Assessment of the Stability of Propellants Modified with Eco-Friendly Plasticizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Triethyl 2-acetylcitrate (ATEC) (Sigma Aldrich, Poznań, Poland);
- Tributyl 2-acetylcitrate (ATBC) (Sigma Aldrich, Poznań, Poland);
- Ethanol with 98% purity (Hurtownia Odczynników Chemicznych Butra, Skierniewice, Poland);
- 2-Nitrodiphenylamine (2-NDPA), 99.8% purity, synthesized at the Department of High-Energetic Materials, Warsaw University of Technology.
- Acetonitrile (ACN) (Sigma Aldrich, Poznań, Poland);
- Methanol (MeOH) (Sigma Aldrich, Poznań, Poland);
- Anhydrous calcium chloride (CaCl2) (Avantor Performance Materials Poland S.A. Zabrze, Poland).
2.2. Modification Process
2.3. Research Methods
3. Results and Discussion
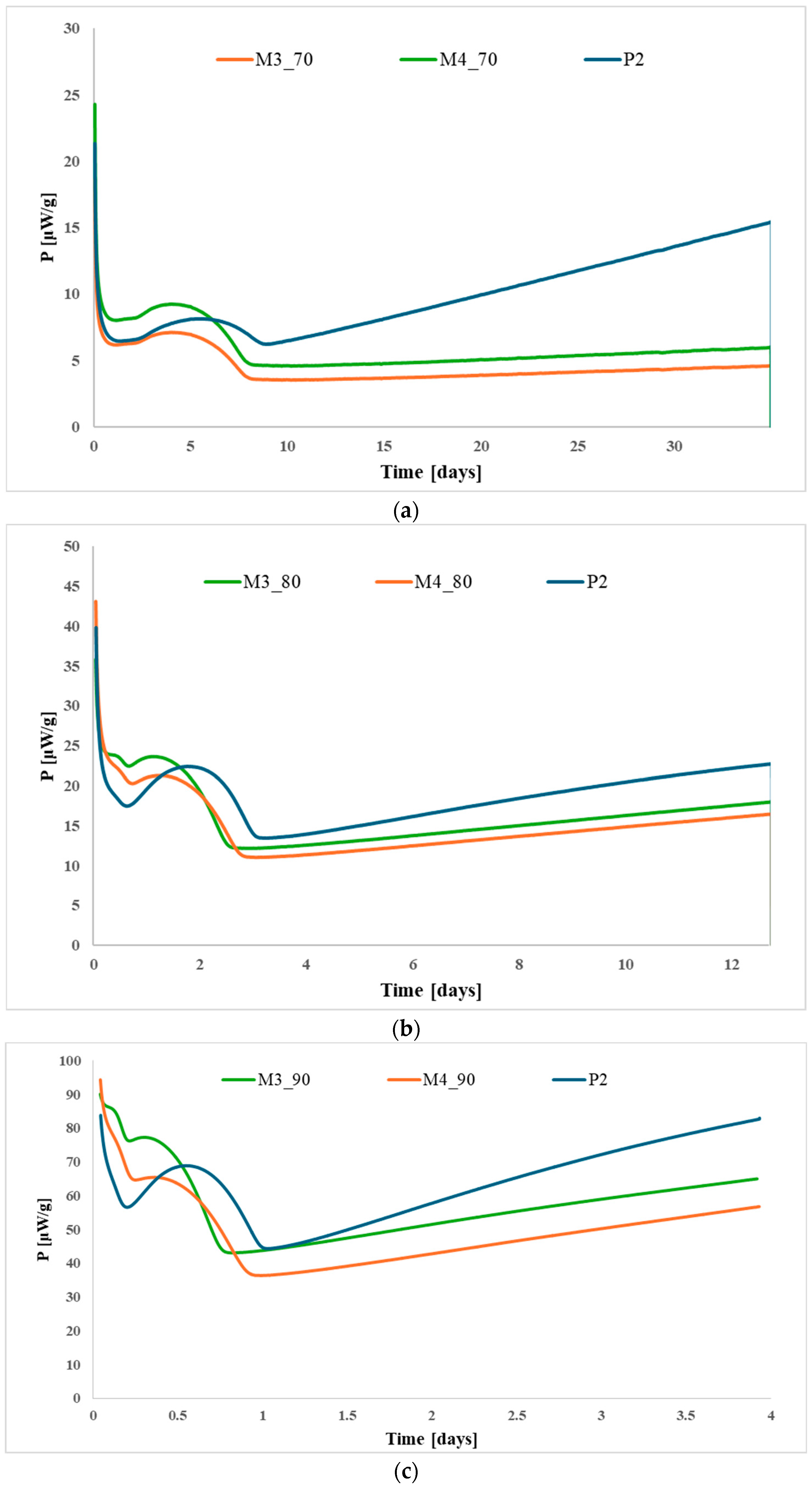
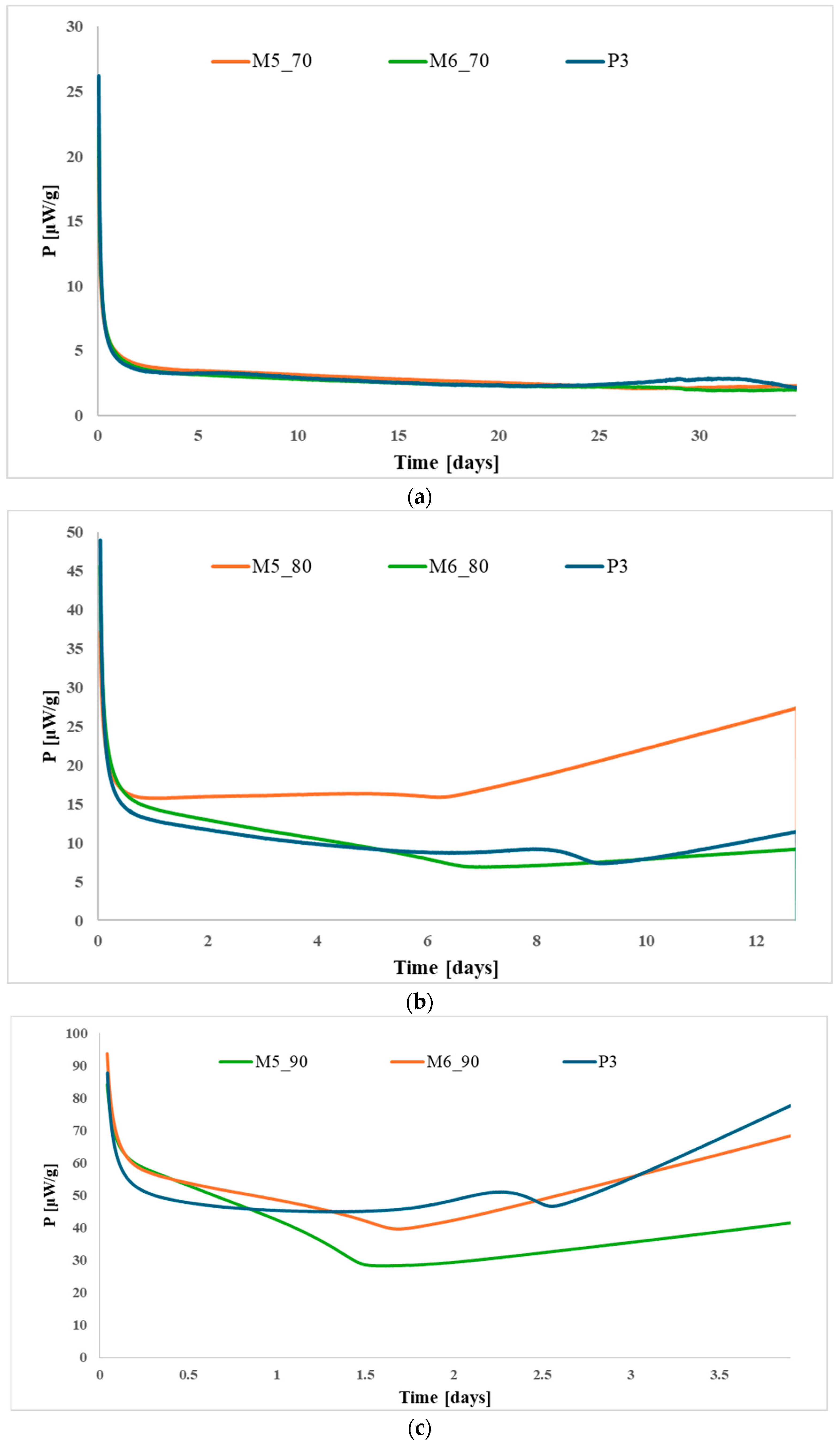
3.1. Thermal Stability Assessment
3.2. Stabilizer Depletion Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DBP | Dibutyl phthalate |
| ATEC | Triethyl 2-acetylcitrate |
| ATBC | Tributyl 2-acetylcitrate |
| CI | N,N′-Diethyl-N,N′-diphenylurea |
| HPLC | High-performance liquid chromatography |
| HFC | Heat flow microcalorimetry |
References
- Anastas, P.T.; Warner, J.C. Principles of Green Chemistry. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Regulation (EC) No 1907/2006 of The European Parliament and of the Council of 18 December 2006 the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC 2006. Available online: https://eur-lex.europa.eu/eli/reg/2006/1907/oj (accessed on 26 October 2025).
