Mechanochemically Synthesized PEG-OTs as a Green Corrosion Inhibitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ball Mill Machine
2.3. Synthesis
2.4. Static Corrosion Tests
2.5. Electrochemical Tests
2.6. SEM Analysis
2.7. Contact Angle Measurements
3. Results and Discussions
3.1. Synthesis of PEG-OTs
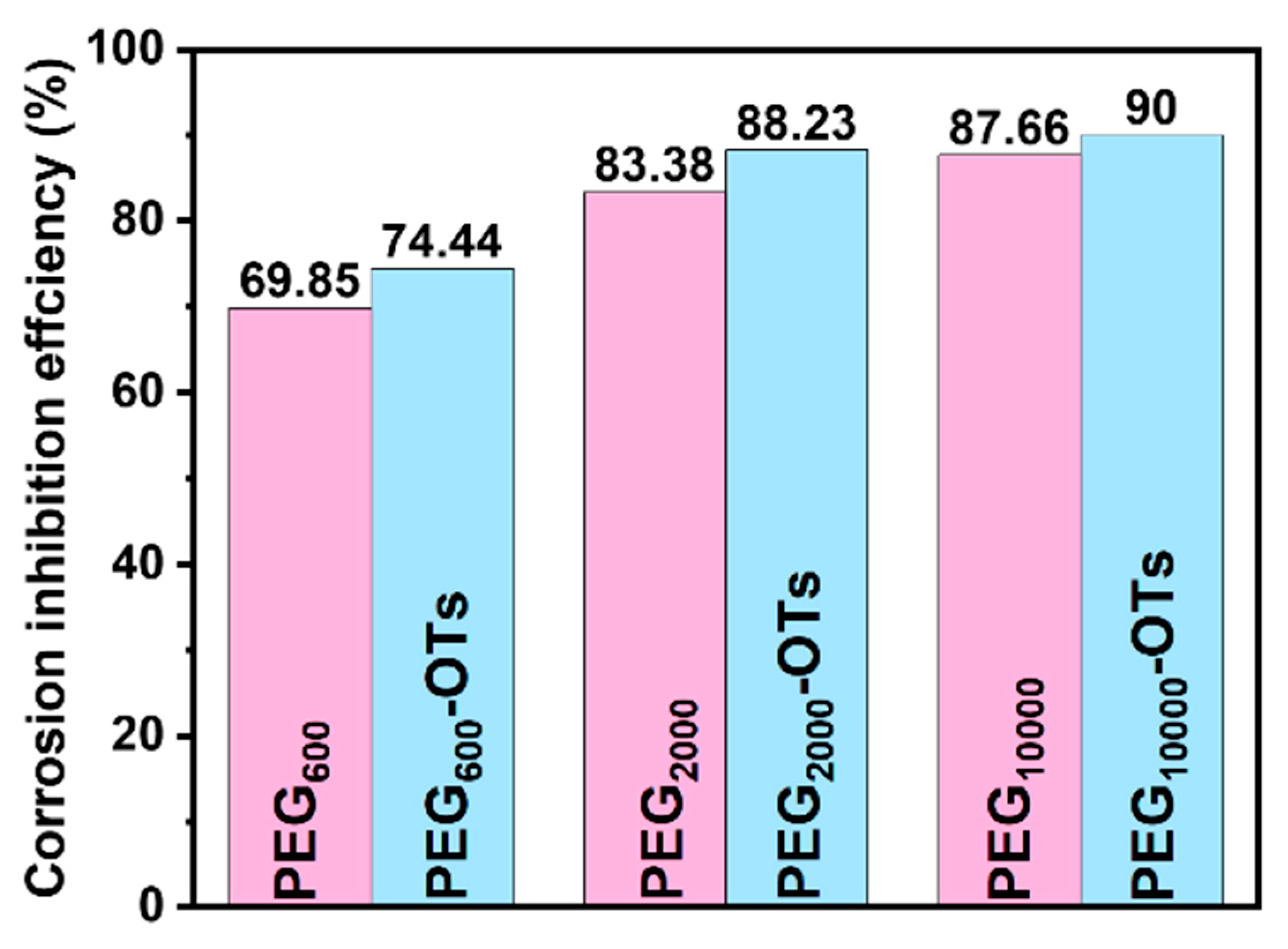
3.2. Static Weight-Loss
3.3. Electrochemical Results
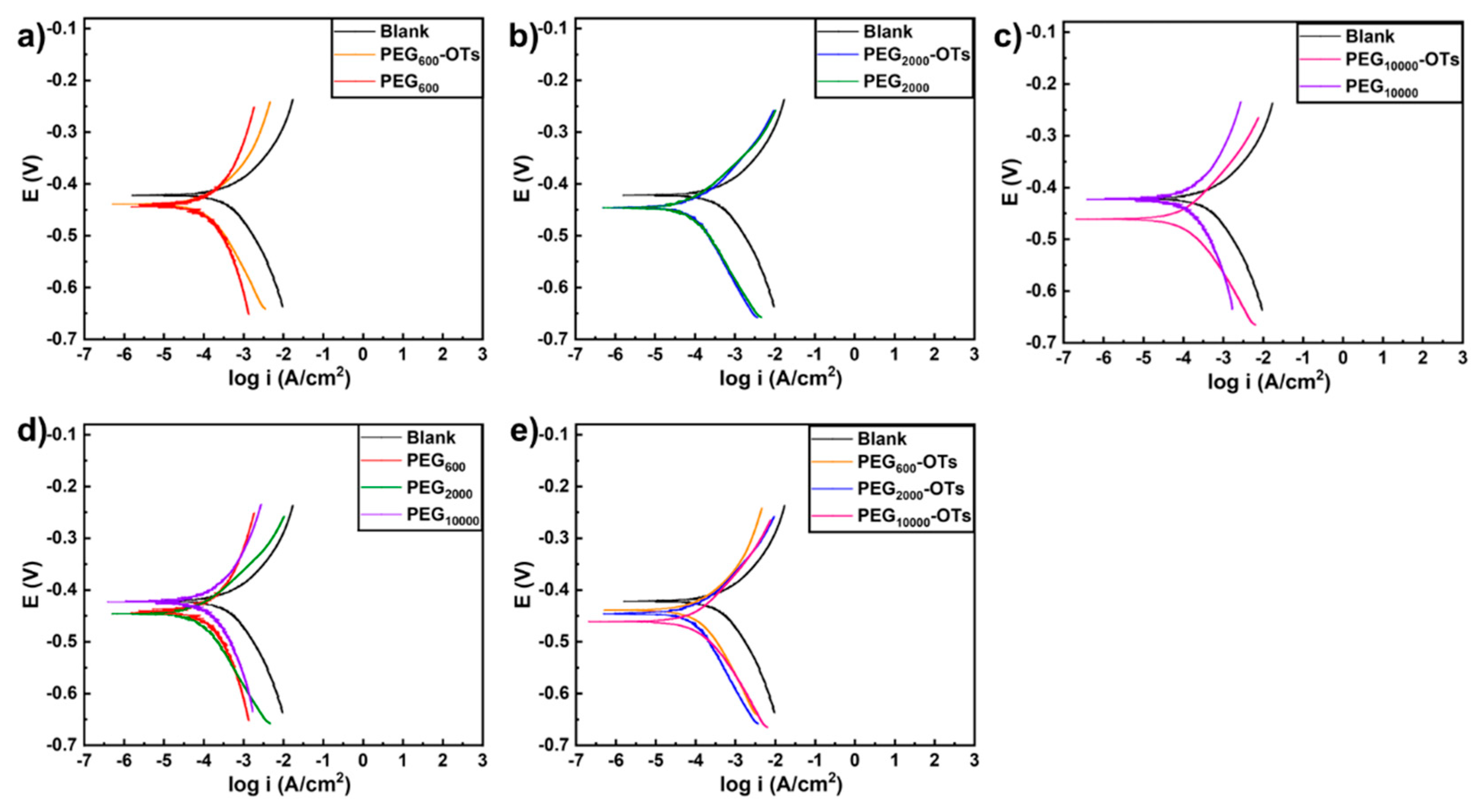
3.4. Tafel Polarization Results
3.5. Morphological Characteristics
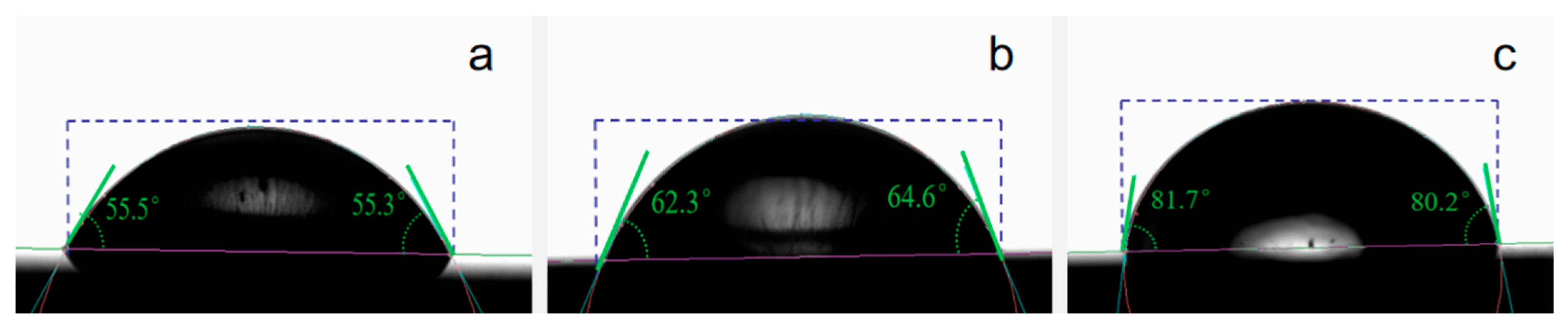
3.6. Contact Angle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EIS | Electrochemical Impedance Spectroscopy |
| PEG | Poly(ethylene) glycol |
References
- Dong, L.; Ma, Y.; Jin, X.; Feng, L.; Zhu, H.; Hu, Z.; Ma, X. High-efficiency corrosion inhibitor of biomass-derived high-yield carbon quantum dots for Q235 carbon steel in 1 M HCl solution. ACS Omega 2023, 8, 46934–46945. [Google Scholar] [CrossRef] [PubMed]
- Jalab, R.; Ali, A.B.; Khaled, M.; Abouseada, M.; AlKhalil, S.; Al-Suwaidi, A.; Hamze, S.; Hussein, I. Novel polyepoxysuccinic acid-grafted polyacrylamide as a green corrosion inhibitor for carbon steel in acidic solution. ACS Omega 2023, 8, 16673–16686. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Lgaz, H.; Chung, I.M. Comprehensive investigation of steel corrosion inhibition at macro/micro level by ecofriendly green corrosion inhibitor in 15% HCl medium. J. Colloid Interface Sci. 2020, 560, 225–236. [Google Scholar] [CrossRef]
