Colorless Polyimides with Low Linear Coefficients of Thermal Expansion and Their Controlled Soft Adhesion/Easy Removability on Glass Substrates: Role of Modified One-Pot Polymerization Method
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
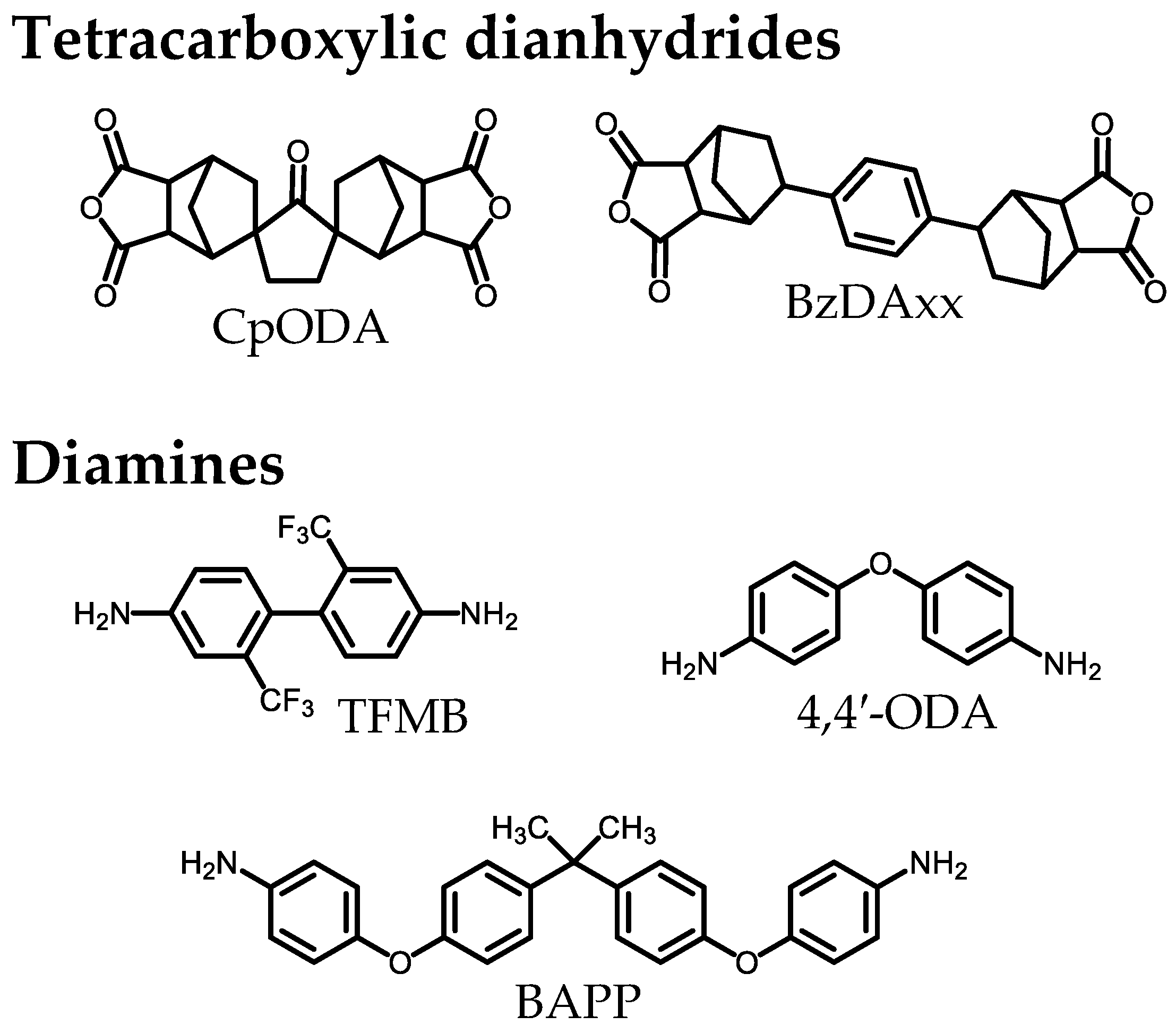
2.1.1. Monomers
2.1.2. Model Compounds
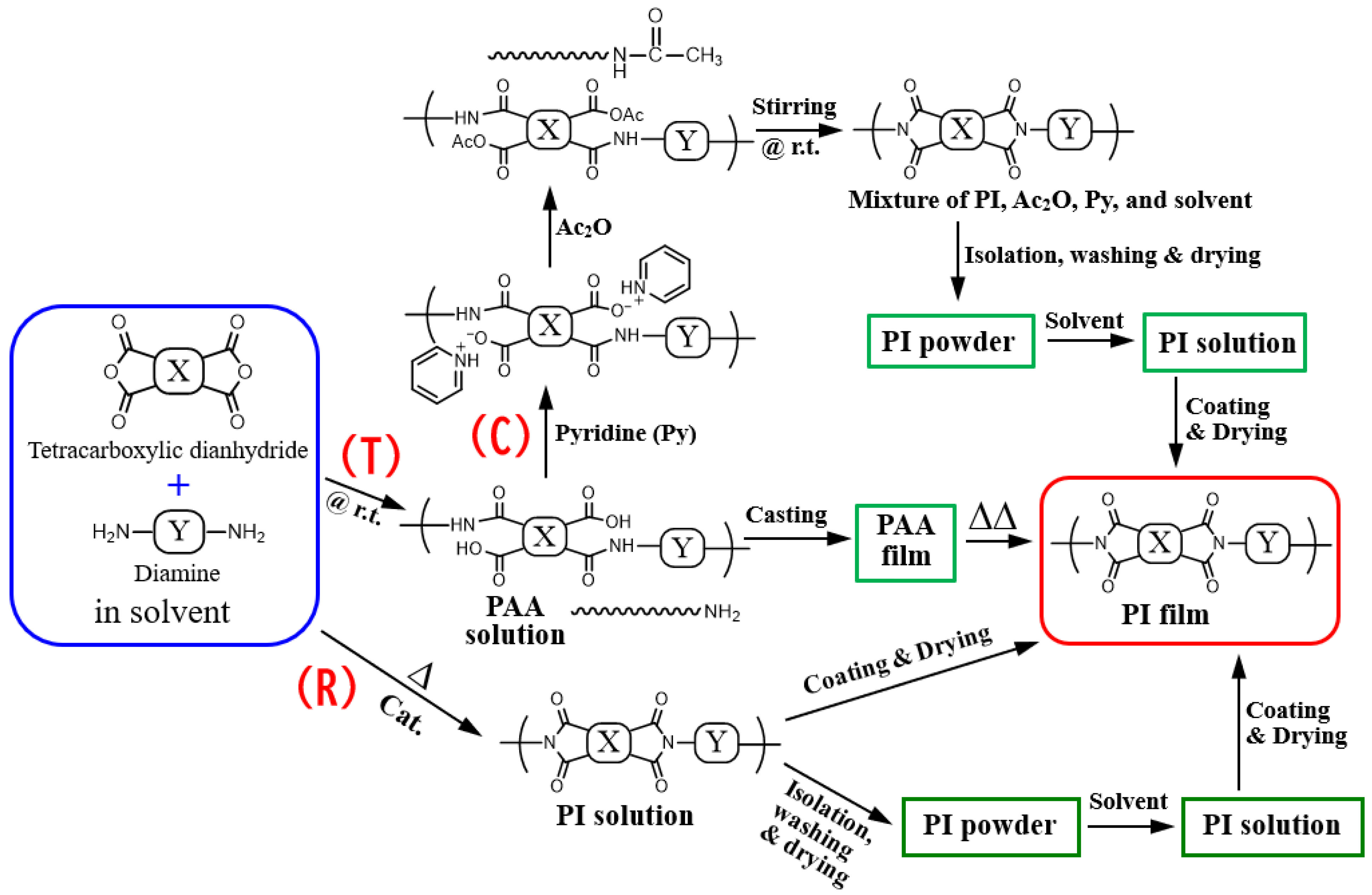
2.1.3. Polymerization and Film Preparation
2.2. Measurements
2.2.1. Structural Characterization
2.2.2. Inherent Viscosities and Molecular Weights
2.2.3. Linear Coefficients of Thermal Expansion (CTE)
- (1)
- Residual strain;
- (2)
- Adsorbed water;
- (3)
- Incomplete imidization and residual solvents;
- (4)
- Crystallization and orientational relaxation (at T > Tg).
