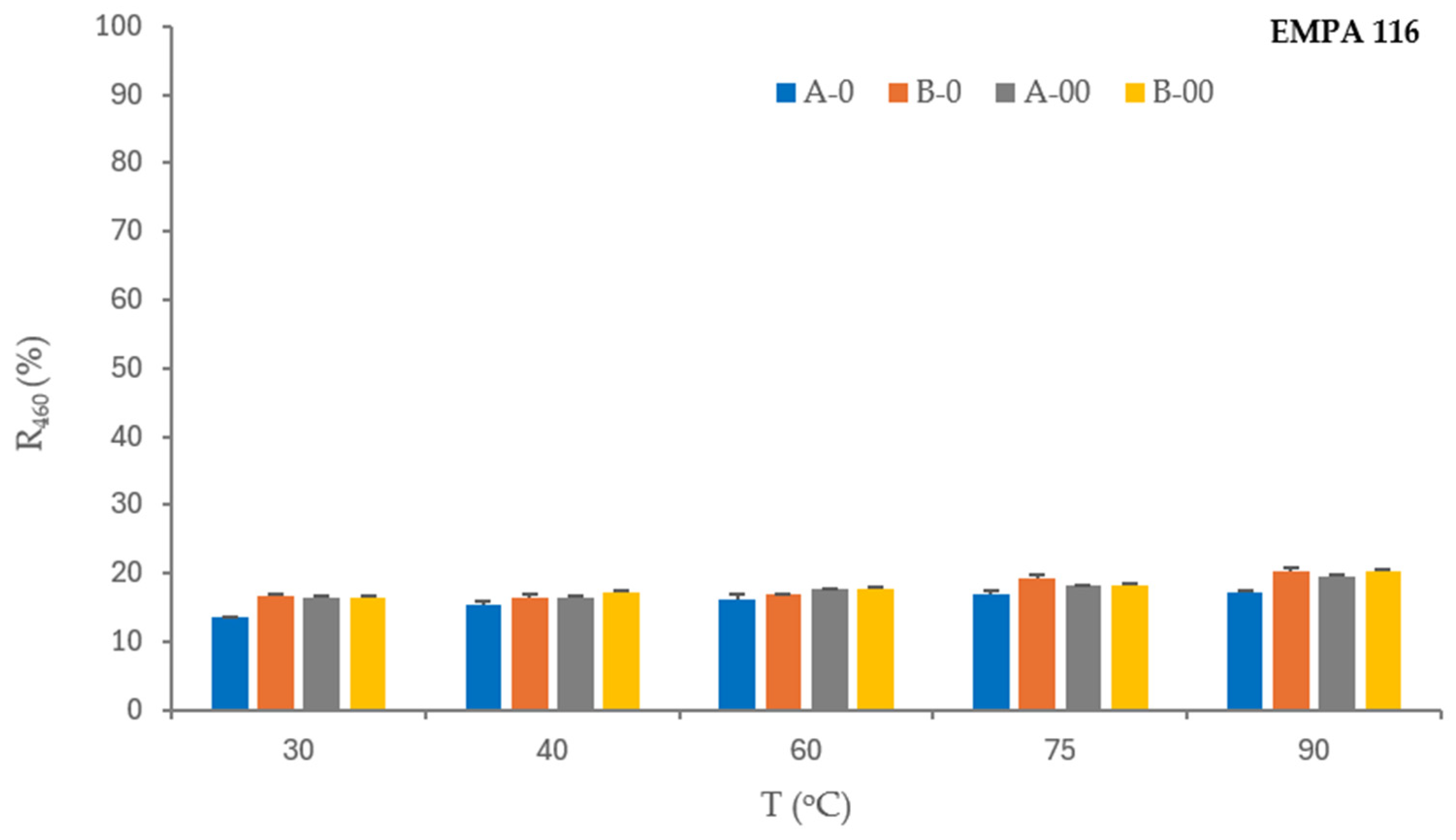3.1. Stain Removal
The influence of detergent and bleaching agent concentration in formulations (A, B) and their ozone-mediated effect (A-O, A-OO, B-O, B-OO) on stain removal from standard monitors—cotton fabrics colored with tea (WFK 10 J), red wine (WFK 90 LI), and blood/milk/ink (EMPA 116) at temperatures of 30 °C, 40 °C, 60 °C, 75 °C, and 90 °C is shown in
Figure 1,
Figure 2 and
Figure 3.