- Singh, P.; Ebenso, E.E.; Olasunkanmi, L.O.; Obot, I.B.; Quraishi, M.A. Electrochemical, theoretical, and surface morphological studies of corrosion inhibition effect of green naphthyridine derivatives on mild steel in hydrochloric acid. J. Phys. Chem. C 2016, 120, 3408–3419. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Sorour, A.A.; Saha, S.K.; Banerjee, P. Triazole-modified chitosan: A biomacromolecule as a new environmentally benign corrosion inhibitor for carbon steel in a hydrochloric acid solution. RSC Adv. 2019, 9, 14990–15003. [Google Scholar] [CrossRef]
- Cao, S.; Liu, D.; Ding, H.; Wang, J.; Lu, H.; Gui, J. Task-specific ionic liquids as corrosion inhibitors on carbon steel in 0.5 M HCl solution: An experimental and theoretical study. Corros. Sci. 2019, 153, 301–313. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Amin, S.; Mohamed, A.A. Current and emerging trends of inorganic, organic and eco-friendly corrosion inhibitors. RSC Adv. 2024, 14, 31877–31920. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Advincula, R.C. Polymeric corrosion inhibitors for the oil and gas industry: Design principles and mechanism. React. Funct. Polym. 2015, 95, 25–45. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Zhang, Y. Organic compounds as corrosion inhibitors for carbon steel in HCl solution: A comprehensive review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef]
- Oung, J.; Shih, H.C. Evaluating film-forming corrosion inhibitors for carbon steel in gas-well environments. Corros. Prev. Control 1997, 44, 81–89. [Google Scholar]
- Dandia, A.; Gupta, S.; Singh, P.; Quraishi, M.A. Ultrasound-assisted synthesis of pyrazolo [3, 4-b] pyridines as potential corrosion inhibitors for mild steel in 1.0 M HCl. ACS Sustain. Chem. Eng. 2013, 1, 1303–1310. [Google Scholar] [CrossRef]
- Manh, T.D.; Huynh, T.L.; Thi, B.V.; Lee, S.; Yi, J.; Nguyen Dang, N. Corrosion inhibition of mild steel in hydrochloric acid environments containing sonneratia caseolaris leaf extract. ACS Omega 2022, 7, 8874–8886. [Google Scholar] [CrossRef]
