Enhanced Flame-Retardant Properties of PVDF Using a Multiphase Synergistic Approach with Phytate-Chitosan-Modified Boron Nitride
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BN@PA-CS and PVDF/BN@PA-CS Composite Materials
2.3. Characterization
3. Results and Discussion
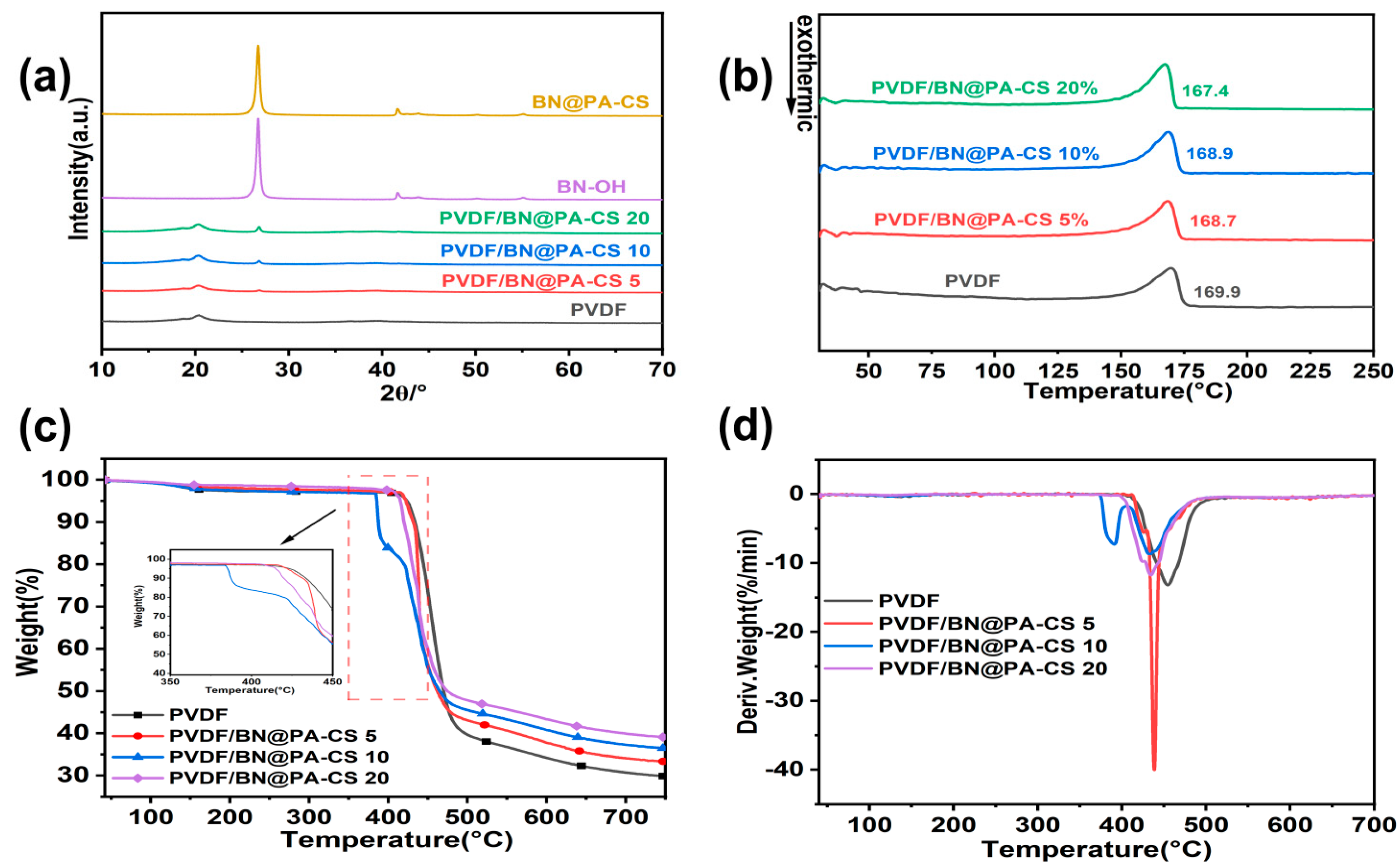
3.1. Structure and Morphology of BN@PA-CS
3.2. Structure and Thermal Performance of PVDF/BN@PA-CS
3.3. Morphology and Mechanical Property Analysis
3.4. Flame Retardancy of PVDF Composite Films and SEM Images of Residual Carbon
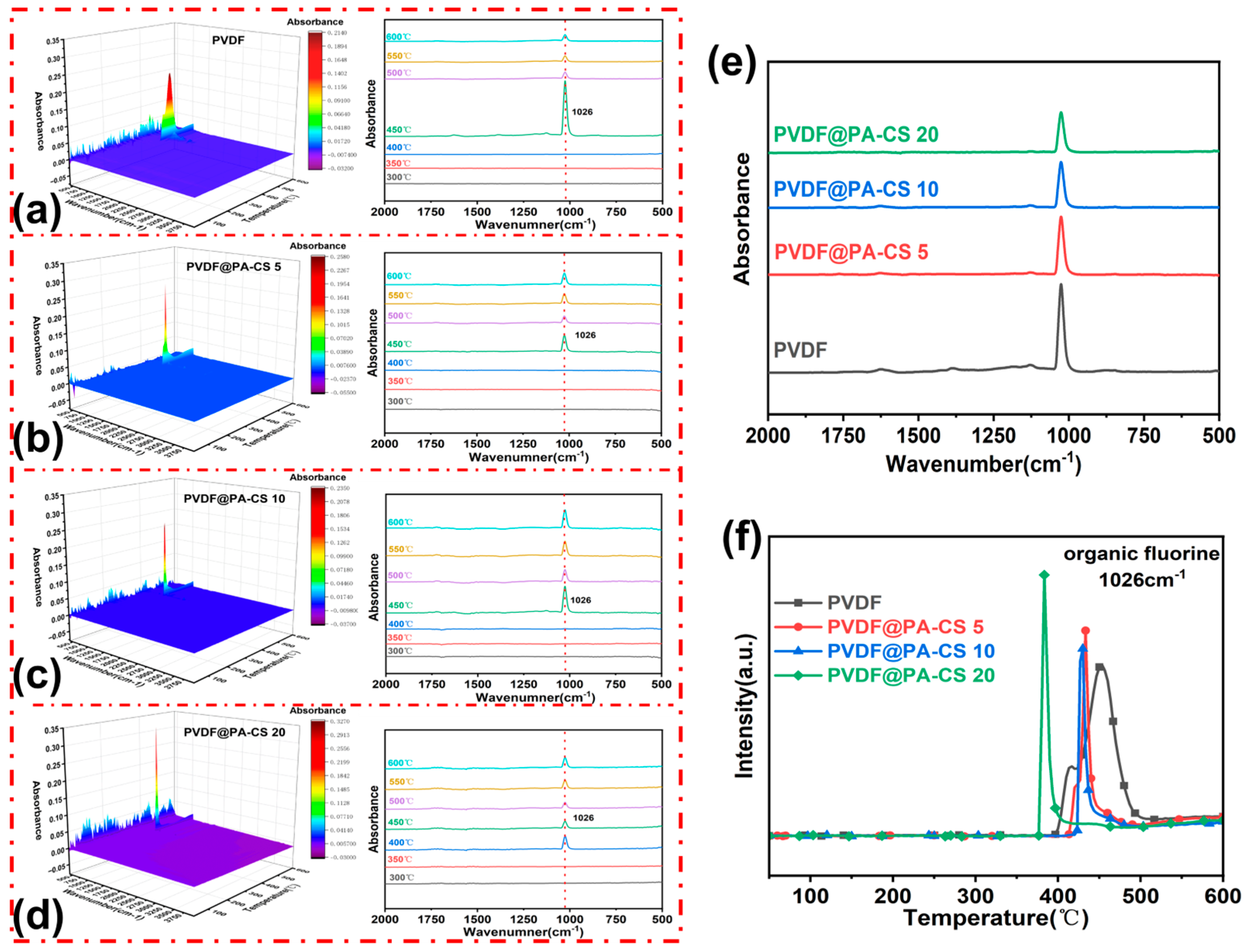
3.5. Condensed Phase Analysis
3.6. Gas Phase Analysis
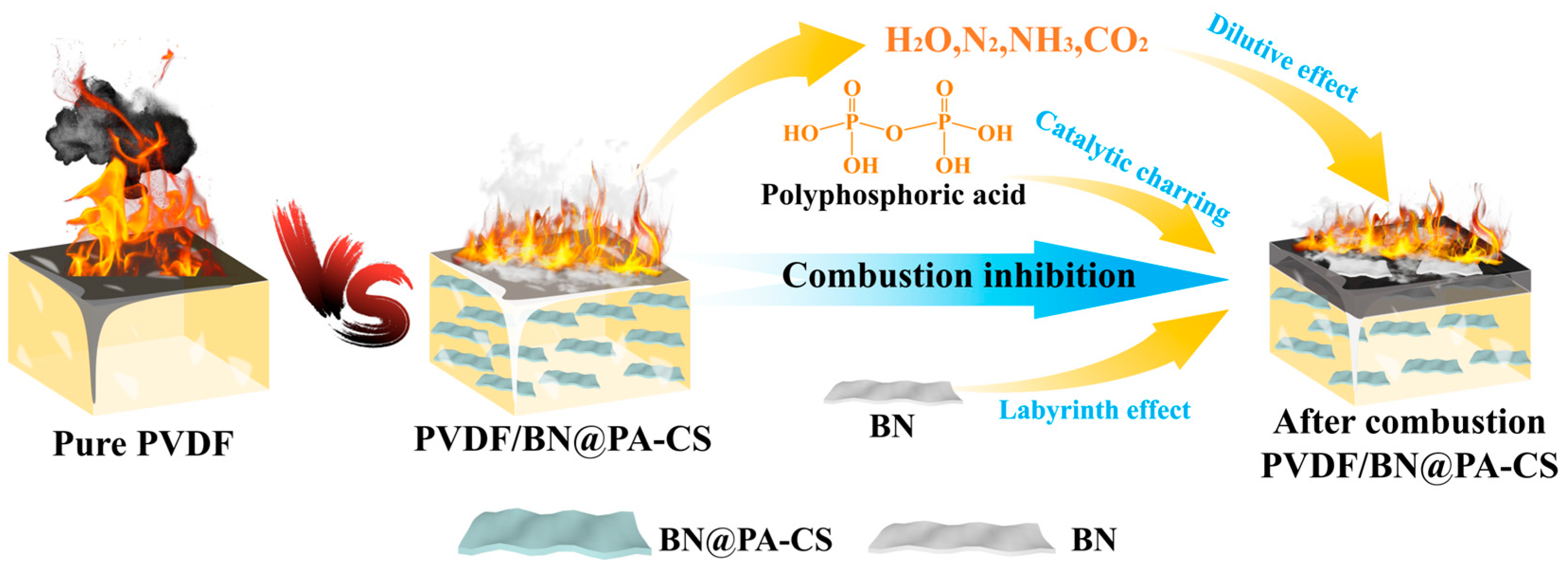
3.7. Proposed Flame-Retardant Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, Y.; Yang, C.; Hei, J.; Cheng, L.; Li, L.; Yin, X.; Zhang, Y.; Ma, Z. Preparation and Combustion Performance of PVDF@Al/CuO Self-Supporting Energetic Films. J. Mater. Eng. Perform. 2025, 1–10. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, H.; Liang, Z.; Qiao, R.; Wang, F.; Luo, X. Enhanced Electrolyte-Wettability and Thermal Stability of Hydrogen-Bonded PVDF-HFP-Coated Paper-Based Separators Fabricated by a Simple Dip Coating for Lithium-Ion Battery. ChemistrySelect 2025, 10, e02528. [Google Scholar] [CrossRef]
- Park, J.-H. Preparation and Performance Study of Heat-Resistant and Flame-Retardant Lithium-Ion Battery Separators. Doctoral Dissertation, Harbin Institute of Technology, Harbin, China, 2023. [Google Scholar]
- Han, B.; Wang, K. Enhancing the safety and ion transport performance of lithium-ion batteries using a parallel electropunk Sb2O3@PMIA/TPP@PVDF-CTFE separator. Guangzhou J. Chem. 2024, 49, 12–17. [Google Scholar]
- Wang, F.; Wang, F.L.; Xu, Y.; Fang, Y. Current Research Status on Safety Technology of Lithium-ion Batteries. Ship Electr. Eng. 2025, 45, 16–20. [Google Scholar]
- Chen, B.; Fang, Y.; Si, Y.; Wang, F.; Shao, B.; Song, Y.; Wang, Q. Layer-by-layer self-assembly of ammonium polyphosphate-chitosan/boron nitride on the surface of plywood and its flame-retardant performance. J. Compos. Mater. 2021, 38, 1252–1261. [Google Scholar]
