Investigation of Metallo(organo)siloxane—Polydimethylsiloxane Composites with a High Metallosiloxane Component Content
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Metallosiloxane Oligomers Synthesis
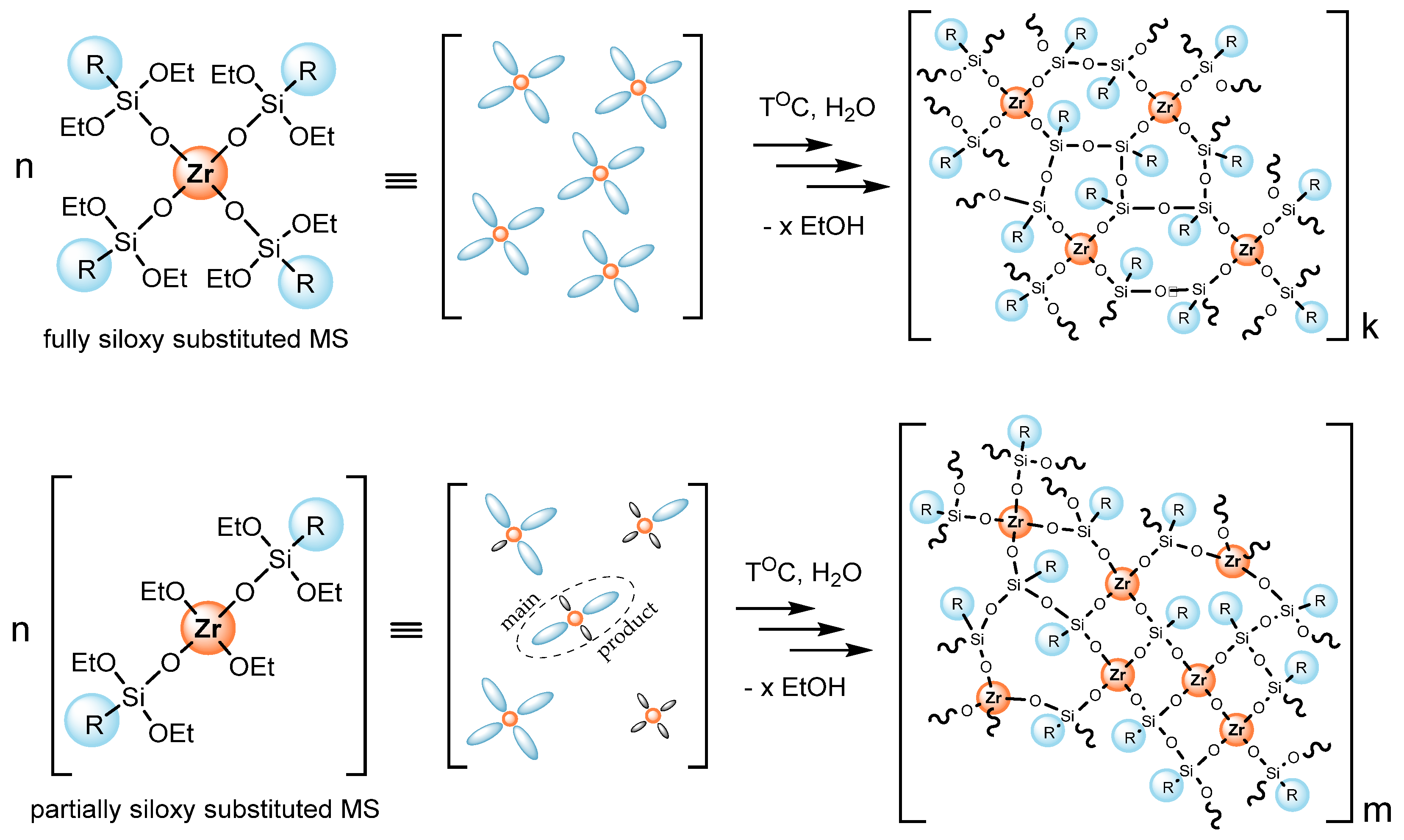
3.2. Obtaining the Composites
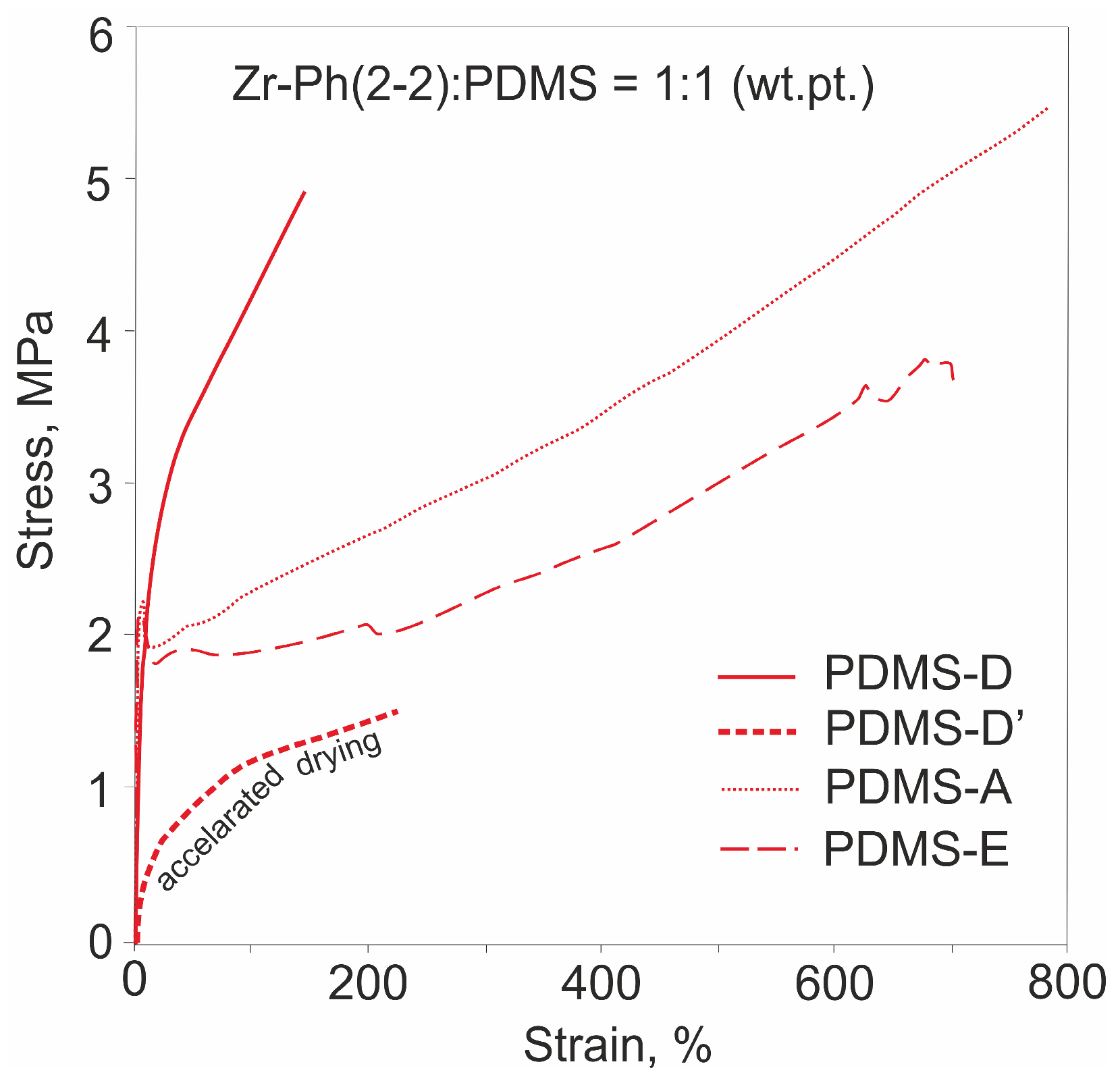
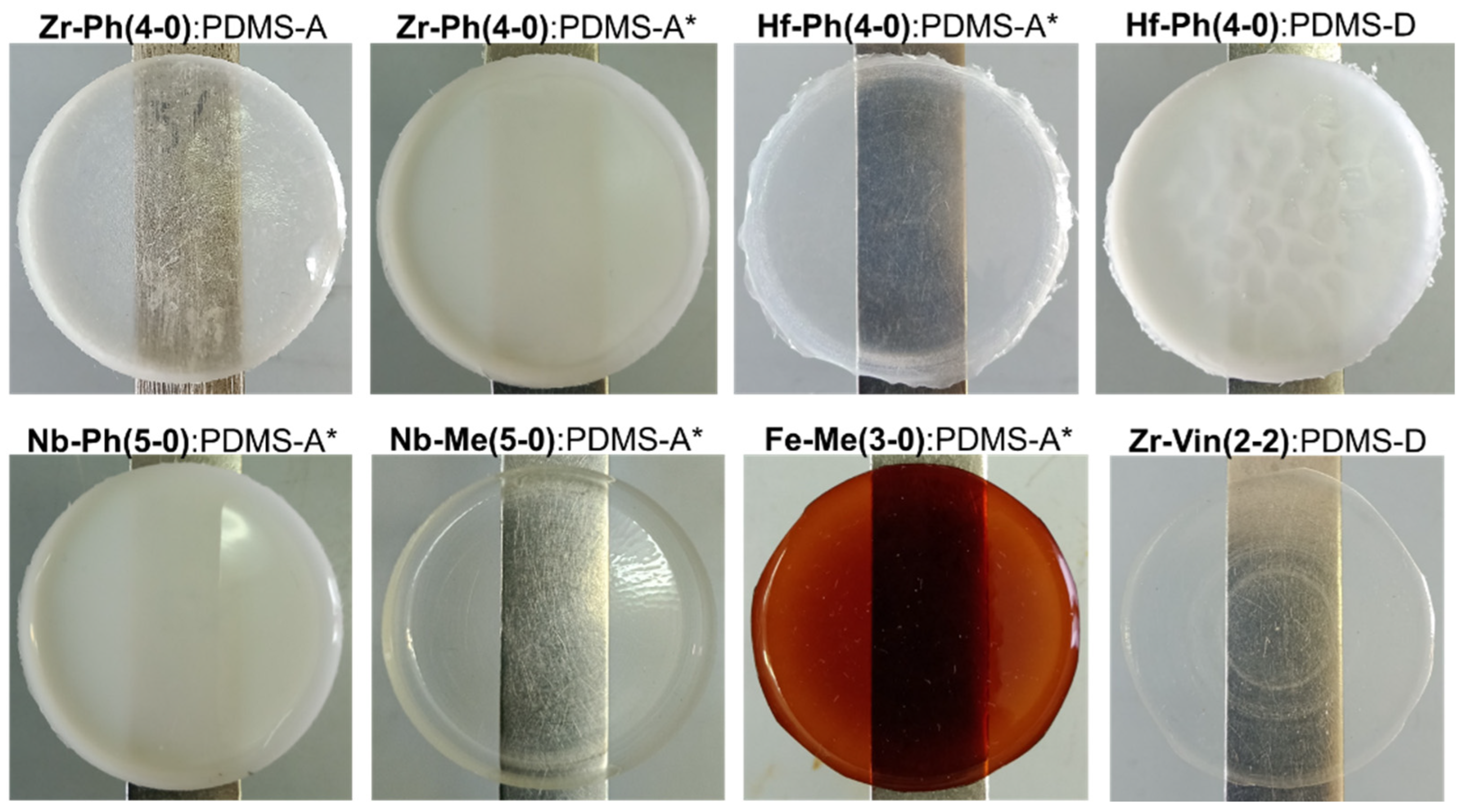
3.3. Mechanical Properties and Morphology of Composites
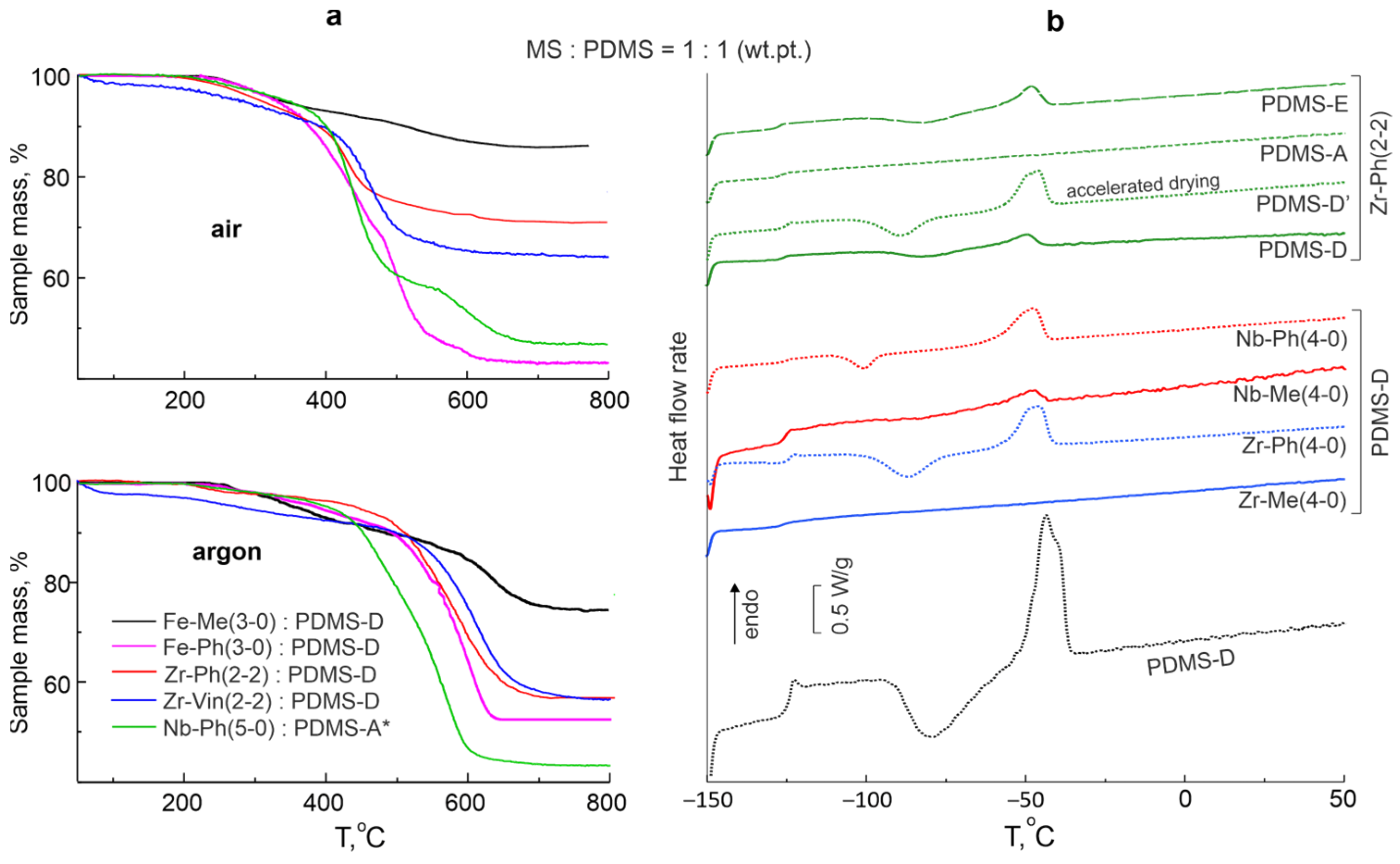
3.4. Thermophysical Properties of Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Polmanteer, K.E. Silicone rubber, its development and technological progress. Rubber Chem. Technol. 1988, 61, 470–502. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; He, J.; Liu, W.; Wang, Y.; Huang, Z.; Pang, H.; Chen, Y. Functional PDMS Elastomers: Bulk composites, surface engineering, and precision fabrication. Adv. Sci. 2023, 10, 2304506. [Google Scholar] [CrossRef]
- Sumi, D.; Dhanabalan, A.; Thimmappa, B.H.S.; Krishnamurthy, S. Effect of colloidal silica dispersions on the properties of PDMS-colloidal silica composites. J. Appl. Polym. Sci. 2012, 125 (Suppl. S1), E515. [Google Scholar] [CrossRef]
- Cordoba, A.; Cauich-Rodríguez, J.V.; Vargas-Coronado, R.F.; Velázquez-Castillo, R.; Esquivel, K. A novel in situ sol-gel synthesis method for PDMS composites reinforced with silica nanoparticles. Polymers 2024, 16, 1125. [Google Scholar] [CrossRef]
- Mohd, A.A.; Sufiah, M.Y.; Sufizar, A.; Hariati, T. Tensile behaviour and fracture characteristics of polydimethylsiloxane (PDMS) filled silica composites. Adv. Mater. Res. 2015, 1087, 6–10. [Google Scholar] [CrossRef]
- Sobhani, S.; Bakhshandeh, E.; Jafari, R.; Momen, G. Mechanical properties, icephobicity, and durability assessment of HT-PDMS nanocomposites: Effectiveness of sol-gel silica precipitation content. J. Sol-Gel Sci. Technol. 2023, 105, 348–359. [Google Scholar] [CrossRef]
