Wood Bio-Adhesives Made by Polymerizing Oxidized Starch with Deep Eutectic Solvent-Modified Lignin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
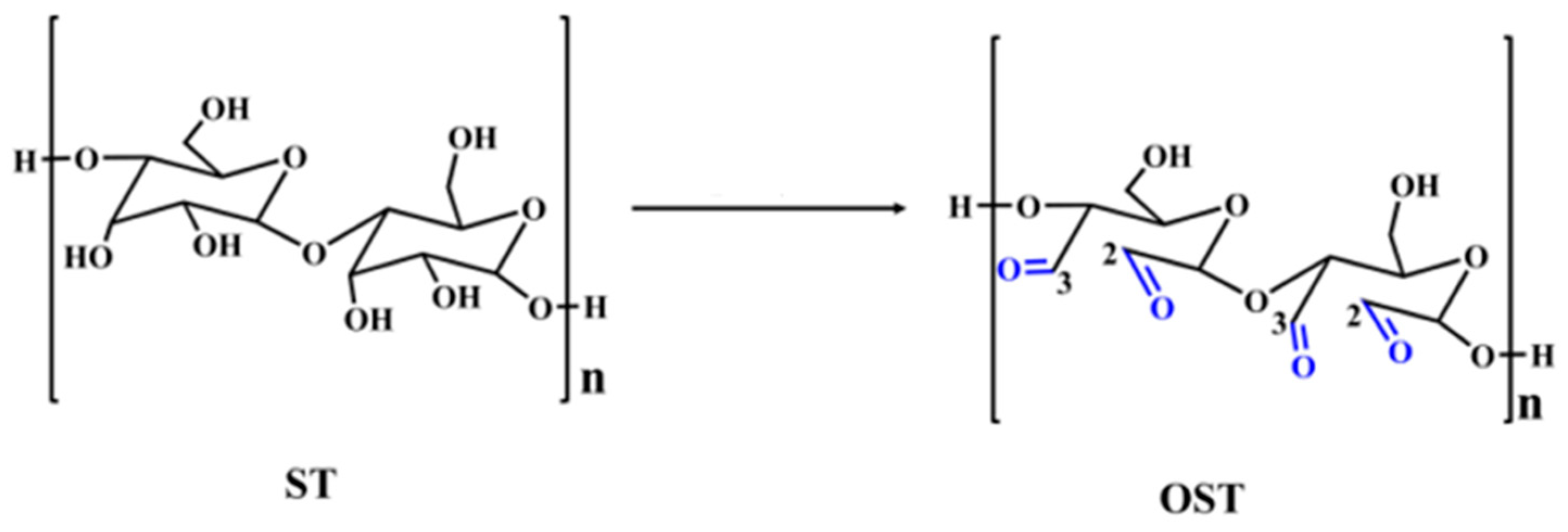
2.2.1. Oxidation of Starch
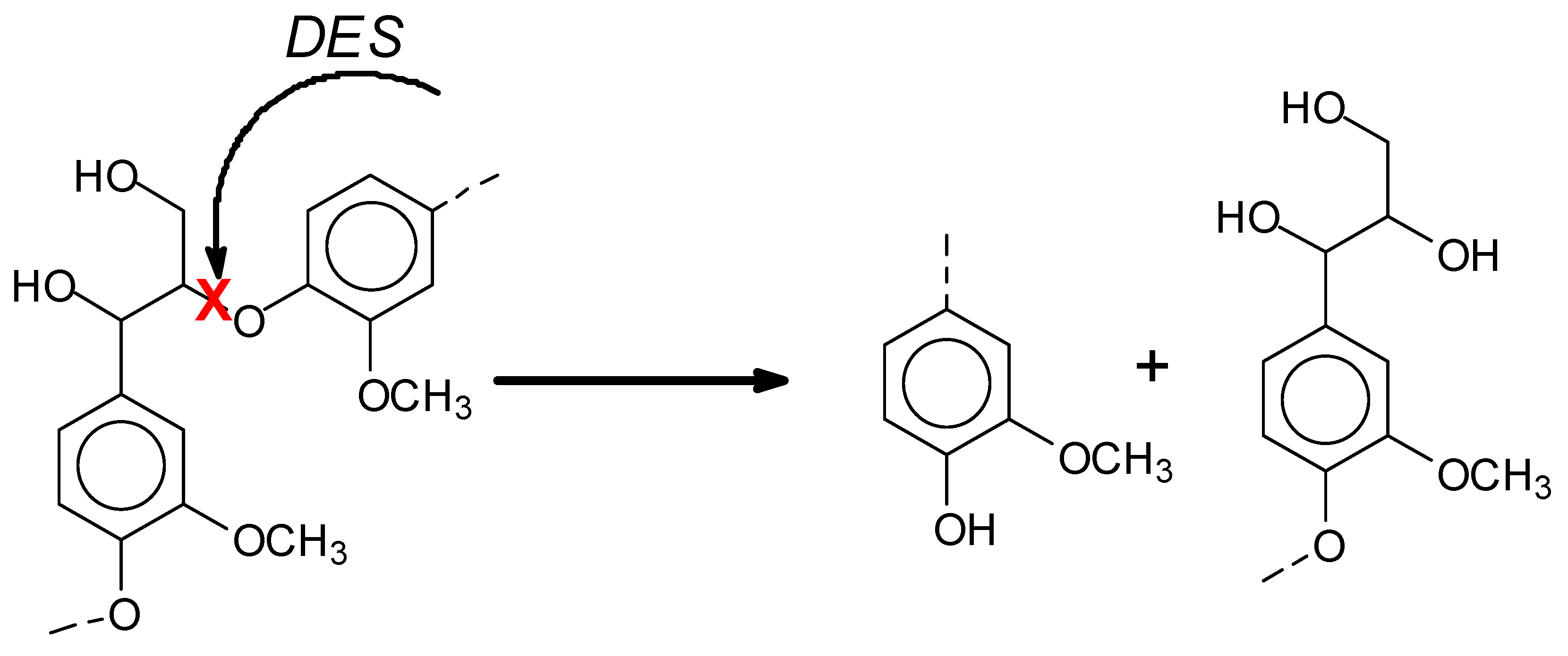
2.2.2. Modification of Lignin
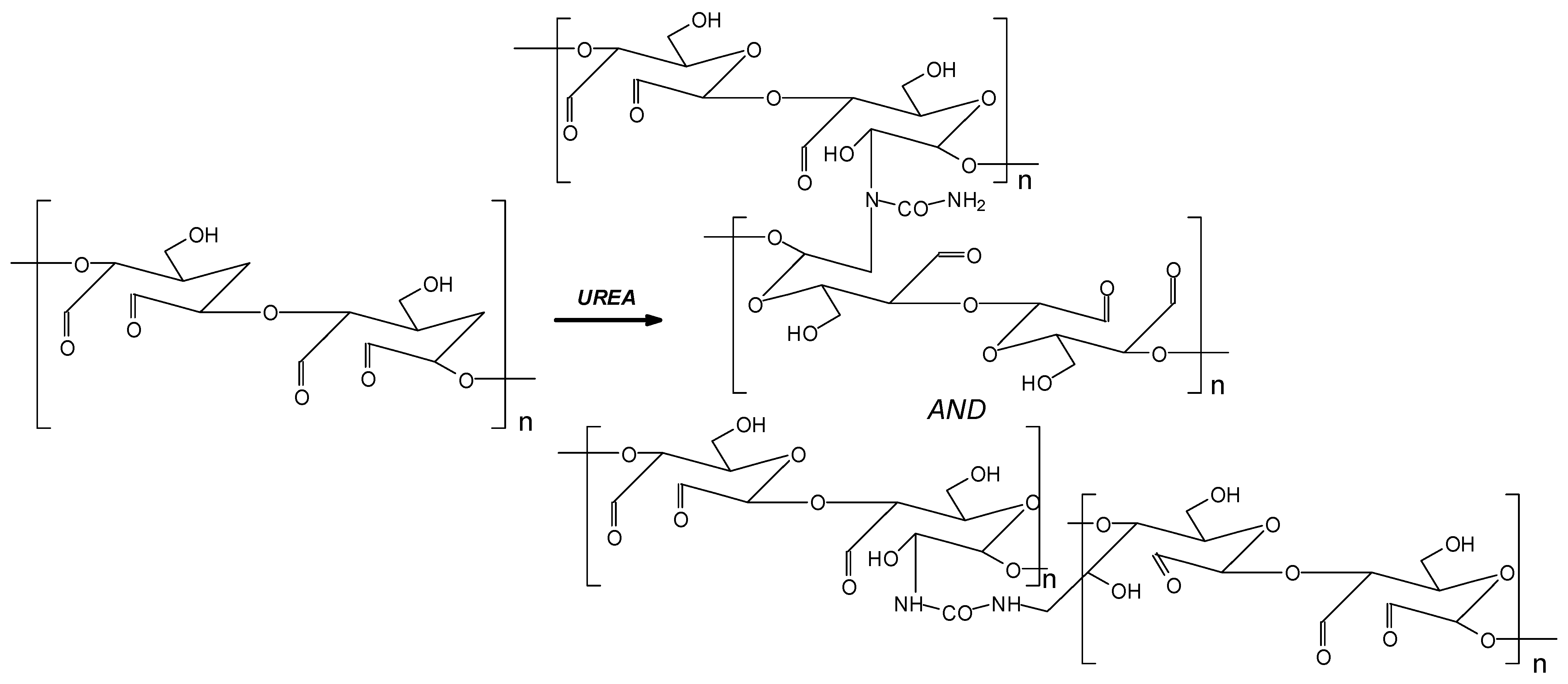