- Chavez, D.E. The Development of Environmentally Sustainable Manufacturing Technologies for Energetic Materials. Green Energetic Mater. 2014, 9, 235–258. [Google Scholar] [CrossRef]
- Boulkadid, M.K.; Lefebvre, M.H.; Jeunieau, L.; Dejeaifve, A. Assessment of the Migration of Combustion Moderator in Nitrocellulose-Based Propellant. In Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2021; pp. 123–132. [Google Scholar] [CrossRef]
- Gańczyk-Specjalska, K.; Cieślak, K.; Jakubczak, M.; Drożdżewska-Szymańska, K.; Tomaszewski, W.; Prasuła, P. The Effect of Citrate Plasticizers on the Properties of Nitrocellulose Granules. Propellants Explos. Pyrotech. 2023, 48, e202200305. [Google Scholar] [CrossRef]
- Cao, X.; Nan, F.; Fan, W.; Gao, H.; Chen, L.; Guo, Y.; He, W. Preparation, Formation Mechanism, and Performance of Nitrocellulose Aqueous Coating Based on the Anti-Solvent Method. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133214. [Google Scholar] [CrossRef]
- Mendonça-Filho, L.G.; Rodrigues, R.L.B.; Rosato, R.; Galante, E.B.F.; Nichele, J. Combined Evaluation of Nitrocellulose-Based Propellants: Toxicity, Performance, and Erosivity. J. Energetic Mater. 2019, 37, 293–308. [Google Scholar] [CrossRef]
- Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers Derived from Biomass Resources: A Short Review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Muobom, S.S.; Umar, A.-M.S.; Brolin, A.-P.; Soongseok, Y. A Review on Plasticizers and Eco-Friendly Bioplasticizers: Biomass Sources and Market. Int. J. Eng. Res. Technol. 2020, 9, 1138–1144. [Google Scholar] [CrossRef]
- Krutko, I.; Yavir, K.; Kaulin, V.; Strankowski, M. Effect of Antioxidants on the Stability of Pitch-Based Polymer to Thermo-Oxidative Action. Chem. Chem. Technol. 2018, 12, 109–113. [Google Scholar] [CrossRef]
- Rusly, S.N.A.; Jamal, S.H.; Samsuri, A.; Mohd Noor, S.A.; Abdul Rahim, K.S. Stabilizer Selection and Formulation Strategies for Enhanced Stability of Single Base Nitrocellulose Propellants: A Review. Energetic Mater. Front. 2024, 5, 52–69. [Google Scholar] [CrossRef]
- Rusly, S.N.A.; Jamal, S.H.; Samsuri, A.; Mohd Noor, S.A.; Abdul Rahim, K.S. A Green Stabilizer for Nitrate Ester-Based Propellants: An Overview. Heliyon 2024, 10, e39631. [Google Scholar] [CrossRef]
- Chebbah, M.; Tarchoun, A.F.; Benaliouche, F.; Abdelaziz, A.; Trache, D. Advancing Nitrocellulose Thermal Stability through the Incorporation of Ion-Exchanged ZSM-5 Zeolite for Enhanced Performance. FirePhysChem 2025, 5, 209–222. [Google Scholar] [CrossRef]
- Cieślak, K.; Gańczyk-Specjalska, K.; Drożdżewska-Szymańska, K.; Królikowska, M.; Jakubczak, M. Physicochemical Properties and Thermal Behavior of Nitrocellulose Granules with Eutectic Mixtures of Stabilizers. J. Therm. Anal. Calorim. 2022, 147, 7421–7430. [Google Scholar] [CrossRef]
- Qi, X.; Li, H.; Zhao, Y.; Yan, N. Comparison of the Structural and Physical Properties of Nitrocellulose Plasticized by N-Butyl-N-(2-Nitroxy-Ethyl) Nitramine and Nitroglycerin: Computational Simulation and Experimental Studies. J. Hazard. Mater. 2019, 362, 303–310. [Google Scholar] [CrossRef]
- Yang, L.; Wu, X.; Li, J.; Chen, T.; Liu, M.; He, Q. Structure and Property of Propellant Based on Nitroglycerine/Glycerol Triacetate Mixed Plasticizers: Molecular Dynamics Simulation and Experimental Study. R. Soc. Open Sci. 2021, 8, 211033. [Google Scholar] [CrossRef]
- Agrawal, J.P. High Energy Materials: Propellants, Explosives and Pyrotechnics; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- De Klerk, W.P.C. Assessment of Stability of Propellants and Safe Lifetimes. Propellants Explos. Pyrotech. 2015, 40, 388–393. [Google Scholar] [CrossRef]
- Cieślak, K.; Tomaszewski, W.; Gańczyk-Specjalska, K. Study of the Effect of Accelerated Ageing on the Properties of Modified Nitrocellulose Propellants. High Energy Mater. 2023, 15, 48–60. Available online: https://wydawnictwa.ipo.lukasiewicz.gov.pl/hig-en-mat-vol/vol-15/?lang=en (accessed on 26 October 2025).
- STANAG 4582 (Ed. 1); Explosives, Nitrocellulose Based Propellants, Stability Test Procedure And Requirments Using Heat Flow Calorimetry. European Defence Agency: Brussels, Belgium, 2007.
- AOP-48 (Ed. 2); Explosives, Nitrocellulose-Based Propellants, Stability Test Procedures And Requirements Using Stabilizer Depletion. NATO: Washington, DC, USA, 2008.
