Abstract
The development of layer-by-layer polyelectrolyte microcapsules (PMCs) with defined buffer capacity (BC) is a key task for creating stable systems in biomedicine and materials science. Manganese carbonate (MnCO3), which shares properties with CaCO3 and the ability to form hollow structures, represents a promising alternative. However, its interaction with polyelectrolytes and its influence on BC remain insufficiently studied. This research focuses on determining the BC of PMCs templated on MnCO3 cores under varying ionic strength (0.22–3 M NaCl) and temperature (60–90 °C), as well as comparing the results with PMCs templated on CaCO3 and PS cores. It was found that MnCO3-based PMCs (PMCMn) exhibit hybrid behavior between CaCO3- and PS-based PMCs: the BC dynamics of PMCMn and CaCO3-based PMCs (PMCCa) in water are identical. At different ionic strength at pH < 5, the BC of PMCMn and PS-based PMCs (PMCPS) remains unchanged, while at pH > 8.5, the BC of PMCMn increases only at 3 M NaCl. The BC of PMCMn remains stable under heating, whereas the BC of PMCCa and PMCPS decreases. These results confirm that the choice of core material dictates PMC functionality, paving the way for adaptive systems in biosensing and controlled drug delivery.
1. Introduction
The polyelectrolyte microcapsules (PMCs) have been known as the multifunctional nanostructures fabricated via layer-by-layer (LbL) deposition of oppositely charged poly-electrolytes onto the spherical sacrificial templates, followed by core dissolution [1]. Those microcapsules have had some broad applications in medicine, food technology, and smart-materials due to their tunable physicochemical properties [1,2,3,4,5]. A critical parameter governing PMC functionality has been its buffer capacity (BC), which reflects the system’s ability to maintain the stable hydrogen ion concentrations in the solution [6,7]. That property has been essential for preserving the activity of the encapsulated molecules, such as enzymes [8,9,10], fluorescent dyes [11,12], and catalytic nanoparticles [13,14], particularly under fluctuating pH [15], ionic strength, and temperature [16,17,18,19].
Previous studies have demonstrated that BC significantly depends on the template/core type [20]. PMCs templated on polystyrene (PS) and calcium carbonate (CaCO3) exhibit dis-tinct BC profiles in response to ionic strength and temperature changes. CaCO3-based PMCs (PMCCa) display BC across a wide pH range (5.5–9), whereas PS-based PMCs (PMCPS) ex-hibit BC changes only at pH > 8. Increasing NaCl concentration gradually enhances BC in PMCPS up to 3 M, while PMCCa show maximal BC enhancement at 1 M NaCl. These differences are attributed to shell morphology: PS templates form dense polyelectrolyte layers with abundant ion pairs, whereas CaCO3-based PMCs possess a porous structure with channel-like pores [21,22,23].
However, differences in the buffer capacity (BC) may also arise from the chemical in-teractions between the template material and the polyelectrolyte shell of the polyelectrolyte microcapsules (PMCs) [20]. Therefore, this study proposes manganese carbonate (MnCO3) as an alternative template (core) due to its similarity to CaCO3 as an insoluble carbonate of a divalent cation, sharing comparable chemical properties. Additionally, MnCO3 enables the formation of hollow PMCs, akin to those templated on polystyrene (PS) cores [24,25]. Despite similarities to these two PMC types, capsules templated on MnCO3 (PMCMn) may exhibit a unique BC modulation pattern. The use of MnCO3 cores significantly alters the assembly of polyelectrolyte layers, as evidenced by shifts in zeta potential during layer-by-layer deposi-tion, and modifies the density of proton-active amine groups in the poly(allylamine hydro-chloride) (PAH)/poly(styrene sulfonate) (PSS) shell [26]. Unlike Ca2+ ions, Mn2+ ions released during core dissolution can form stable complexes with PAH amine groups in the PMC shell [27]. This interaction affects the protonation/deprotonation equilibrium of functional groups, potentially modifying the buffering properties of the capsules. For instance, in acidic envi-ronments (pH < 5), Mn2+ stabilizes the positive charge of PAH via electrostatic binding, en-hancing BC, whereas under alkaline conditions (pH > 8), deprotonated amine groups form a more flexible network capable of reversible structural rearrangements [26]. Morphologically, MnCO3-templated PMCs differ from other capsules Figure 1.
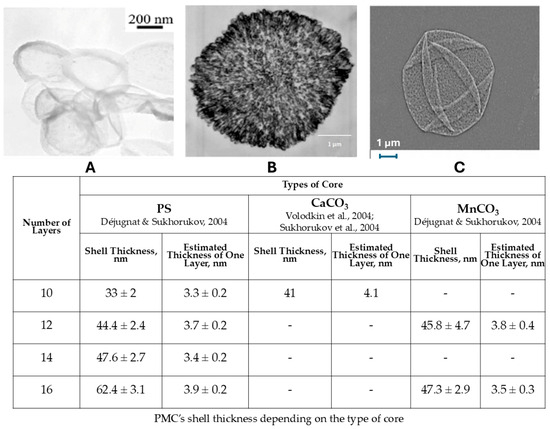
Figure 1.
Electron microscopy image of polyelectrolyte microcapsules, formed on polystyrene [28] (A), CaCO3 [29] (B) and MnCO3 [30] (C). PMC’s shell thickness depending on the type of core (adapted from [23]).