- Jumrus, N.; Suttanon, N.; Sroila, W.; Tippo, P.; Panthawan, A.; Thongpan, W.; Kumpika, T.; Sroila, W.; Rianyoi, R.; Singjai, P.; et al. Durability and photocatalytic activity of superhydrophobic gypsum boards coated with PDMS/MTCS-modified SiO2-TiO2 NPs. Mater. Lett. 2023, 330, 133342. [Google Scholar] [CrossRef]
- Do, V.-T.; Chun, D.-M. Fabrication of large-scale, flexible, and robust superhydrophobic composite films using hydrophobic fumed silica nanoparticles and polydimethylsiloxane. Polymer 2022, 244, 124630. [Google Scholar] [CrossRef]
- Erdene-Ochir, O.; Do, V.-T.; Chun, D.-M. Facile fabrication of durable and flexible superhydrophobic surface with polydimethylsiloxane and silica nanoparticle coating on a polyethylene terephthalate film by hot-roll lamination. Polymer 2022, 255, 125158. [Google Scholar] [CrossRef]
- Cai, G.; Ni, H.; Li, X.; Wang, Y.; Zhao, H. Eco-friendly fabrication of highly stable silica aerogel microspheres with core–shell structure. Polymers 2023, 15, 1882. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Liu, J.; Wang, G.; Yao, P.; Dang, L.; Liu, Z.; Lu, J.; Li, W. Robust UV/moisture dual curable PDMS-microcapsule-silica functional material for self-healing, antifouling, and antibacterial applications. Nano Res. 2023, 16, 7810–7819. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Cho, J.; Kim, Y.-O.; Lim, S.; Youn, S.; Jung, Y.C.; Kim, S.Y.; Seong, D.G. Super-insulating, flame-retardant, and flexible poly(dimethylsiloxane) composites based on silica aerogel. Compos. Part A Appl. Sci. Manuf. 2019, 123, 108. [Google Scholar] [CrossRef]
- Gutierrez, D.B.; Caldona, E.B.; Yang, Z.; Suo, X.; Cheng, X.; Dai, S.; Espiritu, R.D.; Advincula, R.C. PDMS-silica composite gas separation membranes by direct ink writing. J. Appl. Polym. Sci. 2023, 140, e54277. [Google Scholar] [CrossRef]
- Flackett, D. Sealants. In Silicon Compounds: Silanes and Silicones, 2nd ed.; Gelest Inc.: Glen Rock, PA, USA, 2008; pp. 433–439. [Google Scholar]
- Chen, X.; Yu, B.; Yuan, H.; Cong, H.L.; Xu, X.D.; Yang, S.J.; Peng, Q.H.; Yang, R.X.; Gao, X.H. The synthesis and property of neutral silicone glass sealant. Adv. Mater. Res. 2015, 1118, 67–71. [Google Scholar] [CrossRef]