WFK 10 J is a reference monitor—cotton fabric stained with tea of unknown composition. According to [
32], many bioactive compounds such as polyphenols, pigments, polysaccharides, alkaloids, free amino acids, and saponins have been identified in tea, where the quantity of these ingredients depend on the tea category.
Stains (e.g., a natural dye from tea, wine, or fruit juice) from the surface are broken in a redox process enabled by a bleaching agent [
32,
33].
The reflectance values of WFK 10 J before (U) and after washing (A, B, A-O, A-OO, B, B-O, B-OO) in combination with the temperatures are shown in
Table 5.
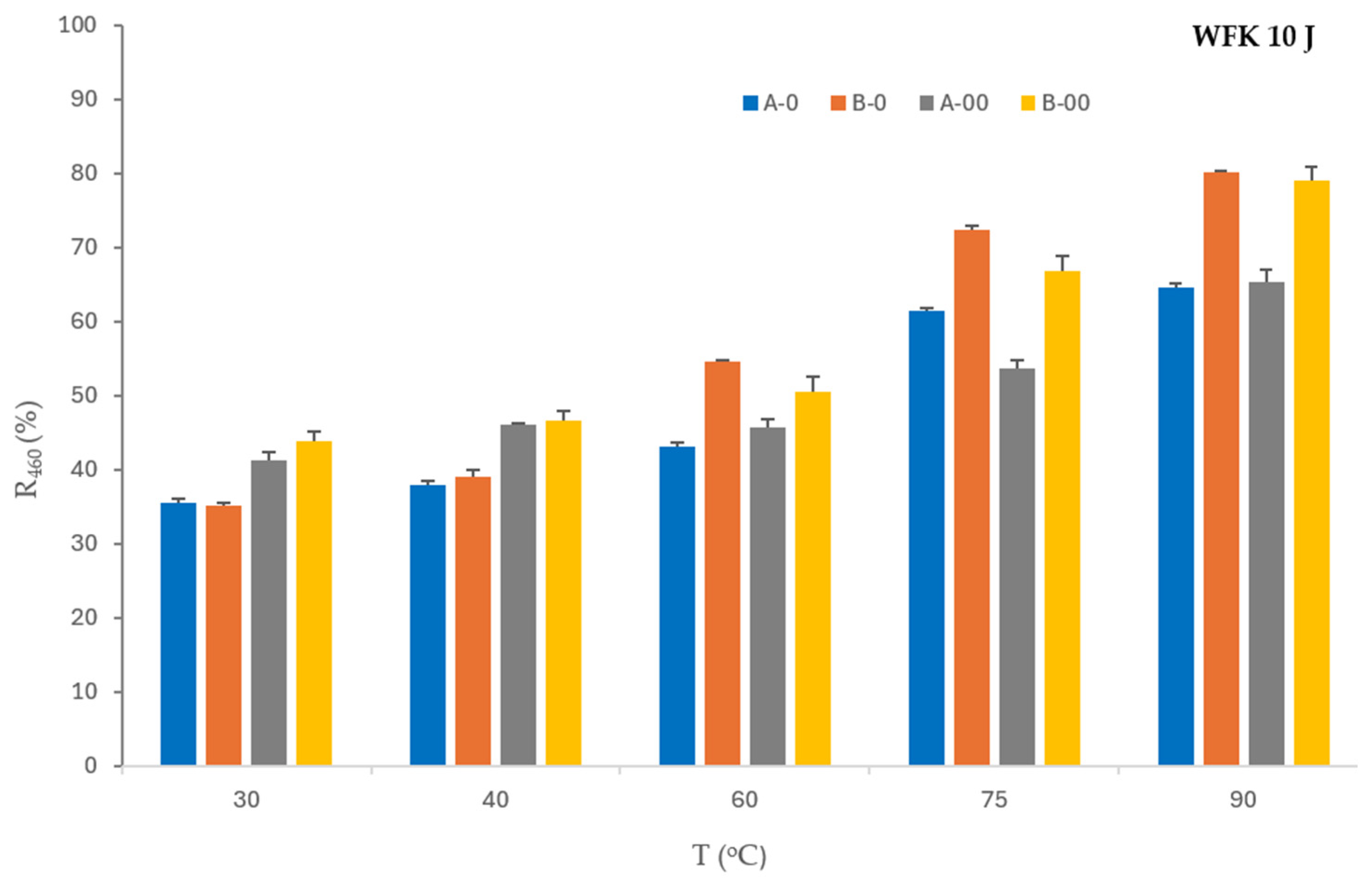
The reflectance values show that a higher concentration of detergent and bleaching agent (B) improves the removal of tea from a monitor at all temperatures compared to lower concentrations of detergent and bleaching agent (A). The washing process with a lower concentration of detergent and bleach, supported by a lower concentration of ozone (A-O), enhances the removal from the analyzed monitor only at 75 °C. The washing process with a high concentration of detergent and a lower concentration of ozone (B-O) does not improve the performance compared to the washing process with a high concentration of detergent and bleach without ozone (B). The effect of a higher concentration of ozone (A-OO, B-OO) improves the removal of soiling from tea at 30 °C and 40 °C compared to A-O and B-O.
Figure 2 shows results of the influence of ozone on the reflectance values of the washed WFK 10 J monitor in dependence of temperatures.
Figure 1 shows the influence of lower (O) and higher concentrations of ozone (OO) with lower (A) and higher concentrations of detergent and bleaching agent (B). The reflectance values of WFK 10 J of the washed monitor confirm that both concentrations of ozone are an equally good mediator at both concentrations of detergent and bleach at lower temperatures (30 °C and 40 °C). Accordingly, it was not necessary to increase the dosage of D and BA for this type of stain monitor. The figure clearly shows that the increased concentration of ozone (OO) with lower and higher concentrations of detergent