As can be seen from Figure 1, morphologically, MnCO3-templated PMCs differ from other capsules, it has a linear shell thickness growth (~3.8 nm/layer). Furthermore, while PMCs templated on MnCO3 cores, like those formed on polystyrene cores, are hollow inside, their shell thickness differs significantly. In contrast, capsules templated on CaCO3 cores have an internal structure filled with a polyelectrolyte complex. Also, according to our previous review article, there was a sharp size increase in alkaline media (pH > 12.5) [23].
The aim of this study is to evaluate the buffer capacity of polyelectrolyte microcapsules synthesized on MnCO3 cores using acid-base titration and differential analysis (Equation (1)) and to analyze the effects of ionic strength and temperature on the buffer capacity. The research hypothesis posits that MnCO3-templated PMCs will combine characteristics of both CaCO3- and PS-based microcapsules. While prior studies have characterized BC in PMCs templated on CaCO3 [6] and PS [20], the role of MnCO3 cores remains unexplored. This work addresses this gap by systematically evaluating BC in MnCO3-templated PMCs under variable ionic strength and temperature, revealing hybrid behavior not reported elsewhere. The findings will advance the understanding of the core (template) role in determining the functional properties of PMCs and enable the optimization of their applications in dynamic environments, such as drug delivery or biosensors.
2. Materials and Methods
2.1. Materials
Sodium polystyrene sulfonate (PSS) and polyallylamine hydrochloride (PAH) (MW = 70 kDa, residual monomer < 10%, Sigma-Aldrich, St. Louis, MO, USA, Cat. # 243051 and 283223) served as the polyelectrolytes. Manganese(II) chloride tetrahydrate (MnCl2·4H2O, ≥99.9%, Reakhim, Moscow, Russia), sodium carbonate (Na2CO3, anhydrous, ≥99.9%, Reakhim), sodium chloride (NaCl, ≥99.9%, Reakhim, Moscow, Russia), sodium hydroxide (NaOH, pellets, ≥99.9%, Reakhim, Moscow, Russia), hydrochloric acid (HCl, 37%, ≥99.9%, Reakhim, Moscow, Russia), and ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA-Na2, ≥99.0%, Sigma-Aldrich, St. Louis, MO, USA, Cat. # E4884) were used as received. Ultrapure water (Milli-Q, 18.2 MΩ·cm) was used for all solutions and washings.
2.2. Synthesis of MnCO3 Microtemplates
Spherical MnCO3 microparticles were precipitated by rapid mixing of equimolar solutions [31]. Briefly, 50 mL of 0.33 M MnCl2 solution was injected within 5 s into 50 mL of vigorously stirred (800 rpm) 0.33 M Na2CO3 solution at ambient temperature (23 ± 2 °C). Stirring was halted precisely 30 s post-mixing. The suspension was aged quiescently for 60 min to allow for complete particle precipitation and crystal ripening. Rapid mixing creates high supersaturation, favoring instantaneous nucleation over growth. Quiescent aging enables Oswald ripening where smaller crystals dissolve and reprecipitate onto larger ones, reducing surface energy. This yields monodisperse spheres, which are critical for uniform capsule properties. Ripening progression was monitored periodically using bright-field optical microscopy (Olympus CX23, Olympus Corporation, Tokyo, Japan). The supernatant was then decanted, and the settled microspherulites were washed three times with ultrapure water via centrifugation–resuspension cycles (10,000 rpm, 1 min). The resulting monodisperse particles exhibited an average diameter of 4.5 ± 1.0 µm, determined statistically from micrographs.
2.3. Fabrication of Polyelectrolyte Microcapsules (PMCs)
Hollow MnCO3-templated PMCs were fabricated via sequential layer-by-layer (LbL) assembly [24,32]. Washed MnCO3 particles were alternately exposed to 2 mg/mL solutions of the polyelectrolytes (PAH or PSS) dissolved in 0.5 M NaCl. Adsorption steps (10 min per layer) were followed by triple washing with 0.5 M NaCl solution (centrifugation at 3000 rpm for 5 min) to ensure removal of unbound polymer chains. The shell architecture consisted of a base sequence (PSS/PAH)n, terminated with an outer PAH layer. Following deposition of the desired n bilayers, the sacrificial MnCO3 cores were dissolved by incubating the coated particles in 0.2 M ethylenediaminetetraacetic acid (EDTA) solution (pH 7.0) for 12 h under gentle agitation. The liberated hollow capsules were purified by three cycles of centrifugation (2500 rpm, 10 min) and resuspension in ultrapure water. The final PMC diameter was 3.0 ± 0.5 µm, verified using dynamic light scattering (Zetasizer Nano ZS, Malvern Panalytical, London, UK; measurement angle 173°, temperature 25 °C).
2.4. Thermal Stability Assessment
PMC thermal resilience was probed by incubating suspensions in tightly sealed polypropylene tubes within a calibrated thermostatic bath (Lauda Ecoline RE 112, Lauda-Königshofen, Germany). Samples were exposed to target temperatures (60 °C or 90 °C) for 60 min. Post-incubation, suspensions were passively cooled to an ambient laboratory temperature (23 ± 1 °C) for 120 min prior to buffer capacity measurements to eliminate transient thermal effects [20].