- Konoplin, A.Y.; Pushkarev, A.V.; Shakurov, A.V.; Baurova, N.I. Evaluation of resistance of organosilicon sealant to ultra-low temperatures. Polym. Sci. D 2021, 14, 335–339. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Guo, L.; Xie, W.; Zhao, B.; Liu, M.; Yin, C.; Li, Q. Preparation and performance of ceramifiable flame-retardant silicone sealant. J. Appl. Polym. Sci. 2024, 141, e54860. [Google Scholar] [CrossRef]
- Norouznezhad, M.; Janani, H. Cross-linking network topology and durability in silicone rubber sealants for PEMFCs: The impact of curing systems and nanosilica interactions. J. Polym. Res. 2024, 31, 59. [Google Scholar] [CrossRef]
- Dinkar, S.; Dhanabalan, A.; Thimmappa, B.H.S. Preparation and properties of poly(dimethyl siloxane)–colloidal silica/functionalized colloidal silica nanocomposites. J. Appl. Polym. Sci. 2012, 126, 1585–1592. [Google Scholar] [CrossRef]
- Nagasarvari, G.; Nair, N.M.; Ranade, S.D.; Neelakantan, L.; Swaminathan, P. PDMS-metal oxide nanocomposites as transparent encapsulants for flexible electronic devices. Flex. Print. Electron. 2023, 8, 045004. [Google Scholar] [CrossRef]
- Qi, R.; Wang, Y.; Chen, J.; Li, J.; Zhu, S. Pervaporative desulfurization of model gasoline with Ag2O-filled PDMS membranes. Separ. Purif. Technol. 2007, 57, 170–175. [Google Scholar] [CrossRef]
- Sulym, I.; Goncharuk, O.; Sternik, D.; Terpilowski, K.; Derylo-Marczewska, A.; Borysenko, M.V.; Gun’ko, V.M. Nanooxide/polymer composites with Silica@PDMS and ceria–zirconia–Silica@PDMS: Textural, morphological, and hydrophilic/hydrophobic features. Nanoscale Res. Lett. 2017, 12, 152. [Google Scholar] [CrossRef]
- Rosales, A.; Mandujano, H.; Cervantes-Chávez, J.A.; Esquivel, K. Antimicrobial hydrophobic SiO2-TiO2-PDMS films: Effect of indirect ultrasonic irradiation on the synthesis process. J. Compos. Sci. 2024, 8, 104. [Google Scholar] [CrossRef]