- Zeng, L.; Li, C.; Luo, C.; Xu, W.; Mu, J. Preparation and performance of chitosan/gelatin/phytic acid composite flame-retardant coatings. J. Beijing For. Univ. 2024, 46, 112–122. [Google Scholar]
- Li, D.; Gou, X.; Wu, D.; Guo, Z. A robust and stretchable superhydrophobic PDMS/PVDF@KNFs membrane for oil/water separation and flame retardancy. Nanoscale 2018, 10, 6695–6703. [Google Scholar] [CrossRef]
- Shen, X.; Li, Z.; Deng, N.; Fan, J.; Wang, L.; Xia, Z.; Liu, Y.; Liu, J. Rational designing of tree-like polymer gel membrane based on PVDF/lamellar organic montmorillonite nanofiber with excellent flame retardancy and superior ion conductivity for high-performance lithium-ion capacitor. Chem. Eng. J. 2021, 422, 130116. [Google Scholar] [CrossRef]
- Zhao, T.; Gai, Q.; Deng, X.; Ma, J.; Gao, H. A new type of LATP doped PVDF-HFP based electrolyte membrane with flame retardancy and long cycle stability for solid state batteries. J. Energy Storage 2023, 73, 108576. [Google Scholar] [CrossRef]
- Guan, L.; Zhao, X.; Ji, Z.; Jiang, M.; Cui, Y.; Weng, L.; Wang, X.; Liu, J. Preparation of 3D BN-BT/PVDF skeleton structure composites for high thermal conductivity and energy storage. Polym. Test. 2023, 125, 108126. [Google Scholar] [CrossRef]
- Meng, G.; She, J.; Wang, C.; Wang, W.; Pan, C.; Cheng, Y. Sandwich-Structured h-BN/PVDF/h-BN Film with High Dielectric Strength and Energy Storage Density. Front. Chem. 2022, 10, 910305. [Google Scholar] [CrossRef]
- Zhu, C.; Yin, J.; Feng, Y.; Li, J.; Li, Y.; Zhao, H.; Yue, D.; Liu, X. Enhanced Energy Storage Performance of PVDF-Based Composites Using BN@PDA Sheets and Titania Nanosheets. Materials 2022, 15, 4370. [Google Scholar] [CrossRef]
- Pratihar, S.; Chandran, A.M.; Mridha, N.; Yella, A.; Mural, P.K.S. Tunable Self-Poled 2D h-BN/PVDF Nanofibers for Enhanced Electromechanical and Electrochemical Applications. ACS Appl. Electron. Mater. 2025, 7, 2751–2766. [Google Scholar] [CrossRef]
