Xylitol Modification of Electrospun Polymer Scaffolds: Impact on Physicochemical and Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- -
- CA pellets with an average molecular weight (Mw) of 30,000 (Sigma Aldrich, St. Louis, MO, USA) were dissolved in acetone (Sigma Aldrich, St. Louis, MO, USA);
- -
- PLLA pellets with a Mw of 106,000 (Musashino Chemical Laboratory, Tokyo, Japan) were added to chloroform (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan);
- -
- PCL pellets with an average molecular weight (Mn) of 80,000 (Sigma Aldrich, St. Louis, MO, USA) were dissolved in a mixture of chloroform/acetone in a ratio of 3/1.
2.2. Morphological, Physical, and Chemical Characterization
2.3. Mechanical Tests
2.4. Thermal Characterization
2.5. In Vitro Test
2.6. Bacterial Characterization
2.7. Statistical Analysis
3. Results
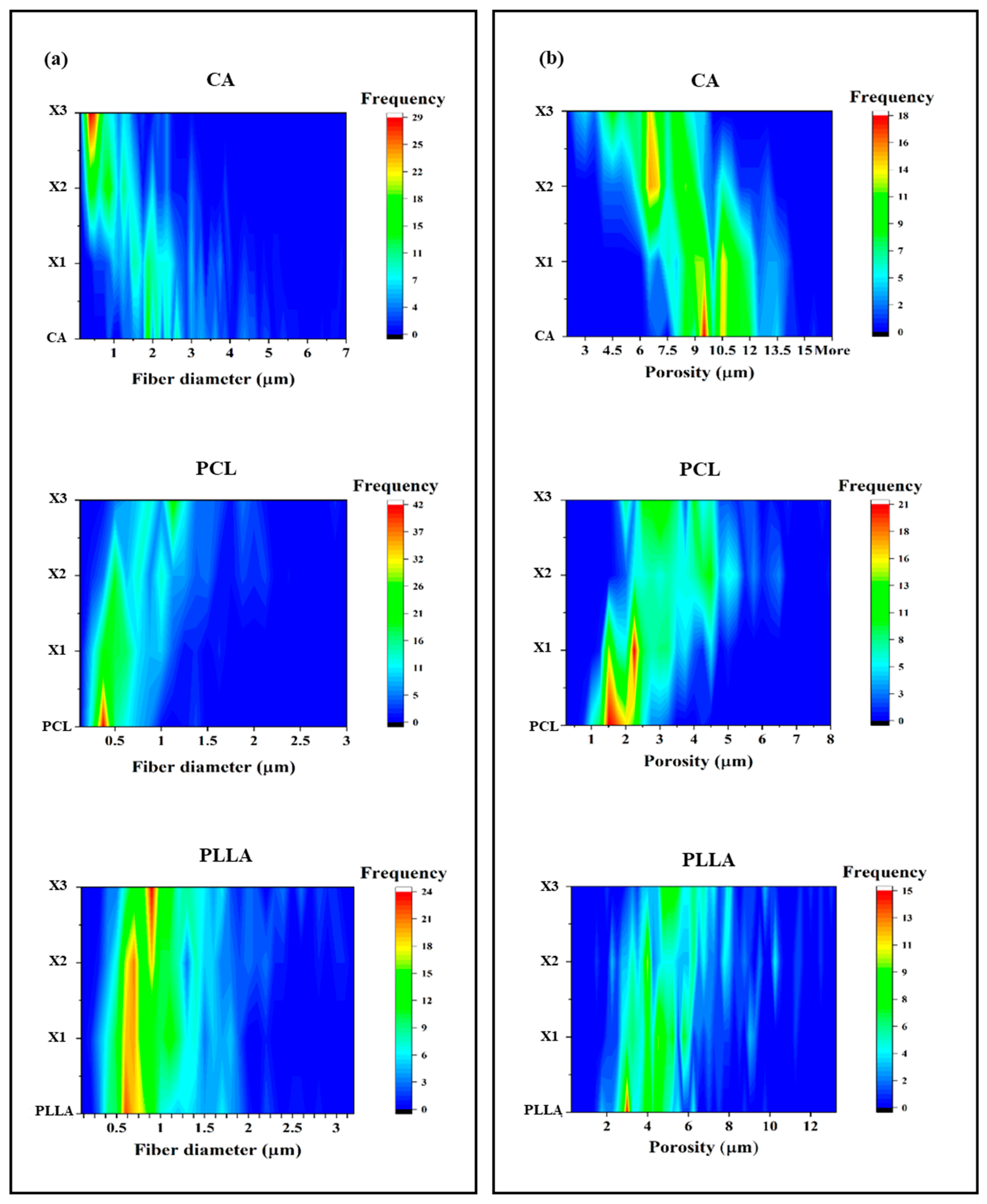
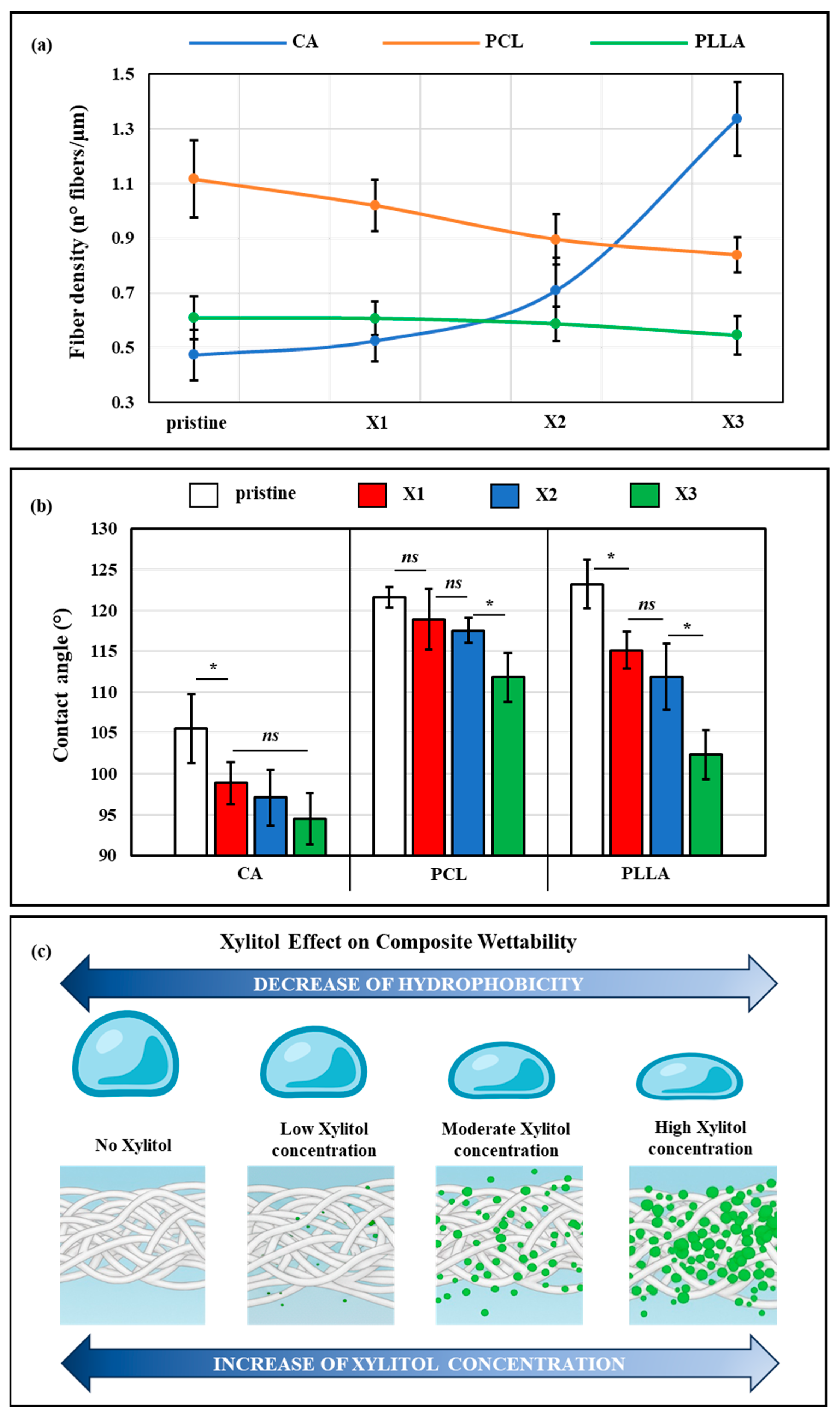
3.1. Effect of Xylitol on Fiber Morphology
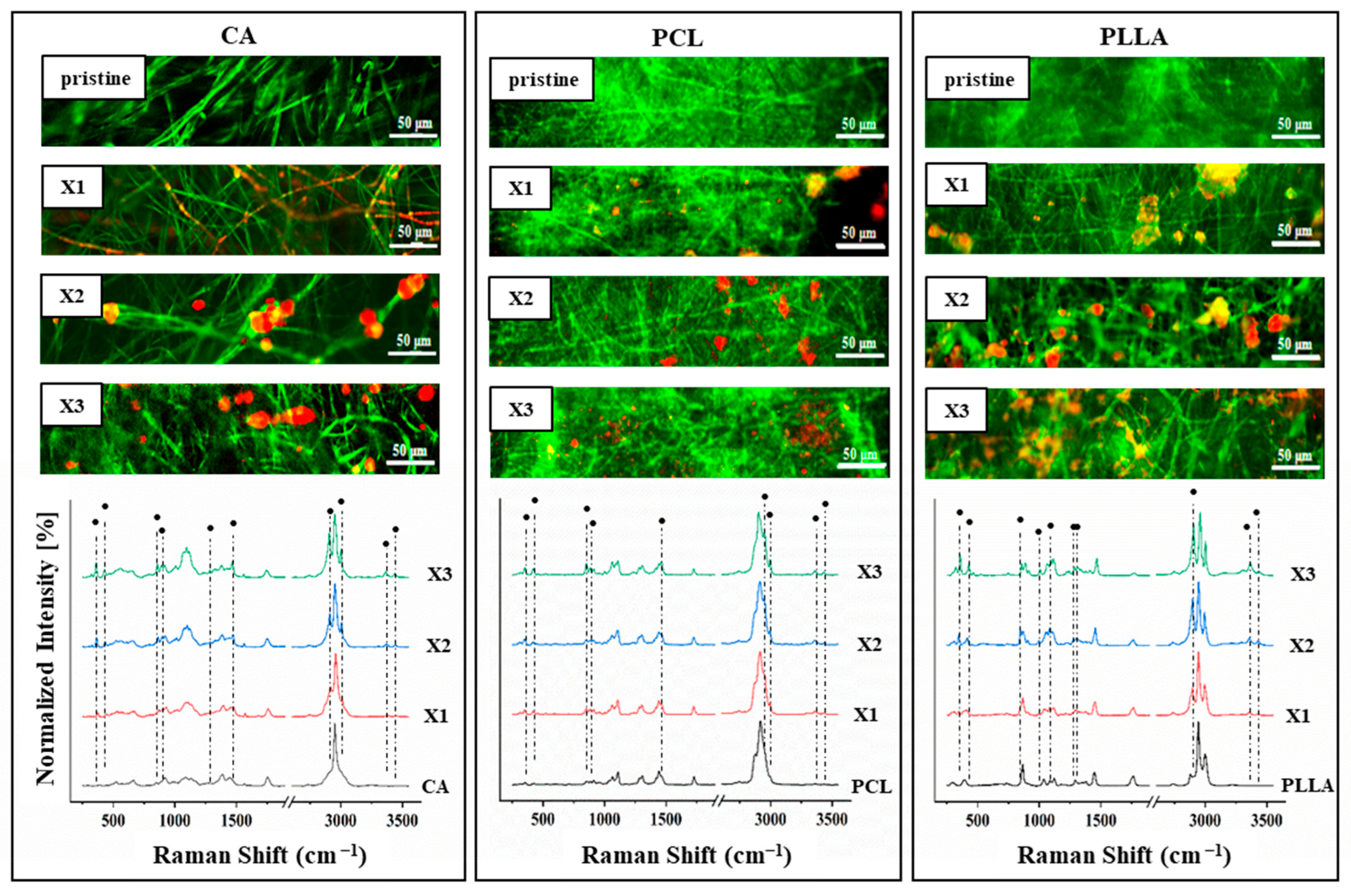
3.2. Raman Characterization and Xylitol Location
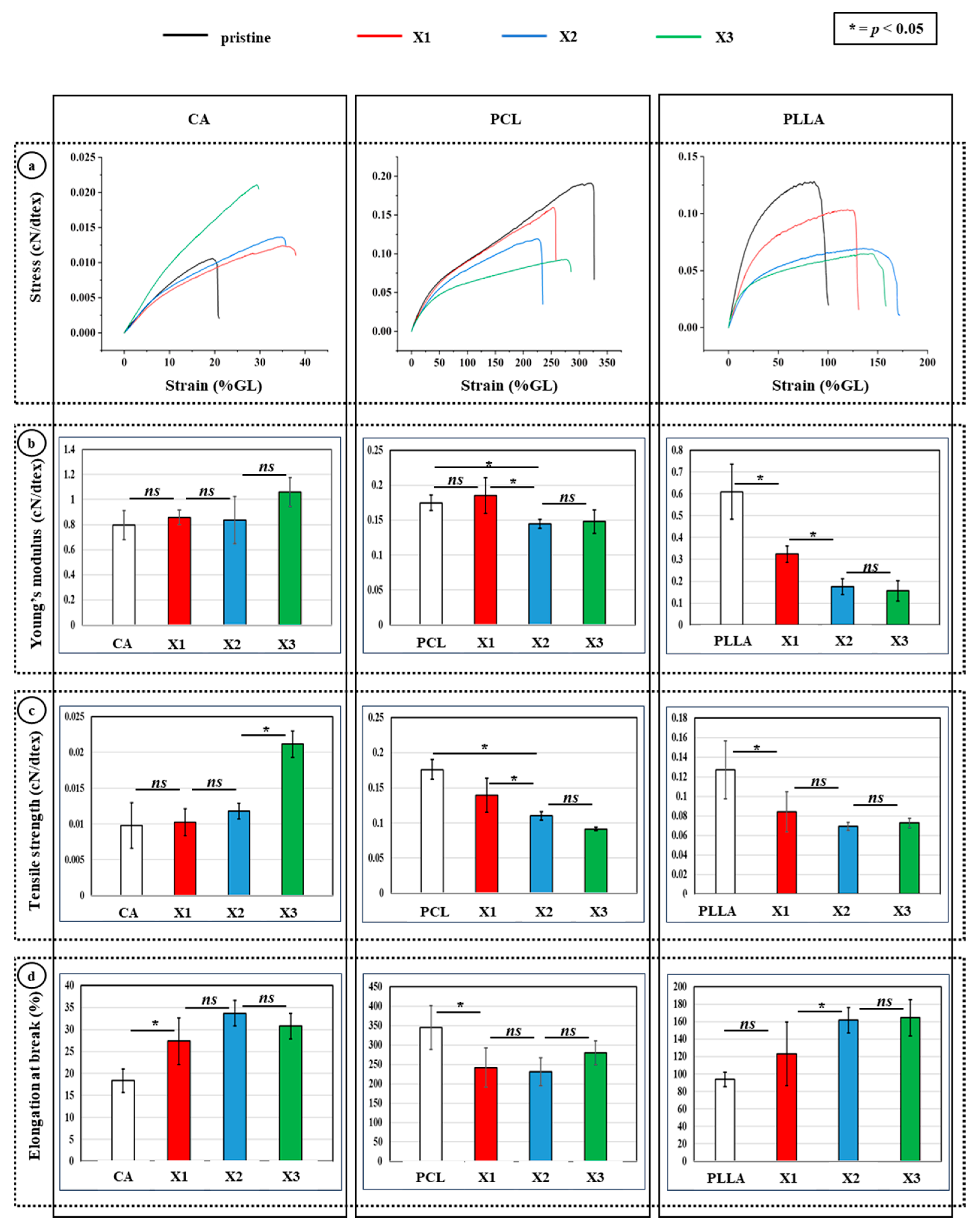
3.3. Mechanical Tests of the Composites to Evaluate Xylitol Incorporation
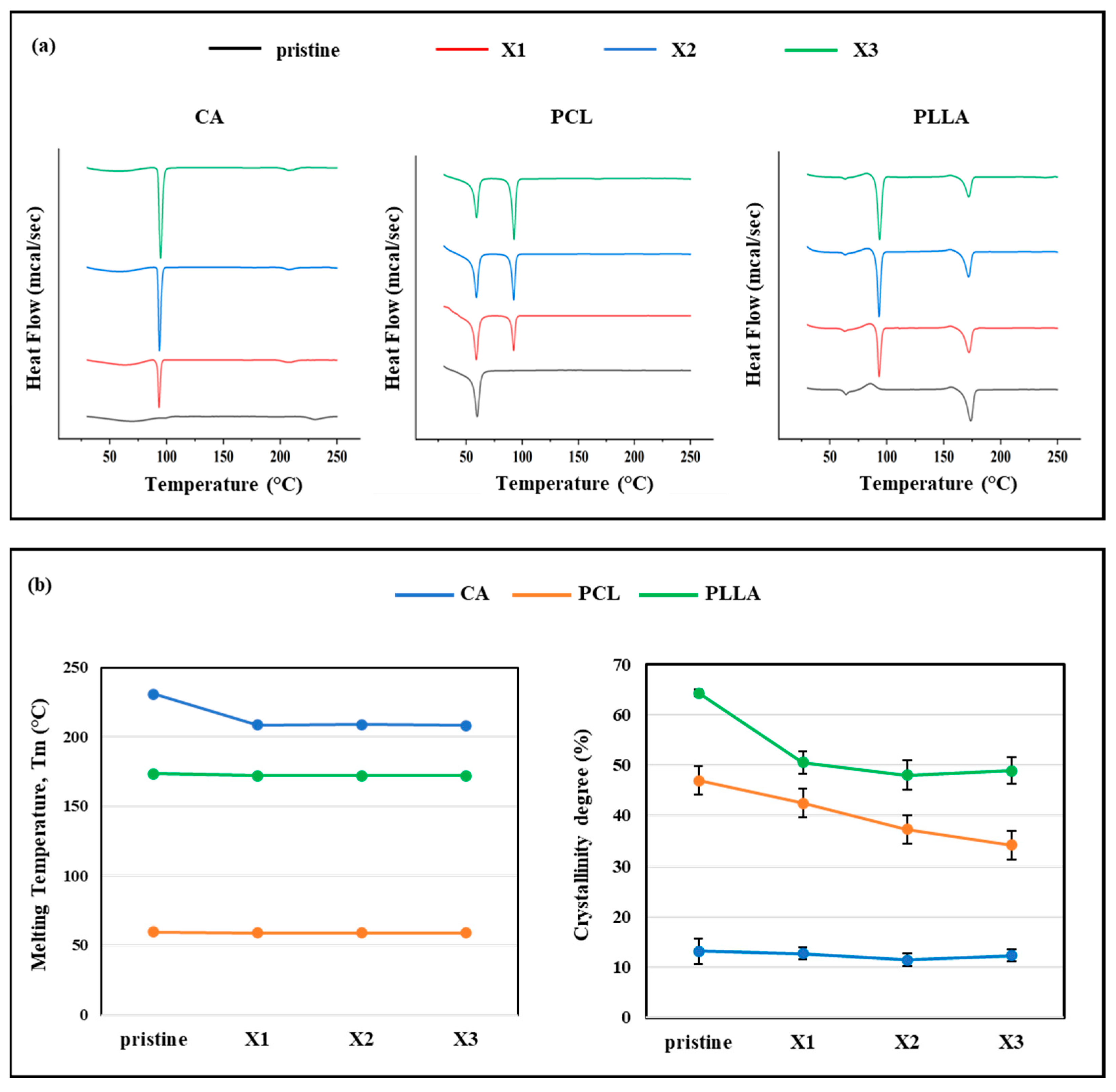
3.4. DSC Analysis
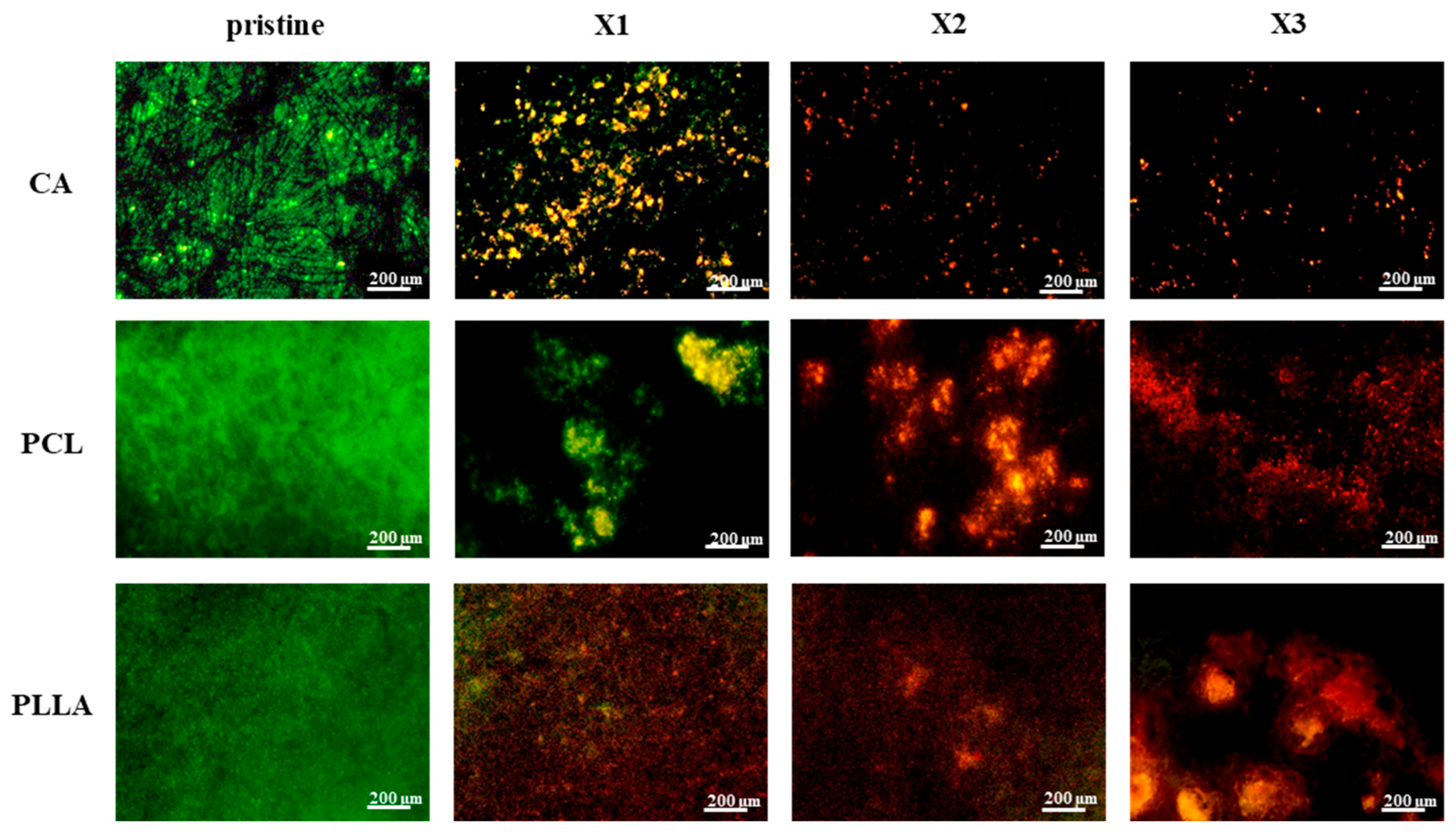
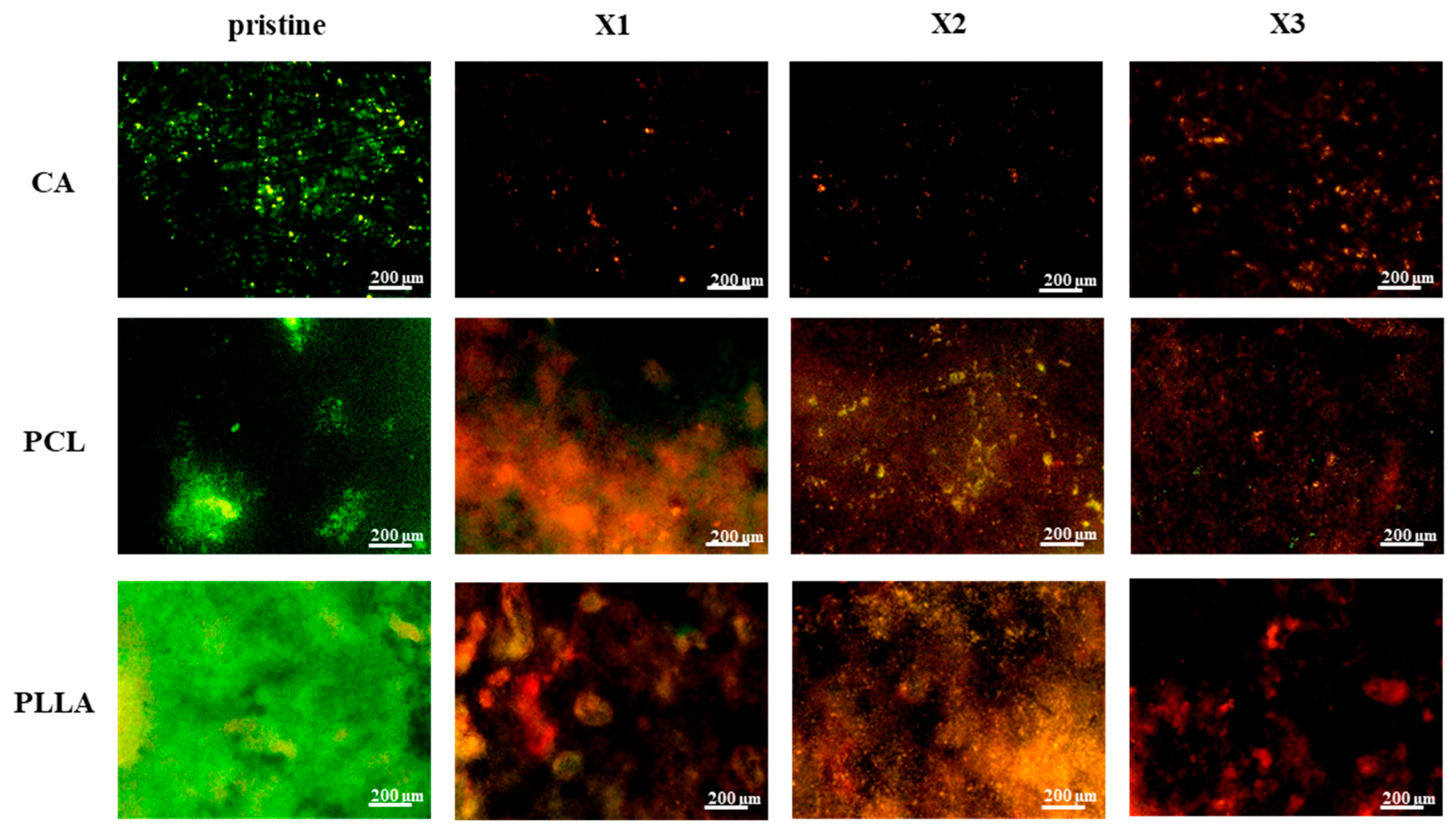
3.5. In Vitro Bacterial Testing
4. Discussion
4.1. Xylitol Content Strongly Affects the Composite Structures and Performances
4.2. Xylitol Decreased Crystallinity, and Influenced Mechanical Properties
4.3. Significant and Stable Antibacterial Effect Provided by the Xylitol Against S. aureus and E. coli over Time
4.4. Use and Limitations of Xylitol-Based Scaffolds
4.5. Notes on the Stability of Xylitol in Physiological Environments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Metter, R.B.; Ifkovits, J.L.; Hou, K.; Vincent, L.; Hsu, B.; Wang, L.; Mauck b, R.L.; Burdick, J.A. Biodegradable Fibrous Scaffolds with Diverse Properties by Electrospinning Candidates from a Combinatorial Macromer Library. Acta Biomater. 2009, 6, 1219. [Google Scholar] [CrossRef]