2.5. Buffer Capacity (BC) Quantification
BC was assessed by acid-base titration of standardized PMC suspensions. A suspension containing 6.6 × 109 capsules in 8 mL ultrapure water was titrated at 25 °C using either 0.001 M HCl or 0.005 M NaOH titrants. pH was continuously monitored with a calibrated glass electrode (HI1131B, Hanna Instruments, Smithfield, RI, USA). Titrant additions were manually controlled to induce pH shifts ≥ 0.02 units per step. The instantaneous buffer capacity (BC, mmol/L/pH unit) at each titration point (i) was computed from the differential change in the added base () relative to the corresponding pH change using the central difference formula [20,33]:
where is the number of moles of NaOH added and (i − 1) and (i + 1) denote the immediately preceding and succeeding titration points, respectively. This differential approach (Equation (1)) captures nonlinear BC responses to pH shifts, which is essential for quantifying how ionic strength and temperature alter protonation equilibria in the PAH/PSS shell.
2.6. Data Analysis and Statistics
Each experimental condition (pH, ionic strength, temperature) was independently replicated five times (n = 5). Due to inherent titration variability preventing exact pH replication across runs, BC values from all replicates were binned into 0.1 pH unit intervals across the measured range (pH 4–9). Reported BC values represent the mean ± standard deviation (SD) within each pH bin. Error bars in figures denote SD for both the mean pH (X-axis) and the mean BC (Y-axis) within each binned interval. Statistical analysis and graphing were performed using OriginPro 2024b (OriginLab Corporation, Northampton, MA, USA) [20].
3. Results and Discussion
The buffer capacity (BC) of polyelectrolyte microcapsules (PMCs) serves as an indicator of their ability to regulate local hydrogen proton concentrations—a critical property for preserving the structural and functional integrity of encapsulated objects. Although previous studies have established pH-dependent buffering behavior in PMCs templated on calcium carbonate (CaCO3) [7], literature data suggest that the chemical composition and dissolution kinetics of the core material influence electrostatic interactions, layer permeability, and ultimately, the buffering efficiency of the resulting microcapsules [26]. In this study, we shifted focus to manganese carbonate (MnCO3), which shares the advantages of CaCO3, enabling the synthesis of hollow PMCs. Unlike PMCs templated on polystyrene particles (PMCPS), MnCO3-based PMCs allow the encapsulation of biological molecules via coprecipitation without organic solvents or significant pH shifts that could compromise the functionality of encapsulated molecules [23,34,35].
In this work, we evaluated the BC of polyelectrolyte microcapsules synthesized on MnCO3 cores and analyzed the effects of ionic strength and temperature on their BC dynamics. The study employed microcapsules with a (PAH/PSS)3/PAH composition, fabricated via layer-by-layer adsorption of polyelectrolytes—polyallylamine (PAH) and polystyrene sulfonate (PSS)—onto MnCO3 particles. The MnCO3 core was subsequently removed through selective dissolution. The fabrication scheme of the polyelectrolyte microcapsules is illustrated in Figure 2.
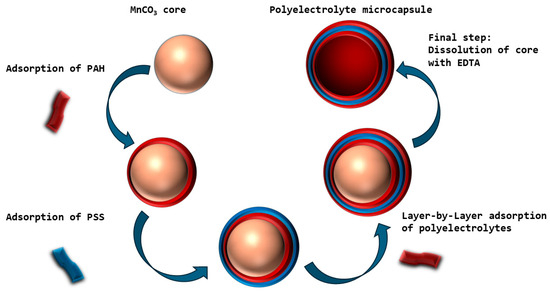
Figure 2.
Schematic of the preparation of polyelectrolyte microcapsules on MnCO3 particles. The size distribution of the polyelectrolyte microcapsules (PMCs), formed on an MnCO3 core (abbreviated as PMCMn), was subsequently determined (Figure 3).
The optical microscopy images of PMCs (Figure 3B) demonstrate their morphological homogeneity. The microcapsules exhibited an average diameter of 3 μm with a polydispersity index of 1.01% (Figure 3A).
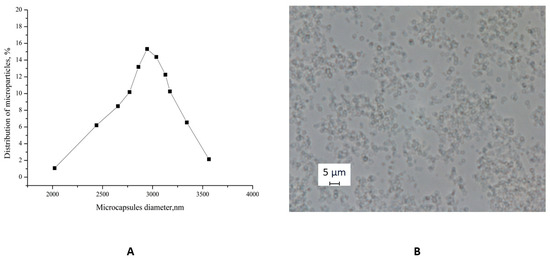
Figure 3.
(A) Diameter distribution function of PMCs. (B) Optical microscopy images of PMCs.
The synthesized polyelectrolyte microcapsules (PMCs) templated on MnCO3 particles (abbreviated as PMCMn) were used to assess their buffer capacity (BC). For this purpose, a PMC suspension (6.7 × 109 microcapsules in 8 mL of water) was titrated with acid or base across a pH range of 4–10, and the pH changes were monitored using a pH meter. The results of the BC determination for the pH range 4–10 are presented in Figure 4.
