Polyethylenimine Carriers for Drug and Gene Delivery
Abstract
1. Introduction
2. Polymerization of Polyethylenimine (PEI)
2.1. Polymerization of Linear PEI
2.2. Polymerization of Branched PEI
3. Processing and Structure–Property of PEI Carriers
3.1. Nanoparticles
3.2. Coatings
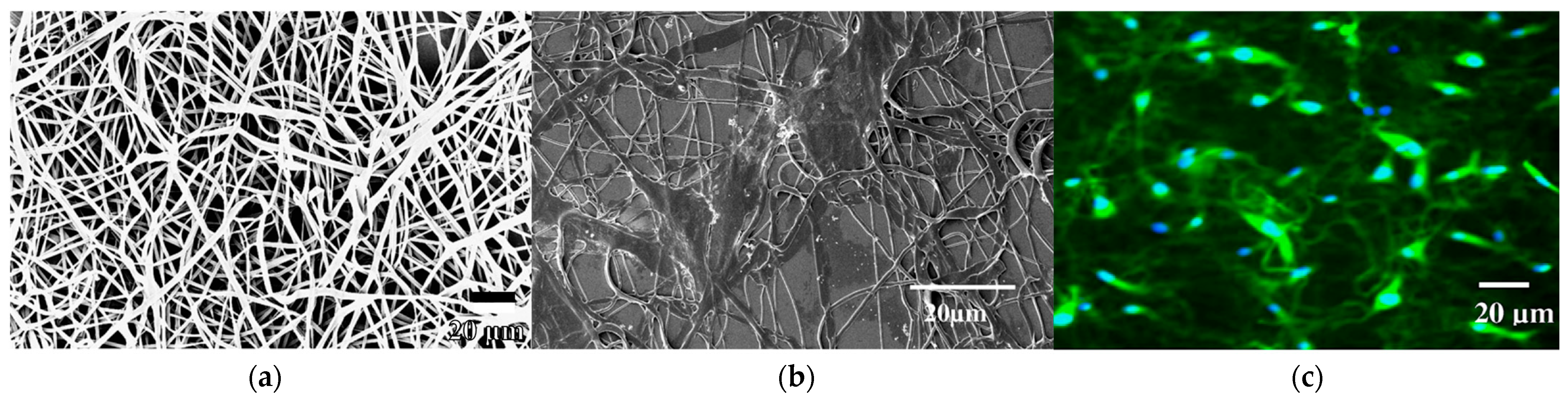
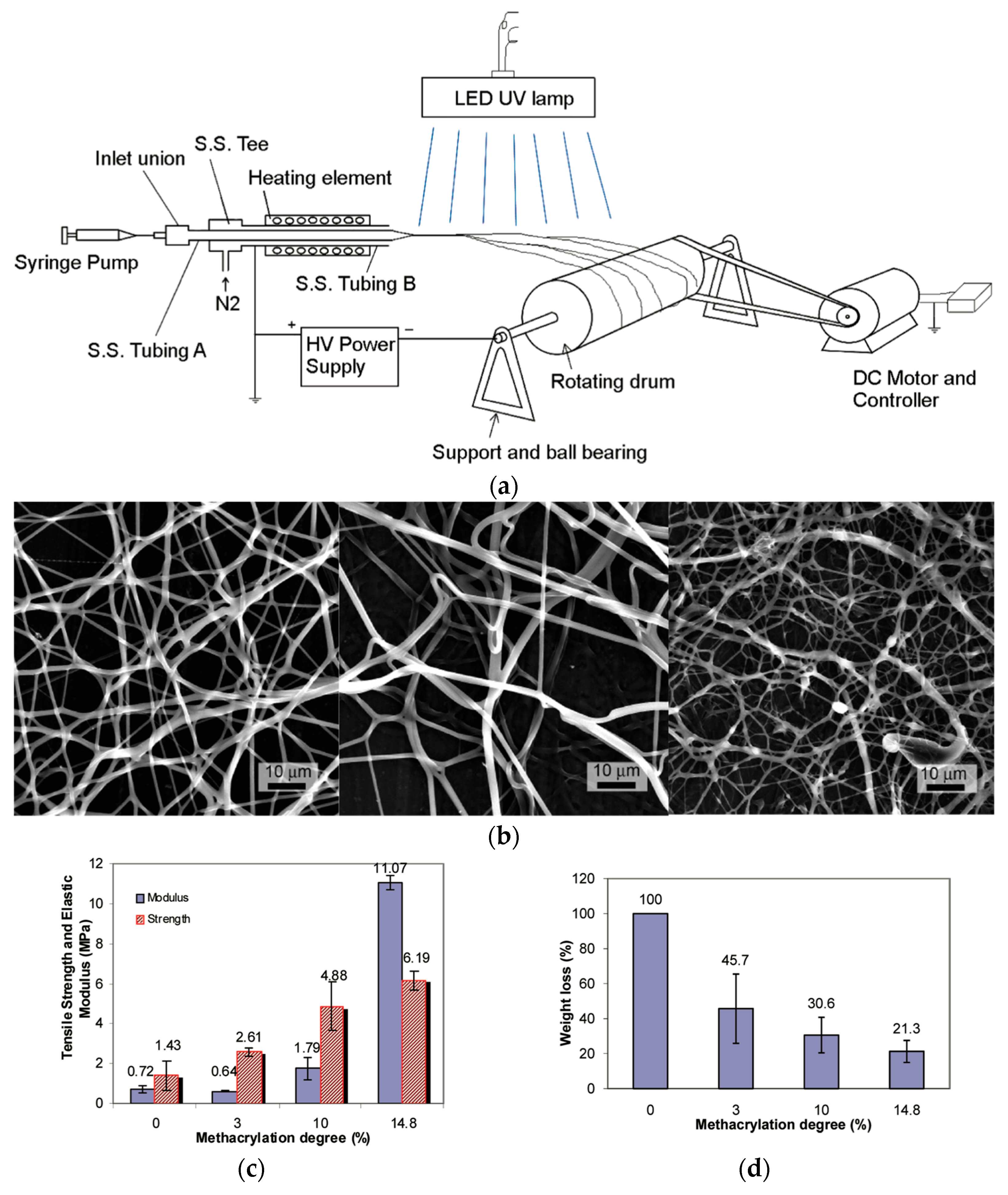
3.3. Fibers
3.4. Hydrogels
3.5. Films
4. Biomedical Applications of Polyethylenimine Carriers
4.1. Applications
4.1.1. Drug Delivery
4.1.2. Gene Therapy
4.2. Obstacles for Transitioning to Clinical Use
4.2.1. High Cytotoxicity
4.2.2. Lack of Biodegradability
4.2.3. Non-Specific Interactions with Serum Components
4.2.4. Poor Systemic Stability and Short Circulation Time
4.2.5. Limited Success in Clinical Trials
5. Conclusions
- (1)
- Advancing PEI’s synthesis and structural tuning, such as gaining exact control over its molecular weight, branching degree, and grafting density, will be essential to balance its activity and safety. This can be carried out through controlled ring-opening polymerization of 2-oxazolines for LPEI and temperature- or pH-regulated aziridine polymerization for BPEI, both influencing transfection efficiency and cytotoxicity.
- (2)
- Utilizing multifunctional PEI composites has demonstrated great potential for intelligent drug and gene delivery, particularly for those that incorporate targeting ligands, biodegradable linkers, or stimuli-responsive moieties.