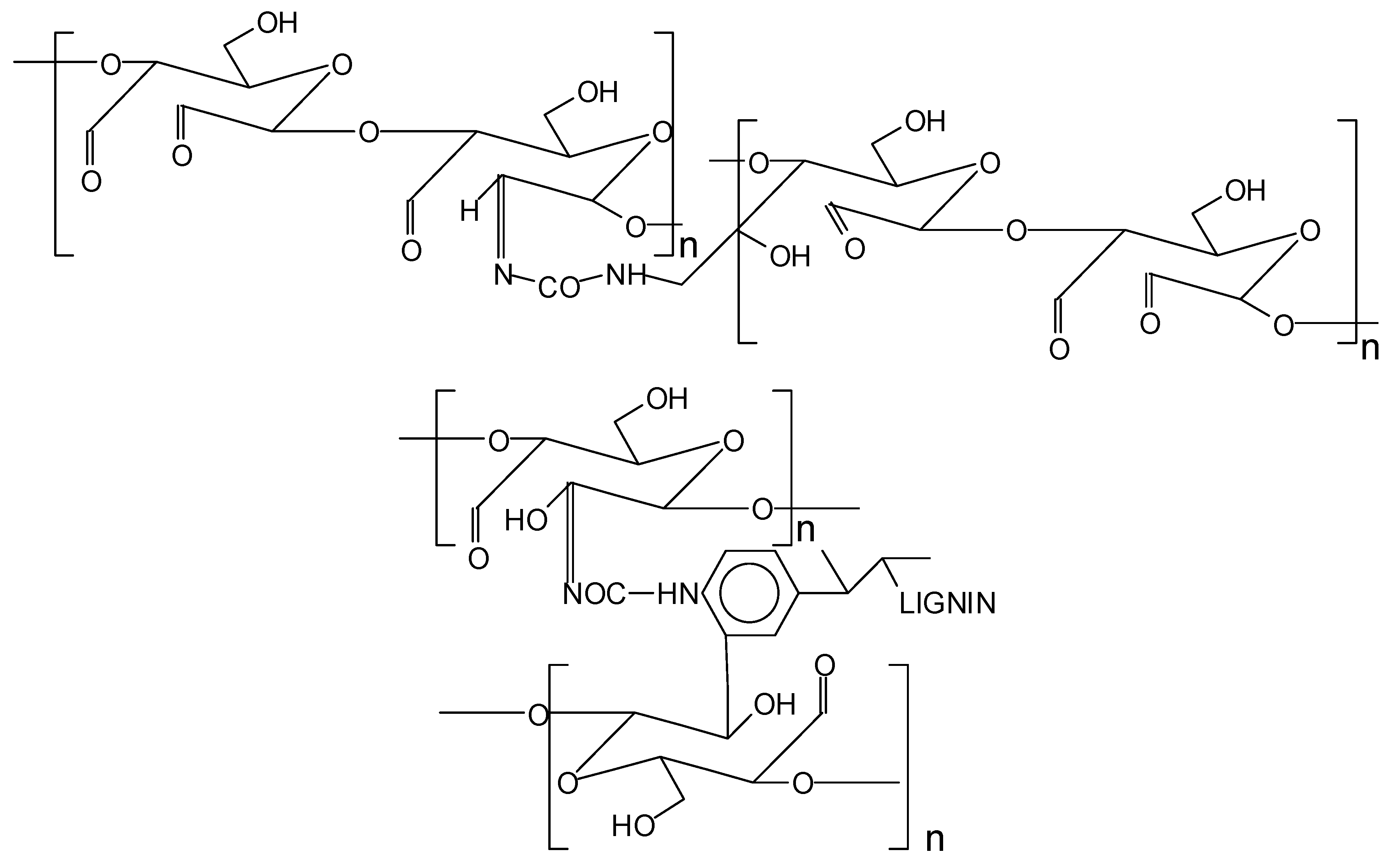
2.2.3. Preparation of Oxidized Starch–Lignin (OSTL) Wood Adhesives
2.2.4. Physicochemical Properties of the Synthesized Resin
2.2.5. Fourier Transform Infrared Spectrometry (FTIR)
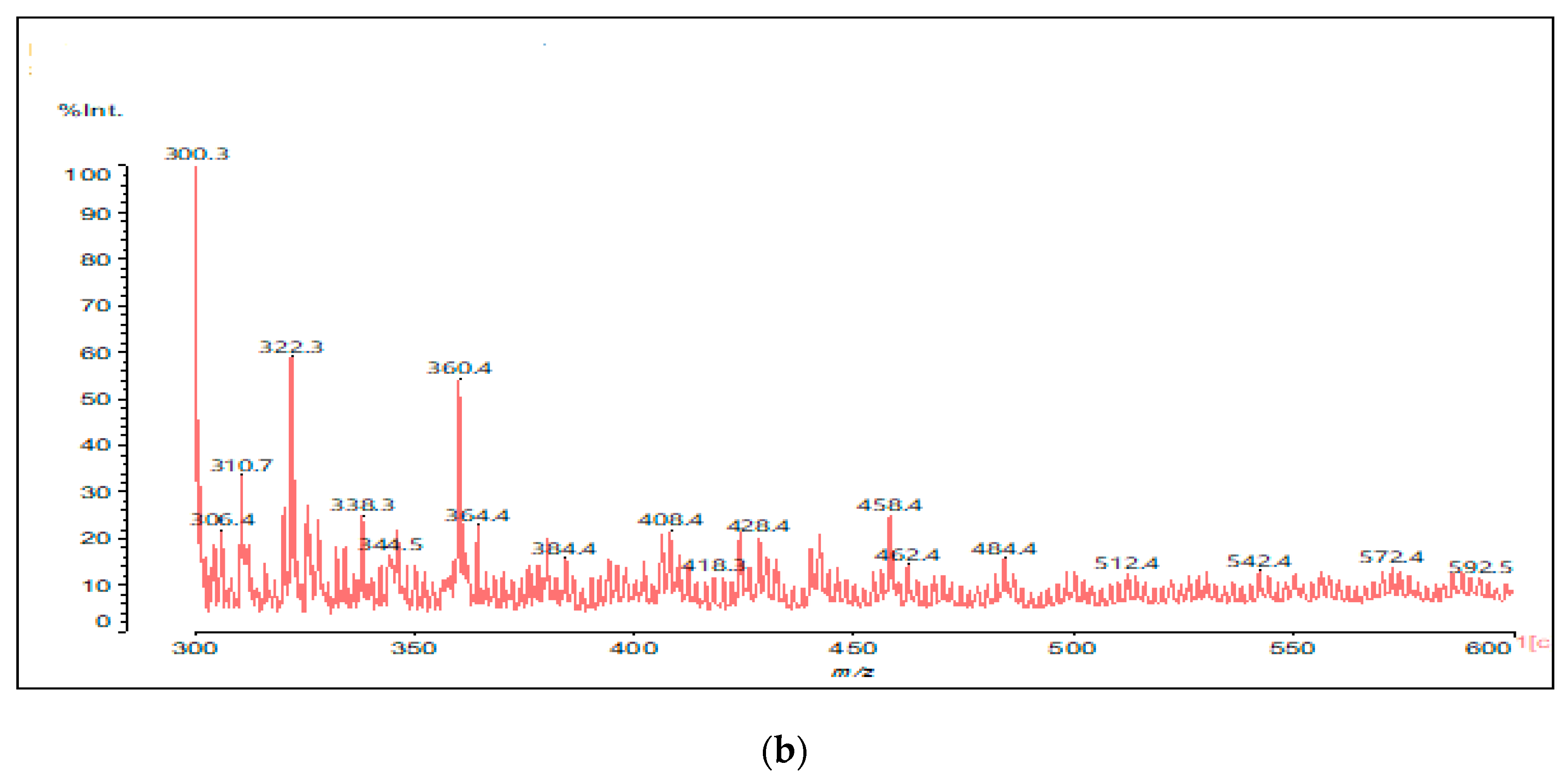
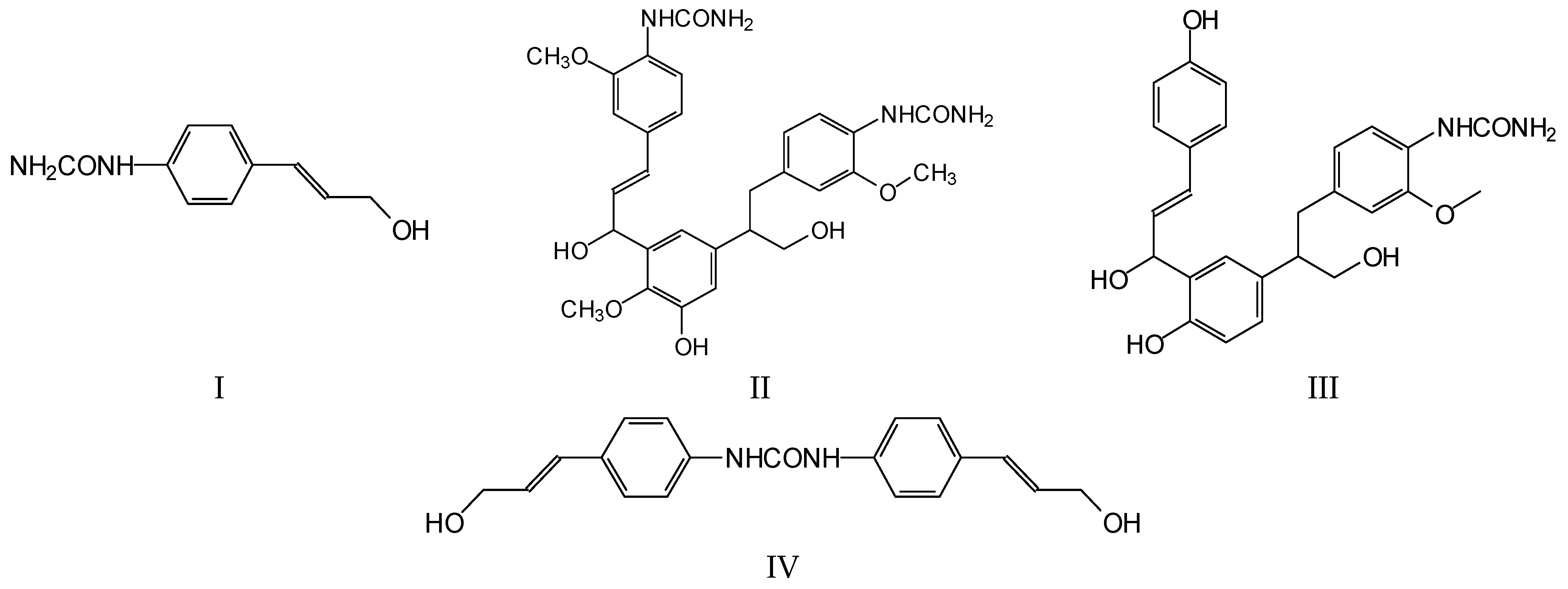