2.2.4. Heat Resistance
2.2.5. Optical Transparency
2.2.6. Birefringence and Optically Estimated Dielectric Constant
2.2.7. Mechanical Properties
2.2.8. Water Uptake
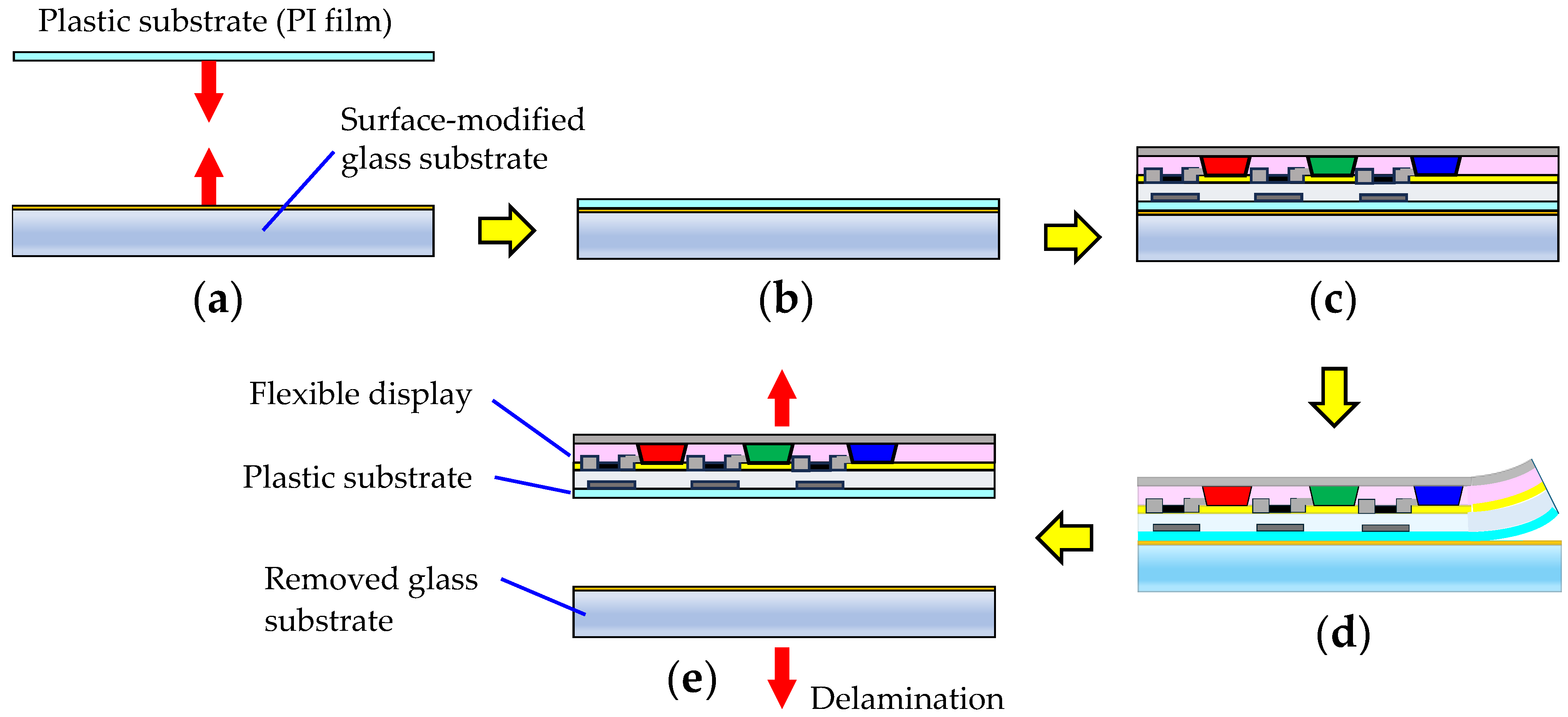
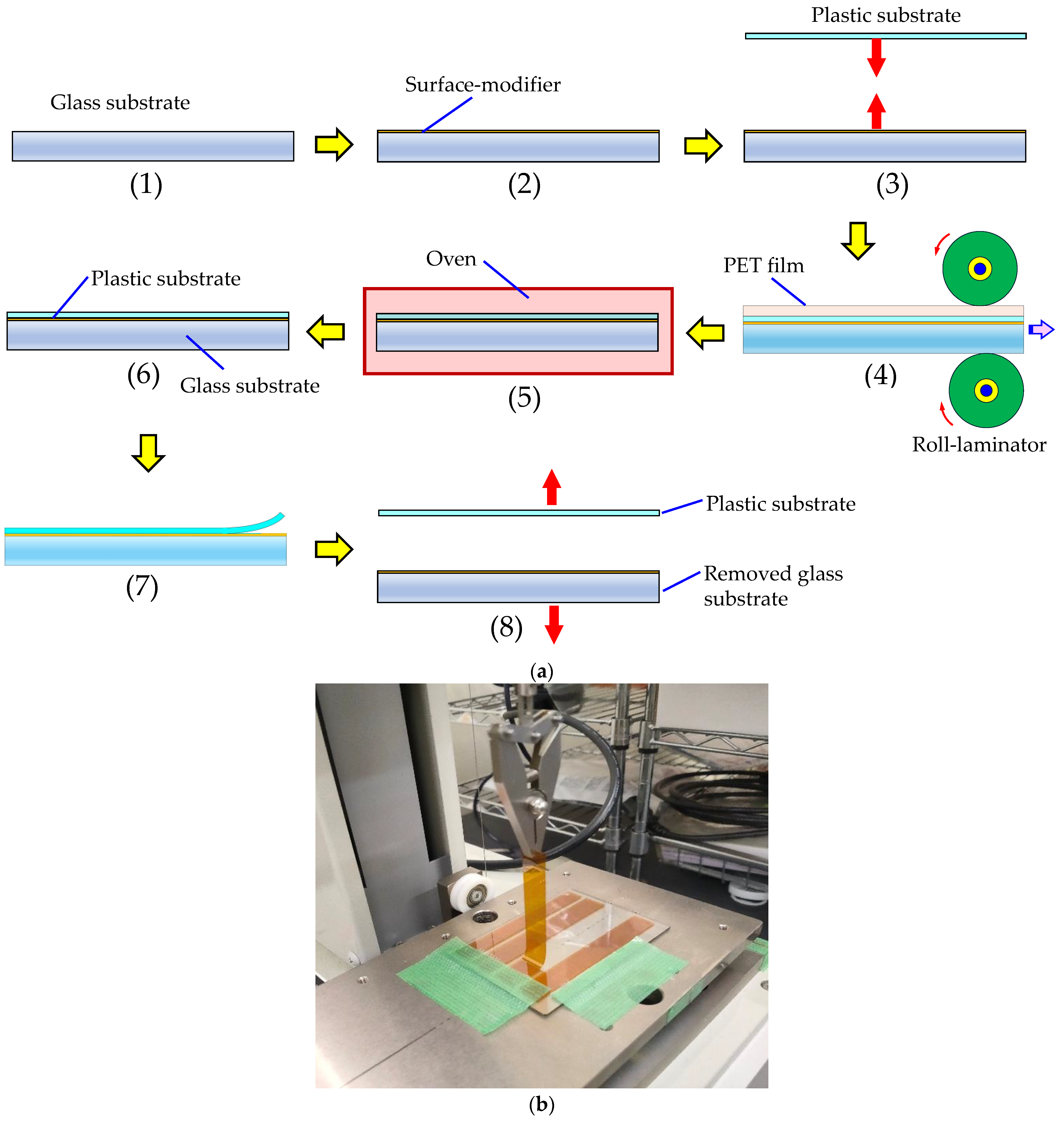