- Lindblom, T. Reactions in Stabilizer and Between Stabilizer and Nitrocellulose in Propellants. Propellants Explos. Pyrotech. 2002, 27, 197–208. Available online: https://onlinelibrary.wiley.com/doi/10.1002/1521-4087(200209)27:4%3C197::AID-PREP197%3E3.0.CO;2-W (accessed on 26 October 2025). [CrossRef]
- Boers, M.N.; De Klerk, W.P.C. Lifetime Prediction of EC, DPA, Akardite II and MNA Stabilized Triple Base Propellants, Comparison of Heat Generation Rate and Stabilizer Consumption. Propellants Explos. Pyrotech. 2005, 30, 356–362. [Google Scholar] [CrossRef]
- Chajistamatiou, A.S.; Bakeas, E.B. A Rapid Method for the Identification of Nitrocellulose in High Explosives and Smokeless Powders Using GC–EI–MS. Talanta 2016, 151, 192–201. [Google Scholar] [CrossRef]
- Mazur, I.; Kasprzak, P.; Borkowski, J. Safety Monitoring of Storage and Use of Solid Homogeneous Rocket Propellants Through the Chemical Composition Analysis. Def. Sci. J. 2025, 75, 401–410. [Google Scholar] [CrossRef]
- Wilker, S.; Heeb, G.; Vogelsanger, B.; Petržílek, J.; Skládal, J. Triphenylamine—A “new” Stabilizer for Nitrocellulose Based Propellants—Part I: Chemical Stability Studies. Propellants Explos. Pyrotech. 2007, 32, 135–148. [Google Scholar] [CrossRef]
- Fryš, O.; Bajerová, P.; Eisner, A.; Skládal, J.; Ventura, K. Utilization of New Non-Toxic Substances as Stabilizers for Nitrocellulose-Based Propellants. Propellants Explos. Pyrotech. 2011, 36, 347–355. [Google Scholar] [CrossRef]
- Rodrigues, R.L.B.; Gomes Buitrago, P.A.; Nakano, N.L.; Peixoto, F.C.; Lemos, M.F.; França, T.C.C.; Mendonça Filho, L.G. Can Green Nitrocellulose-Based Propellants Be Made through the Replacement of Diphenylamine by the Natural Product Curcumin? J. Energetic Mater. 2022, 40, 218–241. [Google Scholar] [CrossRef]
- Gańczyk-Specjalska, K. Conventional and Alternative Nitrocellulose Stabilisers Used in Gun Propellants. High Energy Mater. 2019, 11, 73–82. Available online: https://wydawnictwa.ipo.lukasiewicz.gov.pl/hig-en-mat-vol/tom-11-2/?lang=en (accessed on 26 October 2025). [CrossRef] [PubMed]
- Jones, A.; Warrender, G.; Porter, D.; Barber, N. A Reduced Toxicity Deterrent for Single Base Propellants. In Proceedings of the IMEMTS 2015, Rome, Italy, 18–20 May 2015. [Google Scholar]
- Cieślak, K.; Gołofit, T.; Tomaszewski, W.; Chmielarek, M.; Maksimowski, P.; Pawłowski, W. Modification of the Burning Layer of Nitrocellulose Powders with Liquid Nitroesters. High Energy Mater. 2021, 13, 48–58. Available online: https://wydawnictwa.ipo.lukasiewicz.gov.pl/hig-en-mat-vol/tom-13-2/?lang=en (accessed on 26 October 2025).
- Liu, B.; Ma, F.S.; Bian, X.Y.; Tian, D.Q.; Wang, Q.L.; Lv, H. Research on the Performance of Deterred-Coating DIANP Gun Propellant. In Journal of Physics: Conference Series; Institute of Physics: London, UK, 2023; Volume 2478. [Google Scholar]
- Cieślak, K.; Gańczyk-Specjalska, K. Methods of Modifying Single Base Propellants Using Centralite I, Dibutyl Phthalate and Rosin. High Energy Mater. 2022, 14, 107–116. Available online: https://wydawnictwa.ipo.lukasiewicz.gov.pl/hig-en-mat-vol/vol-14/?lang=en (accessed on 26 October 2025).