2.2.6. Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry (MALDI ToF)
2.2.7. Differential Scanning Calorimetry (DSC) Analysis
2.2.8. Particleboard Manufacturing
2.2.9. Panel Testing
2.2.10. Statistical Analysis
3. Results and Discussion
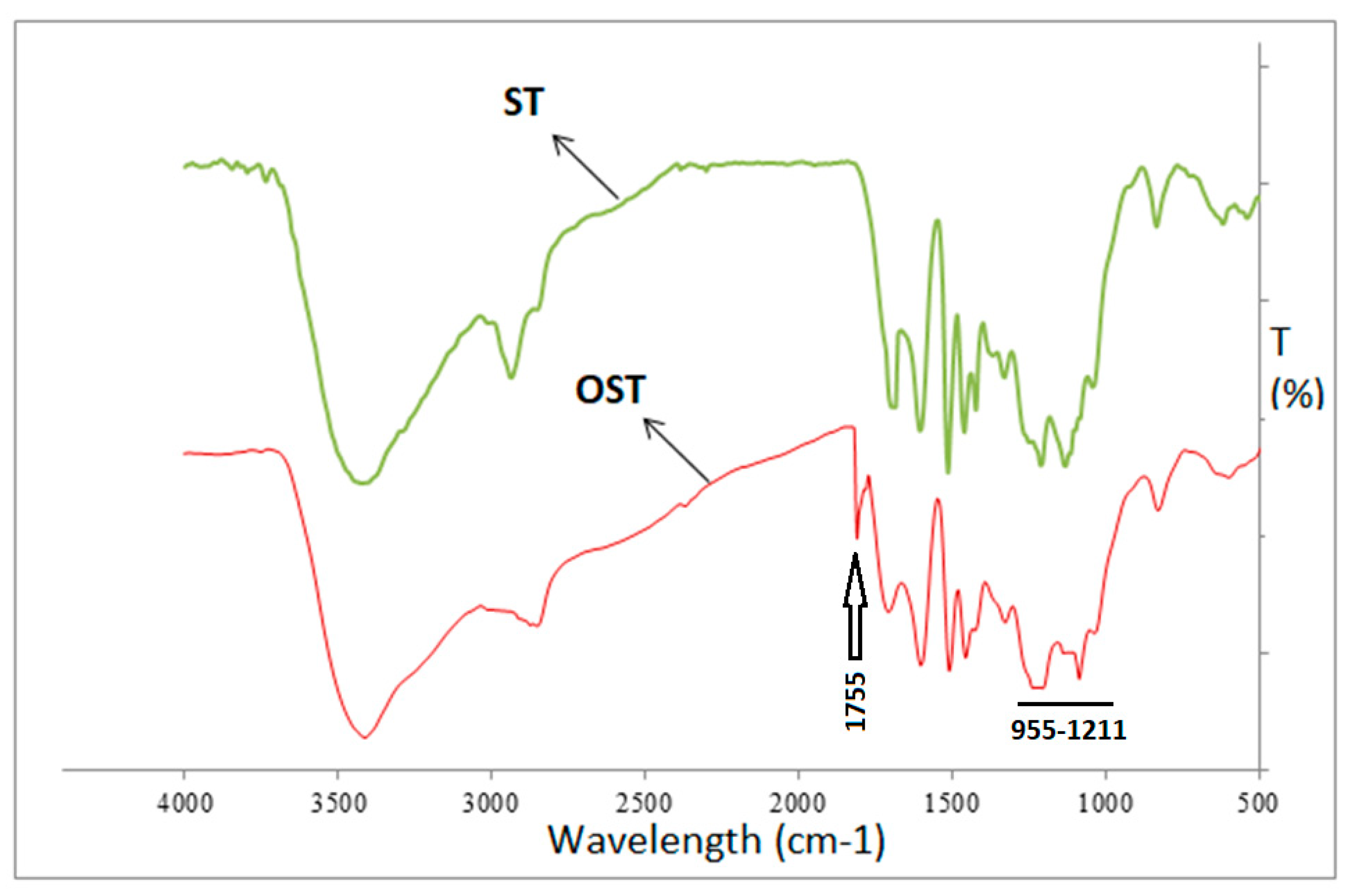
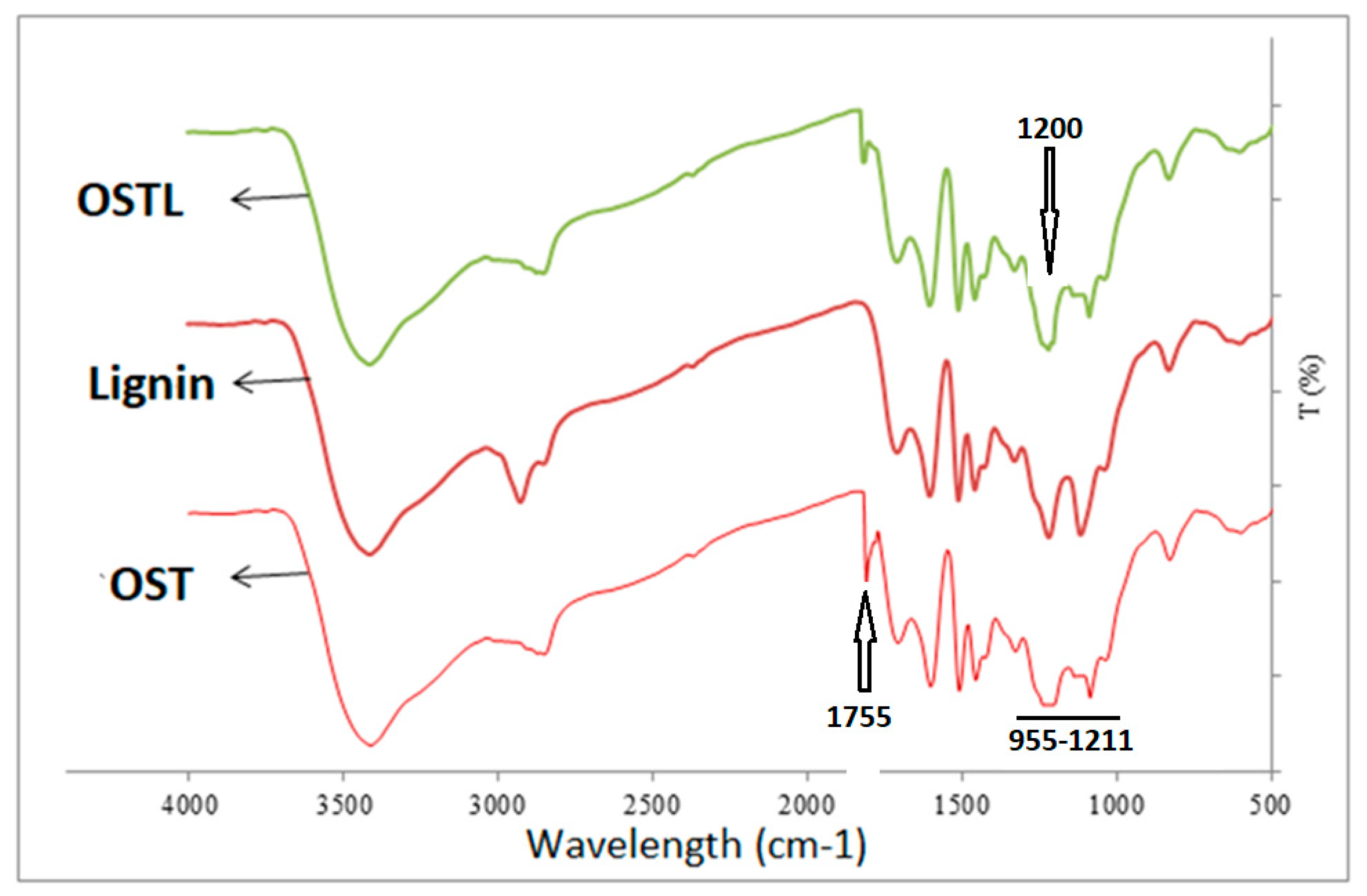
