3.1. Particle Size and Zeta Potential of the Latexes
Latex stability is a key factor in emulsion polymerization, particularly when reactive monomers such as GMA and VTES are involved. The epoxy rings (oxirane) of GMA and the alkoxysilane groups of VTES are highly susceptible to side reactions such as hydrolysis, condensation, or nucleophilic ring-opening, which can trigger premature crosslinking, gel formation, and coagulation during synthesis. To suppress these undesired reactions and ensure the formation of stable polymer dispersions, the polymerization was carefully controlled. All reactions were carried out in a buffered medium to maintain a slightly alkaline medium, which stabilizes the ionic environment and reduces the risk of uncontrolled hydrolysis. A slow monomer feed strategy and moderate reaction temperatures were employed to further minimize localized concentration gradients and thermal hotspots that could promote gelation. Under these optimized conditions, the final solid content of both latexes was maintained at approximately 25%, allowing efficient polymerization without compromising colloidal stability.
Using this approach, two types of reactive acrylic copolymer dispersions were successfully synthesized: G0, containing GMA as the epoxy-functional monomer, and V0, incorporating VTES as the silane-functional monomer. Both dispersions were obtained as stable, coagulum-free latexes with high polymerization yields exceeding 98%, demonstrating the effectiveness of the controlled process in preventing premature crosslinking.
The particle size distributions of the dispersions were characterized by dynamic light scattering (DLS), and the results are summarized in
Figure 1 and
Table 4. The G0 dispersion exhibited a Z-average particle diameter of 82 nm with a polydispersity index (PDI) of 0.145, while the V0 dispersion showed a smaller average particle size of 53 nm with a PDI of 0.088. These data indicate that both systems consist of fine particles with narrow and relatively uniform size distributions, confirming the successful preparation of stable, nanoscale reactive latexes suitable for subsequent PA incorporation and leather finishing applications.
The markedly smaller mean particle size measured for the V0 (VTES-containing) dispersion (53 nm, PDI 0.088) compared with G0 (GMA-containing, 82 nm, PDI 0.145) can be ascribed to differences in monomer chemistry that govern nucleation and interfacial behavior during seeded emulsion polymerization. The higher hydrophobicity and lower aqueous solubility of VTES favor the generation of a greater number of primary nuclei, producing a larger particle population with smaller diameters. Moreover, the possible partial hydrolysis of alkoxysilane groups during polymerization generates silanol moieties that increase hydrophilicity and facilitate hydrogen bonding with water molecules. This phenomenon enhances colloidal stabilization by reducing interfacial tension and promoting nucleation over particle growth, ultimately yielding smaller and more narrowly distributed latex particles. Similar effects of silane-induced stabilization have been reported for vinyl silane and organosilane-modified acrylic systems [
36,
37,
38].
Zeta potential analysis was performed to further assess the electrostatic stability and effect of phytic acid addition on the colloidal stability of the reactive acrylic dispersions. The measured zeta potential values are presented in
Table 5. For the GMA-containing series, the zeta potential decreased progressively from –51.0 mV (G0) to –27.4 mV (G3) with increasing phytic acid concentration. A similar decreasing trend was observed for the VTES-based series, where the potential dropped from –65.0 mV (V0) to –35.4 mV (V2).
The reduction in surface charge magnitude indicates partial neutralization of the negatively charged latex particles by the acidic phosphate groups of phytic acid. This charge screening effect reduces the electrostatic repulsion among particles, thereby decreasing colloidal stability at higher PA loadings. The results are consistent with the observed sedimentation tendency in formulations containing ≥10 wt% PA, particularly in the VTES system, where silanol condensation and reduced surface charge may jointly contribute to instability.
Overall, the zeta potential data confirm that the latexes exhibit sufficient electrostatic stabilization (|ζ| > 30 mV) up to 10 wt% phytic acid addition, beyond which the dispersion stability begins to decline significantly.