- Gniesmer, S.; Sonntag, S.R.; Gapeeva, A.; Cojocaru, A.; Kaps, S.; Adelung, R.; Sewing, J.; Tura, A.; Grisanti, S.; Grisanti, S. In vivo evaluation of a nanotechnology-based microshunt for filtering glaucoma surgery. Sci. Rep. 2024, 14, 4452. [Google Scholar] [CrossRef]
- Paul, D.R.; Mark, J.E. Fillers for polysiloxane (“silicone”) elastomers. Progr. Polym. Sci. 2010, 35, 893–901. [Google Scholar] [CrossRef]
- Tebeneva, N.A.; Meshkov, I.B.; Tarasenkov, A.N.; Polshchikova, N.V.; Кalinina, A.A.; Buzin, M.I.; Serenko, O.A.; Zubavichus, Y.V.; Katsoulis, D.E.; Muzafarov, A.M. Polyfunctional branched metallosiloxane oligomers and composites based on them. J. Organomet. Chem. 2018, 868, 112–121. [Google Scholar] [CrossRef]
- Tarasenkov, A.N.; Tebeneva, N.A.; Parshina, M.S.; Meshkov, I.B.; Vasilenko, N.G.; Cherkaev, G.V.; Goncharuk, G.P.; Katsoulis, D.E.; Muzafarov, A.M. New functional metallosiloxanes with partially siloxy substituted metall atom and their use in silicone compositions. J. Organomet. Chem. 2020, 906, 121034. [Google Scholar] [CrossRef]
- Murugavel, R.; Voigt, A.; Walawalkar, M.G.; Roesky, H.W. Hetero- and Metallasiloxanes Derived from silanediols, disilanols, silanetriols, and trisilanols. Chem. Rev. 1996, 96, 2205–2236. [Google Scholar] [CrossRef]
- Roux, S.; Audebert, P.; Pagetti, J.; Roche, M. Electrochemical growth of conducting polymer into zirconium oxopolymers sol-gel coatings. J. Sol-Gel Sci. Technol. 2003, 26, 435–439. [Google Scholar] [CrossRef]
- Tarasenkov, A.N.; Parshina, M.S.; Tebeneva, N.A.; Borisov, K.M.; Goncharuk, G.P.; Shevchenko, V.G.; Muzafarov, A.M. Metalloalkoxysiloxanes-cured polydimethylsiloxane compositions filled with silica component for special applications: Dielectric and mechanical properties. Expr. Polym. Lett. 2022, 16, 846–870. [Google Scholar] [CrossRef]
- Andropova, U.; Serenko, O.; Tebeneva, N.; Tarasenkov, A.; Askadskii, A.; Afanasyev, E.; Novikov, L.; Chernik, V.; Voronina, E.; Muzafarov, A. New oligomeric metallosiloxane-polyimide nanocomposites for anti-atomic-oxygen erosion. Polym. Degrad. Stab. 2021, 183, 109424. [Google Scholar] [CrossRef]