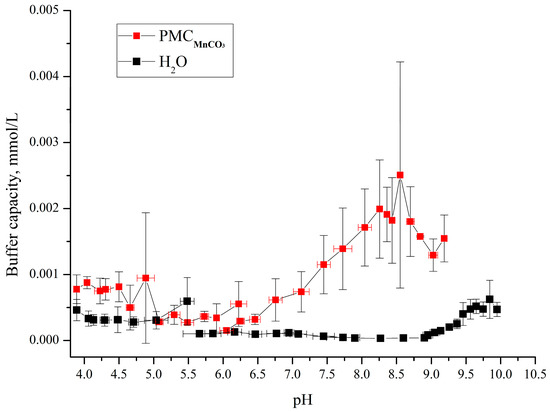
Figure 4.
Buffer capacity of PMCs formed on MnCO3 with composition (PAH/PSS)3/PAH in the pH range from 3.5 to 10.
As shown in the figure, the buffer capacity (BC) of the microcapsules templated on MnCO3 particles differs from that of water at pH > 6.2 and pH < 5. In our previous work, we determined the BC of polyelectrolyte microcapsules templated on CaCO3 cores (abbreviated as PMCCa) [6] and polystyrene cores (PMCPS) [20]. Comparative analysis of the BC across these three capsule types revealed significant differences: PMCCa and PMCPS exhibit distinct BC profiles across the entire pH range compared to PMCMn. Results from our prior studies are summarized in Figure 5.
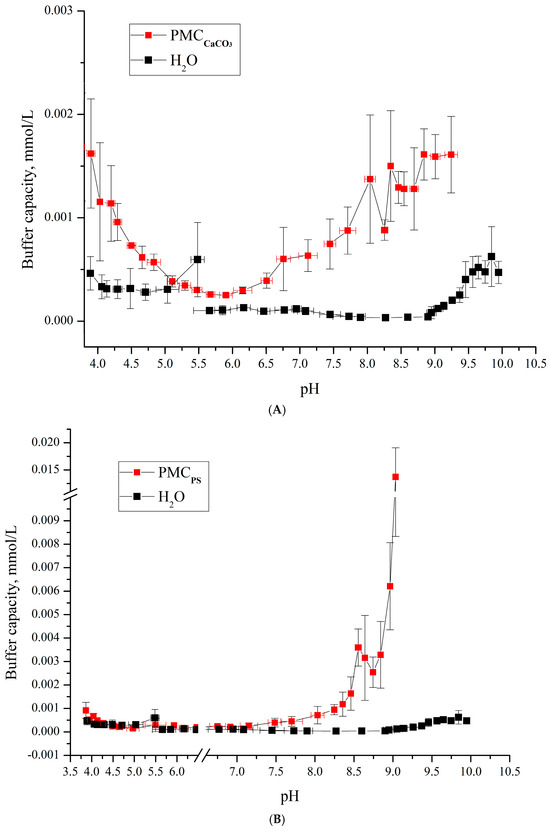
Figure 5.
Buffer capacity of microcapsules PMCCa (PSS/PAH)3 and water in the pH range from 4 to 9. (A) PMCCaCO3 Adapted from [6]; (B) PMCPs Adapted from [20].
A comparison of the results presented in Figure 4 and Figure 5 reveals that PMCMn exhibits buffer capacity (BC) behavior analogous to PMCCa: BC increases at pH > 6.2 under alkaline conditions and at pH < 5 under acidic conditions. This suggests that the use of carbonate cores (e.g., MnCO3 and CaCO3) leads to a consistent model of BC dynamics.
However, to draw definitive conclusions, it is critical to investigate the effects of ionic strength and temperature on the BC of PMCMn, as these factors influence interpolyelectrolyte interactions [36,37]. In the first stage, we analyzed the impact of ionic strength on the BC of PMCMn within the pH range of 6.5–9. The results are shown in Figure 6.
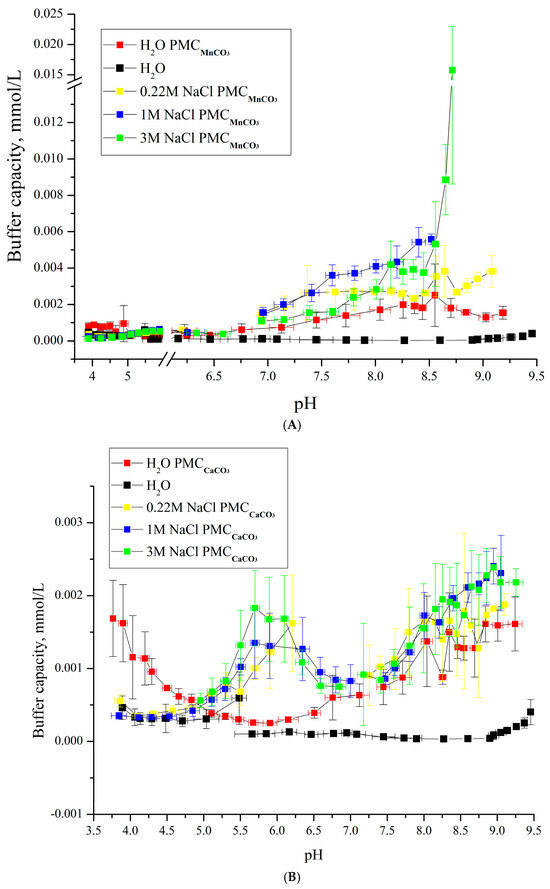
Figure 6.