3.2. Structural Analyses of Reactive Copolymers and Crosslinked Films
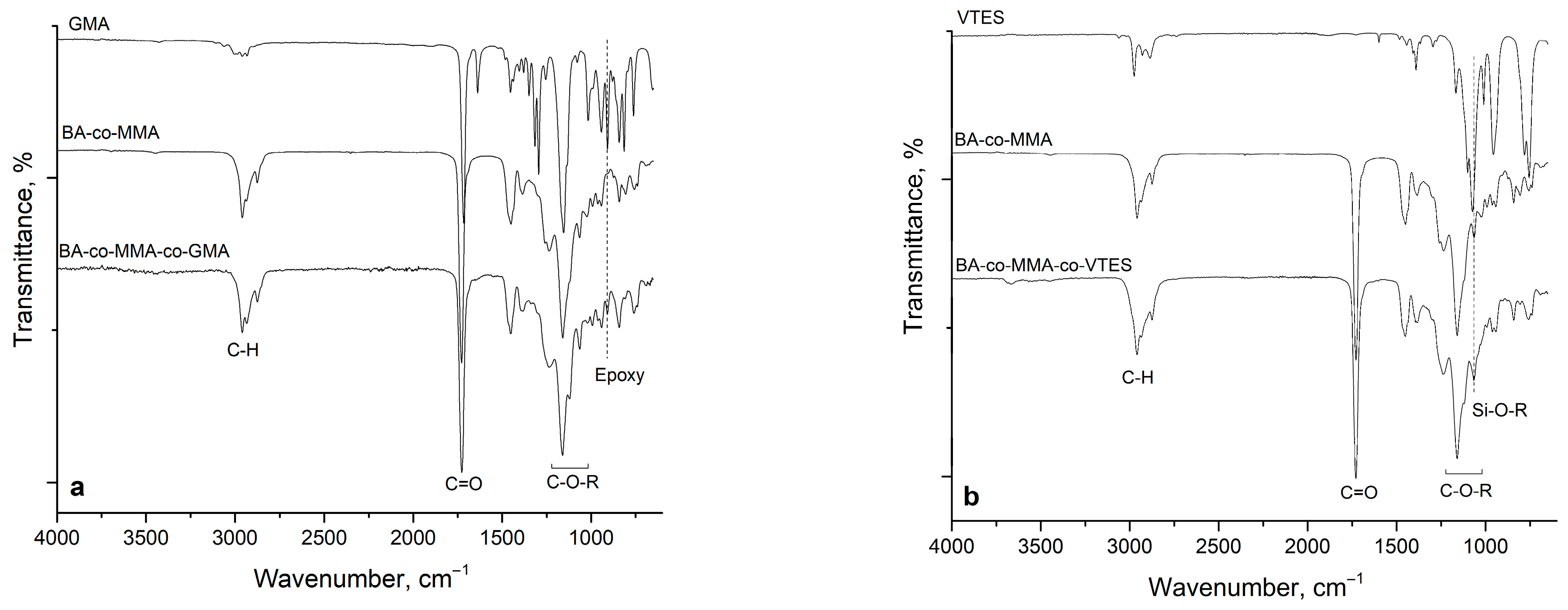
The FTIR spectra of the copolymers and their corresponding reactive monomers are presented in
Figure 2. To verify the incorporation of the reactive monomers into the copolymer structure, a reference BA-co-MMA copolymer was synthesized under identical conditions without any reactive monomer. Its spectrum was recorded for comparison alongside those of the functional monomers.
The spectra exhibit the characteristic absorption bands of poly(acrylate) structures, including the asymmetric and symmetric C–H stretching vibrations of CH
2 and CH
3 groups at 2959, 2934, and 2875 cm
−1; the ester carbonyl (C=O) stretching at 1728 cm
−1; and the CH
2/CH
3 deformation bands at 1452 and 1385 cm
−1 [
39]. The C–O–C stretching vibrations of the ester moiety appear at 1160 and 1066 cm
−1, confirming the successful formation of the poly(acrylate) backbone [
40]. Importantly, no absorption was detected in the 1608–1634 cm
−1 region corresponding to the vinyl C=C bonds of unreacted acrylate monomers, indicating nearly complete double-bond conversion and effective emulsion polymerization.
Distinct features attributable to the reactive comonomers further validate their incorporation. The characteristic oxirane-ring absorption of GMA is observed at 908 cm
−1, which also appears in the spectrum of the epoxy-functional copolymer (G0), demonstrating that the epoxide groups remain largely intact and available for subsequent crosslinking [
41]. Similarly, the strong Si–O–R stretching band of VTES, centered at ≈1073 cm
−1, is retained in the V0 copolymer, contributing to an increased intensity of the adjacent C–O stretching band near 1066 cm
−1 [
42]. These spectral signatures confirm the intended copolymer compositions and the preservation of the targeted reactive functionalities in both latex systems.