- (3)
- Managing PEI’s advantages by extending its application beyond drug and gene delivery to related fields, including bioimaging, diagnostics, and environmental cleanup.
- (4)
- Developing new and additional strategies to improve PEI’s long-term toxicity, metabolic destiny, and immunogenicity to gain regulatory approval that translates basic fundamental research to clinical use.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Q.; Li, S.; Lu, X.; Zhao, Y.; Guan, J.-S.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. Hydroxybutyrate methyl ester as a potential drug against Alzheimer’s disease via mitochondria protection mechanism. Biomaterials 2013, 34, 7552–7562. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’ht, F.; Zanta, M.A.; Mergnyt, D.; Schermant, D.; Demeneixt, B.; Behr, J.-P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Lungwitz, U.; Breunig, M.; Blunk, T.; Göpferich, A. Polyethylenimine-based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 2005, 60, 247–266. [Google Scholar] [CrossRef]
- Fattahi, N.; Gorgannezhad, L.; Masoule, S.F.; Babanejad, N.; Ramazani, A.; Raoufi, M.; Sharifikolouei, E.; Foroumadi, A.; Khoobi, M. PEI-based functional materials: Fabrication techniques, properties, and biomedical applications. Adv. Colloid Interface Sci. 2024, 325, 103119. [Google Scholar] [CrossRef]
- Mees, M.A.; Hoogenboom, R. Full and partial hydrolysis of poly(2-oxazoline)s and the subsequent post-polymerization modification of the resulting polyethylenimine (co)polymers. Polym. Chem. 2018, 9, 4968–4978. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, B. Polyethyleneimine-based drug delivery systems for cancer theranostics. J. Funct. Biomater. 2023, 14, 12. [Google Scholar] [CrossRef]
- Al-Absi, A.A.; Ogungbenro, A.E.; Benneker, A.M.; Mahinpey, N. Review of polyethylenimine through ring-opening polymerization reactions and its application in CO2 capture. J. Environ. Chem. Eng. 2024, 12, 112739. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, L.; Liu, L.; Liu, F.; Maruyama, A.; Tian, H.; Chen, X. Ionic-crosslinked polysaccharide/PEI/DNA nanoparticles for stabilized gene delivery. Carbohydr. Polym. 2018, 201, 246–256. [Google Scholar] [CrossRef]
- Park, J.S.; Yi, S.W.; Kim, H.J.; Park, K.-H. Receptor-mediated gene delivery into human mesenchymal stem cells using hyaluronic acid-shielded polyethylenimine/pDNA nanogels. Carbohydr. Polym. 2016, 136, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Adams, F.; Möller, J.; Isert, L.; Zimmermann, C.M.; Keul, D.; Merkel, O.M. Synthesis and application of low molecular weight PEI-based copolymers for siRNA delivery with smart polymer blends. Macromol. Biosci. 2023, 23, e2200409. [Google Scholar] [CrossRef] [PubMed]
- Bonner, D.K.; Zhao, X.; Buss, H.; Langer, R.; Hammond, P.T. Crosslinked linear polyethylenimine enhances delivery of DNA to the cytoplasm. J. Control. Release 2013, 167, 101–107. [Google Scholar] [CrossRef]
- Tamaddon, A.; Abolmaali, S.S.; Yousefi, G.; Javidnia, K.; Dinarvand, R. Sequential optimization of methotrexate encapsulation in micellar nano-networks of polyethyleneimine ionomer containing redox-sensitive cross-links. Int. J. Nanomed. 2014, 9, 2833–2848. [Google Scholar] [CrossRef] [PubMed]
- Ratanajanchai, M.; Soodvilai, S.; Pimpha, N.; Sunintaboon, P. Polyethylenimine-immobilized core–shell nanoparticles: Synthesis, characterization, and biocompatibility test. Mater. Sci. Eng. C 2014, 34, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhao, L.; Shen, M.; Zhao, J.; Shi, X. A multifunctional polyethylenimine-based nanoplatform for targeted anticancer drug delivery to tumors in vivo. J. Mater. Chem. B 2017, 5, 1542–1550. [Google Scholar] [CrossRef]