- Meshkov, I.B.; Kalinina, A.A.; Gorodov, V.V.; Bakirov, A.V.; Krasheninnikov, S.V.; Chvalun, S.N.; Muzafarov, A.M. New principles of polymer composite preparation. MQ Copolymers as an active molecular filler for polydimethylsiloxane rubbers. Polymers 2021, 13, 2848. [Google Scholar] [CrossRef]
- Bakirov, A.V.; Krasheninnikov, S.V.; Shcherbina, M.A.; Meshkov, I.B.; Kalinina, A.A.; Gorodov, V.V.; Tatarinova, E.A.; Muzafarov, A.M.; Chvalun, S.N. True Molecular Composites: Unusual structure and properties of PDMS-MQ resin blends. Polymers 2023, 15, 48. [Google Scholar] [CrossRef]
- Pletnev, S.I.; Parshina, M.S.; Tarasenkov, A.N.; Muzafarov, A.M. Effect of metal catalysis and functionality of a metallosiloxane during the formation of metallosiloxane-epoxy materials. INEOS OPEN 2024, 7, 52. [Google Scholar] [CrossRef]
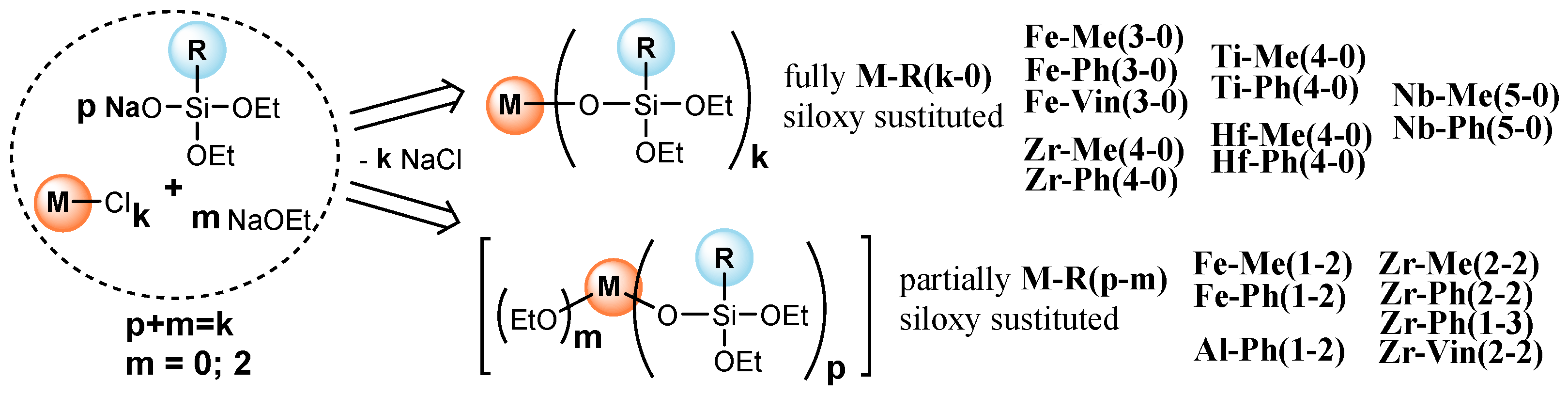


| # | MS | PDMS | x, wt.pt. | ω, % wt. | MPa/% | Eo ± ΔEo, MPa | Characteristics |
|---|---|---|---|---|---|---|---|
| 1 | Fe-Me(3-0) | D | 0.5 | 36 | 6.0 ± 0.8 7 ± 1 | 161.9 ± 8.9 | Dark brown, transparent, homogeneous |
| 2 | D c | 36 | 5.2 ± 0.3 605 ± 73 b | 74.4 ± 2.9 | Dark brown, transparent, homogeneous, “grain” of the surface | ||
| 3 | A * | 35 | 4.6 ± 0.4 296 ± 86 b | 71.6 ± 6.5 | Dark brown, transparent, homogeneous | ||
| 4 | Fe-Ph(3-0) | D | 0.5 | 40 | 2.1 ± 0.1 138 ± 27 | 5.3 ± 0.7 | Dark brown, opaque, uniform |
| 5 | Fe-Vin(3-0) | D | 0.5 | 37 | 4.5 ± 0.8 3 ± 1 | 206.5 ± 10.9 | Dark brown, transparent, homogeneous |
| 6 | Fe-Me(1-2) | D | 0.5 | 39 | 5.9 ± 1.3 4 ± 1 | 261.7 ± 25.6 | Dark brown, opaque, uniform |
| 7 | Fe-Ph(1-2) | D | 0.5 | 37 | 5.8 ± 0.6 152 ± 64 | 125.2 ± 10.1 | |
| 8 | D | 0.6 | 47 | 10.3 ± 0.3 12 ± 2 a |
| # | MS | PDMS | x, wt.pt. | ω, % wt. | MPa/% | Eo ± ΔEo, MPa | Characteristics |
|---|---|---|---|---|---|---|---|
| 1 | Zr-Me(4-0) | D | 0.5 | 36 | 6.3 ± 0.1 16 ± 1 a | 135.4 ± 8.1 | Colorless, transparent, homogeneous |