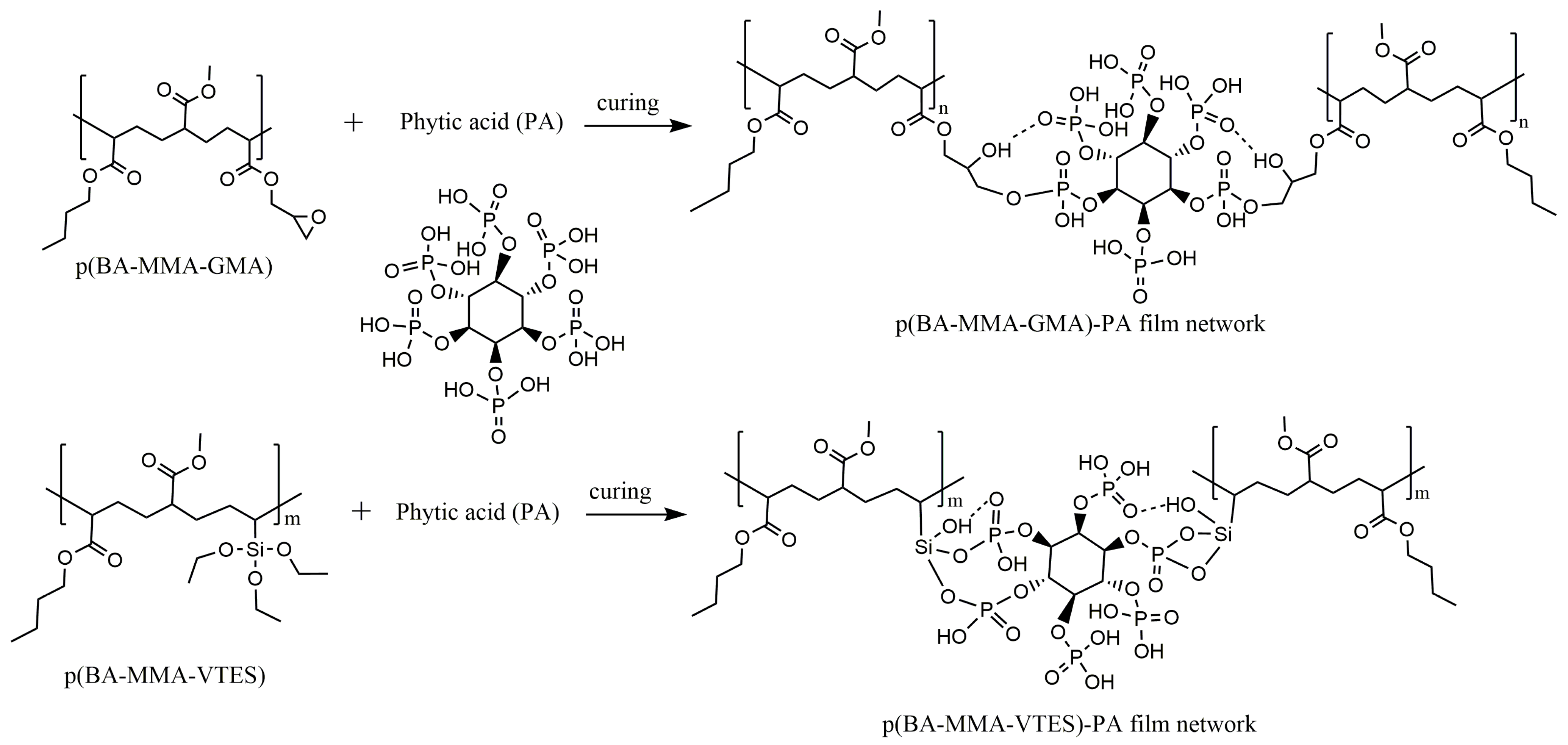
The multifunctional phosphate groups of phytic acid are capable of forming both covalent and non-covalent interactions with the reactive functionalities present in the acrylic copolymers.
Scheme 1 explains the crosslinking mechanism of phytic acid with functional polymer moieties. The phosphate oxygens can act as nucleophiles, attacking the electrophilic carbon of the epoxy ring in GMA units, leading to ring opening and formation of stable P–O–C bonds. Simultaneously, hydrogen bonding between the phosphate and hydroxyl groups can contribute to secondary network reinforcement. In VTES-containing systems, partial hydrolysis of alkoxy groups generates silanol (Si–OH) sites that can undergo condensation with phosphate groups of PA, yielding Si–O–P or Si–O–C linkages. These interactions are expected to enhance crosslinking density and interfacial adhesion, thereby improving the mechanical robustness, rub fastness, and thermal stability of the resulting coating films.
To further evaluate the post-crosslinking behavior of the reactive functionalities, the reactive latexes were mixed with phytic acid with the ratios given at
Table 3. The mixtures were then dried at room temperature for 3 days and FTIR analyses were conducted on films containing GMA and VTES, both with and without phytic acid, before and after thermal curing at 100 °C for 30 min.