- Rodriguez-Cabello, J.C.; De Torre, I.G.; González-Pérez, M.; González-Pérez, F.; Montequi, I. Fibrous Scaffolds From Elastin-Based Materials. Front. Bioeng. Biotechnol. 2021, 9, 652384. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Li, W.J.; Cooper, J.A. Fibrous Scaffolds for Tissue Engineering. In Biomaterials for Tissue Engineering Applications: A Review of the Past and Future Trends; Springer: Vienna, Austria, 2011; pp. 47–73. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.G.; Wang, K. Electrospun Nanofiber Membranes for Air Filtration: A Review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Senthamizhan, A.; Balusamy, B.; Uyar, T. Electrospinning: A versatile processing technology for producing nanofibrous materials for biomedical and tissue-engineering applications. In Electrospun Materials for Tissue Engineering and Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2017; pp. 3–41. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- CeCe, R.; Jining, L.; Islam, M.; Korvink, J.G.; Sharma, B. An Overview of the Electrospinning of Polymeric Nanofibers for Biomedical Applications Related to Drug Delivery. Adv. Eng. Mater. 2024, 26, 2301297. [Google Scholar] [CrossRef]
- Wang, L.; Ryan, A.J. Introduction to electrospinning. In Electrospinning for Tissue Regeneration; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–33. [Google Scholar] [CrossRef]
- Fair, E.; Bornstein, J.; Lyons, T.; Sgobba, P.; Hayes, A.; Rourke, M.; Macwan, I.; Haghbin, N. Evaluating the efficacy of uniformly designed square mesh resin 3D printed scaffolds in directing the orientation of electrospun PCL nanofibers. Sci. Rep. 2024, 14, 22722. [Google Scholar] [CrossRef] [PubMed]
- Šišková, A.O.; Bučková, M.; Kroneková, Z.; Kleinová, A.; Nagy, S.; Rydz, J.; Opálek, A.; Sláviková, M.; Andicsová, A.E. The Drug-Loaded Electrospun Poly(ε-Caprolactone) Mats for Therapeutic Application. Nanomaterials 2021, 11, 922. [Google Scholar] [CrossRef]