- Seo, S.-J.; Lee, S.-Y.; Choi, S.-J.; Kim, H.-W. Tumor-targeting co-delivery of drug and gene from tempera-ture-triggered micelles. Macromol. Biosci. 2015, 15, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, F.; Feng, L.; Li, M.; Zhang, J.; Zhang, N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials 2013, 34, 2547–2564. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Qiu, X.; Winnik, F.M.; Benderdour, M.; Zhang, X.; Dai, K.; Shi, Q. Linear polyethylenimine produced by partial acid hydrolysis of poly(2-ethyl-2-oxazoline) for DNA and siRNA delivery in vitro. Int. J. Nanomed. 2013, 8, 4091–4102. [Google Scholar] [CrossRef][Green Version]
- Tauhardt, L.; Kempe, K.; Knop, K.; Altuntaş, E.; Jäger, M.; Schubert, S.; Fischer, D.; Schubert, U.S. Linear polyethyleneimine: Optimized synthesis and characterization—On the way to “Pharmagrade” batches. Macromol. Chem. Phys. 2011, 212, 1918–1924. [Google Scholar] [CrossRef]
- Dick, C.R.; Ham, G.E. Characterization of Polyethylenimine. J. Macromol. Sci. Part A—Chem. 1970, 4, 1301–1314. [Google Scholar] [CrossRef]
- Brodie, C.N.; Goodfellow, A.S.; Andrews, M.J.; Owen, A.E.; Bühl, M.; Kumar, A. Direct synthesis of partially ethoxylated branched polyethylenimine from ethanolamine. Nat. Commun. 2024, 15, 6253. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, D.; Wang, X.; Xie, X. Insight into the synthesis of branched polyethylenimine from 2-haloethylamine via a one-pot two-stage process. Polymer 2022, 255, 125113. [Google Scholar] [CrossRef]
- Gleede, T.; Reisman, L.; Rieger, E.; Mbarushimana, P.C.; Rupar, P.A.; Wurm, F.R. Aziridines and azetidines: Building blocks for polyamines by anionic and cationic ring-opening polymerization. Polym. Chem. 2019, 10, 3257–3283. [Google Scholar] [CrossRef]
- Zou, Y.; Li, D.; Shen, M.; Shi, X. Polyethylenimine-based nanogels for biomedical applications. Macromol. Biosci. 2019, 19, 1900272. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Demirci, S. Degradable tannic acid/polyethyleneimine polyplex particles with highly antioxidant and antimicrobial effects. Polym. Degrad. Stab. 2016, 133, 152–161. [Google Scholar] [CrossRef]
- Peng, H.; Rübsam, K.; Jakob, F.; Schwaneberg, U.; Pich, A. Tunable enzymatic activity and enhanced stability of cellulase immobilized in biohybrid nanogels. Biomacromolecules 2016, 17, 3619–3631. [Google Scholar] [CrossRef]
- Sun, W.; Yang, J.; Zhu, J.; Zhou, Y.; Li, J.; Zhu, X.; Shen, M.; Zhang, G.; Shi, X. Immobilization of iron oxide nanoparticles within alginate nanogels for enhanced MR imaging applications. Biomater. Sci. 2016, 4, 1422–1430. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, L.; Dong, Z.; Chen, S.; Yu, X.-Y.; Tang, S. Intracellular delivery of proteins into living cells by low-molecular-weight polyethyleneimine. Int. J. Nanomed. 2021, 16, 4197–4208. [Google Scholar] [CrossRef]
- Pandey, A.P.; Sawant, K.K. Polyethylenimine: A versatile, multifunctional non-viral vector for nucleic acid delivery. Mater. Sci. Eng. C 2016, 68, 904–918. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Sidorov, S.N.; Gourkova, A.Y.; Valetsky, P.M.; Hartmann, J.; Breulmann, M.; Cölfen, H.; Antonietti, M. Interaction of metal compounds with ‘double-hydrophilic’ block copolymers in aqueous medium and metal colloid formation. Inorg. Chim. Acta 1998, 280, 348–354. [Google Scholar] [CrossRef]
- Hu, R.; Wang, L.; Xu, S.; Lu, Y.; Zhou, S. Silica nanospheres-encapsulated polymer ligands-bound Pd nanoparticles as highly efficient and selective catalysts for semi-hydrogenations of alkynes. Microporous Mesoporous Mater. 2024, 377, 113213. [Google Scholar] [CrossRef]