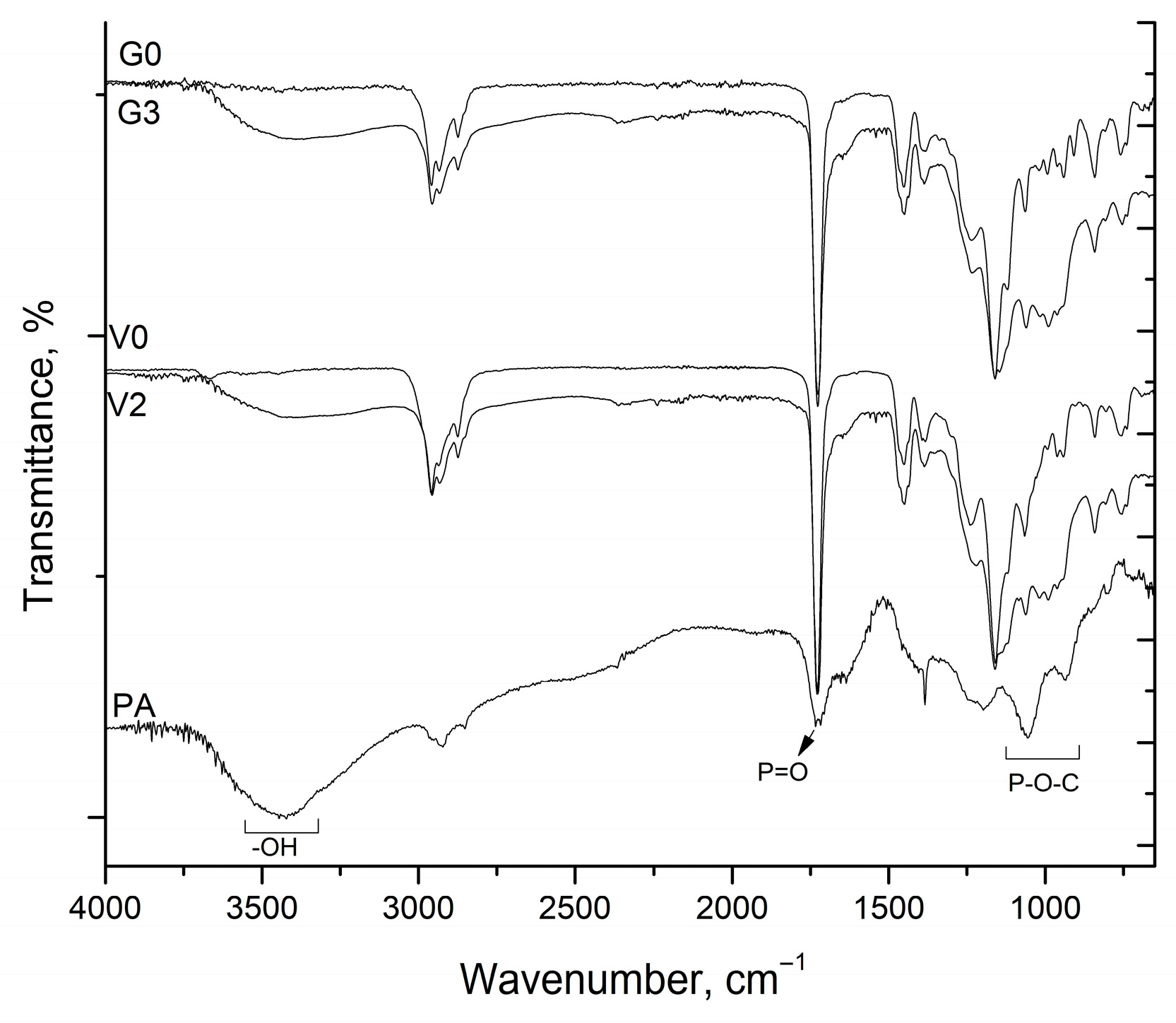
The FTIR spectra of pure phytic acid (PA), unmodified copolymers (G0 and V0), and representative PA-modified copolymers (G3 and V2) are shown in
Figure 3. The spectrum of PA exhibited characteristic bands at 3430, 1727, 1193, 1055, and 939 cm
−1, corresponding to O–H stretching, P=O stretching, P–O–C asym, P-O-C sym stretching and P–O-H stretching vibrations of phosphate groups, respectively. Upon incorporation of PA into the reactive acrylic dispersions, the spectra of G3 and V2 displayed increased band intensities in the regions 3450 cm
−1 due to the O-H stretching, 1200–900 cm
−1, particularly around 1055 cm
−1, which can be attributed to the overlapping of P–O–C and P–O–H vibrations from PA with the C–O stretching of the acrylic backbone. These intensity enhancements confirm the successful incorporation of PA within the polymer matrices.