- Nayl, A.; Abd-Elhamid, A.I.; Awwad, N.S.; Abdelgawad, M.A.; Wu, J.; Mo, X.; Gomha, S.M.; Aly, A.A.; Bräse, S. Recent Progress and Potential Biomedical Applications of Electrospun Nanofibers in Regeneration of Tissues and Organs. Polymers 2022, 14, 1508. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, Y.; Pang, X.; Chen, X. Poly (Lactic Acid): Recent Stereochemical Advances and New Materials Engineering. Adv. Mater. 2024, 37, 2412185. [Google Scholar] [CrossRef]
- De Luca, S.; Milanese, D.; Gallichi-Nottiani, D.; Cavazza, A.; Sciancalepore, C. Poly(lactic acid) and Its Blends for Packaging Application: A Review. Clean Technol. 2023, 5, 1304–1343. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly (lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef]
- Konwarh, R.; Karak, N.; Misra, M. Electrospun cellulose acetate nanofibers: The present status and gamut of biotechnological applications. Biotechnol. Adv. 2013, 31, 421–437. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of sustainable polymers from biomass for active food packaging. Sustain. Food Technol. 2024, 2, 1266–1296. [Google Scholar] [CrossRef]
- Oldal, D.G.; Topuz, F.; Holtzl, T.; Szekely, G. Green Electrospinning of Biodegradable Cellulose Acetate Nanofibrous Membranes with Tunable Porosity. ACS Sustain. Chem. Eng. 2023, 11, 994–1005. [Google Scholar] [CrossRef]
- Sabarees, G.; Velmurugan, V.; Tamilarasi, G.P.; Alagarsamy, V.; Raja Solomon, V. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers 2022, 14, 3994. [Google Scholar] [CrossRef] [PubMed]
- Samokhin, Y.; Varava, Y.; Diedkova, K.; Yanko, I.; Korniienko, V.; Husak, Y.; Iatsunskyi, L.; Grebnevs, V.; Bertiņs, M.; Banasiuk, R.; et al. Electrospun Chitosan/Polylactic Acid Nanofibers with Silver Nanoparticles: Structure, Antibacterial, and Cytotoxic Properties. ACS Appl. Bio Mater. 2025, 8, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Reyes-Guzmán, V.L.; Villarreal-Gómez, L.J.; Vázquez-Mora, R.; Méndez-Ramírez, Y.I.; Paz-González, J.A.; Zizumbo-López, A.; Borbón, H.; Lizarraga-Medina, E.G.; Cornejo-Bravo, J.M.; Pérez-González, G.L.; et al. Integrating an antimicrobial nanocomposite to bioactive electrospun fibers for improved wound dressing materials. Sci. Rep. 2024, 14, 25118. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Jhatial, A.K.; Kumelachew, D.M. A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Jin, E.; Wu, M.; Wang, S.; Qiao, Z.; Li, M.; Linghu, W. Preparation and application performance of graft-quaternization double modified chitosan electrospun antibacterial nanofibers. Mater. Today Commun. 2022, 31, 103712. [Google Scholar] [CrossRef]
- Li, M.; Zhu, S.; Li, X.; Jin, E. Effects of the molecular structure of graft-quaternization double modified chitosan on the functional properties of electrospun antibacterial nanofiber. Iran. Polym. J. 2025, 34, 355–371. [Google Scholar] [CrossRef]
- Hadipour-Goudarzi, E.; Montazer, M.; Latifi, M.; Aghaji, A.A.G. Electrospinning of chitosan/sericin/PVA nanofibers incorporated with in situ synthesis of nano silver. Carbohydr. Polym. 2014, 113, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Zhou, J.; Chatterton, N.P.; Li, Y.; Huang, J.; Wang, X. Polyacrylonitrile nanofibers coated with silver nanoparticles using a modified coaxial electrospinning process. Int. J. Nanomed. 2012, 7, 5725–5732. [Google Scholar] [CrossRef] [PubMed]
- Salaeh, S.; Thongnuanchan, B.; Bueraheng, Y.; Das, A.; Kaus, N.H.M.; Wießner, S. The utilization of glycerol and xylitol in bio-based vitrimer-like elastomer: Toward more environmentally friendly recyclable and thermally healable crosslinked rubber. Eur. Polym. J. 2023, 198, 112422. [Google Scholar] [CrossRef]
- Miao, J.; Ma, H.; Wu, X.; Fang, S.; Zou, L.; Zhu, M.; Ma, L.; Liu, W.; Chen, L. Glycerol-regulated interfacial adsorption behavior of whey protein isolate for stable high internal phase emulsion gels: Impact on lipid oxidation and curcumin bioaccessibility. Food Hydrocoll. 2025, 163, 111159. [Google Scholar] [CrossRef]
- Ben, Z.Y.; Samsudin, H.; Yhaya, M.F. Glycerol: Its properties, polymer synthesis, and applications in starch based films. Eur. Polym. J. 2022, 175, 111377. [Google Scholar] [CrossRef]