- Sidorov, S.N.; Bronstein, L.M.; Valetsky, P.M.; Hartmann, J.; Cölfen, H.; Schnablegger, H.; Antonietti, M. Stabilization of metal nanoparticles in aqueous medium by polyethyleneoxide–polyethyleneimine block copolymers. J. Colloid Interface Sci. 1999, 212, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Lächelt, U.; Bartek, J.; Wagner, E.; Moghimi, S.M. Polyplex evolution: Understanding biology, optimizing performance. Mol. Ther. 2017, 25, 1476–1490. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Shi, X.; Shen, M. Cancer nanomedicine based on polyethylenimine-mediated multifunctional nanosystems. Prog. Mater. Sci. 2022, 124, 100871. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, X.; Jia, H.R.; Zhu, Y.X.; Guo, Y.; Gao, G.; Li, Y.H.; Wu, F.G. Water-dispersible candle soot–derived carbon nano-onion clusters for imaging-guided photothermal cancer therapy. Small 2019, 15, 1804575. [Google Scholar] [CrossRef]
- Sun, X.; Dong, S.; Wang, E. One-step synthesis and characterization of polyelectrolyte-protected gold nanoparticles through a thermal process. Polymer 2004, 45, 2181–2184. [Google Scholar] [CrossRef]
- Gorejová, R.; Podrojková, N.; Sisáková, K.; Shepa, J.; Shepa, I.; Kovalčíková, A.; Šišoláková, I.; Kaľavský, F.; Oriňaková, R. Interaction of thin polyethyleneimine layer with the iron surface and its effect on the electrochemical behavior. Sci. Rep. 2022, 12, 3460. [Google Scholar] [CrossRef]
- Khanam, N.; Mikoryak, C.; Draper, R.K.; Balkus, K.J. Electrospun linear polyethyleneimine scaffolds for cell growth. Acta Biomater. 2007, 3, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, J.-F.; Fan, Y. Fabrication of cross-linked polyethyleneimine microfibers by reactive electrospinning within situ photo-cross-linking by UV radiation. Biomacromolecules 2010, 11, 2283–2289. [Google Scholar] [CrossRef]
- Englert, C.; Tauhardt, L.; Hartlieb, M.; Kempe, K.; Gottschaldt, M.; Schubert, U.S. Linear poly(ethylene imine)-based hydrogels for effective binding and release of DNA. Biomacromolecules 2014, 15, 1124–1131. [Google Scholar] [CrossRef]
- Chang, C.-W.; Ho, H.-O.; Lo, Y.-J.; Lee, S.-Y.; Yang, Y.-R.; Sheu, M.-T. Development of swellable local implants of a polyethyleneimine-poly(vinyl pyrrolidone) (PEI-PVP) hydrogel as a socket filler. J. Biomater. Sci. Polym. Ed. 2012, 23, 2171–2184. [Google Scholar] [CrossRef]
- Avais, M.; Chattopadhyay, S. Waterborne pH responsive hydrogels: Synthesis, characterization and selective pH responsive behavior around physiological pH. Polymer 2019, 180, 121701. [Google Scholar] [CrossRef]
- Wu, M.-B.; Fan, X.-L.; Yang, H.-C.; Yang, J.; Zhu, M.-M.; Ren, K.-F.; Ji, J.; Xu, Z.-K. Ultrafast formation of pyrogallol/polyethyleneimine nanofilms for aqueous and organic nanofiltration. J. Membr. Sci. 2019, 570–571, 270–277. [Google Scholar] [CrossRef]
- Wen, H.; Liu, Z.; Lu, Z.; Yang, Y.; Chen, J.P. High-performance PEI-based nanofiltration membrane by MXene-regulated interfacial polymerization reaction: Design, fabrication and testing. J. Membr. Sci. 2025, 717, 123568. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Liu, Y.; Zheng, B.; Meng, L.; Lee, R.J.; Xie, J.; Teng, L. Non-covalent complexes of folic acid and oleic acid conjugated polyethylenimine: An efficient vehicle for antisense oligonucleotide delivery. Colloids Surf. B 2015, 135, 274–282. [Google Scholar] [CrossRef]
- Ding, G.-B.; Meng, X.; Yang, P.; Li, B.; Stauber, R.H.; Li, Z. Integration of Polylactide into Polyethylenimine facilitates the safe and effective intracellular siRNA delivery. Polymers 2020, 12, 445. [Google Scholar] [CrossRef]