- Alhaffar, M.T.; Umoren, S.A.; Obot, I.B.; Ali, S.A. Isoxazolidine derivatives as corrosion inhibitors for low carbon steel in HCl solution: Experimental, theoretical and effect of KI studies. RSC Adv. 2018, 8, 1764–1777. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Guo, L.; Yang, H.; Zhang, F.; El Bakri, Y. Synergistic effect of potassium iodide and sodium dodecyl sulfonate on the corrosion inhibition of carbon steel in HCl medium: A combined experimental and theoretical investigation. RSC Adv. 2020, 10, 15163–15170. [Google Scholar] [CrossRef] [PubMed]
- Gadow, H.S.; Motawea, M.M. Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv. 2017, 7, 24576–24588. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Quadri, T.W.; Sherif, E.S.M.; Ebenso, E.E. Gravimetric, electrochemical, surface morphology, DFT, and Monte Carlo simulation studies on three N-substituted 2-aminopyridine derivatives as corrosion inhibitors of mild steel in acidic medium. J. Phys. Chem. C 2018, 122, 11870–11882. [Google Scholar] [CrossRef]
- Radojčić, I.; Berković, K.; Kovač, S.; Vorkapić-Furač, J. Natural honey and black radish juice as tin corrosion inhibitors. Corros. Sci. 2008, 50, 1498–1504. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, S.; Li, W.; Gong, M.; Yin, L. Experimental and theoretical studies of two imidazolium-based ionic liquids as inhibitors for mild steel in sulfuric acid solution. Corros. Sci. 2015, 95, 168–179. [Google Scholar] [CrossRef]
- Olasunkanmi, L.O.; Obot, I.B.; Kabanda, M.M.; Ebenso, E.E. Some quinoxalin-6-yl derivatives as corrosion inhibitors for mild steel in hydrochloric acid: Experimental and theoretical studies. J. Phys. Chem. C 2015, 119, 16004–16019. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Do, J.L.; Friščić, T. Mechanochemistry: A force of synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Amrute, A.P.; Zibrowius, B.; Schüth, F. Mechanochemical grafting: A solvent-less highly efficient method for the synthesis of hybrid inorganic–organic materials. J. Mater. Chem. 2020, 32, 4699–4706. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W. Mechanochemistry: Opportunities for New and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Guan, X.; Liu, H.; Yang, J.; Li, S.; Wang, Q.; Huang, X.; Wang, Y.; Yu, Q.; Li, M. Solvent-free lignin modification through ball-milled CuAAc. ACS Sustain. Chem. Eng. 2024, 12, 13700–13710. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Sun, Z.; Qian, P.; Han, P.; Yu, B.; Ye, S. A novel, solvent-free mechanochemistry approach for gold extraction from anode slime. ACS Sustain. Chem. Eng. 2019, 7, 11415–11425. [Google Scholar] [CrossRef]
- Tan, D.; Garcia, F. Main group mechanochemistry: From curiosity to established protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. [Google Scholar] [CrossRef]
- Nič, M.; Jirát, J.; Košata, B.; Jenkins, A.; McNaught, A. IUPAC Compendium of Chemical Terminology; IUPAC: Research Triagle Park, NC, USA, 2009; Volume 10. [Google Scholar]
- Guan, X.; Sang, Y.; Liu, H. Ball-milled click chemistry: A solvent-free green chemistry. Prog. Chem. 2024, 36, 401–415. [Google Scholar]
- Porcheddu, A.; Colacino, E.; De Luca, L.; Delogu, F. Metal-mediated and metal-catalyzed reactions under mechanochemical conditions. ACS Catal. 2020, 10, 8344–8394. [Google Scholar] [CrossRef]
- Li, J.; Nagamani, C.; Moore, J.S. Polymer mechanochemistry: From destructive to productive. Acc. Chem. Res. 2015, 48, 2181–2190. [Google Scholar] [CrossRef]