3.1. FTIR Analysis
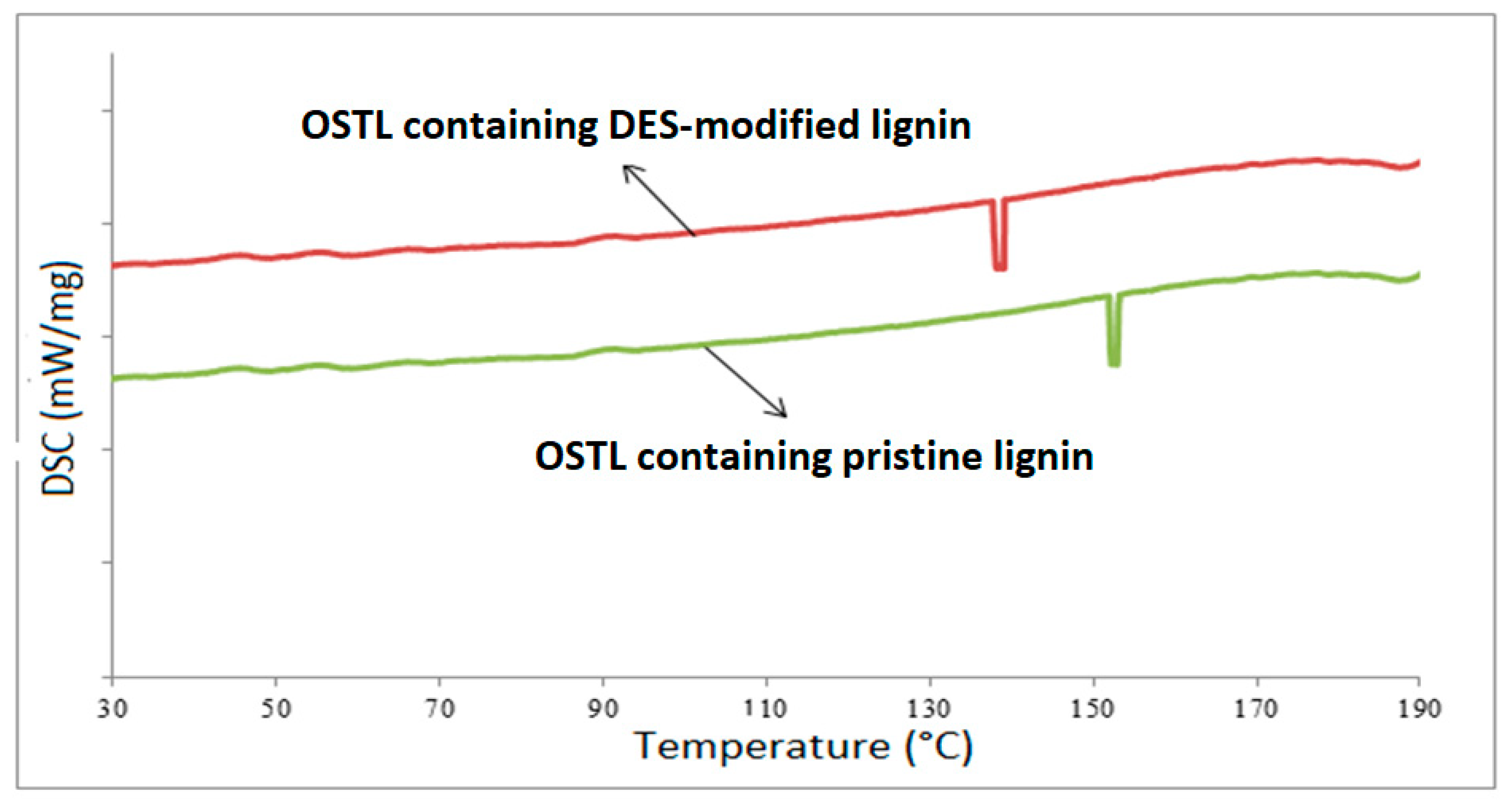
3.2. DSC Analysis
3.3. Mechanical and Physical Properties of the Particleboards Prepared
3.4. Water Absorption and Thickness Swelling
4. Conclusions
- The poor dimensional stability and weak mechanical strength of the particleboard panels bonded with the lignin-oxidized starch resin could be improved by modifying lignin with DES in the formulation.
- FTIR analysis showed that the content of carboxyl groups at the 1200 cm−1 band increased after curing the OSTL resin. Even the content of carbonyl groups in the OSTL resin was lower than that in the OST resin.
- DSC analysis indicated that the curing temperature of the OSTL resin containing DES-modified lignin was lower than that with unmodified lignin.
- The panels containing the OSTL resin with DES-modified lignin presented higher dimensional stability and mechanical strength compared to those prepared with unmodified lignin.