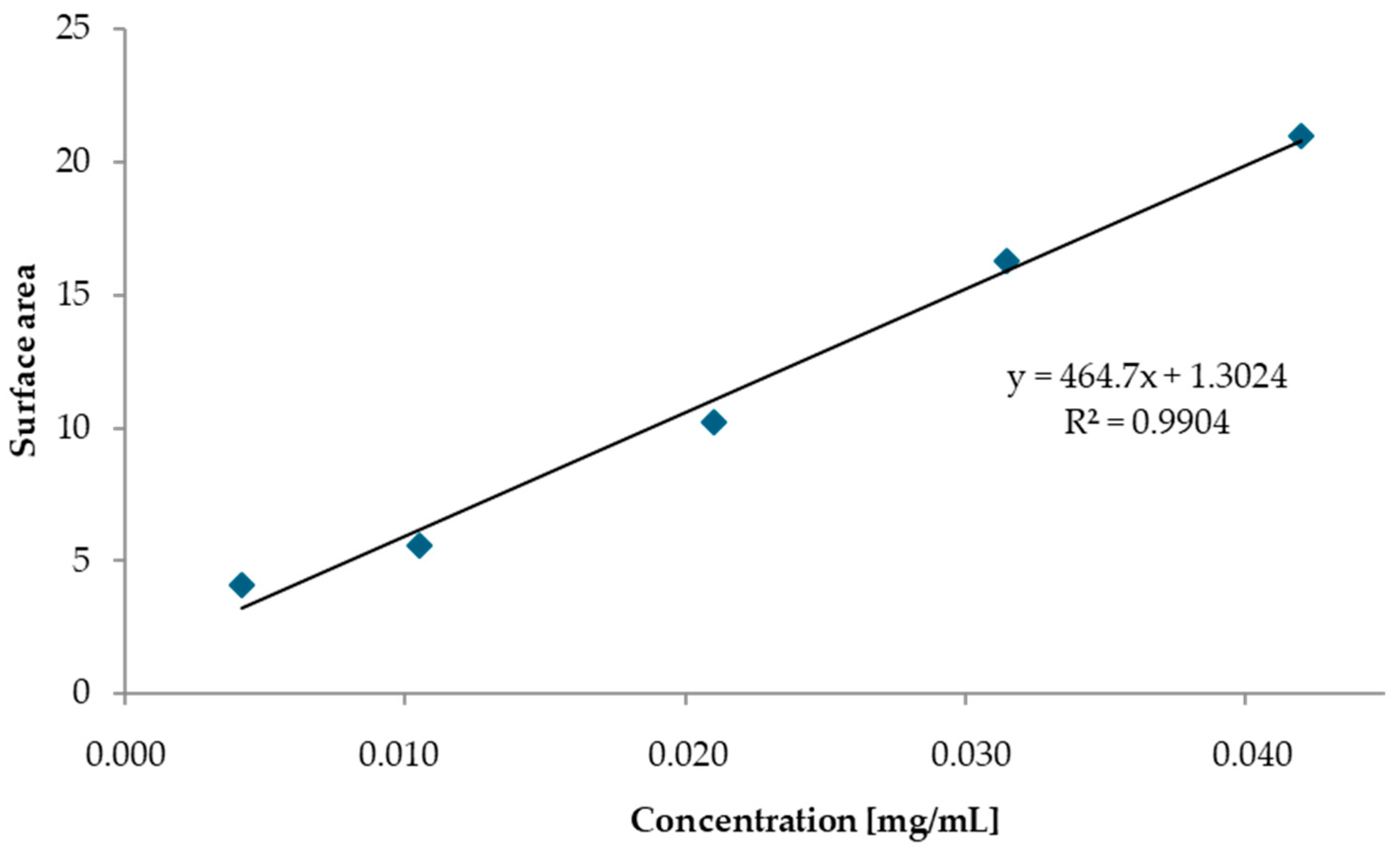
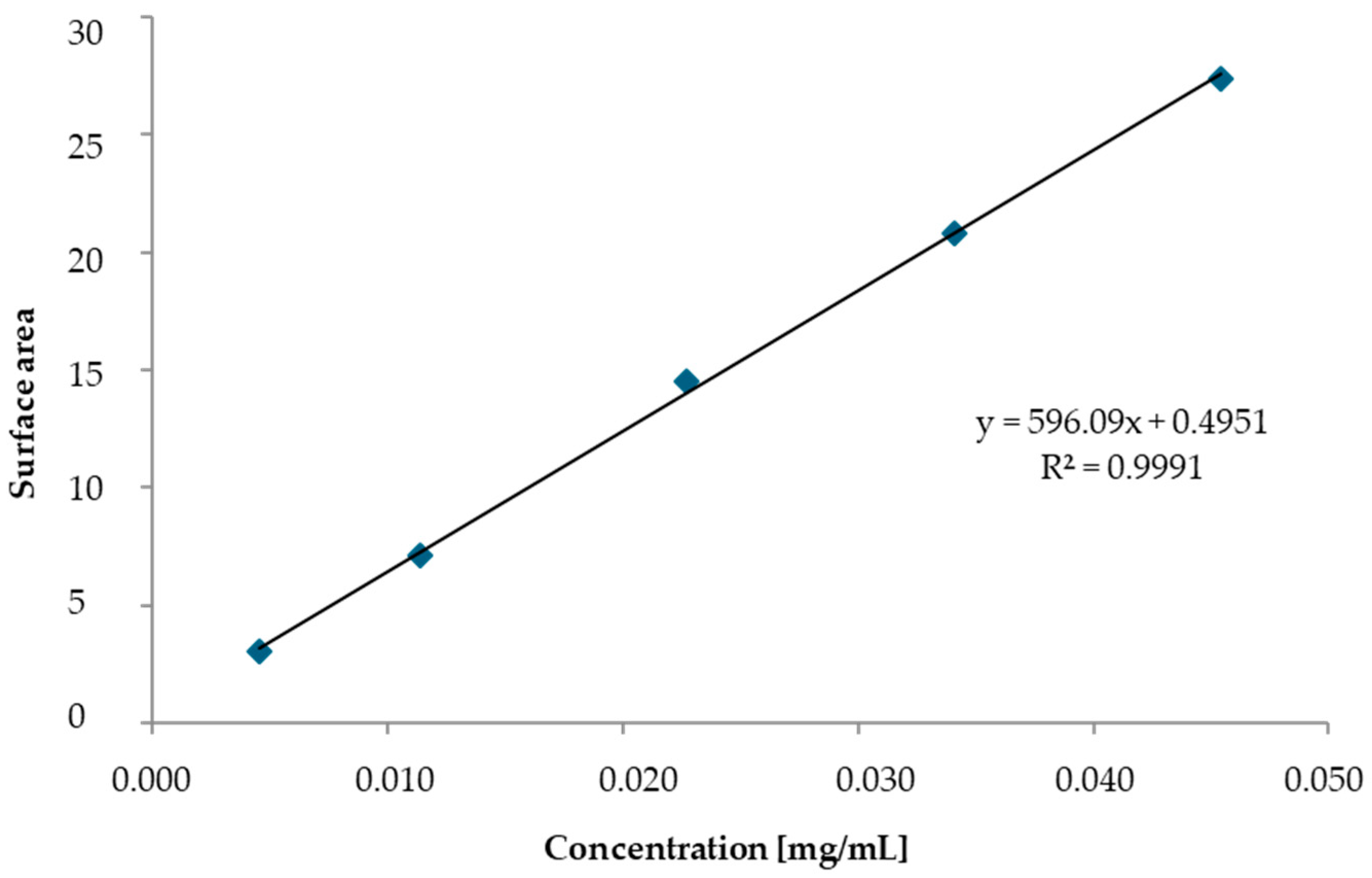
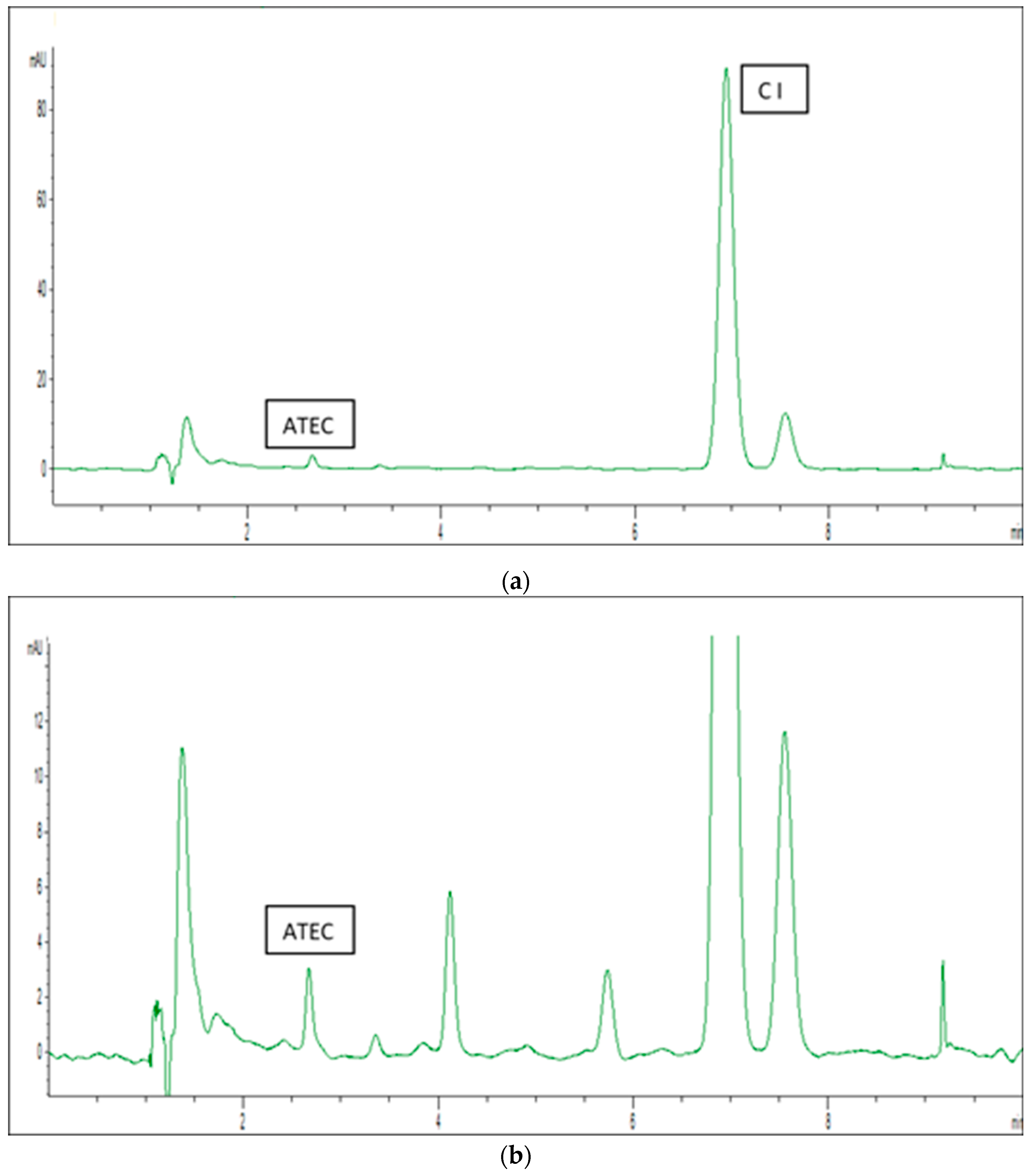

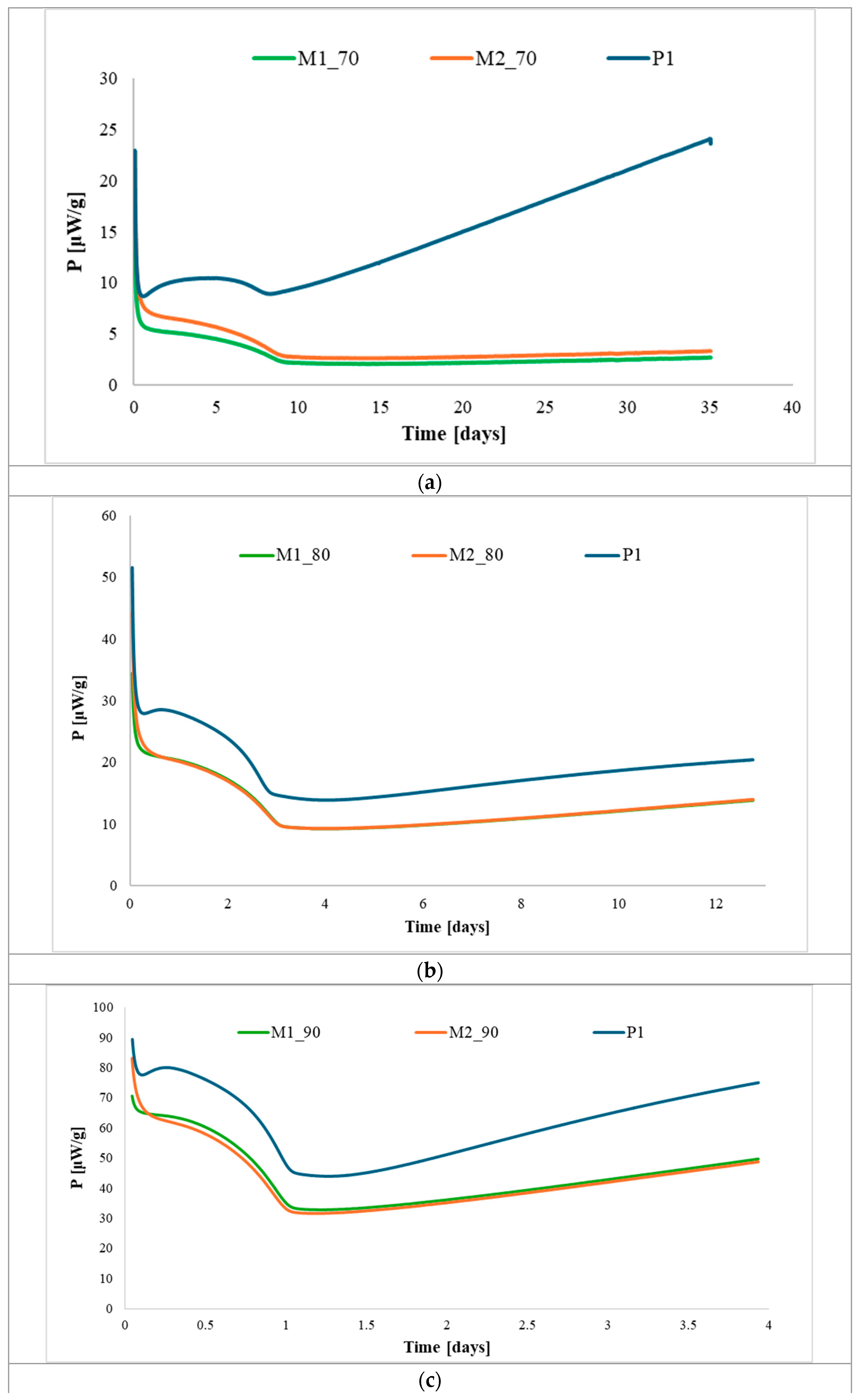

| Base Propellant | Type of Nitrocellulose | Nitrogen Content [%] |
|---|---|---|
| P1 | wood base | 13.01 |
| P2 | wood base | 13.26 |
| P3 | cotton-based (linter) | 13.26 |
| Code | Base Propellant | Type of Modifier |
|---|---|---|
| M1 | P1 | ATBC |
| M2 | P1 | ATEC |
| M3 | P2 | ATBC |
| M4 | P2 | ATEC |
| M5 | P3 | ATBC |
| M6 | P3 | ATEC |
| Solution | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Concentration of ATBC [mg/mL] | 0.004 | 0.011 | 0.021 | 0.032 | 0.042 |
| Concentration of ATEC [mg/mL] | 0.005 | 0.011 | 0.023 | 0.034 | 0.045 |
| Analyte | Column Temperature [°C] | Mobile-Phase Composition | Flow Rate [mL/min] | Injection Volume [μL] | Wavelength [nm] |
|---|---|---|---|---|---|
| CI | 50 | 14% ACN, 40% MeOH 46% water | 1.5 | 10 | 210 |