- Hajiahmadi, M.; Faghri, J.; Salehi, Z.; Heidari, F. Comparative Evaluation of Antibacterial Effect of Propolis and Aloe Vera, Xylitol, and Cpp-Acp Gels on Streptococcus mutans and Lactobacillus in Vitro. Int. J. Dent. 2021, 2021, 5842600. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Silva-Paes-Leme, A.; Raposo, N.; da Silva, S. By passing microbial resistance: Xylitol controls microorganisms growth by means of its anti-adherence property. Curr. Pharm. Biotechnol. 2015, 16, 35–42. [Google Scholar] [CrossRef]
- Nayak, P.A.; Nayak, U.A.; Khandelwal, V. The effect of xylitol on dental caries and oral flora. Clin. Cosmet. Investig. Dent. 2014, 6, 89. [Google Scholar] [CrossRef]
- Loesche, W.J. Microbiology of Dental Decay and Periodontal Disease. 1996. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8259/ (accessed on 9 April 2025).
- Effect of Xylitol on an In Vitro Model of Oral Biofilm|Quintessence Publishing Germany|Quintessenz Verlags-GmbH. 2008. Available online: https://www.quintessence-publishing.com/deu/en/article/841613 (accessed on 2 October 2025).
- Marttinen, A.M.; Ruas-Madiedo, P.; Hidalgo-Cantabrana, C.; Saari, M.A.; Ihalin, R.A.; Söderling, E.M. Effects of xylitol on xylitol-sensitive versus xylitol-resistant streptococcus mutans strains in a three-species in vitro biofilm. Curr. Microbiol. 2012, 65, 237–243. [Google Scholar] [CrossRef]
- Azarpazhooh, A.; Lawrence, H.P.; Shah, P.S. Xylitol for preventing acute otitis media in children up to 12 years of age. Cochrane Database Syst. Rev. 2016, 2016, CD007095. [Google Scholar] [CrossRef] [PubMed]
- Anglenius, H.; Tiihonen, K. Evaluation of xylitol as an agent that controls the growth of skin microbes: Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes. Microbiol. Soc. Korea 2020, 56, 54–58. [Google Scholar] [CrossRef]
- Salli, K.; Lehtinen, M.J.; Tiihonen, K.; Ouwehand, A.C. Xylitol’s Health Benefits beyond Dental Health: A Comprehensive Review. Nutrients 2019, 11, 1813. [Google Scholar] [CrossRef]
- Cerqueira, D.A.; Filho, G.R.; Assunção, R.M.N. A new value for the heat of fusion of a perfect crystal of cellulose acetate. Polym. Bull. 2006, 56, 475–484. [Google Scholar] [CrossRef]