- Li, R.; Pei, J.; Xiong, C. Preparation and electric property of PA/PVDF blend for energy storage material. Polym. Sci. Ser. A 2016, 58, 765–775. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.; Liu, S.; Zhao, J.; Hu, Z.; Song, W. A Green Triboelectric Nano-Generator Composite of Degradable Cellulose, Piezoelectric Polymers of PVDF/PA6, and Nanoparticles of BaTiO3. Sensors 2020, 20, 506. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, H.; Zhao, X.; Li, X.; Gu, X.; Li, Y. Flame-retarding nanoparticles as the compatibilizers for immiscible polymer blends: Simultaneously enhanced mechanical performance and flame retardancy. J. Mater. Chem. A 2019, 7, 4903–4912. [Google Scholar] [CrossRef]
- Halder, B.; Padwal, M.; Chandrasekhar, P.; Elumalai, P. A Flame Retardant PVDF-HFP-Based Composite Electrolyte Enabled by Yttrium-Doped NASICON for Stable Lithium Metal Batteries. ACS Appl. Energy Mater. 2025, 8, 12815–12825. [Google Scholar] [CrossRef]
- Cao, J.-P.; Zhao, X.; Zhao, J.; Zha, J.-W.; Hu, G.-H.; Dang, Z.-M. Improved Thermal Conductivity and Flame Retardancy in Polystyrene/Poly(vinylidene fluoride) Blends by Controlling Selective Localization and Surface Modification of SiC Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 6915–6924. [Google Scholar] [CrossRef]
- Zhang, Y.; He, R.; Li, Y.; Liu, H.; Liu, H.; Zhang, X.-X. Fabrication of coaxially electrospun PEI@PVDF-HFP fibrous membrane as flame-retardant and anti-shrink separator for lithium-ion battery. Mater. Lett. 2024, 361, 136063. [Google Scholar] [CrossRef]
- Lv, Q.; Song, Y.; Wang, B.; Wang, S.; Wu, B.; Jing, Y.; Ren, H.; Yang, S.; Wang, L.; Xiao, L.; et al. Bifunctional flame retardant solid-state electrolyte toward safe Li metal batteries. J. Energy Chem. 2023, 81, 613–622. [Google Scholar] [CrossRef]
- Huang, S.; Hong, S.; Su, Y.; Jiang, Y.; Fukushima, S.; Gill, T.M.; Yilmaz, N.E.D.; Tiwari, S.; Nomura, K.-I.; Kalia, R.K.; et al. Enhancing combustion performance of nano-Al/PVDF composites with β-PVDF. Combust. Flame 2020, 219, 467–477. [Google Scholar] [CrossRef]
- Qin, Y.; Yu, H.; Wang, D.; Song, Y.; Li, F.; Liu, J. Preparation and characterization of energetic composite films with mutual reactions based on B/PVDF mosaic structure. Chem. Eng. J. 2023, 451, 138792. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Kim, H.S.; Jo, S.M.; Kim, S.Y.; Yang, B.; Cho, J.; Lee, S.; Cha, J.E. Thermally insulating, fire-retardant, smokeless and flexible polyvinylidene fluoride nanofibers filled with silica aerogels. Chem. Eng. J. 2018, 351, 473–481. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, T.; Hao, X.; Zhu, T.; Zang, J.; Li, Y.; Wang, W. Ingenious utilization of Sb-graphite composite and PVDF binder as flame-retardant and performance-improved electrode for safer lithium-ion batteries. J. Alloys Compd. 2024, 1004, 175871. [Google Scholar] [CrossRef]
- Liu, S.; Ye, H.; Zhou, Y.; He, J.; Jiang, Z.; Zhao, J.; Huang, X. Study on flame-retardant mechanism of polycarbonate containing sulfonate-silsesquioxane-fluoro retardants by TGA and FTIR. Polym. Degrad. Stab. 2006, 91, 1808–1814. [Google Scholar] [CrossRef]
- Malucelli, G. Flame-Retardant Systems Based on Chitosan and Its Derivatives: State of the Art and Perspectives. Molecules 2020, 25, 4046. [Google Scholar] [CrossRef]
- Han, C.; Cao, Y.; Zhang, S.; Bai, L.; Yang, M.; Fang, S.; Gong, H.; Tang, D.; Pan, F.; Jiang, Z.; et al. Separator with Nitrogen–Phosphorus Flame-Retardant for LiNixCoyMn1−x−yO2 Cath-ode-Based Lithium-Ion Batteries. Small 2023, 19, 2207453. [Google Scholar] [CrossRef]
- Fang, C.; Huang, K.; Zhao, J.; Tian, S.; Dou, H.; Zhang, X. Dual-filler reinforced PVDF-HFP based polymer electrolyte enabling high-safety design of lithium metal batteries. Nano Res. 2024, 17, 5251–5260. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, M.; Huang, H.; Dai, Z.; Cheng, B.; Hao, S. A phytic acid-based chelating coordination embedding structure of phosphorus–boron–nitride synergistic flame retardant to enhance durability and flame retardancy of cotton. Cellulose 2020, 27, 4817–4829. [Google Scholar] [CrossRef]
- Xia, L.; Wang, X.; Ren, T.; Luo, L.; Li, D.; Dai, J.; Xu, Y.; Yuan, C.; Zeng, B.; Dai, L. Green construction of multi-functional fire resistant epoxy resins based on boron nitride with core-shell structure. Polym. Degrad. Stab. 2022, 203, 110059. [Google Scholar] [CrossRef]
- Wang, C.; Yu, B.; Zhao, T.; Yang, F.; Chen, M.; Zhu, X.; Cai, Z.; Fu, B. Chitosan cryogels incorporated with phytic acid-modified UiO-66-NH2 for enhanced flame-retardant performance. Carbohydr. Polym. 2025, 353, 123259. [Google Scholar] [CrossRef]
