Raman and AFM Techniques. Part. Sci. Technol. Int. J. 2015, 33, 429–435. [Google Scholar] [CrossRef]
- Öztürk, E.K.; Nemli, S.K.; Bal, B.T.; Güngör, M.B. Evaluation of the optical and surface properties of monolithic CAD-CAM ceramics after simulated tooth-brushing. J. Prosthet. Dent. 2024, 132, 1325.e1–1325.e8. [Google Scholar] [CrossRef]
- Choi, W.; Yoo, L.; Kim, Y.; Jung, B. Mechanical properties of CAD/CAM polylactic acid as a material for interim restoration. Heliyon 2023, 9, e15314. [Google Scholar] [CrossRef] [PubMed]
- Schepke, U.; Filius, D.; Lohbauer, U.; la Bastide-van Gemert, S.; Gresnigt, M.; Cune, M. Dimensional changes of CAD/CAM polymer crowns after water aging—An in vitro experiment. J. Mech. Behav. Biomed. Mater. 2022, 128, 105109. [Google Scholar] [CrossRef]
- Jrab, B.; Saleh, A.; Al-Jadaa, A.; Jurado, C.; Saeed, M.; Afrashtehfar, K. Fracture resistance of CAD/CAM tooth-colored versus cast metal post-and-core restorations in root filled teeth: An in vitro study. Saudi Dent. J. 2024, 36, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Prause, E.; Hey, J.; Beuer, F.; Yassine, J.; Hesse, B.; Weitkamp, T. Gerber and F. Schmidt. Microstructural investigation of hybrid CAD/CAM restorative dental materials by micro-CT and SEM. Dent. Mater. 2024, 40, 930–940. [Google Scholar] [CrossRef]
- Jassim, S.; Majeed, M. Effect of plasma surface treatment of three different CAD/CAM materials on the micro shear bond strength with resin cement (A comparative in vitro study). Heliyon 2023, 9, e17790. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Guo, X.; Chen, J.; Zhao, Y.; Song, J.; Alshawwa, H.; Zou, X.; Zhao, H.; Zhang, Z. Biodegradation of Urethane Dimethacrylate-based materials (CAD/CAM resin-ceramic composites) and its effect on the adhesion and proliferation of Streptococcus mutans. J. Mech. Behav. Biomed. Mater. 2024, 150, 106280. [Google Scholar] [CrossRef]
- Ferrini, F.; Paolone, G.; Di Domenico, G.L.; Pagani, N.; Gherlone, E.F. SEM Evaluation of the Marginal Accuracy of Zirconia, Lithium Disilicate, and Composite Single Crowns Created by CAD/CAM Method: Comparative Analysis of Different Materials. Materials 2023, 16, 2413. [Google Scholar] [CrossRef]


| Sample No | Commercial Name (Manufacturer) | Composition |
|---|---|---|
| #1 | Trinia (SHOFU, Kyoto, Japan) | Glass fiber: 55 wt% + epoxy matrix resin: 45 wt% |
| #2 | Coritec ZrO (IMES-ICORE GMBH—Eiterfeld, Germany) | ZrO2 + Y2O3 + HfO2 > 99 wt%, Al2O3 ˂ 0.5 wt%, other oxides 0.25 wt% |
| #3 | Vita Enamic (Vita Zahnfabrik, Bad Sackingen, Germany), | UDMA, TEGDMA. Filler: Feldspar ceramic enriched with aluminum oxide, 86 wt% |
| #1 (Trinia) | #2 (Coritec) | #3 (Vita) | Assignment | Ref. |
|---|---|---|---|---|
| 316 cm−1 | Tetragonal ZrO2 (symmetry B1g) | [41] | ||
| 343 cm−1 | C-C aliphatic chain | [42] | ||
| 392 cm−1 | 383 cm−1 | Si-O stretch in glass | [42] | |
| 462 cm−1 | Tetragonal ZrO2 (symmetry Eg) | [41] | ||
| 608 cm−1 | Cubic lattice of the ZrO2 | [43] | ||
| 639 cm−1 | 643 cm−1 | Tetragonal ZrO2 (symmetry Eg)—#2 | [41] | |
| 668 cm−1 | C-H deformation (-CH=CH-) cis | [44] | ||
| 706 cm−1 | Mono-substituted C-H deformation out-of-plane | [44] | ||
| 736 cm−1 | CH3 aliphatic/-CH2- rocking of organic polymer * | [45] | ||
| 810 cm−1 | C-H deformation out-of-plane of the organic polymer | [44] | ||
| 820 cm−1 | C-H deformation out-of-plane of the organic polymer | [44] | ||
| 914 cm−1 | O-H deformation (aromatic carboxylic acids) | [44] | ||
| 967 cm−1 | C-H deformation | [44] | ||
| 1010 cm−1 | C-O-C stretch in alkyl-aryl ethers | [44] | ||
| 1113 cm−1 | 1118 cm−1 | Si-O-CH2 stretch | [44] | |
| 1186 cm−1 | P=O stretch/P-O-C strech | [44] | ||
| 1253 cm−1 | P=O stretch | [44] | ||
| 1288 cm−1 | Si-CH3 deformation | [44] | ||
| 1349 cm−1 | S=O (anti-symmetrical) | [44] | ||
| 1401 cm−1 | 1401 cm−1 | Polynuclear aromatic polymers/C=O stretch (sym.) | [44] | |
| 1428 cm−1 | C-O stretch combined with O-H deformation in aromatic carboxylic acids | [44] | ||
| 1536 cm−1 | 1536 cm−1 | In-plane ring deformation | [44] | |
| 1608 cm−1 | C=C aromatic | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatin, E.; Iordache, S.; Iordache, A.M.; Totan, A.; Moldovan, A.; Luculescu, C. A Preliminary Stability Assessment of Three State-of-the-Art CAD/CAM Materials Under Human Gingival Cell Culture. Polymers 2025, 17, 221. https://doi.org/10.3390/polym17020221
Gatin E, Iordache S, Iordache AM, Totan A, Moldovan A, Luculescu C. A Preliminary Stability Assessment of Three State-of-the-Art CAD/CAM Materials Under Human Gingival Cell Culture. Polymers. 2025; 17(2):221. https://doi.org/10.3390/polym17020221
Chicago/Turabian StyleGatin, Eduard, Stefan Iordache, Ana Maria Iordache, Alexandra Totan (Ripsvki), Antoniu Moldovan, and Catalin Luculescu. 2025. "A Preliminary Stability Assessment of Three State-of-the-Art CAD/CAM Materials Under Human Gingival Cell Culture" Polymers 17, no. 2: 221. https://doi.org/10.3390/polym17020221
APA StyleGatin, E., Iordache, S., Iordache, A. M., Totan, A., Moldovan, A., & Luculescu, C. (2025). A Preliminary Stability Assessment of Three State-of-the-Art CAD/CAM Materials Under Human Gingival Cell Culture. Polymers, 17(2), 221. https://doi.org/10.3390/polym17020221