- Fernández-Tena, A.; Pérez-Camargo, R.A.; Coulembier, O.; Sangroniz, L.; Aranburu, N.; Guerrica-Echevarria, G.; Liu, G.; Wang, D.; Cavallo, D.; Müller, A.J. Effect of Molecular Weight on the Crystallization and Melt Memory of Poly(ϵ-caprolactone) (PCL). Macromolecules 2023, 56, 4602–4620. [Google Scholar] [CrossRef]
- Boonying, S.; Sutapun, W.; Supakarn, N.; Ruksakulpiwat, Y. Crystallization behavior of vetiver grass fiber- polylactic acid composite. Adv. Mat. Res. 2012, 410, 55–58. [Google Scholar] [CrossRef]
- Han, S.O.; Youk, J.H.; Min, K.D.; Kang, Y.O.; Park, W.H. Electrospinning of cellulose acetate nanofibers using a mixed solvent of acetic acid/water: Effects of solvent composition on the fiber diameter. Mater. Lett. 2008, 62, 759–762. [Google Scholar] [CrossRef]
- Mosquera, M.; Orozco, F.; Benítez, R.; Martin, J.; Rojas, G. Controlled branching by step-growth polymerization of xylitol and succinic acid via microwave irradiation. ACS Omega 2021, 6, 13987–13994. [Google Scholar] [CrossRef]
- Dong, W.; Li, T.; Xiang, S.; Ma, P.; Chen, M. Influence of glutamic acid on the properties of poly(xylitol glutamate sebacate) bioelastomer. Polymers 2013, 5, 1339–1351. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; Santos, D.C.D.; Motta, J.F.G.; Santos, R.R.D.; Chávez, D.W.H.; de Melo, N.R. Structure and functional properties of cellulose acetate films incorporated with glycerol. Carbohydr. Polym. 2019, 209, 190–197. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pagaduan, J.N.M.; Melgar, Z.K.A.; Seitz, S.; Kan, K.; Ajiro, H. Glycerol-modified poly(ε-caprolactone): An biocatalytic approach to improve the hydrophilicity of poly(ε-caprolactone). Polym. Bull. 2019, 76, 1915–1928. [Google Scholar] [CrossRef]
- Satriyatama, A.; Rochman, V.A.A.; Adhi, R.E. Study of the Effect of Glycerol Plasticizer on the Properties of PLA/Wheat Bran Polymer Blends. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 012020. [Google Scholar] [CrossRef]
- Lv, S.; Liu, X.; Gu, J.; Jiang, Y.; Tan, H.; Zhang, Y. Effect of glycerol introduced into PLA based composites on the UV weathering behavior. Constr. Build. Mater. 2017, 144, 525–531. [Google Scholar] [CrossRef]