Figure 4 represents IR spectra G-series reactive copolymer-PA blends before and after curing process. For the unmodified binder (G0), the FTIR spectrum exhibits a distinct absorption band at 908 cm
−1, attributed to the epoxy ring stretching of glycidyl methacrylate units. This peak remains visible even after the curing process at 100 °C for 30 min, indicating that thermal treatment alone is not sufficient to induce complete epoxy ring opening in the absence of reactive species. In contrast, the PA-incorporated systems (G1–G3) show a significant reduction or disappearance of the epoxy signal both before and after curing. This observation suggests that phytic acid facilitates partial ring opening reactions, likely through nucleophilic attack of phosphate oxygen atoms on the epoxy groups, leading to the formation of phosphate–ether linkages even at room temperature. Upon curing, a further consolidation of these interactions occurs, as evidenced by increased band intensity in the P–O and C–O stretching region (1050–950 cm
−1). These spectral changes support the presence of chemical crosslinking between phytic acid and epoxy functionalities, enhancing network formation and contributing to improved film performance.
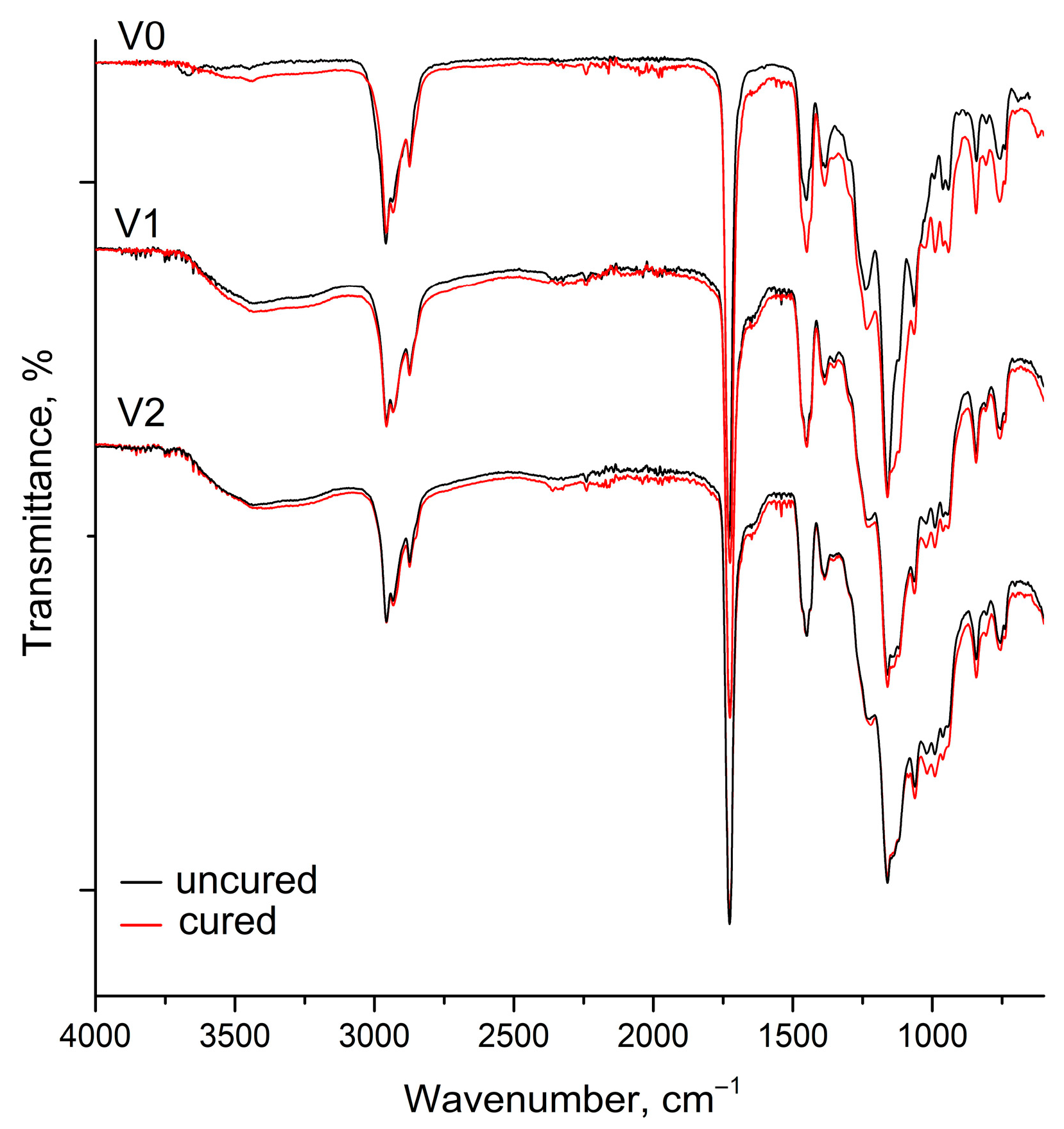
The IR spectra of V-series reactive copolymer-PA blends before and after curing process is shown in
Figure 5. In the unmodified VTES-containing binder (V0), curing results in a general increase in the absorption intensity within the 1450–750 cm
−1 region, particularly around 1100 cm
−1, corresponding to Si–O–Si stretching vibrations. This indicates that alkoxysilane hydrolysis and condensation reactions are activated during the curing process, forming a siloxane network that reinforces the polymer matrix. In PA-modified samples (V1 and V2), this region becomes broader and more intense, suggesting the coexistence of Si–O–Si, Si–O–C, and P–O–Si vibrations. The observed spectral evolution implies that phytic acid participates in silanol–phosphate coordination or condensation reactions, resulting in a denser and more crosslinked hybrid network. Consequently, these chemical interactions are expected to enhance both the structural cohesion and the environmental resistance of the resulting coating films.