- Pandi, J.S.; Pavadai, P.; Babkiewicz, E.; Panneerselvam, T.; Maszczyk, P.; Sankaranarayanan, M.; Mohan, R.; Kunjiappan, S. Targeted delivery of thymoquinone-encapsulated polyethyleneimine/poly (lactic acid) nanoparticles into breast cancer cells. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Xiao, Y.; Fan, Y.; Tu, W.; Ning, Y.; Zhu, M.; Liu, Y.; Shi, X. Multifunctional PLGA microfibrous rings enable MR imaging-guided tumor chemotherapy and metastasis inhibition through prevention of circulating tumor cell shedding. Nano Today 2021, 38, 101123. [Google Scholar] [CrossRef]
- Zhou, B.; Xiong, Z.; Zhu, J.; Shen, M.; Tang, G.; Peng, C.; Shi, X. PEGylated polyethylenimine-entrapped gold nanoparticles loaded with Gadolinium for dual-mode Ct/Mr imaging applications. Nanomedicine 2016, 11, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Ringsdorf, H.; Satchi-Fainaro, R. Polymer therapeutics—Polymers as drugs, drug and protein conjugates and gene delivery systems: Past, present and future opportunities. J. Drug Target. 2006, 14, 337–341. [Google Scholar] [CrossRef]
- Kamruzzaman Selim, K.M.; Ha, Y.-S.; Kim, S.-J.; Chang, Y.; Kim, T.-J.; Lee, G.; Kang, I.-K. Surface modification of magnetite nanoparticles using lactobionic acid and their interaction with hepatocytes. Biomaterials 2007, 28, 710–716. [Google Scholar] [CrossRef]
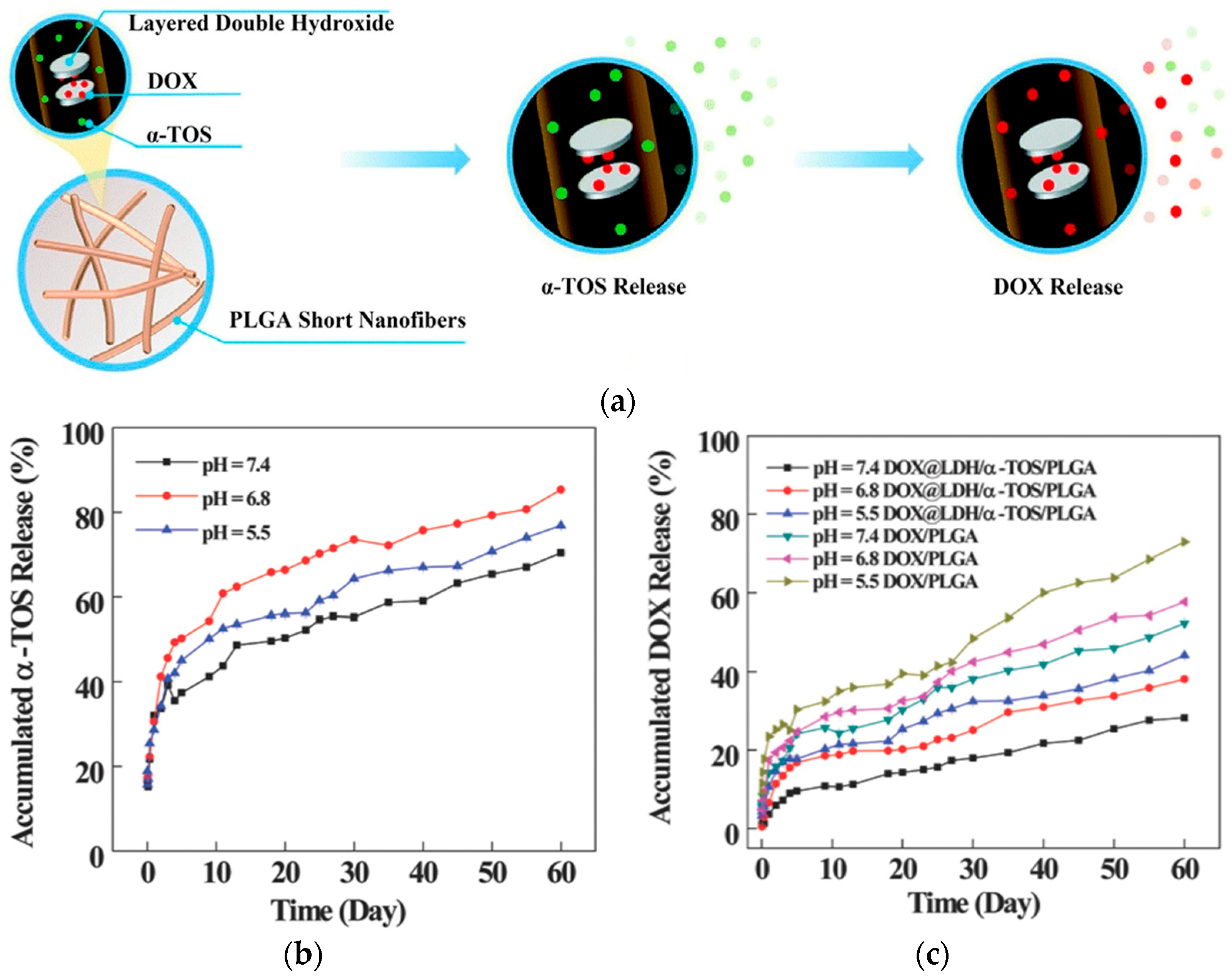
- Huang, Y.; Zhan, M.; Shen, M.; Zhang, L.; Shi, X. Electrospun short fibers: A new platform for cancer nanomedicine applications. Explor. Drug Sci. 2023, 1, 454–467. [Google Scholar] [CrossRef]
- Ma, Y.; Li, D.; Xiao, Y.; Ouyang, Z.; Shen, M.; Shi, X. LDH-doped electrospun short fibers enable dual drug loading and multistage release for chemotherapy of drug-resistant cancer cells. New J. Chem. 2021, 45, 13421–13428. [Google Scholar] [CrossRef]
- de Souza, A.P.; Bastos, A.P.; da Fonseca, F.N.; Pandolfi, J.R.; Costamilan, C.A.D.V.L.R.; Marques, M.G. Polyethyleneimine-mediated gene transfection in porcine fetal fibroblasts. Anim. Reprod. 2024, 21, e20240026. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Kim, B.K.; Ke, X.; Yeo, Y. In-vitro and in-vivo difference in gene delivery by lithocholic acid-polyethyleneimine conjugate. Biomaterials 2019, 217, 119296. [Google Scholar] [CrossRef]
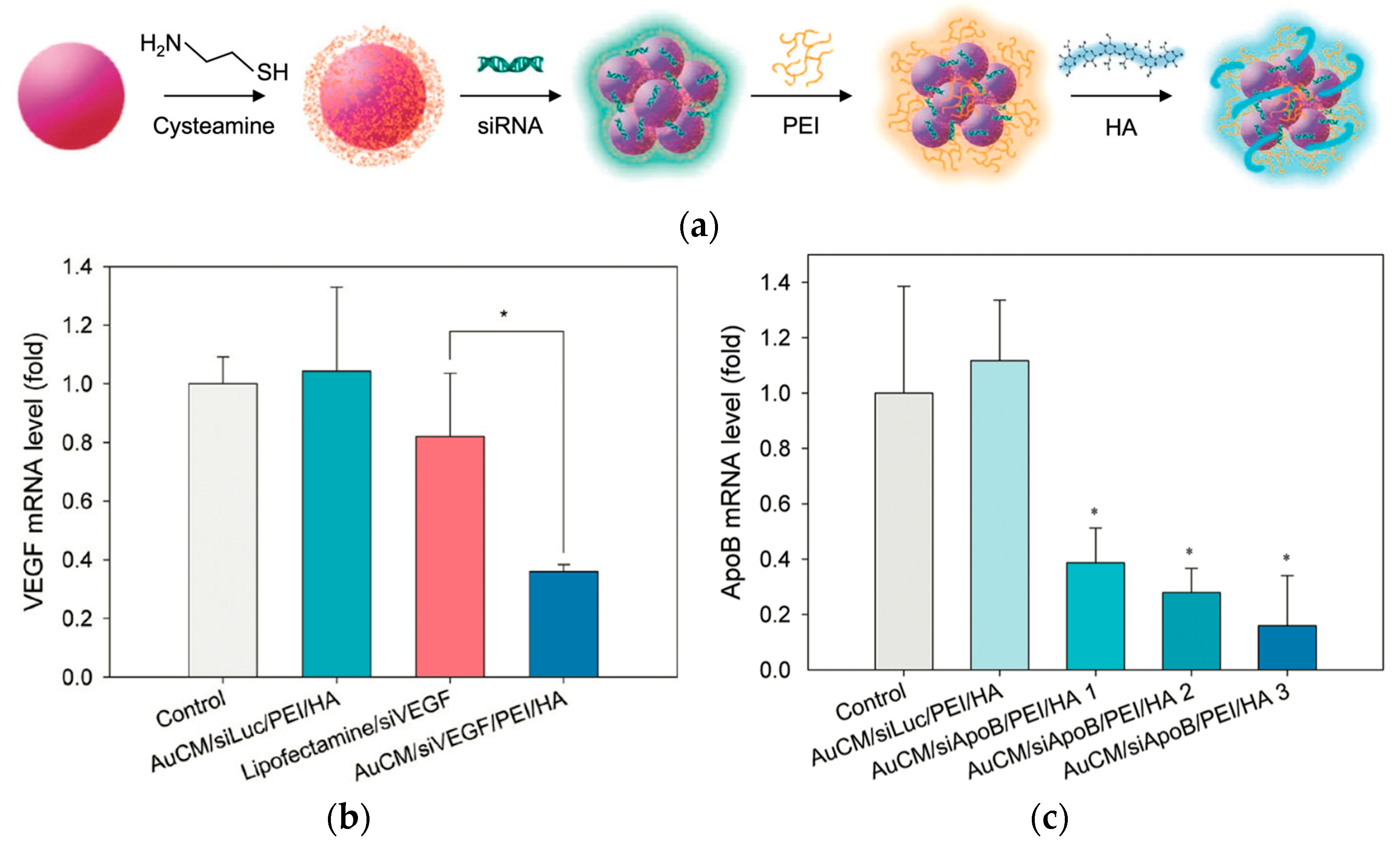
- Lee, M.Y.; Park, S.J.; Park, K.; Kim, K.S.; Lee, H.; Hahn, S.K. Target-specific gene silencing of layer-by-layer assembled gold-cysteamine/siRNA/PEI/HA nanocomplex. ACS Nano 2011, 5, 6138–6147. [Google Scholar] [CrossRef]
- Dehshahri, A.; Alhashemi, S.H.; Jamshidzadeh, A.; Sabahi, Z.; Samani, S.M.; Sadeghpour, H.; Mohazabieh, E.; Fadaei, M. Comparison of the effectiveness of polyethylenimine, polyamidoamine and chitosan in transferring plasmid encoding interleukin-12 gene into hepatocytes. Macromol. Res. 2013, 21, 1322–1330. [Google Scholar] [CrossRef]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in gene therapy: Current status and clinical applications. J. Control. Release 2023, 362, 667–691. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yao, L.; Song, Q.; Zhu, L.; Xia, Z.; Xia, H.; Jiang, X.; Chen, J.; Chen, H. The association of autophagy with polyethylenimine-induced cytotoxicity in nephritic and hepatic cell lines. Biomaterials 2011, 32, 8613–8625. [Google Scholar] [CrossRef]
- Lin, C.W.; Jan, M.S.; Kuo, J.H.S.; Hsu, L.J.; Lin, Y.S. Protective role of autophagy in branched polyethylenimine (25K)- and poly(L-lysine) (30–70K)-induced cell death. Eur. J. Pharm. Sci. 2012, 47, 865–874. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Islam, M.A.; Xing, L.; Firdous, J.; Cao, W.; He, Y.-J.; Zhu, Y.; Cho, K.-H.; Li, H.-S.; Cho, C.-S. Degradable polyethylenimine-based gene carriers for cancer therapy. Top. Curr. Chem. 2017, 375, 34. [Google Scholar] [CrossRef]