Buffer capacity of PMC composition (PAH/PSS)3/PAH at different pH values in water, 0.22 M, 1 M, and 3 M NaCl solutions. (A) PMCMn; (B) PMCCa Adapted from [6].
As shown in Figure 6A,B, the buffer capacity (BC) dynamics of PMCMn and PMCCa differ in acidic pH ranges: at pH < 5, the BC of PMCCa decreases, while that of PMCMn remains unchanged. Under alkaline conditions, the BC behavior of PMCMn and PMCCa also diverges. For PMCCa, BC remains consistent across ionic strengths (0.22–3 M NaCl) in the pH range of 6.5–8, but gradually increases at pH > 8, with similar enhancement trends at 1 M and 3 M NaCl. In contrast, PMCMn exhibits BC growth with increasing NaCl concentration up to 1 M. At 3 M NaCl, a sharp BC increase occurs at pH > 8.5, a behavior characteristic of PMCPS. The sharp BC surge at 3 M NaCl (Figure 6A) exemplifies threshold-driven nonlinearity, where ionic screening collapses electrostatic barriers, enabling rapid shell reorganization—analogous to percolation transitions in Sun’s colloidal wave model [38]. This finding challenges the initial hypothesis, suggesting that PMCMn’s BC dynamics may vary not only with ionic strength but also under temperature changes.
In the next stage, we investigated the effect of temperature on the BC of PMCMn within the pH range of 6.5–9. The results are presented in Figure 7.
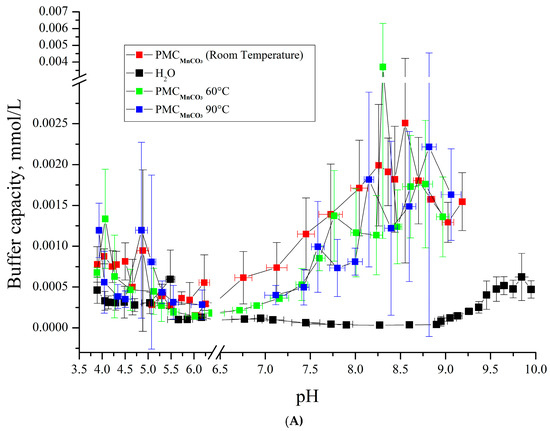
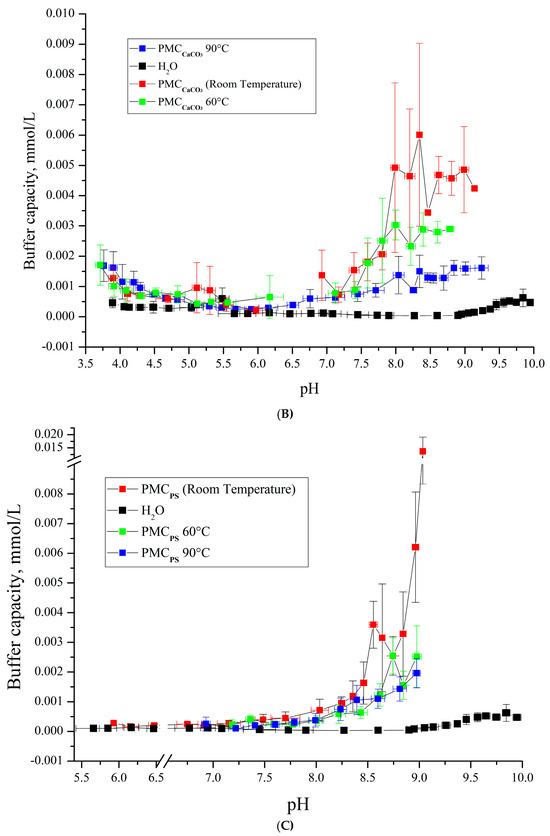
Figure 7.
Buffer capacity of PMC composition (PAH/PSS)3/PAH at different pH in water, 0.22 M, 1 M, and 3 M NaCl solution. (A) PMCMn; (B) PMCCaCO3 Adapted from [6]; (C) PMCPs Adapted from [20].
As shown in Figure 7A,B, the buffer capacity (BC) dynamics of PMCMn and PMCCa align under acidic pH conditions. However, their BC profiles diverge in alkaline pH ranges: PMCCa exhibits a gradual decline in BC with increasing incubation temperature, while PMCMn remains thermally stable. This BC behavior under heating parallels that of PMCPS (Figure 7C). Notably, at pH > 9, PMCPS retains its BC without thermal treatment, whereas PMCMn shows no temperature-dependent BC variation. For clarity, the key findings are summarized in Table 1.

Table 1.
Comparison of buffer capacity dynamics in polyelectrolyte microcapsules templated on different core materials.
Based on the results presented above (Table 1), it can be unequivocally concluded that the use of carbonate cores (MnCO3 and CaCO3) leads to distinct models of buffer capacity (BC) dynamics in polyelectrolyte microcapsules (PMCs). For instance, the presence of residual ions from the carbonate core, which influence the microcapsule microenvironment, has been discussed in studies of polyelectrolyte capsules templated on CaCO3 and MnCO3 particles, including their dissolution and ion release during core removal [23,25,36,39]. Specifically, Mn2+ ions form stronger complexes with polyelectrolyte functional groups, altering protonation equilibria and BC [40,41].