3.4. Water and Solvent Absorption Behavior
The water absorption and acetone swelling behavior of the films are summarized in
Table 6 and
Table 7. The unmodified copolymer films (G0 and V0) exhibited high water uptake, reaching 35.6 wt% and 41.0 wt%, respectively, after 24 h of immersion. Incorporation of phytic acid (PA) significantly influenced the hydrophilicity and network density of the coatings. At moderate PA loadings (G1–G2 and V1), the overall water absorption decreased, particularly at short immersion times, indicating that PA contributed to the formation of denser polymer networks through covalent/hydrogen bonding or ionic interactions between phosphate groups and reactive functional sites (epoxy or silanol). This denser structure limited the diffusion of water molecules within the film matrix.
At higher PA concentrations, however, the opposite trend was observed. The G3 and V2 samples displayed a marked increase in water uptake (38.1 wt% and 88.3 wt% after 24 h, respectively), suggesting that excessive PA addition introduces unbound hydrophilic phosphate groups and disrupts colloidal uniformity, thereby facilitating water penetration. The strong hydrophilicity of PA and its partial incompatibility at high contents may lead to phase heterogeneity and enhanced water sorption.
Acetone swelling tests further supported these findings. The control samples (G0 and V0) were completely disintegrated in acetone, indicating poor solvent resistance due to the absence of sufficient crosslinking. Upon PA incorporation, all films exhibited a considerable reduction in acetone-induced dissolution, instead showing measurable swelling degrees that gradually reached equilibrium after 6 h. Among the G-series, G1 and G2 displayed moderate swelling ratios (~277–291 wt%), while G3 reached over 320 wt%, consistent with the excessive hydrophilic character at high PA loadings. The V-series showed a similar pattern, with V1 exhibiting the lowest swelling degree (≈239 wt%) and V2 the highest (≈279 wt%).
Overall, these results confirm that moderate incorporation of phytic acid improves film compactness and solvent resistance through controlled interfacial crosslinking, while excessive amounts lead to over-hydrophilization and structural loosening of the polymer network.
3.5. Finishing Application Results
3.5.1. Visual Assessment of Finished Leathers
The synthesized reactive acrylic polymer dispersions were applied as finishing coatings onto shoe upper leathers. Each polymer was used individually as the sole binder in simplified finishing formulations to enable a clear assessment of its independent coating performance.
The finishing mixtures were prepared by blending the reactive binders with pigment, wax, isopropyl alcohol (IPA), and aqueous PA solutions at various concentrations. During formulation, it was observed that a high PA content (15 wt% based on polymer solids) caused precipitation in the VTES-containing dispersion; therefore, only 5 and 10 wt% PA levels (samples V2 and V3) were employed for this system. The instability observed in VTES-based dispersions at phytic acid (PA) concentrations above 10 wt% can be attributed to pH-induced hydrolysis and condensation of the silane groups. The introduction of PA lowers the system pH, which accelerates the hydrolysis of the triethoxysilane moieties to silanols. These reactive silanol groups may undergo uncontrolled condensation (Si–O–Si crosslinking) both within and between latex particles, leading to partial aggregation and loss of colloidal stability. Additionally, the high ionic strength introduced by the phosphate groups of PA may disrupt the electrostatic stabilization of the polymer particles, further promoting coagulation. In contrast, the GMA-based dispersions showed no such instability and were formulated at all PA levels. All mixtures were filtered prior to application and sprayed onto the leathers using a hand-held spray gun.
After application, the coatings produced a uniform color distribution with a soft, smooth hand and an attractive medium gloss, indicating good film formation on the leather surface. To ensure completion of any latent reactions, the finished leathers were rested for a short period, heat-treated at 100 °C for 30 min, and then reconditioned under standard testing conditions before being subjected to physical performance evaluations.
3.5.2. Color Fastness to Rubbing
Color fastness to rubbing is a critical parameter for evaluating the performance of leather finishes, as it simulates the potential color transfer that may occur when finished leathers come into contact with other surfaces (e.g., cotton or synthetic fabrics, felt) during use.