- Nouri, F.; Sadeghpour, H.; Heidari, R.; Dehshahri, A. Preparation, characterization, and transfection efficiency of low molecular weight polyethylenimine-based nanoparticles for delivery of the plasmid encoding CD200 gene. Int. J. Nanomed. 2017, 12, 5557–5569. [Google Scholar] [CrossRef]
- Yu, H.; Russ, V.; Wagner, E. Influence of the molecular weight of bioreducible oligoethylenimine conjugates on the polyplex transfection properties. AAPS J. 2009, 11, 445–455. [Google Scholar] [CrossRef][Green Version]
- Meng, F.; Hennink, W.E.; Zhong, Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef]
- Forrest, M.L.; Koerber, J.T.; Pack, D.W. A degradable polyethylenimine derivative with low toxicity for highly efficient gene delivery. Bioconjug. Chem. 2003, 14, 934–940. [Google Scholar] [CrossRef]
- Russ, V.; Elfberg, H.; Thoma, C.; Kloeckner, J.; Ogris, M.; Wagner, E. Novel degradable oligoethylenimine acrylate ester-based pseudodendrimers for in vitro and in vivo gene transfer. Gene Ther. 2008, 15, 18–29. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Su, J.; Wu, F.; Lu, P.; Yuan, L.F.; Yuan, W.E.; Sheng, J.; Jin, T. Biscarbamate cross-linked polyethylenimine derivative with low molecular weight, low cytotoxicity, and high efficiency for gene delivery. Int. J. Nanomed. 2012, 7, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, J.; Yang, Q.; Zhuo, R.; Jiang, X. Disulfide-containing brushed polyethylenimine derivative synthesized by click chemistry for nonviral gene delivery. Bioconjug. Chem. 2012, 23, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Zeng, F.; Yu, C.; Wu, S. Low molecular weight PEIs modified by hydrazone-based crosslinker and betaine as improved gene carriers. Colloids Surf. B 2014, 122, 472–481. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, J.H.; Lee, M.; Kim, Y.-H.; Park, T.G.; Kim, S.W. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J. Control. Release 2005, 103, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Ochrimenko, S.; Vollrath, A.; Tauhardt, L.; Kempe, K.; Schubert, S.; Schubert, U.S.; Fischer, D. Dextran-graft-linear poly(ethylene imine)s for gene delivery: Importance of the linking strategy. Carbohydr. Polym. 2014, 113, 597–606. [Google Scholar] [CrossRef]
- Xie, B.; Peng, J.; Wang, S.; Zhang, X.; Nie, H. Investigation of the sequential actions of doxorubicin and p53 on tumor cell growth via branched polyethylenimine-β-cyclodextrin conjugates. Ann. Biomed. Eng. 2016, 44, 3372–3383. [Google Scholar] [CrossRef]
- Yamada, H.; Loretz, B.; Lehr, C.-M. Design of starch-graft-PEI polymers: An effective and biodegradable gene delivery platform. Biomacromolecules 2014, 15, 1753–1761. [Google Scholar] [CrossRef]
- Yue, J.; Wu, J.; Liu, D.; Zhao, X.; Lu, W.W. BMP2 gene delivery to bone mesenchymal stem cell by chitosan-g-PEI nonviral vector. Nanoscale Res. Lett. 2015, 10, 203. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, H.I.; Choi, J.S. Novel glycol chitosan-based polymeric gene carrier synthesized by a Michael addition reaction with low molecular weight polyethylenimine. Carbohydr. Polym. 2016, 137, 669–677. [Google Scholar] [CrossRef]
- Liu, K.; Hui, L.; Qing, Z.; Kewu, L.; Manman, Z.; Wenfang, Z.; Yuan, M. Coupling of a bifunctional peptide R13 to OTMCS-PEI copolymer as a gene vector increases transfection efficiency and tumor targeting. Int. J. Nanomed. 2014, 9, 1311–1322. [Google Scholar] [CrossRef][Green Version]