Furthermore, it can be hypothesized that BC also depends on the spatial orientation of polyelectrolyte layers within the PMCs. Unlike PMCCa, PMCPS and PMCMn possess an internal cavity unfilled by interpolyelectrolyte complexes. Additionally, the PAH/PSS shells of PMCPS and PMCMn exhibit greater charge compensation due to their significantly thinner shell structure compared to PMCCa [24,42,43,44].
4. Conclusions
This study demonstrates that polyelectrolyte microcapsules (PMCs) synthesized on manganese carbonate (MnCO3) cores exhibit unique hybrid buffering properties, combining characteristics of both CaCO3- and polystyrene (PS)-templated microcapsules. It was established that in acidic environments (pH < 5), the buffer capacity (BC) of MnCO3-based PMCs resembles that of CaCO3-based analogs, attributed to the electrostatic stabilization of poly(allylamine hydrochloride) (PAH) amine groups by Mn2+ ions. Under alkaline conditions (pH > 8), their behavior aligns with PS-based PMCs, showing a sharp BC increase at high ionic strength (3 M NaCl), driven by morphological changes in the shell and interpolyelectrolyte interactions. Furthermore, MnCO3-based PMCs retain BC across a wide temperature range (60–90 °C), unlike CaCO3-based capsules, where heating reduces BC. This thermal stability underscores the role of the core’s chemical nature: Mn2+ ions form stronger complexes with polyelectrolytes, reinforcing shell integrity.
These findings broaden the potential applications of MnCO3-based PMCs in biomedicine and materials science, particularly in drug delivery systems and microsensors requiring precise pH control under variable ionic strength and temperature. The study confirms that core material selection is a critical factor in designing functional polyelectrolyte microcapsules, paving the way for their optimization for specific applications.
Author Contributions
Conceptualization, A.V.D., A.L.K. and S.A.T.; methodology, A.V.D., A.L.K. and S.A.T.; investigation, A.V.D. and S.A.T.; data curation, A.L.K.; writing—original draft, A.L.K.; writing—review and editing, A.V.D., A.L.K. and S.A.T.; visualization, A.L.K.; supervision, S.A.T.; project administration, S.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the P.L. Kapitsa grant program (third round) and the State Assignment of the Russian Federation No. 075-00223-25-03.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The experimental part of this work with temperature treated PMCs, formed on MnCO3, preparation of polyelectrolyte microcapsules, data processing and analysis was carried out with the financial support of the Moscow Polytechnic University within the framework of the P.L. Kapitsa grant program (third round). The experimental part of this work with NaCl treated PMCs, formed on CaCO3 and MnCO3, preparation of polyelectrolyte microcapsules and microspherulites was performed with the financial support of the State Assignment of the Russian Federation N° 075-00223-25-03 of the Institute of Theoretical and Experimental Biophysics Russian Academy of Science.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel Hollow Polymer Shells by Colloid-Templated Assembly of Polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- De Geest, B.G.; Skirtach, A.G.; Mamedov, A.A.; Antipov, A.A.; Kotov, N.A.; De Smedt, S.C.; Sukhorukov, G.B. Ultrasound-Triggered Release from Multilayered Capsules. Small 2007, 3, 804–808. [Google Scholar] [CrossRef]
- De Geest, B.G.; Sanders, N.N.; Sukhorukov, G.B.; Demeester, J.; De Smedt, S.C. Release Mechanisms for Polyelectrolyte Capsules. Chem. Soc. Rev. 2007, 36, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Skirtach, A.G.; Yashchenok, A.M.; Möhwald, H. Encapsulation, Release and Applications of LbL Polyelectrolyte Multilayer Capsules. Chem. Commun. 2011, 47, 12736. [Google Scholar] [CrossRef]
- Sukhorukov, G.; Fery, A.; Möhwald, H. Intelligent Micro- and Nanocapsules. Proc. Prog. Polym. Sci. 2005, 30, 885–897. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Kim, A.L.; Musin, E.V.; Ramazanov, B.R.; Tikhonenko, S.A. The Discovery of the Buffer Capacity of Various Types of Polyelectrolyte Microcapsules. Polymers 2021, 13, 4026. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Kim, A.L.; Musin, E.V.; Tikhonenko, S.A. A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Ionic Environment and Incubation Temperature. Int. J. Mol. Sci. 2022, 23, 6608. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.V.; Larionova, N.I.; Sukhorukov, G.B. Protein Encapsulation via Porous CaCO3 Microparticles Templating. Biomacromolecules 2004, 5, 1962–1972. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Q.; Anzai, J. Horseradish Peroxidase Microcapsules Based on Layer-by-Layer Polyelectrolyte Deposition. J. Environ. Sci. 2009, 21, S135–S138. [Google Scholar] [CrossRef]