| ATBC | 65 | 30% ACN, 40% MeOH 30% water | 1.5 | 25 | 222 |
| ATEC | 50 | 14% ACN, 40% MeOH 46% water | 1.5 | 25 | 222 |
| Propellant | Modifier | Heat of Combustion [J/g] | Density [g/cm3] | The Thickness of the Combustible Layer [mm] |
|---|---|---|---|---|
| P1 | - | 4163 ± 1 | 1.659 ± 0.001 | - |
| M1 | ATBC | 3841 ± 11 | 1.645 ± 0.001 | 0.035 ± 0.006 |
| M2 | ATEC | 3933 ± 8 | 1.632 ± 0.001 | 0.036 ± 0.008 |
| P2 | - | 4150 ± 7 | 1.656 ± 0.001 | - |
| M3 | ATBC | 3902 ± 11 | 1.630 ± 0.001 | 0.040 ± 0.011 |
| M4 | ATEC | 3949 ± 1 | 1.627 ± 0.001 | 0.039 ± 0.005 |
| P3 | - | 4122 ± 2 | 1.644 ± 0.001 | - |
| M5 | ATBC | 3821 ± 2 | 1.625 ± 0.001 | 0.038 ± 0.004 |
| M6 | ATEC | 3860 ± 6 | 1.631 ± 0.001 | 0.041 ± 0.005 |
| Propellant | Modifier | Determined Amount [mg per 100 mg of Propellant] |
|---|---|---|
| M1 | ATBC | 1.00 ± 0.05 |
| M2 | ATEC | 1.10 ± 0.05 |
| M3 | ATBC | 0.96 ± 0.05 |