- Nam, J.-P.; Nah, J.-W. Target gene delivery from targeting ligand conjugated chitosan–PEI copolymer for cancer therapy. Carbohydr. Polym. 2016, 135, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Jiao, Y.; Zhang, Y.; Zhang, W.; Li, N.; Zuo, Q.; Wang, Q.; Xue, W.; Liu, Z. Effects of the gene carrier polyethyleneimines on structure and function of blood components. Biomaterials 2013, 34, 294–305. [Google Scholar] [CrossRef]
- Ogris, M.; Brunner, S.; Schüller, S.; Kircheis, R.; Wagner, E. PEGylated DNA/transferrin–PEI complexes: Reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999, 6, 595–605. [Google Scholar] [CrossRef]
- Toy, R.; Pradhan, P.; Ramesh, V.; Di Paolo, N.C.; Lash, B.; Liu, J.; Blanchard, E.L.; Pinelli, C.J.; Santangelo, P.J.; Shayakhmetov, D.M.; et al. Modification of primary amines to higher order amines reduces in vivo hematological and immunotoxicity of cationic nanocarriers through TLR4 and complement pathways. Biomaterials 2019, 225, 119512. [Google Scholar] [CrossRef]
- Mehrizi, T.Z. Hemocompatibility and hemolytic effects of functionalized nanoparticles on red blood cells: A recent review study. Nano 2021, 16, 2130007. [Google Scholar] [CrossRef]
- Goula, D.; Becker, N.; Lemkine, G.F.; Normandie, P.; Rodrigues, J.; Mantero, S.; Levi, G.; Demeneix, B.A. Rapid crossing of the pulmonary endothelial barrier by polyethylenimine/DNA complexes. Gene Ther. 2000, 7, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Chollet, P.; Favrot, M.C.; Hurbin, A.; Coll, J. Side-effects of a systemic injection of linear polyethylenimine–DNA complexes. J. Gene Med. 2002, 4, 84–91. [Google Scholar] [CrossRef]
- Merkel, O.M.; Urbanics, R.; Bedőcs, P.; Rozsnyay, Z.; Rosivall, L.; Toth, M.; Kissel, T.; Szebeni, J. In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers. Biomaterials 2011, 32, 4936–4942. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, G.; Griffin, J.I.; Brenneman, B.; Banda, N.K.; Holers, V.M.; Backos, D.S.; Wu, L.; Moghimi, S.M.; Simberg, D. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat. Nanotechnol. 2017, 12, 387–393. [Google Scholar] [CrossRef]
- Parhiz, H.; Hashemi, M.; Hatefi, A.; Shier, W.T.; Amel Farzad, S.; Ramezani, M. Arginine-rich hydrophobic polyethylenimine: Potent agent with simple components for nucleic acid delivery. Int. J. Biol. Macromol. 2013, 60, 18–27. [Google Scholar] [CrossRef]
- Lam, A.K.; Moen, E.L.; Pusavat, J.; Wouters, C.L.; Panlilio, H.; Ferrell, M.J.; Houck, M.B.; Glatzhofer, D.T.; Rice, C.V. PEGylation of polyethylenimine lowers acute toxicity while retaining anti-biofilm and β-lactam potentiation properties against antibiotic-resistant pathogens. ACS Omega 2020, 5, 26262–26270. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Ma, K.; Hu, M.; Qi, Y.; Qiu, L.; Jin, Y.; Yu, J.; Li, B. Structure–transfection activity relationships with glucocorticoid–polyethyl-enimine conjugate nuclear gene delivery systems. Biomaterials 2009, 30, 3780–3789. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Luo, X.; Wu, H.; Zhang, Q.; Wang, K.; Sun, M.; Oupicky, D. Combined hydrophobization of polyethylenimine with cholesterol and perfluorobutyrate improves siRNA delivery. Bioconjug. Chem. 2020, 31, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.W.; Kalinichenko, V.V.; Shi, D. Highly efficient in vivo targeting of the pulmonary endothelium using novel modifications of polyethylenimine: An importance of charge. Adv. Healthc. Mater. 2018, 7, 1800876. [Google Scholar] [CrossRef] [PubMed]
- Heller, P.; Birke, A.; Huesmann, D.; Weber, B.; Fischer, K.; Reske-Kunz, A.; Bros, M.; Barz, M. Introducing PeptoPlexes: Polylysine-block-polysarcosine based polyplexes for transfection of HEK 293T cells. Macromol. Biosci. 2014, 14, 1380–1395. [Google Scholar] [CrossRef] [PubMed]