- Bolm, C.; Hernández, J.G. Mechanochemistry of gaseous reactants. Angew. Chem. Int. Ed. Engl. 2019, 58, 3285–3299. [Google Scholar] [CrossRef]
- Bolt, R.R.A.; Leitch, J.A.; Jones, A.C.; Nicholson, W.I.; Browne, D.L. Continuous flow mechanochemistry: Reactive extrusion as an enabling technology in organic synthesis. Chem. Soc. Rev. 2022, 51, 4243–4260. [Google Scholar] [CrossRef] [PubMed]
- Jafter, O.F.; Lee, S.; Park, J.; Cabanetos, C.; Lungerich, D. Navigating ball mill specifications for theory-to-practice reproducibility in mechanochemistry. Angew. Chem. Int. Ed. Engl. 2024, 63, e202409731. [Google Scholar] [CrossRef] [PubMed]
- Colacino, E.; Carta, M.; Pia, G.; Porcheddu, A.; Ricci, P.C.; Delogu, F. Processing and investigation methods in mechanochemical kinetics. ACS Omega 2018, 3, 9196–9209. [Google Scholar] [CrossRef] [PubMed]
- Malca, M.Y.; Ferko, P.O.; Friščić, T.; Moores, A. Solid-state mechanochemical omega-functionalization of poly(ethylene glycol). Beilstein J. Org. Chem. 2017, 13, 1963–1968. [Google Scholar] [CrossRef]
- Glassner, M.; Maji, S.; de la Rosa, V.R.; Vanparijs, N.; Ryskulova, K.; De Geest, B.G.; Hoogenboom, R. Solvent-free mechanochemical synthesis of a bicyclononyne tosylate: A fast route towards bioorthogonal clickable poly(2-oxazoline)s. Polym. Chem. 2015, 6, 8354–8359. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Guo, Z.; Luo, J.; Zhang, C.; Zhang, G. Heterocyclic group end-capped polyethylene glycol: Synthesis and used as corrosion inhibitors for mild steel in HCl medium. J. Mol. Liq. 2023, 388, 122779. [Google Scholar] [CrossRef]
- Chen, B.M.; Cheng, T.L.; Roffler, S.R. Polyethylene glycol immunogenicity: Theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies. ACS Nano 2021, 15, 14022–14048. [Google Scholar] [CrossRef]