In this study, the rub fastness of the coated leathers was assessed according to international standards using both dry and wet rubbing tests. In each test, a white felt pad was rubbed back and forth across the finished leather surface under a defined load and number of cycles. After testing, the felt was examined for color transfer, while the leather surface itself was inspected not only for color change but also for possible physical defects such as gloss loss, surface dulling, or film peeling. All color changes were rated using the ISO Grey Scale under a daylight-simulated light cabinet. The results are summarized in
Table 8.
In the dry rubbing test, all samples withstood up to 2000 cycles. The epoxy-containing control sample (G0) received a rating of 3 (leather) and 3/4 (felt) after 2000 dry cycles. The incorporation of PA further enhanced dry rub resistance, and a similar trend was observed for the VTES-based coatings.
Performance differences became more pronounced in the wet rubbing test (
Figure 7). At 50 wet cycles, all samples exhibited good resistance. After 250 wet cycles, the G0 sample showed ratings of 3 (leather) and 2/3 (felt), which increased markedly with the addition of PA up to 10 wt%. However, further increases in PA content led to a gradual decline in wet rub fastness. A comparable behavior was observed for the VTES-based finishes. For example, the fastness level of V0 improved from 2/3 (leather) and 3 (felt) to 4 (leather) and 3/4 (felt) with the addition of 5 wt% PA, but higher PA loadings resulted in reduced ratings, dropping as low as 1/2.
Overall, the incorporation of 5 wt% PA provided the most balanced improvement in rub resistance for both systems. Additionally, epoxy-based coatings consistently exhibited higher rub fastness values than the silane-containing finishes under both dry and wet conditions. It should be noted that the coatings exhibited superior resistance under both dry and wet rubbing conditions—enduring up to 2000 and 250 cycles, respectively, which exceed the standard test cycles typically used for footwear leathers (500 dry, 50 wet). This superior rub fastness is likely the result of a synergistic effect between covalent and secondary interactions. During curing, phosphate groups of PA may react with epoxy and silanol moieties, yielding C–O–P or Si–O–P linkages, while simultaneously forming hydrogen bonds and ionic associations with polar sites naturally present in the collagen matrix and/or with the chromium complex of tanned leather. These combined effects improve interfacial adhesion and film cohesion, leading to enhanced mechanical durability. However, at higher PA levels, the excessive hydrophilicity and potential plasticizing effect of unreacted PA likely compromise network compactness and reduce film cohesion, leading to slightly decreased wet rub fastness.
3.5.3. Flex Resistance of Finished Leathers
The results of the flexometer (bending) test are summarized in
Table 9. Flex testing is a key performance evaluation for shoe upper leathers, as it simulates the repeated bending and stretching that occurs during walking and assesses the resistance of the finish layer to cracking or other mechanical damage. Each sample was first subjected to 50,000 flex cycles, followed by an extended test up to 100,000 cycles.
Across all formulations, no peeling, blistering, or visible color change was observed on the finished surfaces even after 100,000 cycles. Minor surface wrinkling was noted in some samples during flexing, and this effect became slightly more pronounced with increasing PA content. The flexometer test provides a standardized assessment of visible defects and failures in the leather finish through qualitative evaluation of surface integrity. However, developing instrumental approaches for quantifying flex damage (e.g., optical or microscopic surface analysis) could be an interesting direction for future research. Nevertheless, all coated leathers met the flex performance requirements, demonstrating that both the epoxy- and silane-based reactive binders provided adequate flexibility and durability for demanding footwear applications.