- Noga, M.; Edinger, D.; Rödl, W.; Wagner, E.; Winter, G.; Besheer, A. Controlled shielding and deshielding of gene delivery polyplexes using hydroxyethyl starch (HES) and alpha-amylase. J. Control. Release 2012, 159, 92–103. [Google Scholar] [CrossRef] [PubMed]


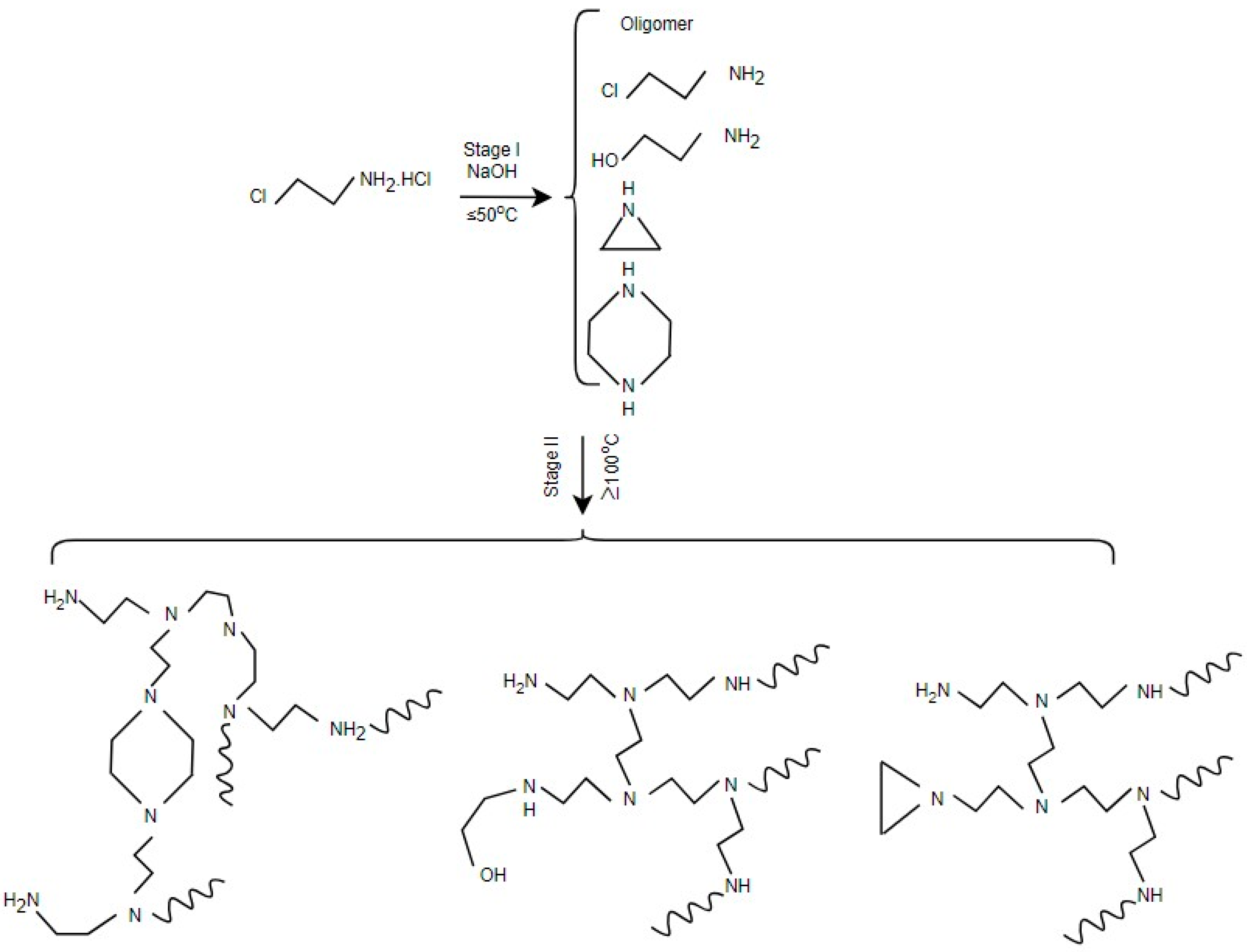

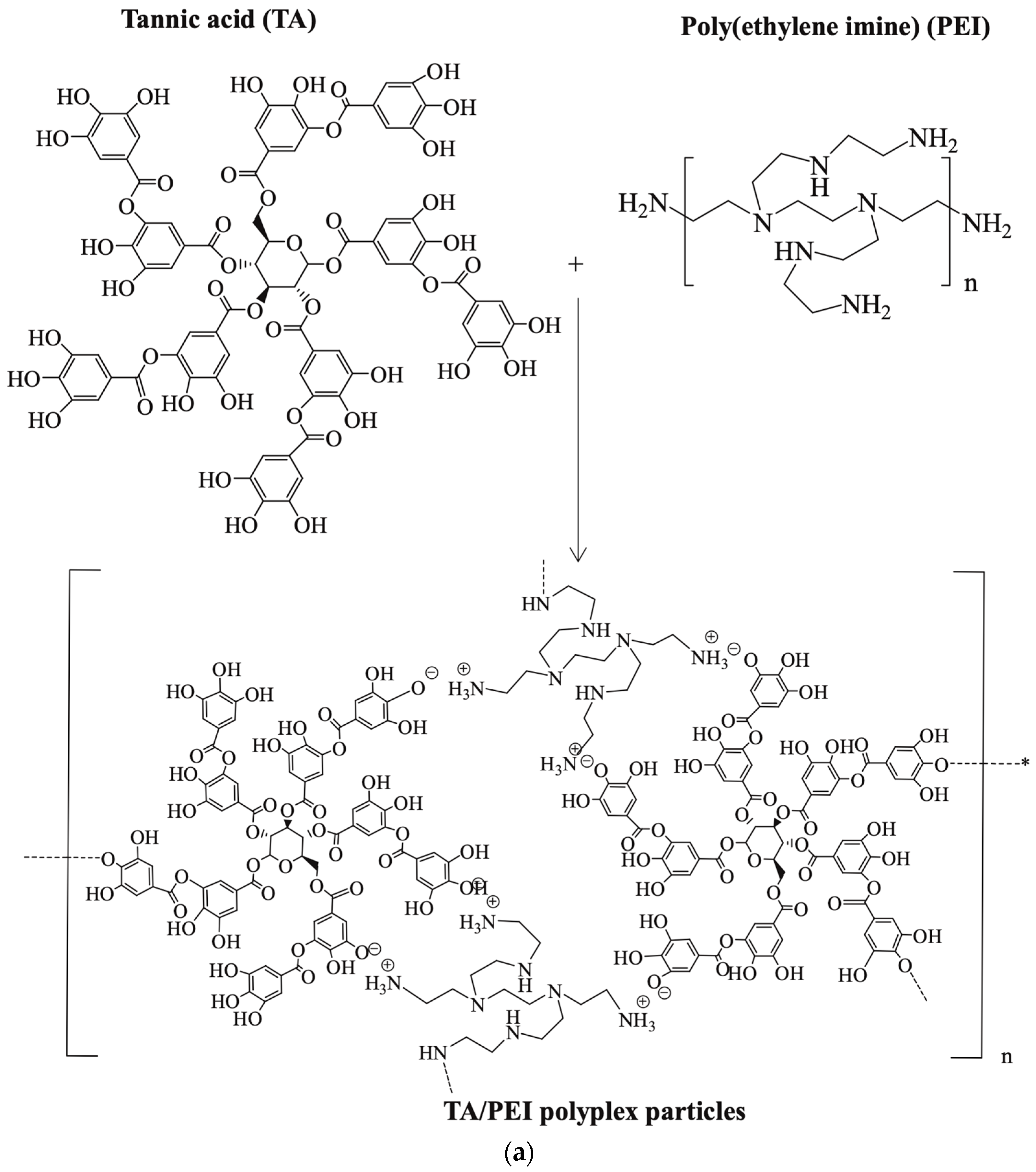

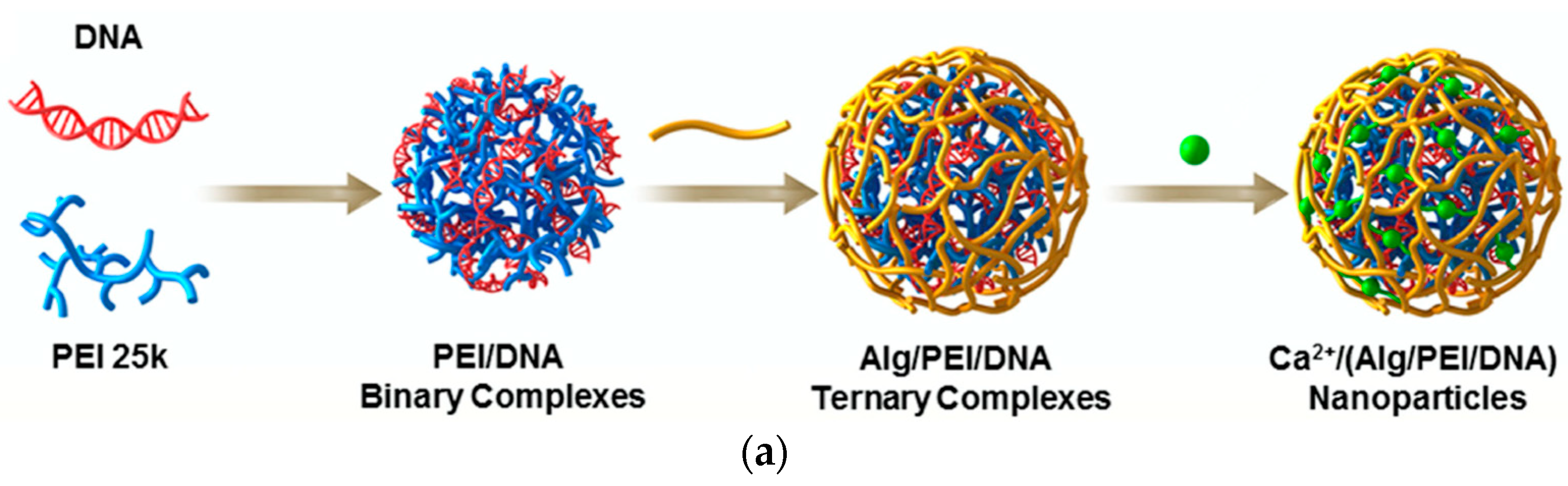
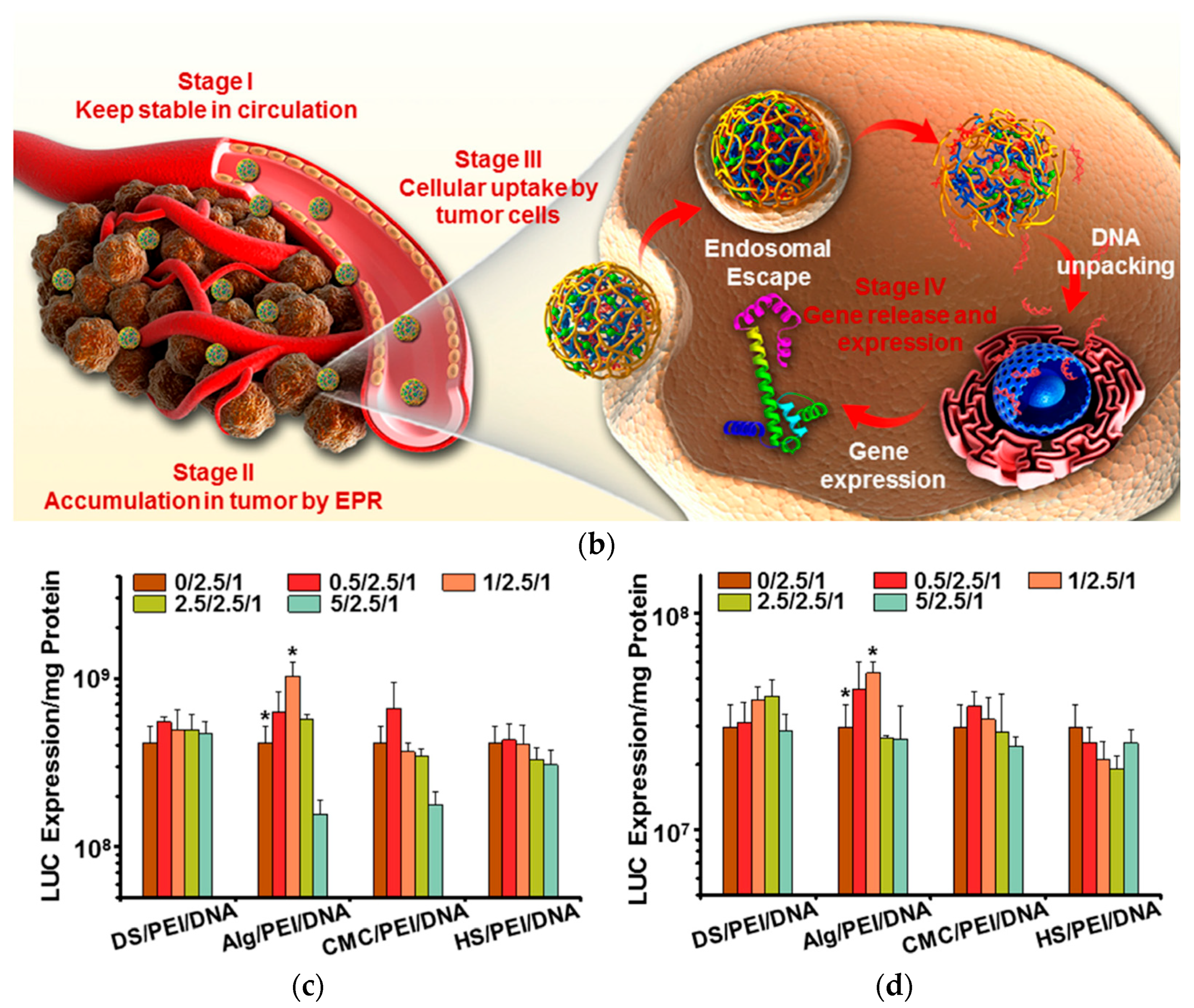

| Applications | LPEI | BPEI | Ref. |
|---|---|---|---|
| Gene Delivery | Lower cytotoxicity; more controllable transfection; favorable for in vivo applications | Higher transfection efficiency due to strong proton sponge effect but with cytotoxicity | [16,17,18,19] |
| Drug Delivery | Forms stable nanocomplexes with tunable release; lower toxicity | High drug loading due to dense amine content; stronger interactions | [20,21,22] |
| Co-Delivery (Gene + Drug) | Enables dual delivery of nucleic acids and hydrophobic drugs with reduced toxicity; effective for in vivo applications with PEGylation or FA-targeting | Higher loading efficiency and strong complexation; often used with ligands or PEG to reduce cytotoxicity | [22,23,24] |
| PEI Form | PEI MW | Transfection Efficiency | Cell Viability | Remarks | Ref. | |
|---|---|---|---|---|---|---|
| LMW PEI | 1.8 kDa | ~20–30% | >95% @ 10 µg/mL | Minimal cytotoxicity but ineffective transfection | [18] | |
| BPEI (25 kDa) | 25 kDa | ~70–95% | ~60% @ 10 µg/mL | Highly effective but significantly toxic; poor safety profile for therapeutic applications | [18] | |
| PEI–PCL–PEI + PEG–PCL (2 µL added) | 5–4 kDa | ~70–80% | ~85–90% @ 10 µg/mL | High performance, enhanced uptake and reduced toxicity due to PEG shielding, and improved stability | [18] | |
| Moderately crosslinked LPEI (x-LPEI-2) | ~22 kDa | ~70–80% | ~90% @ 20 µg/mL | Retained high transfection efficiency while dramatically reducing cytotoxicity | [19] | |
| BPEI | 25 kDa | ~40–50% | ~50–60% @ 20 µg/mL | Lower transfection efficiency and higher toxicity compared to crosslinked LPEI (xLPEI-2) | [19] | |
| Free PEI | 10 kDa | ~40–50% | ~50–60% @ 20 µg/mL | Unmodified PEI; effective but highly toxic | [41] | |
| PPS NPs (PEI + PDA + siRNA) | 10 kDa | ~55–60% | ~65–70% @ 20 µg/mL | Intermediate formulation without MnO2/FA, moderately safe, and moderately effective | [41] | |
| PPSM NPs (PEI + PDA + siRNA + MnO2 + FA) | 10 kDa | ~70–80% | ~80–90% @ 20 µg/mL | Optimized nanoparticle with FA and MnO2; best balance of efficiency and safety | [41] | |
| CNOC-PEI-PEG | 600 Da | N/A | >90% @ ≤100 µg/mL | Very low toxicity due to low-MW PEI | [42] | |
| BPEI | 25 kDa | ~60–70% | ~40–50% @ 10 µg/mL | Strong condensation and delivery, but high cytotoxicity at >5 µg/mL | [45] | |
| LPEI | 2.5 kDa | ~20–30% | ~80–90% @ 10 µg/mL | Poor gene delivery alone; considered safer but less potent | [45] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, A.; Chou, S.-F. Polyethylenimine Carriers for Drug and Gene Delivery. Polymers 2025, 17, 2150. https://doi.org/10.3390/polym17152150
Ismail A, Chou S-F. Polyethylenimine Carriers for Drug and Gene Delivery. Polymers. 2025; 17(15):2150. https://doi.org/10.3390/polym17152150
Chicago/Turabian StyleIsmail, Ahmed, and Shih-Feng Chou. 2025. "Polyethylenimine Carriers for Drug and Gene Delivery" Polymers 17, no. 15: 2150. https://doi.org/10.3390/polym17152150
APA StyleIsmail, A., & Chou, S.-F. (2025). Polyethylenimine Carriers for Drug and Gene Delivery. Polymers, 17(15), 2150. https://doi.org/10.3390/polym17152150