- Pastorino, L.; Dellacasa, E.; Noor, M.R.; Soulimane, T.; Bianchini, P.; D’Autilia, F.; Antipov, A.; Diaspro, A.; Tofail, S.A.M.; Ruggiero, C. Multilayered Polyelectrolyte Microcapsules: Interaction with the Enzyme Cytochrome C Oxidase. PLoS ONE 2014, 9, e112192. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Shabarchina, L.I.; Sukhorukov, G.B. Co-Encapsulation of Enzyme and Sensitive Dye as a Tool for Fabrication of Microcapsule Based Sensor for Urea Measuring. Proc. Phys. Chem. Chem. Phys. 2011, 13, 11110–11117. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.; Serra, V.V.; Paulo, P.M.R.; Andrade, S.M.; Costa, S.M.B. Encapsulation of Photoactive Porphyrinoids in Polyelectrolyte Hollow Microcapsules Viewed by Fluorescence Lifetime Imaging Microscopy (FLIM). RSC Adv. 2015, 5, 79050–79060. [Google Scholar] [CrossRef]
- Pavlov, A.M.; Saez, V.; Cobley, A.; Graves, J.; Sukhorukov, G.B.; Mason, T.J. Controlled Protein Release from Microcapsules with Composite Shells Using High Frequency Ultrasound—Potential for In Vivo Medical Use. Soft Matter 2011, 7, 4341–4347. [Google Scholar] [CrossRef]
- Nifontova, G.; Efimov, A.; Agapova, O.; Agapov, I.; Nabiev, I.; Sukhanova, A. Bioimaging Tools Based on Polyelectrolyte Microcapsules Encoded with Fluorescent Semiconductor Nanoparticles: Design and Characterization of the Fluorescent Properties. Nanoscale Res. Lett. 2019, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Peyratout, C.S.; Dähne, L. Tailor-Made Polyelectrolyte Microcapsules: From Multilayers to Smart Containers. Angew. Chem. Int. Ed. 2004, 43, 3762–3783. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Shabarchina, L.I.; Anastasova, S.; Pavlov, A.M.; Vadgama, P.; Skirtach, A.G.; Sukhorukov, G.B. Chemosensors and Biosensors Based on Polyelectrolyte Microcapsules Containing Fluorescent Dyes and Enzymes. Anal. Bioanal. Chem. 2013, 405, 1559–1568. [Google Scholar] [CrossRef]
- Mak, W.C.; Cheung, K.Y.; Trau, D. Influence of Different Polyelectrolytes on Layer-by-Layer Microcapsule Properties: Encapsulation Efficiency and Colloidal and Temperature Stability. Chem. Mater. 2008, 20, 5475–5484. [Google Scholar] [CrossRef]
- Prevot, M.; Déjugnat, C.; Möhwald, H.; Sukhorukov, G.B. Behavior of Temperature-Sensitive PNIPAM Confined in Polyelectrolyte Capsules. ChemPhysChem 2006, 7, 2497–2502. [Google Scholar] [CrossRef]
- Shen, H.-J.; Shi, H.; Ma, K.; Xie, M.; Tang, L.-L.; Shen, S.; Li, B.; Wang, X.-S.; Jin, Y. Polyelectrolyte Capsules Packaging BSA Gels for PH-Controlled Drug Loading and Release and Their Antitumor Activity. Acta Biomater. 2013, 9, 6123–6133. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Kim, A.L.; Tikhonenko, S.A. The Buffer Capacity of Polyelectrolyte Microcapsules Depends on the Type of Template. Polymers 2024, 16, 2261. [Google Scholar] [CrossRef]
- Heuvingh, J.; Zappa, M.; Fery, A. Salt Softening of Polyelectrolyte Multilayer Capsules. Langmuir 2005, 21, 3165–3171. [Google Scholar] [CrossRef]
- Kim, B.; Vinogradova, O.I. PH-Controlled Swelling of Polyelectrolyte Multilayer Microcapsules. J. Phys. Chem. B 2004, 108, 8161–8165. [Google Scholar] [CrossRef]
- Kim, A.L.; Musin, E.V.; Chebykin, Y.S.; Tikhonenko, S.A. Characterization of Polyallylamine/Polystyrene Sulfonate Polyelectrolyte Microcapsules Formed on Solid Cores: Morphology. Polymers 2024, 16, 1521. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Shchukin, D.G.; Dong, W.; Möhwald, H.; Lulevich, V.V.; Vinogradova, O.I. Comparative Analysis of Hollow and Filled Polyelectrolyte Microcapsules Templated on Melamine Formaldehyde and Carbonate Cores. Macromol. Chem. Phys. 2004, 205, 530–535. [Google Scholar] [CrossRef]
- Antipov, A.A.; Shchukin, D.; Fedutik, Y.; Petrov, A.I.; Sukhorukov, G.B.; Möhwald, H. Carbonate Microparticles for Hollow Polyelectrolyte Capsules Fabrication. Colloids Surf. A Physicochem. Eng. Asp. 2003, 224, 175–183. [Google Scholar] [CrossRef]
- Déjugnat, C.; Sukhorukov, G.B. PH-Responsive Properties of Hollow Polyelectrolyte Microcapsules Templated on Various Cores. Langmuir 2004, 20, 7265–7269. [Google Scholar] [CrossRef]