- Ju, Y.; Lee, W.S.; Pilkington, E.H.; Kelly, H.G.; Li, S.; Selva, K.J.; Wragg, K.M.; Subbarao, K.; Nguyen, T.H.O.; Rowntree, L.C.; et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. ACS Nano 2022, 16, 11769–11780. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Ghalebsaz-Jeddi, N.; Hashemzadeh, F.; Jahani, H. Corrosion inhibition of carbon steel in hydrochloric acid by some polyethylene glycols. Electrochim. Acta 2006, 51, 3848–3854. [Google Scholar] [CrossRef]
- Ding, J.; Peng, W.; Zhao, H.; Yu, H. Facile preparation of AT-PEG polymer and its corrosion inhibition performance. Int. J. Electrochem. Sci. 2017, 12, 5058–5071. [Google Scholar] [CrossRef]
- Kwon, S.K.; Kim, D.H. Effect of process parameters of UV-assisted gas-phase cleaning on the removal of PEG (polyethyleneglycol) from a Si substrate. J. Korean Phys. Soc. 2006, 49, 1421. [Google Scholar]
- Mazari, S.A.; Abro, R.; Bhutto, A.W.; Saeed, I.M.; Ali, B.S.; Jan, B.M.; Ghalib, L.; Ahmed, M.A.; Mubarak, N.M. Thermal degradation kinetics of morpholine for carbon dioxide capture. J. Environ. Chem. Eng. 2020, 8, 103814. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Chen, X. 2-Benzylsulfanyl-1H-benzimidazole and its mixture as highly efficient corrosion inhibitors for carbon steel under dynamic supercritical CO2 flow conditions. Corros. Sci. 2024, 235, 112170–112190. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Gu, L.; Su, S.; Yu, H. A novel waterborne epoxy coating with anticorrosion properties on rusty steel. Int. J. Electrochem. Sci. 2016, 11, 7066–7075. [Google Scholar] [CrossRef]


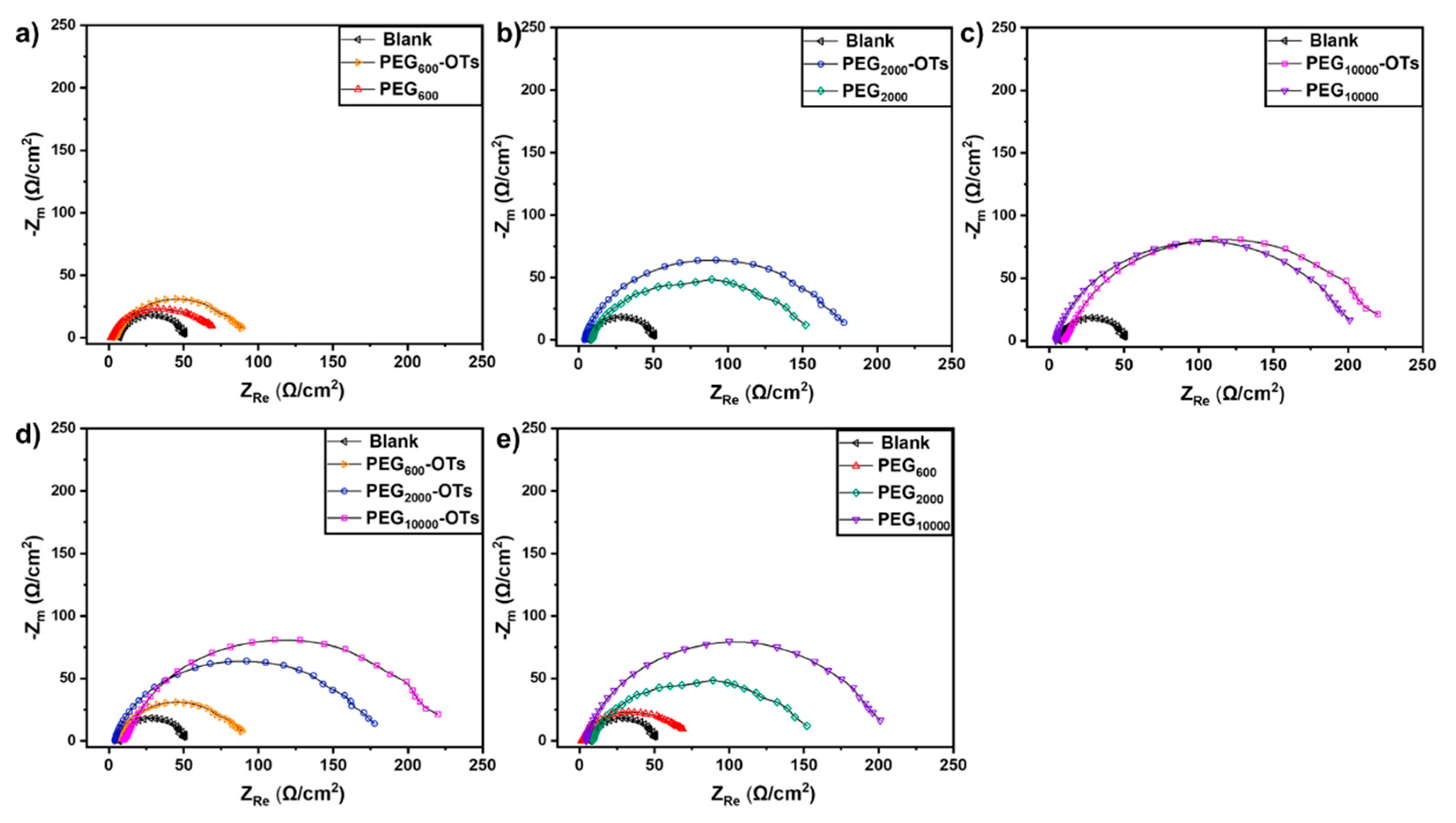

| Sample | Rs (Ω·cm2) | Cf (μF·cm−2) | nf | Rf (Ω·cm2) | Cd (μF·cm−2) | nd | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|
| blank | 0.0185 | 255.7 | 0.851 | 4.8 | 184.3 | 0.887 | 51.6 |