| 2 | 0.6 | 46 | 6.9 ± 0.7 7 ± 1 | ||||
| 3 | Zr-Ph(4-0) | D | 0.5 | 41 | 1.4 ± 0.1 458 ± 54 | 0.3 ± 0.03 | Yellowish, opalescent, heterogeneous morphology |
| 4 | A | 40 | 2.1 ± 0.4 222 ± 55 | 1.0 ± 0.01 | |||
| 5 | E | 41 | - | - | Yellowish, inhomogeneous, cracked surface | ||
| 6 | A * | 40 | 2.6 ± 0.3 256 ± 63 | 1.5 ± 0.1 | White, homogeneous | ||
| 7 | Zr-Me(2-2) | D | 0.5 | 35 | 3.2 ± 0.5 4 ± 1 | 117.2 ± 6.8 | Colorless, transparent, homogeneous |
| 8 | Zr-Ph(2-2) | D | 0.5 | 39 | 4.9 ± 0.4 140 ± 31 | 56.3 ± 3.3 | |
| 9 | D c | 39 | 1.4 ± 0.1 209 ± 15 | 1.7 ± 0.1 | Whitish, cloudy, homogeneous | ||
| 10 | A | 38 | 5.2 ± 0.4 820 ± 71 b | 101.9 ± 2.8 | Colorless, transparent, homogeneous | ||
| 11 | E | 39 | 3.6 ± 0.2 637 ± 61 b | 77.9 ± 6.4 | Colorless, opalescent, homogeneous | ||
| 12 | D | 0.6 | 49 | 7.4 ± 0.6 14 ± 6 | Colorless, transparent, homogeneous | ||
| 13 | Zr-Ph(1-3) | D | 0.5 | 37 | 4.5 ± 0.3 7 ± 1 a | 138.2 ± 4.2 | |
| 14 | Zr-Vin(2-2) | D | 0.5 | 36 | 4.7 ± 0.1 280 ± 49 b | 102.6 ± 2.5 | Yellowish, transparent, homogeneous |
| 15 | D c | 36 | 2.8 ± 0.1 19 ± 7 b | 80.3 ± 1.9 | Yellowish, transparent, homogeneous, “grain” of the surface | ||
| 16 | A | 36 | 4.3 ± 0.1 184 ± 22 b | 85.4 ± 3.5 | Yellowish, transparent, homogeneous | ||
| 17 | E | 36 | 4.8 ± 0.1 114 ± 31 b | 124.4 ± 6.4 |
| # | MS | PDMS | x, wt.pt. | ω, % wt. | MPa/% | Eo ± ΔEo, MPa | Characteristics |
|---|---|---|---|---|---|---|---|
| 1 | Ti-Me(4-0) | D | 0.5 | 35 | 1.5 ± 0.1 1 ± 1 | 187.2 ± 16.9 | Colorless, transparent, homogeneous |
| 2 | Ti-Ph(4-0) | D | 0.5 | 40 | |||
| 3 | D c | 40 | Yellowish, opalescent, heterogeneous morphology | ||||
| 4 | A * | 38 | 3.2 ± 0.2 260 ± 71 | 3.8 ± 0.1 | |||
| 5 | Hf-Me(4-0) | D | 0.5 | 38 | 5.8 ± 0.3 22 ± 2 a | 101.0 ± 2.8 | Yellowish, inhomogeneous, cracked surface |
| 6 | Hf-Ph(4-0) | D | 0.5 | 41 | 3.3 ± 0.2 421 ± 25 | 3.0 ± 0.4 | White, homogeneous |
| 7 | A * | 41 | 5.9 ± 1.6 610 ± 179 | 4.5 ± 0.8 | Colorless, transparent, homogeneous | ||