- Higher dimensional stability and mechanical strength could be achieved by increasing the amount of DES-modified lignin in the OSTL wood adhesive from 10 to 30 wt%.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oktay, S.; Pizzi, A.; Koken, N.; Bengu, B. Tannin-based wood panel adhesives. Int. J. Adhes. Adhes. 2024, 130, 103621. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, Z.; Xi, X.; Lei, H.; Li, C.; Chen, Z.; Shi, J.; Du, G. A bio-based soy wood adhesive modified by dual-crosslinking strategy with excellent mechanical strength and water-resistance. Ind. Crops Prod. 2024, 222, 119417. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, Z.; Wang, Y.; Wang, J.; Chen, H.; Jiang, S.; Han, X. Technology of lignin modification: Progress in specialty and green adhesives’ mechanical properties. Green Chem. 2025, 27, 13577–13606. [Google Scholar] [CrossRef]
- Balk, M.; Sofia, P.; Neffe, A.T.; Tirelli, A. Lignin, the lignification process, and advanced, lignin-based materials. Int. J. Mol. Sci. 2023, 24, 11668. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A.; Erfani, S.; Amiri, M. The synthesis of maleated nanolignin–polyvinyl alcohol–hexamine resin as a cold-setting wood adhesive. Eur. J. Wood Prod. 2024, 82, 159–166. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A. Preparation and properties of a modified corn flour-lignin-glyoxal as a Green wood adhesive. Int. Wood Prod. J. 2022, 13, 119–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Li, B.; Udin, S.M.; Maarof, H.; Zhou, W.; Cheng, F.; Yang, J.; Liu, Y.; Alias, H.; et al. Mechanistic insights into the lignin dissolution behavior in amino acid based deep eutectic solvents. Int. J. Biol. Macromol. 2023, 242, 124829. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, H.; He, Y.; Su, Y.; Dong, M.; Wang, B.; Zeng, B.; Wang, J. All-biomass-based material with tunable mechanical and optical properties fabricated by multiscale cellulose and lignin from waste wheat straw. Int. J. Biol. Macromol. 2025, 319, 145422. [Google Scholar] [CrossRef]
- Li, W.; Wu, S. Depolymerization and product evolution of kraft lignin in novel acid deep eutectic solvents (DESs). Ind. Crops Prod. 2025, 225, 120476. [Google Scholar] [CrossRef]
- Du, X.; Zhang, J.; Li, X.; Du, G.; Deng, S. Development of environmentally friendly glyoxal-based adhesives with outstanding water repellency utilizing wheat gluten protein. Int. J. Biol. Macromol. 2024, 273, 133081. [Google Scholar] [CrossRef]
- Chen, Y.; Rao, Y.; Liu, P.; Han, Z.; Xie, F. Facile fabrication of a starch-based wood adhesive showcasing water resistance, flame retardancy, and antibacterial properties via a dual crosslinking strategy. Int. J. Biol. Macromol. 2024, 282, 137180. [Google Scholar] [CrossRef] [PubMed]
- Guigo, N.; Mazeau, K.; Putaux, J.L.; Heux, L. Surface modification of cellulose microfibrils by periodate oxidation and subsequent reductive amination with benzylamine: A topochemical study. Cellulose 2014, 21, 4119–4133. [Google Scholar] [CrossRef]
- Codou, A.; Guigo, N.; Heux, L.; Sbirrazzuoli, N. Partial periodate oxidation and thermal cross-linking for the processing of thermoset all-cellulose composites. Compos. Sci. Technol. 2015, 117, 54–61. [Google Scholar] [CrossRef]
- Frihart, C.R.; Lorenz, L.F. Specific oxidants improve the wood bonding strength of soy and other plant flours. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1017–1023. [Google Scholar] [CrossRef]
- Frihart, C.R.; Pizzi, A.; Xi, X.; Lorenz, L.F. Reactions of soy flour and soy protein by non-volatile aldehydes generation by specific oxidation. Polymers 2019, 11, 1478. [Google Scholar] [CrossRef]
- Zhang, B.; Pizzi, A.; Petrissans, M.; Petrissans, A.; Baptiste, C. A Melamine–Dialdehyde Starch Wood Particleboard Surface Finish without Formaldehyde. J. Renew. Mater. 2023, 11, 3867–3889. [Google Scholar] [CrossRef]
- Liu, T.; Du, G.; Wu, Y.; Liu, C.; Yang, H.; Ni, K.; Yin, C.; Ran, X.; Gao, W.; Yang, L. Activated wood surface and functionalized cellulose co-building strong chemical wood bonding performance. Carbohydr. Polym. 2023, 305, 120573. [Google Scholar] [CrossRef]
- Song, J.; Chen, S.; Zhang, Q.; Lei, H.; Xi, X.; Du, G.; Pizzi, A. Developing on the well performance and eco-friendly sucrose—Based wood adhesive. Ind. Crops Prod. 2023, 195, 116298. [Google Scholar] [CrossRef]
- Diaz-Baca, J.A.; Fatehi, P. Production and Characterization of Starch-Lignin Based Materials: A Review. J. Biotechnol. Adv. 2024, 70, 108281. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, L.; Wang, H.; Wang, Y.; Zhang, H. Environment-friendly urea-oxidized starch adhesive with zero formaldehyde-emission. Carbohydr. Polym. 2018, 181, 1112–1118. [Google Scholar] [CrossRef]
- Liu, C.; Lin, H.; Zhang, S.; Essawy, H.; Wang, H.; Wu, L.; Chen, X.; Zhou, X.; Papadopoulos, A.; Pizzi, A.; et al. Characterization of Water-Resistant Adhesive Prepared by Cross-Linking Reaction of Oxidized Starch with Lignin. Polymers 2025, 17, 1545. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lian, H.; Sun, X.; Pan, D.; Carranza, A.; Pojman, J.A.; Mota-Morales, J.D. Zinc-based deep eutectic solvent-mediated hydroxylation and demethoxylation of lignin for the production of wood adhesive. RSC Adv. 2016, 6, 89599–89608. [Google Scholar] [CrossRef]
- China National Standard GB/T 14074-2017; Testing Methods for Wood Adhesives and Resins. Beijing COC Tech Co., Ltd.: Beijing, China, 2017.
- Younesi-Kordkheili, H.; Kazemi-Najafi, S.; Behrooz, R. Influence of nanoclay on urea–glyoxalated lignin–formaldehyde resins for wood adhesive. J. Adhes. 2017, 93, 431–443. [Google Scholar] [CrossRef]
- EN 312:2010; Particleboards-Specifications. European Sandardization Committee: Brussel, Belgium, 2010.
- ASTM 4442; Standard Test Method for Direct Moisture Content Measurement of Wood and Wood-Based Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 2003.