| PEG600-OTs | 4.228 | 141.8 | 0.811 | 12.7 | 126.7 | 0.932 | 125.6 |
| PEG600 | 6.182 | 11.94 | 0.498 | 6.9 | 115.1 | 0.845 | 89.5 |
| PEG2000-OTs | 4.911 | 38.2 | 0.916 | 20.2 | 141.5 | 0.613 | 189.2 |
| PEG2000 | 9.68 | 3.15 | 0.896 | 13.3 | 98.92 | 0.813 | 175.2 |
| PEG10,000-OTs | 9.371 | 30.15 | 0.831 | 24.1 | 61.21 | 0.837 | 262.3 |
| PEG10,000 | 4.323 | 62.2 | 0.894 | 19.7 | 162.1 | 0.739 | 238.5 |
| Sample | Ecorr (V) | Icorr (μA·cm−2) | βa (mV·dec−1) | βc (mV·dec−1) | RP (Ω·cm2) | CR (Mpy) | IE% |
|---|---|---|---|---|---|---|---|
| Blank | −0.42 | 257.04 | 85.2 | 89.6 | 52.68 | 84.9 | - |
| PEG600-OTs | 0.465 | 84.077 | 95.5 | 73.5 | 126.68 | 26.8 | 67 |
| PEG600 | 0.443 | 86.961 | 86.3 | 95.6 | 90.58 | 72.3 | 66 |
| PEG2000-OTs | 0.445 | 70.958 | 71.6 | 77.2 | 190.28 | 6.98 | 72 |
| PEG2000 | 0.443 | 74.121 | 88.9 | 109.5 | 176.28 | 9.15 | 71 |
| PEG10,000-OTs | 0.441 | 60.663 | 98.7 | 100.6 | 263.38 | 0.55 | 76 |
| PEG10,000 | 0.422 | 63.782 | 102.3 | 91.8 | 239.58 | 0.83 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Sang, Y.; Yang, J.; Liu, H. Mechanochemically Synthesized PEG-OTs as a Green Corrosion Inhibitor. Polymers 2025, 17, 422. https://doi.org/10.3390/polym17030422
Wang Q, Sang Y, Yang J, Liu H. Mechanochemically Synthesized PEG-OTs as a Green Corrosion Inhibitor. Polymers. 2025; 17(3):422. https://doi.org/10.3390/polym17030422
Chicago/Turabian StyleWang, Qiannian, Yuan Sang, Jiang Yang, and Hailing Liu. 2025. "Mechanochemically Synthesized PEG-OTs as a Green Corrosion Inhibitor" Polymers 17, no. 3: 422. https://doi.org/10.3390/polym17030422
APA StyleWang, Q., Sang, Y., Yang, J., & Liu, H. (2025). Mechanochemically Synthesized PEG-OTs as a Green Corrosion Inhibitor. Polymers, 17(3), 422. https://doi.org/10.3390/polym17030422