- Radtchenko, I.L.; Sukhorukov, G.B.; Leporatti, S.; Khomutov, G.B.; Donath, E.; Möhwald, H. Assembly of Alternated Multivalent Ion/Polyelectrolyte Layers on Colloidal Particles. Stability of the Multilayers and Encapsulation of Macromolecules into Polyelectrolyte Capsules. J. Colloid Interface Sci. 2000, 230, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Deng, Y.; Hao, J. In Situ Fabrication and Electrochemical Behavior of Amino Acid Polyoxometalate Nanoparticles-Embedded Microcapsules. Amino Acids 2010, 39, 1363–1367. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Dubrovskiĭ, A.V.; Moshkov, D.A.; Shabarchina, L.I.; Sukhorukov, B.I. An Electron Microscopy Study of the Structure of Polyelectrolyte Microcapsules Containing Protein and Containing No Protein. Biofizika 2007, 52, 850–854. [Google Scholar] [PubMed]
- Zhu, Y.; Tong, W.; Gao, C.; Möhwald, H. Fabrication of Bovine Serum Albumin Microcapsules by Desolvation and Destroyable Cross-Linking. J Mater Chem 2008, 18, 1153–1158. [Google Scholar] [CrossRef]
- Schmidt, S.; Volodkin, D. Microparticulate Biomolecules by Mild CaCO 3 Templating. J. Mater. Chem. B 2013, 1, 1210–1218. [Google Scholar] [CrossRef]
- Kochetkova, O.Y.; Kazakova, L.I.; Moshkov, D.A.; Vinokurov, M.G.; Shabarchina, L.I. Incorporation of Proteins into Polyelectrolyte Microcapsules by Coprecipitation and Adsorption. Russ. J. Bioorg. Chem. 2013, 39, 504–509. [Google Scholar] [CrossRef]
- Richard, I.; Thibault, M.; De Crescenzo, G.; Buschmann, M.D.; Lavertu, M. Ionization Behavior of Chitosan and Chitosan–DNA Polyplexes Indicate That Chitosan Has a Similar Capability to Induce a Proton-Sponge Effect as PEI. Biomacromolecules 2013, 14, 1732–1740. [Google Scholar] [CrossRef]
- Tong, W.; Dong, W.; Gao, C.; Möhwald, H. Charge-Controlled Permeability of Polyelectrolyte Microcapsules. J. Phys. Chem. B 2005, 109, 13159–13165. [Google Scholar] [CrossRef]
- Tong, W.; Song, H.; Gao, C.; Möhwald, H. Equilibrium Distribution of Permeants in Polyelectrolyte Microcapsules Filled with Negatively Charged Polyelectrolyte: The Influence of Ionic Strength and Solvent Polarity. J. Phys. Chem. B 2006, 110, 12905–12909. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, L.; Bell, M.; Ashwell, R.; Volodkin, D.; Vikulina, A.S. Internal Structure of Matrix-Type Multilayer Capsules Templated on Porous Vaterite CaCO3 Crystals as Probed by Staining with a Fluorescence Dye. Micromachines 2018, 9, 547. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Strand, S.P.; Tribet, C.; Jaeger, W.; Dubin, P.L. Effect of Polyelectrolyte Structure on Protein-Polyelectrolyte Coacervates: Coacervates of Bovine Serum Albumin with Poly(Diallyldimethylammonium Chloride) versus Chitosan. Biomacromolecules 2007, 8, 3568–3577. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y. Open-Ocean Shallow-Water Dynamics via a (2+1)-Dimensional Generalized Variable-Coefficient Hirota-Satsuma-Ito System: Oceanic Auto-Bäcklund Transformation and Oceanic Solitons. China Ocean Eng. 2025, 39, 541–547. [Google Scholar] [CrossRef]
- Böttcher, M.E. Experimental Dissolution of CaCO3-MnCO3 Solid Solutions in CO2-H2O Solutions at 20 °C: I. Synthetic Low-Temperature Carbonates. Solid State Ion. 1997, 101–103, 1263–1266. [Google Scholar] [CrossRef]
- Brugman, S.J.T.; Ottenbros, A.B.; Megens, F.; van Enckevort, W.J.P.; Vlieg, E. Epitaxy of Rhodochrosite (MnCO 3 ) on Muscovite Mica and Its Relation with Calcite (CaCO3). Cryst. Growth Des. 2020, 20, 4802–4810. [Google Scholar] [CrossRef]
- Mlowe, S.; Garje, S.S.; Moyo, T.; Revaprasadu, N. Impact of Monovalent Counter-Ions on the Conformation of Flexible Polyelectrolytes Having Different Molecular Architectures. MRS Adv. 2016, 1, 1841. [Google Scholar] [CrossRef][Green Version]
- Dong, W.-F.; Ferri, J.K.; Adalsteinsson, T.; Schönhoff, M.; Sukhorukov, G.B.; Möhwald, H. Influence of Shell Structure on Stability, Integrity, and Mesh Size of Polyelectrolyte Capsules: Mechanism and Strategy for Improved Preparation. Chem. Mater. 2005, 17, 2603–2611. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Petrov, A.I.; Prevot, M.; Sukhorukov, G.B. Matrix Polyelectrolyte Microcapsules: New System for Macromolecule Encapsulation. Langmuir 2004, 20, 3398–3406. [Google Scholar] [CrossRef] [PubMed]
- Ibarz, G.; Dähne, L.; Donath, E.; Möhwald, H. Resealing of Polyelectrolyte Capsules after Core Removal. Macromol. Rapid Commun. 2002, 23, 474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).