and bleaching agent (A, B) only increases the reflectance values at temperatures of 30 °C and 40 °C, i.e., favors the removal of the tea stain from the cotton monitor. Lower concentrations of detergent, bleach, and ozone (A-O) improve the tea stain removal effect only at 75 °C, while a higher concentration of detergent and bleach with a lower concentration of ozone (B-O) increases the reflectance values in the washing process at 60 °C, 75 °C, and 90 °C.
WFK 90 LI is a standard cotton monitor stained with red wine. The compounds isolated from red wine are pyranoanthocyanins, where the pyran ring stabilizes the structure and provides a more intense color than anthocyanin dyes [
33].
The primary effect in the washing process of cotton fabric stained with red wine is improved by increasing the temperature in almost all analyzed washing process conditions (A, B, A-O, B-O, A-OO, B-OO). A small decrease in the reflectance value was recorded only for WFK 90 LI fabric washed with a smaller concentration of detergent and bleach at 90 °C (A). The influence of a smaller ozone concentration (O) with a high concentration of agents in washing bath B is insignificant. The influence of a higher ozone concentration (OO) with a higher concentration of detergent and bleaching agent compared to B and B-O is favorable only at 30 °C and 40 °C. Lower concentrations of agents (A) supported by a higher concentration of ozone (OO) improves the reflectance values of washed WFK LI fabric at all analyzed temperatures,
Table 6. The washing process with a higher concentration of detergent and bleach with a lower concentration of ozone (B-O) compared to B does not favor the effect of removing wine stains at the analyzed temperatures, while a higher concentration of ozone (B-OO) favors the effect at 30 °C and 40 °C.
Figure 2 shows more clearly that a higher concentration of ozone (OO) with a less concentration of detergent and bleaching agent (A) favors the removal of wine stains only at 30 °C, while a higher concentration of detergent and bleach (B) increases the reflectance values, i.e., favors the removal of wine stains from cotton monitors at temperatures of 30 °C and 40 °C.