- Wang, G.; Li, H.; Shao, Y.; Wu, X.; He, Q.; Niu, K. Preparation method of biogenic phytic acid and chitosan-related epoxy resin flame retardant: A review. J. Polym. Res. 2023, 30, 1–31. [Google Scholar] [CrossRef]
- Yin, L.; Gong, K.; Zhou, K.; Qian, X.; Shi, C.; Gui, Z.; Qian, L. Flame-retardant activity of ternary integrated modified boron nitride nanosheets to epoxy resin. J. Colloid Interface Sci. 2022, 608, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Min-Ji, S. Synthesis and Characterization of Phosphorus-Based Organic and Boron Nitride-Based Inorganic Flame Retardants for Polymers. Ph.D. Thesis, Graduate School of Seoul National University, Seoul, Republic of Korea, 2022. [Google Scholar]
- Zhu, W.; Hao, S.; Yang, M.; Cheng, B.; Zhang, J. A synergistic flame retardant of glycosyl cross-linking boron acid and ammonium salt of phytic acid to enhance durable flame retardancy of cotton fabrics. Cellulose 2020, 27, 9699–9710. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, J.; Jin, L.; Guan, Y.; Wang, W.; Guo, W. Influence of a novel biobased coating decorated with UiO-66/BN in persistent flame-retardant hybrids on the fire safety and thermal degradation of epoxy resin. Front. Chem. Sci. Eng. 2025, 19, 43. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sadiku, E.R.; Ray, S.S.; Mochane, M.J.; Matabola, K.P.; Motloung, M. Flame retardancy efficacy of phytic acid: An overview. J. Appl. Polym. Sci. 2022, 139, e52495. [Google Scholar] [CrossRef]
- Gradys, A.; Sajkiewicz, P. Determination of the melting enthalpy of β phase of poly(vinylidene fluoride). e-Polymers 2013, 13, 019. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, W.; Zhao, X.; Li, Z.; Li, X.; Zhang, Z. Fabrication of Bismuth Oxychloride Nanosheets Decorated with Chitosan and Phytic Acid for Improvement of Flexible Poly(vinyl chloride) Flame Retardancy. Fibers Polym. 2021, 22, 2656–2663. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Liu, Y.; Niu, K. Synthesis of nitrogen and phosphorus-doped chitosan derivatives for enhanced flame retardancy, smoke suppression, and mechanical properties in epoxy resin composites. Int. J. Biol. Macromol. 2024, 283, 137889. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-H.; Jing, C.