- Yang, H.; Liu, J. Recent advances on the preparation conditions, structural characteristics, physicochemical properties, functional properties and potential applications of dialdehyde starch: A review. Int. J. Biol. Macromol. 2024, 259, 129261. [Google Scholar] [CrossRef]
- Oktay, S.; Kizilcan, N.; Bengu, B. Oxidized cornstarch—Urea wood adhesive for interior particleboard production. Int. J. Adhes. Adhes. 2021, 110, 102947. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A. Modification of nanolignin by deep eutectic solvent to improve the properties of phenol-formaldehyde resins. Eur. J. Wood Prod. 2024, 82, 2099–2108. [Google Scholar] [CrossRef]
- Braghiroli, F.; Fierro, V.; Izquierdo, M.T.; Parmentier, J.; Pizzi, A.; Celzard, A. Nitrogen-doped carbon materials produced from hydrothermally treated tannin. Carbon 2012, 50, 5411–5420. [Google Scholar] [CrossRef]
- McMurry, J.E. Organic Chemistry, 3rd ed.; Brooks/Cole Publishing Company: Pacific Grove, CA, USA, 1992. [Google Scholar]
- Ge, H.; Liu, Y.; Zhu, B.; Xu, Y.; Zhou, R.; Xu, H.; Li, B. Machine learning prediction of delignification and lignin structure regulation of deep eutectic solvents pretreatment processes. Ind. Crops Prod. 2023, 203, 117138. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A. Acidic deep eutectic solvent as a novel catalyst for urea–formaldehyde resin curing. Wood Mater. Sci. Eng. 2025, 20, 177–183. [Google Scholar] [CrossRef]
- Chee, S.S.; Jawaid, M.; Sultan, M.T.H.; Alothman, O.Y.; Abdullah, L.C. Effects of nanoclay on physical and dimensional stability of Bamboo/Kenaf/nanoclay reinforced epoxy hybrid nanocomposites. J. Mater. Res. Technol. 2020, 9, 5871–5880. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H. Improving Physical and Mechanical Properties of New Lignin-Urea-Glyoxal Resin by Nanoclay. Eur. J. Wood Prod. 2017, 75, 885–891. [Google Scholar] [CrossRef]
- Yan, G.; Zhou, Y.; Zhao, L.; Wang, W.; Yang, Y.; Zhao, X.; Chen, Y.; Yao, X. Recycling of deep eutectic solvent for sustainable and efficient pretreatment of corncob. Ind. Crops Prod. 2022, 183, 115005. [Google Scholar] [CrossRef]
- Chen, Z.; Reznicek, W.D.; Wan, C. Deep eutectic solvent pretreatment enabling full utilization of switchgrass. Bioresour. Technol. 2018, 263, 40–48. [Google Scholar] [CrossRef]
- Kim, K.H.; Dutta, T.; Sun, J.; Simmons, B.; Singh, S. Biomass pretreatment using deep eutectic solvents from lignin derived phenols. Green Chem. 2018, 20, 809–815. [Google Scholar] [CrossRef]


| Resin | Bending Strength (MPa) | Bending Modulus (MPa) | IB Strength (MPa) |
|---|---|---|---|
| EN312 | >11 | >1800 | ≥0.40 |
| OSTL + 10% Lignin | 8.3 ± 0.01 | 1632 ± 70 | 0.35 ± 0.02 |
| OSTL + 20% Lignin | 7.2 ± 0.02 | 1520 ± 61 | 0.31 ± 0.03 |
| OSTL + 30% Lignin | 5.5 ± 0.03 | 1402 ± 42 | 0.27 ± 0.02 |
| OSTL + 10% DES-modified Lignin | 10 ± 0.05 | 1845 ± 33 | 0.39 ± 0.03 |
| OSTL + 10% DES-modified Lignin | 11.9 ± 0.03 | 2350 ± 51 | 0.41 ± 0.01 |
| OSTL + 10% DES-modified Lignin | 12.2 ± 0.04 | 2593 ± 50 | 0.44 ± 0.03 |
| Resin | Density | Water Absorption | Thickness Swelling |
|---|---|---|---|
| (kg/m3) | (%) | (%) | |
| OSTL + 10% lignin | 750 ± 7 | 57 ± 2 | 33 ± 4 |
| OSTL + 20% lignin | 752 ± 4 | 53 ± 4 | 22 ± 2 |
| OSTL + 30% lignin | 751 ± 2 | 49 ± 3 | 16 ± 3 |
| OSTL + 10% DES-modified lignin | 749 ± 3 | 52 ± 6 | 28 ± 3 |
| OSTL + 20% DES-modified lignin | 750 + 5 | 45 ± 3 | 17 ± 2 |
| OSTL + 30% DES-modified lignin | 752 ± 4 | 35 + 2 | 13 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younesi-Kordkheili, H.; Pizzi, A. Wood Bio-Adhesives Made by Polymerizing Oxidized Starch with Deep Eutectic Solvent-Modified Lignin. Polymers 2025, 17, 3023. https://doi.org/10.3390/polym17223023
Younesi-Kordkheili H, Pizzi A. Wood Bio-Adhesives Made by Polymerizing Oxidized Starch with Deep Eutectic Solvent-Modified Lignin. Polymers. 2025; 17(22):3023. https://doi.org/10.3390/polym17223023
Chicago/Turabian StyleYounesi-Kordkheili, Hamed, and Antonio Pizzi. 2025. "Wood Bio-Adhesives Made by Polymerizing Oxidized Starch with Deep Eutectic Solvent-Modified Lignin" Polymers 17, no. 22: 3023. https://doi.org/10.3390/polym17223023
APA StyleYounesi-Kordkheili, H., & Pizzi, A. (2025). Wood Bio-Adhesives Made by Polymerizing Oxidized Starch with Deep Eutectic Solvent-Modified Lignin. Polymers, 17(22), 3023. https://doi.org/10.3390/polym17223023