The statement already made in the evaluation of the WFK 10J stain monitor that it is not necessary to increase the dose of D and BA for the WFK 90 LI does not apply. This confirms that it is necessary to monitor the influence of ozone in the washing process in combination with a specific stain monitor and it is difficult to draw general conclusions. The best effect is achieved with the B-O washing process at 90 °C. When observing the influence of temperature on the stain removal performance in the analyzed washing processes, it can be seen that the optimal combination depends on temperature, so the A-OO at 30 °C, the B-OO at 40 °C, the B-O at 60 °C, the B-O at 75 °C, and the B-O at 90 °C. As a summary, the best combination for removing wine stains at 60 °C, 75 °C, and 90 °C is a higher concentration of detergent and bleaching agent with a less concentration of ozone (B-O). A higher concentration of ozone favors the removal of wine stains at lower temperatures, 30 °C (A-OO) and 40 °C (B-OO).
Less concentrations of detergent, bleaching agent, and ozone (A-O) improve the tea stain removal effect only at 75 °C, while a high concentration of detergent and bleaching agent with a less concentration of ozone (B-O) increases the reflectance values in the washing process at 60 °C, 75 °C, and 90 °C.
EMPA 116 is a monitor stained with heterogenic mixture of blood/milk/ink for testing detergency and bleach effect in a washing process. The removal performance of blood/milk/ink stains from cotton fabric—reflectance values of EMPA 116 before and after the washing processes as a function of the observed variables is shown in
Table 7. The washing process of cotton fabric stained with a blood/milk/ink mixture (EMPA 116) requires a synergy of components that ensure the detergency and bleaching effect. Blood is a stain that requires a decolorizing agent, although its removal can sometimes be problematic [
5]. Stubborn protein stains on textiles from sources such as milk, cocoa, blood, egg, egg yolk, and grass are difficult to remove with non-enzymatic detergents, especially when the stains have dried and aged. Ink is a solution or suspension of suitable dyes or pigments [
34] that must be decolorized during the washing process.
The stain removal from the standard monitor EMPA 116 (cotton fabric soiled with a mixture of blood, milk, and ink) is extremely poor under all washing process conditions. From the reflectance values in
Table 7, it is evident that the bleaching agent in both concentrations was not efficient. It means that the protein component (blood/milk) predominates over the ink as a colorant, which requires proteolytic enzyme activity in the washing process that liquid detergents do not contain. The results obtained are consistent with earlier research findings, where it is proved that all tested standard soils, with the exception of EMPA 116, were washed better with hydrogen peroxide [
10].
The one-way ANOVA focusses on the evaluation effects of temperature for each washing process. Results of the ANOVA analysis,
Table 8, show that there is a statistically significant difference in relation to the temperature during the washing process,
p value < 0.05, and F value > F crit.
The analysis of the sum of reflectance values (ΣR
460) of all three washed monitors (WFK 90 LI, WFK 10 J and EMPA 116) is shown in
Table 9.
A higher ozone concentration combined with less concentrations of detergent and bleaching agent (A-OO) favors the achievement of the overall effect in the washing process at temperatures of 30 °C, 40 °C, 60 °C, and 90 °C. A higher ozone concentration in combination with a higher concentration of detergent and bleach (B-OO) is suitable at 30 °C and 40 °C. The washing process conducted with a higher concentration of detergent and bleach mediated by less ozone concentration (B-O) at 60 °C and 90 °C is the best combination.