| 8 | Nb-Me(5-0) | D | 0.5 | 36 | 9.2 ± 0.5 20 ± 3 | 87.1 ± 2.7 | |
| 9 | D c | 36 | 4.6 ± 0.1 80 ± 18 b | 83.6 ± 3.4 | Whitish, cloudy, homogeneous | ||
| 10 | A | 36 | 5.9 ± 0.1 73 ± 4 | 46.5 ± 1.8 | Colorless, transparent, homogeneous | ||
| 11 | A * | 35 | 4.8 ± 0.3 19 ± 2 | 62.3 ± 1.4 | Colorless, opalescent, homogeneous | ||
| 12 | E | 36 | 5.0 ± 0.5 573 ± 103 b | 57.9 ± 0.3 | Colorless, transparent, homogeneous | ||
| 13 | D | 0.6 | 46 | 5.0 ± 1.4 3 ± 1 | |||
| 14 | Nb-Ph(5-0) | D | 0.5 | 40 | Colorless, transparent, homogeneous, fragile | ||
| 15 | A * | 40 | 2.5 ± 0.2 249 ± 18 | 0.7 ± 0.1 | Yellowish, transparent, homogeneous, “grain” of the surface | ||
| 16 | A * | 0.6 | 50 | 2.4 ± 0.1 320 ± 44 | Yellowish, transparent, homogeneous | ||
| 17 | A * | 0.7 | 61 | 1.9 ± 0.1 85 ± 13 |
| # | MS | PDMS | TGA | DSC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T5%, °C | T10%, °C | Coke, % wt. | Tg, °C | Tm, °C | ΔHm, J/g | ||||||
| Air | Argon | Air | Argon | Air | Argon | ||||||
| 1 | Fe-Me(3-0) | D | 343 | 386 | 531 | 484 | 86 | 75 | −127 | −51 | 1 |
| 2 | D a | −126 | −49 | 8 | |||||||
| 3 | A * | −125 | - | - | |||||||
| 4 | Fe-Me(1-2) | D | 272 | 272 | 337 | 356 | 77 | 73 | −126 | - | - |
| 5 | Fe-Ph(3-0) | D | 329 | 430 | 385 | 535 | 68 | 57 | −125 | −45 | 13 |
| 6 | Fe-Ph(1-2) | D | 315 | 338 | 363 | 485 | 68 | 53 | −125 | −51 | 2 |
| 7 | Zr-Me(4-0) | D | 330 | 390 | 374 | 438 | 75 | 43 | −126 | - | - |
| 8 | Zr-Ph(4-0) | D | 301 | 343 | 369 | 444 | 54 | 47 | −124 | −46 | 13 |
| 9 | A | −126 | −50 | 3 | |||||||
| 10 | A * | −125 | −50 | 10 | |||||||
| 11 | Zr-Me(2-2) | D | 315 | 352 | 374 | 380 | 79 | 53 | −125 | - | - |
| 12 | Zr-Ph(2-2) | D | 315 | 446 | 401 | 523 | 74 | 61 | −126 | −49 | 4 |
| 13 | D a | −125 | −46 | 11 | |||||||
| 14 | A | −126 | - | - | |||||||
| 15 | E | −127 | −48 | 6 | |||||||
| 16 | Zr-Vin(2-2) | D | 310 | 258 | 405 | 485 | 65 | 56 | −126 | −53 | 1 |
| 17 | D a | −125 | - | - | |||||||
| 18 | A | −124 | - | - | |||||||
| 19 | E | −125 | - | - | |||||||