| M4 | ATEC | 1.17 ± 0.05 |
| M5 | ATBC | 1.01 ± 0.05 |
| M6 | ATEC | 1.14 ± 0.05 |
| Temperature [°C] | Aging Time [Days] | Maximum Heat Flow [μW/g] |
| 70 | 34.8 | 34.5 |
| 80 | 10.6 | 114 |
| 90 | 3.43 | 350 |
| Propellant | CI Content [mg per 100 mg of Propellant] | |||
| Before Aging | After Aging T = 90 °C | After Aging T = 80 °C | After Aging T = 70 °C | |
| P1 | 1.16 ± 0.05 | 0.66 ± 0.05 | 0.60 ± 0.05 | 0.78 ± 0.05 |
| M1 | 1.09 ± 0.05 | 0.98 ± 0.05 | 0.90 ± 0.05 | 0.94 ± 0.05 |
| M2 | 1.11 ± 0.05 | 0.96 ± 0.05 | 0.91 ± 0.05 | 0.92 ± 0.05 |
| P2 | 1.11 ± 0.05 | 0.87 ± 0.05 | 0.87 ± 0.05 | 0.89 ± 0.05 |
| M3 | 1.10 ± 0.05 | 0.98 ± 0.05 | 0.96 ± 0.05 | 0.96 ± 0.05 |
| M4 | 1.10 ± 0.05 | 1.00 ± 0.05 | 0.96 ± 0.05 | 0.98 ± 0.05 |
| P3 | 1.31 ± 0.05 | 1.24 ± 0.05 | 1.25 ± 0.05 | 1.20 ± 0.05 |
| M5 | 1.29 ± 0.05 | 1.22 ± 0.05 | 1.20 ± 0.05 | 1.22 ± 0.05 |
| M6 | 1.29 ± 0.05 | 1.22 ± 0.05 | 1.22 ± 0.05 | 1.23 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieślak, K.; Wycech, M.I.; Tomaszewski, W. Assessment of the Stability of Propellants Modified with Eco-Friendly Plasticizers. Polymers 2025, 17, 3033. https://doi.org/10.3390/polym17223033
Cieślak K, Wycech MI, Tomaszewski W. Assessment of the Stability of Propellants Modified with Eco-Friendly Plasticizers. Polymers. 2025; 17(22):3033. https://doi.org/10.3390/polym17223033
Chicago/Turabian StyleCieślak, Katarzyna, Monika Izabella Wycech, and Waldemar Tomaszewski. 2025. "Assessment of the Stability of Propellants Modified with Eco-Friendly Plasticizers" Polymers 17, no. 22: 3033. https://doi.org/10.3390/polym17223033
APA StyleCieślak, K., Wycech, M. I., & Tomaszewski, W. (2025). Assessment of the Stability of Propellants Modified with Eco-Friendly Plasticizers. Polymers, 17(22), 3033. https://doi.org/10.3390/polym17223033