-Y.; Luo, H.; Shi, H.; Wang, D.-Y. A flame retardant coating based on amino acid and phytic acid for cotton fabrics. Polym. Degrad. Stab. 2024, 230, 111069. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, Y.; Wang, J.; Zhang, T.; Jiang, S.; Han, X. Functionalization of chitosan and its application in flame retardants: A review. Int. J. Biol. Macromol. 2025, 295, 139615. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mu, X.; Cai, W.; Song, L.; Ma, C.; Hu, Y. Constructing phosphorus, nitrogen, silicon-co-contained boron nitride nanosheets to reinforce flame retardant properties of unsaturated polyester resin. Compos. Part A Appl. Sci. Manuf. 2018, 109, 546–554. [Google Scholar] [CrossRef]
- Qiu, S.; Hou, Y.; Xing, W.; Ma, C.; Zhou, X.; Liu, L.; Kan, Y.; Yuen, R.K.; Hu, Y. Self-assembled supermolecular aggregate supported on boron nitride nanoplatelets for flame retardant and friction application. Chem. Eng. J. 2018, 349, 223–234. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Cheng, Y.; Li, M.; Cui, Y.; Li, Z. Recent advances in biomass phytic acid flame retardants. Polym. Test. 2023, 124, 108100. [Google Scholar] [CrossRef]
- Barbalini, M.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Phytic Acid and Biochar: An Effective All Bio-Sourced Flame Retardant Formulation for Cotton Fabrics. Polymers 2020, 12, 811. [Google Scholar] [CrossRef]






| Sample | T5% (°C) | Tmax (°C) | Residual Mass (%) |
|---|---|---|---|
| BN | / | / | 97.2 |
| BN@PA-CS | 75.3 ± 0.6 | 257.5 ± 0.4 | 69.1 |
| PA-CS | 66.3 ± 0.5 | 298.7 ± 0.5 | 28.8 |
| Sample | T5% (°C) | Tmax (°C) | Rate of Tmax (wt.% min−1) | Yc (wt%) |
|---|---|---|---|---|
| PVDF | 421.5 ± 0.35 | 454.6 ± 0.25 | −13.24 ± 0.50 | 29.86 ± 0.50 |
| PVDF/BN@PA-CS 5 | 421.8 ± 0.45 | 438.5 ± 0.55 | −40.04 ± 0.50 | 33.34 ± 0.35 |
| PVDF/BN@PA-CS 10 | 385.5 ± 0.50 | 432.2 ± 0.50 | −8.72 ± 0.55 | 36.49 ± 0.45 |
| PVDF/BN@PA-CS 20 | 414.5 ± 0.50 | 434.5 ± 0.45 | −11.75 ± 0.45 | 39.10 ± 0.55 |
| Sample | Melting Point/°C | Melting Enthalpy/(J·g−1) | Crystallinity (%) |
|---|---|---|---|
| PVDF | 169.86 ± 0.50 | 34.89 ± 0.54 | 37.1 ± 0.65 |