| 20 | Ti-Me(4-0) | D | 325 | 420 | 446 | 445 | 82 | 56 | −126 | - | - |
| 21 | Ti-Ph(4-0) | A * | 330 | 352 | 371 | 404 | 55 | 57 | −125 | −50 | 9 |
| 22 | Hf-Me(4-0) | D | 357 | 398 | 406 | 446 | 79 | 50 | −127 | - | - |
| 23 | Hf-Ph(4-0) | D | 292 | 273 | 432 | 468 | 57 | 46 | −125 | −46 | 11 |
| 24 | A * | −124 | −52 | <0.5 | |||||||
| 25 | Nb-Me(5-0) | D | 365 | 418 | 439 | 450 | 85 | 65 | −125 | −49 | 2 |
| 26 | D a | −125 | −49 | 2 | |||||||
| 27 | A | −124 | - | - | |||||||
| 28 | A * | −124 | - | - | |||||||
| 29 | E | −125 | −50 | 4 | |||||||
| 30 | Nb-Ph(5-0) | A * | 325 | 382 | 394 | 445 | 45 | 42 | −125 | −48 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tebeneva, N.A.; Tarasenkov, A.N.; Meshkov, I.B.; Kalinina, A.A.; Buzin, A.I.; Buzin, M.I.; Goncharuk, G.P.; Muzafarov, A.M. Investigation of Metallo(organo)siloxane—Polydimethylsiloxane Composites with a High Metallosiloxane Component Content. Polymers 2025, 17, 3034. https://doi.org/10.3390/polym17223034
Tebeneva NA, Tarasenkov AN, Meshkov IB, Kalinina AA, Buzin AI, Buzin MI, Goncharuk GP, Muzafarov AM. Investigation of Metallo(organo)siloxane—Polydimethylsiloxane Composites with a High Metallosiloxane Component Content. Polymers. 2025; 17(22):3034. https://doi.org/10.3390/polym17223034
Chicago/Turabian StyleTebeneva, Nadezhda A., Alexander N. Tarasenkov, Ivan B. Meshkov, Aleksandra A. Kalinina, Alexander I. Buzin, Mikhail I. Buzin, Galina P. Goncharuk, and Aziz M. Muzafarov. 2025. "Investigation of Metallo(organo)siloxane—Polydimethylsiloxane Composites with a High Metallosiloxane Component Content" Polymers 17, no. 22: 3034. https://doi.org/10.3390/polym17223034
APA StyleTebeneva, N. A., Tarasenkov, A. N., Meshkov, I. B., Kalinina, A. A., Buzin, A. I., Buzin, M. I., Goncharuk, G. P., & Muzafarov, A. M. (2025). Investigation of Metallo(organo)siloxane—Polydimethylsiloxane Composites with a High Metallosiloxane Component Content. Polymers, 17(22), 3034. https://doi.org/10.3390/polym17223034