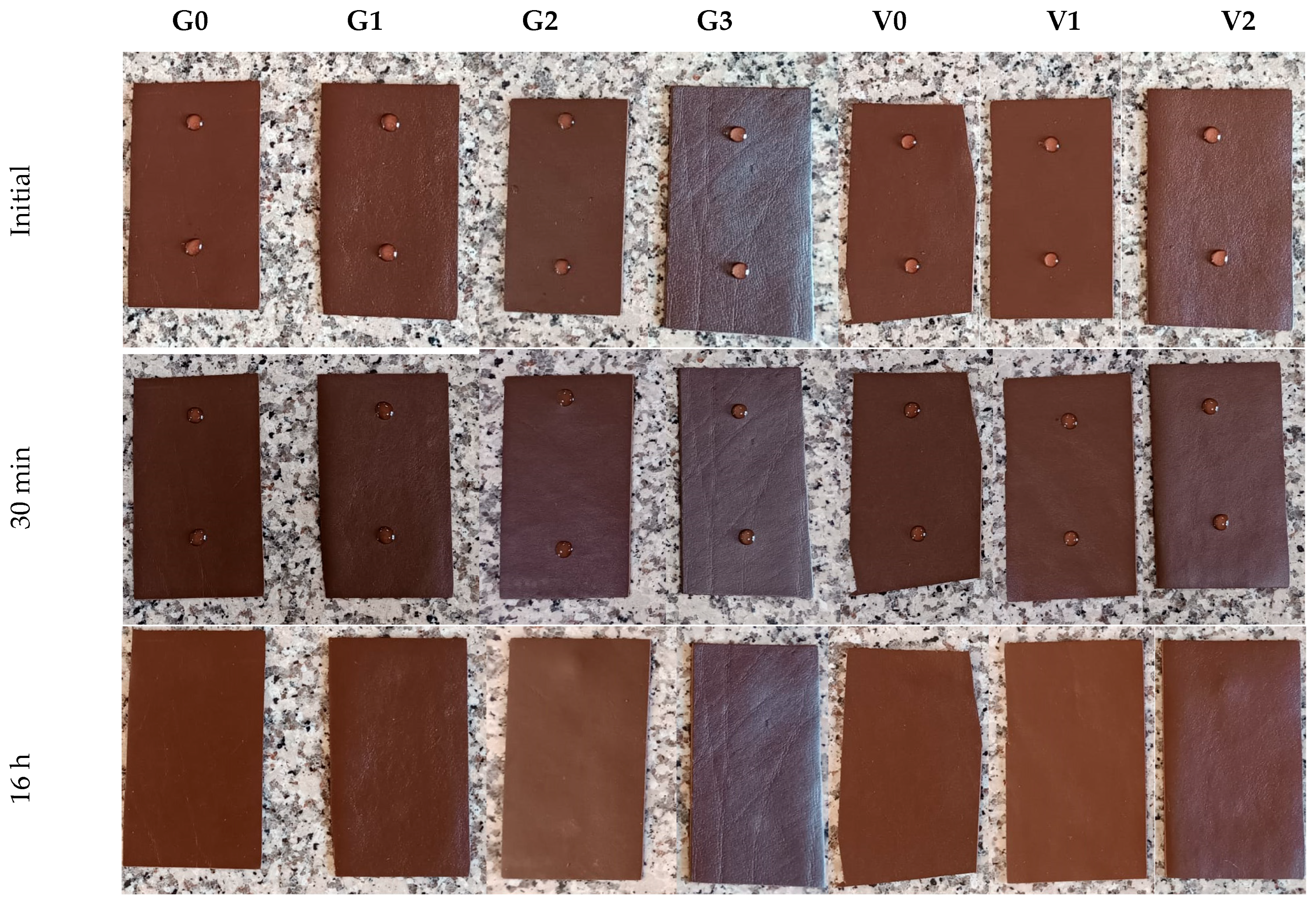
3.5.4. The Water Spotting Test Results
The water spot test evaluates a leather finish’s ability to withstand moisture-related effects such as staining, surface marking, blistering, or color alteration. To simulate prolonged contact with water, two drops of distilled water were placed on the finished leather surface. After 30 min, excess water from one drop was gently removed with filter paper and the surface was inspected for any visible changes. The second drop was left to evaporate overnight, and color variation was assessed using the standard grey scale after 16 h of exposure.
The results of the water drop (water spot) test after 30 min and 16 h are summarized in
Table 10, and representative images taken after 30 min are shown in
Figure 8. Even after the first 30 min of exposure, the water droplets remained on the leather surface without being absorbed, and removal of the initial drop caused no visible physical damage or noticeable surface change. This behavior demonstrates the high water repellency of the finish films across all samples.
After 16 h, no significant color change was detected on any of the finished leathers; only a very light watermark was observed on samples containing PA. Overall, the finished leathers exhibited excellent resistance to water spotting, confirming the strong protective barrier provided by the reactive polymer coatings.
3.5.5. Heat Resistance of Finished Leathers
The heat resistance of the finished leathers was evaluated by applying heated chrome plates at 150, 200, 250, and 300 °C under a pressure of 0.21 kg/cm
2 for 5 s. Following exposure, the samples were examined under a light cabinet using the grey scale to assess color changes and other surface effects. The evaluation results are summarized in
Table 11.
As expected, increasing temperature led to gradual color changes on the leather surfaces. For the GMA-based coatings, the incorporation of PA provided a noticeable improvement in thermal stability, particularly in the formulation containing 10 wt% PA (G2), which showed the best resistance at elevated temperatures. In contrast, PA addition did not produce a significant effect on the thermal resistance of the VTES-based coatings.
Overall, all samples exhibited good heat resistance, with no severe film failure or finish delamination observed even at 300 °C. At this highest temperature, only slight shrinkage of the leather substrate was detected, confirming that the coatings retained their structural integrity under extreme thermal stress.