| PVDF/BN@PA-CS 5 | 168.71 ± 0.54 | 32.00 ± 0.55 | 34.0 ± 0.55 |
| PVDF/BN@PA-CS 10 | 168.93 ± 0.55 | 34.97 ± 0.58 | 37.2 ± 0.55 |
| PVDF/BN@PA-CS 20 | 167.44 ± 0.54 | 35.14 ± 0.45 | 37.4 ± 0.58 |
| Samples |
Breaking
Stress (MPa) |
Breaking
Strain (%) |
Elastic
Modulus (GPa) |
|---|---|---|---|
| PVDF | 25.27 ± 0.65 | 2.78 ± 0.55 | 2.47 ± 0.45 |
| PVDF/BN@PA-CS 5 | 26.74 ± 0.55 | 3.45 ± 0.65 | 2.61 ± 0.65 |
| PVDF/BN@PA-CS 10 | 27.98 ± 0.45 | 4.86 ± 0.55 | 2.67 ± 0.65 |
| PVDF/BN@PA-CS 15 | 29.45 ± 0.55 | 7.01 ± 0.55 | 2.59 ± 0.58 |
| PVDF/BN@PA-CS 20 | 34.13 ± 0.56 | 5.56 ± 0.50 | 2.48 ± 0.57 |
| Sample | PVDF
Content (wt%) | BN@PA-CS Content (wt%) | UL-94 Rating | LOI (%) |
|---|---|---|---|---|
| PVDF | 100 | 0 | NR | 32.6 |
| PVDF/BN@PA-CS 5 | 95 | 5 | V-2 | 34.1 |
| PVDF/BN@PA-CS 10 | 90 | 10 | V-0 | 36.8 |
| PVDF/BN@PA-CS 15 | 85 | 15 | V-0 | 37.2 |
| PVDF/BN@PA-CS 20 | 80 | 20 | V-0 | 38.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, S.; Wang, P.; Wu, S.; Hu, J.; Zhang, J.; Li, L.; Huang, B.; Huang, W.; Guan, X.; Wang, K.; et al. Enhanced Flame-Retardant Properties of PVDF Using a Multiphase Synergistic Approach with Phytate-Chitosan-Modified Boron Nitride. Polymers 2025, 17, 2904. https://doi.org/10.3390/polym17212904
Ming S, Wang P, Wu S, Hu J, Zhang J, Li L, Huang B, Huang W, Guan X, Wang K, et al. Enhanced Flame-Retardant Properties of PVDF Using a Multiphase Synergistic Approach with Phytate-Chitosan-Modified Boron Nitride. Polymers. 2025; 17(21):2904. https://doi.org/10.3390/polym17212904
Chicago/Turabian StyleMing, Shiyi, Piao Wang, Shaoyuan Wu, Jinghan Hu, Jie Zhang, Lianlian Li, Bingyue Huang, Weijiang Huang, Xingyu Guan, Kui Wang, and et al. 2025. "Enhanced Flame-Retardant Properties of PVDF Using a Multiphase Synergistic Approach with Phytate-Chitosan-Modified Boron Nitride" Polymers 17, no. 21: 2904. https://doi.org/10.3390/polym17212904
APA StyleMing, S., Wang, P., Wu, S., Hu, J., Zhang, J., Li, L., Huang, B., Huang, W., Guan, X., Wang, K., & Yan, W. (2025). Enhanced Flame-Retardant Properties of PVDF Using a Multiphase Synergistic Approach with Phytate-Chitosan-Modified Boron Nitride. Polymers, 17(21), 2904. https://doi.org/10.3390/polym17212904