- Masako, K.; Hideyuki, I.; Shigeyuki, O.; Zenro, I. A novel method to control the balance of skin microflora: Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J. Dermatol. Sci. 2005, 38, 197–205. [Google Scholar] [CrossRef]
- Akiyama, H.; Oono, T.; Huh, W.-K.; Yamasaki, O.; Ogawa, S.; Katsuyama, M.; Ichikawa, H.; Iwatsuki, K. Actions of farnesol and xylitol against Staphylococcus aureus. Chemotherapy 2002, 48, 122–128. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Barbosa, N.R.; Júnior, D.R.; da Silva, S.S. In vitro mechanism of xylitol action against Staphylococcus aureus ATCC 25923. In Current Research Topics in Applied Microbiology and Microbial Biotechnology; World Scientific Connect: Singapore, 2009; pp. 505–509. [Google Scholar] [CrossRef]
- da Silva, A.F.; Suzuki, É.Y.; Ferreira, A.S.; Oliveira, M.G.; da Silva, S.S.; Raposo, N.R.B. In vitro inhibition of adhesion of Escherichia coli strains by Xylitol. Braz. Arch. Biol. Technol. 2011, 54, 235–241. [Google Scholar] [CrossRef]
- Galbis, J.A.; García-Martín, M.D.G.; De Paz, M.V.; Galbis, E. Synthetic Polymers from Sugar-Based Monomers. Chem. Rev. 2016, 116, 1600–1636. [Google Scholar] [CrossRef]
- Wan, L.; Lu, L.; Liang, X.; Liu, Z.; Huang, X.; Du, R.; Luo, Q.; Xu, Q.; Zhang, Q.; Jia, X. Citrate-Based Polyester Elastomer with Artificially Regulatable Degradation Rate on Demand. Biomacromolecules 2023, 24, 4123–4137. [Google Scholar] [CrossRef]
- Kovtun, G.; Casas, D.; Cuberes, T. Influence of Glycerol on the Surface Morphology and Crystallinity of Polyvinyl Alcohol Films. Polymers 2024, 16, 2421. [Google Scholar] [CrossRef]
- Lin, C.C.; Chiu, J.Y. Glycerol-modified γ-PGA and gellan composite hydrogel materials with tunable physicochemical and thermal properties for soft tissue engineering application. Polymer 2021, 230, 124049. [Google Scholar] [CrossRef]
- Hong, G.W.; Wan, J.; Yoon, S.E.; Wong, S.; Yi, K.H. Conditions to Consider When Choosing Fillers. J. Cosmet. Dermatol. 2025, 24, e70075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tsigkou, O.; Chen, X. The effect of bioactive glass particle size on viscosity, stickiness and packability of resin composites. Next Mater. 2024, 5, 100265. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nakamura, T.; Shinzato, S.; Mousa, W.F.; Nishio, K.; Ohsawa, K.; Kokubo, T.; Kikutani, T. Effect of bioactive filler content on mechanical properties and osteoconductivity of bioactive bone cement. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 1999, 46, 447–457. [Google Scholar] [CrossRef]
- Bruggeman, J.P.; Bettinger, C.J.; Langer, R. Biodegradable xylitol-based elastomers: In vivo behavior and biocompatibility. J Biomed. Mater. Res. A 2010, 95A, 92–104. [Google Scholar] [CrossRef]
- Bruggeman, J.P.; Bettinger, C.J.; Nijst, C.L.E.; Kohane, D.S.; Langer, R. Biodegradable Xylitol-Based Polymers. Adv. Mater. 2008, 20, 1922–1927. [Google Scholar] [CrossRef]
- de Arruda, P.V.; Milessi, T.S.; Alves-Ferreira, J.; Sene, L.; Carvalheiro, F.; Duarte, L.C.; de Almeida Felipe, M.D.G. Applications of xylitol in food, material, health, and medical sector. In Current Advances in Biotechnological Production of Xylitol: Fermentative Production of Xylitol; Springer International Publishing: Cham, Switzerland, 2022; pp. 205–237. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschetto, F.; Zanocco, M.; Kamei, K.; Xu, H.; Marin, E. Xylitol Modification of Electrospun Polymer Scaffolds: Impact on Physicochemical and Antibacterial Properties. Polymers 2025, 17, 3024. https://doi.org/10.3390/polym17223024
Boschetto F, Zanocco M, Kamei K, Xu H, Marin E. Xylitol Modification of Electrospun Polymer Scaffolds: Impact on Physicochemical and Antibacterial Properties. Polymers. 2025; 17(22):3024. https://doi.org/10.3390/polym17223024
Chicago/Turabian StyleBoschetto, Francesco, Matteo Zanocco, Kaeko Kamei, Huaizhong Xu, and Elia Marin. 2025. "Xylitol Modification of Electrospun Polymer Scaffolds: Impact on Physicochemical and Antibacterial Properties" Polymers 17, no. 22: 3024. https://doi.org/10.3390/polym17223024
APA StyleBoschetto, F., Zanocco, M., Kamei, K., Xu, H., & Marin, E. (2025). Xylitol Modification of Electrospun Polymer Scaffolds: Impact on Physicochemical and Antibacterial Properties. Polymers, 17(22), 3024. https://doi.org/10.3390/polym17223024