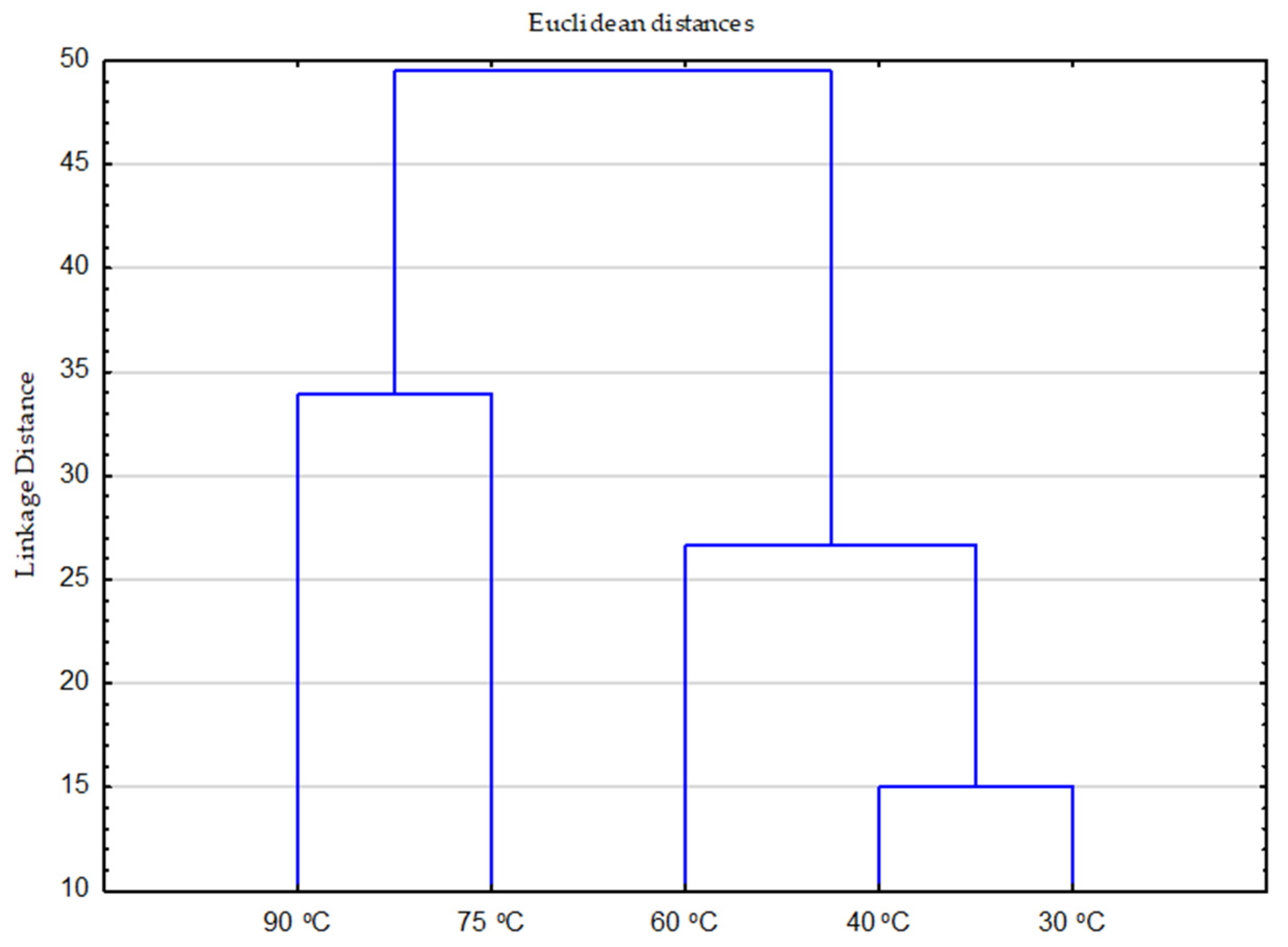
Analysis of the combined effect of all chemistry and temperature parameters on individual stains,
Figure 4 and
Figure 5.
The effect of temperature is illustrated by the expected standard distribution from lower to higher temperatures,
Figure 4.
The influence of chemistry (A, B, A-O, B-O, A-OO, B-OO) on a single stain in
Figure 5 is characterized by a different grouping. For the reference-stained monitor, EMPA 116, small differences in the effects were observed. Furthermore, the influence of chemistry on the WFK 10 J reference monitor is visible in the different distribution and grouping for low and high concentrations of detergent with bleaching agent and for low and high concentrations of ozone. Homogeneous grouping is characteristic for stain reference WFK 90 LI analyzed through the impact of chemistry.