2.2.9. Interfacial Adhesion Strength of PI Film/Glass Substrate Laminates
2.2.10. Contents of Imide and Fluorine Groups in the PI Structures
3. Results and Discussion
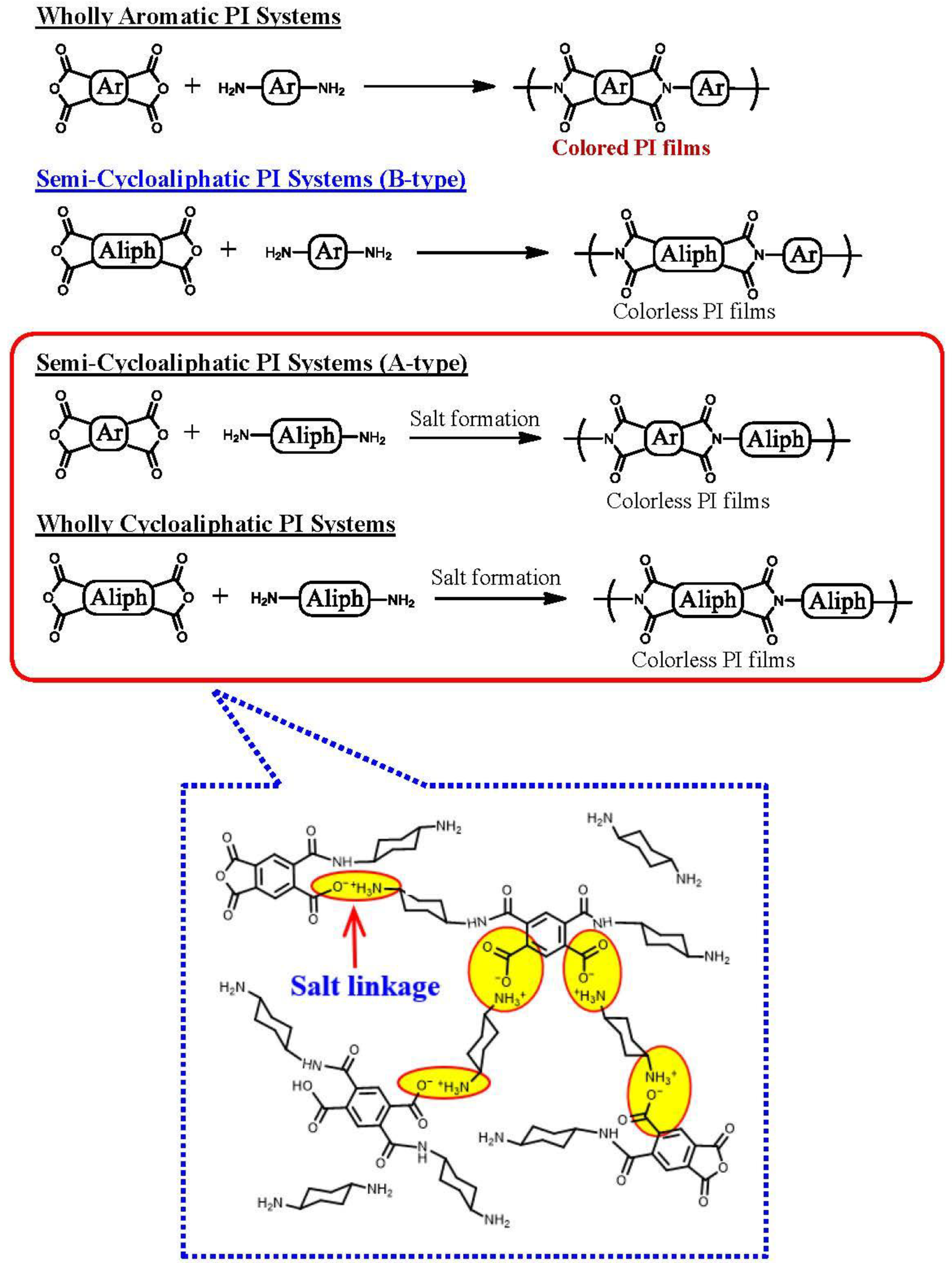
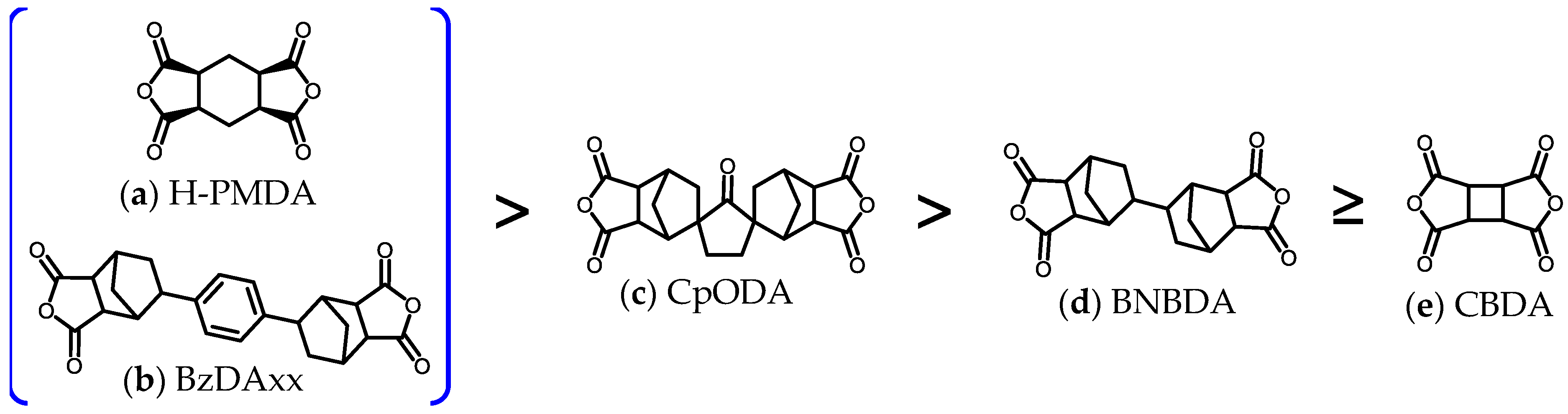
3.1. Difficulty in Simultaneously Achieving the Target Properties and Selection of Optimum Diamine for the Present Aim
3.2. Presumed Steric Structures and the Structural Linearity/Rigidity of CpODA and BzDAxx
3.3. Applicability to Conventional Two-Step Process
3.3.1. Polyaddition Reactivity of CpODA and BzDAxx with TFMB

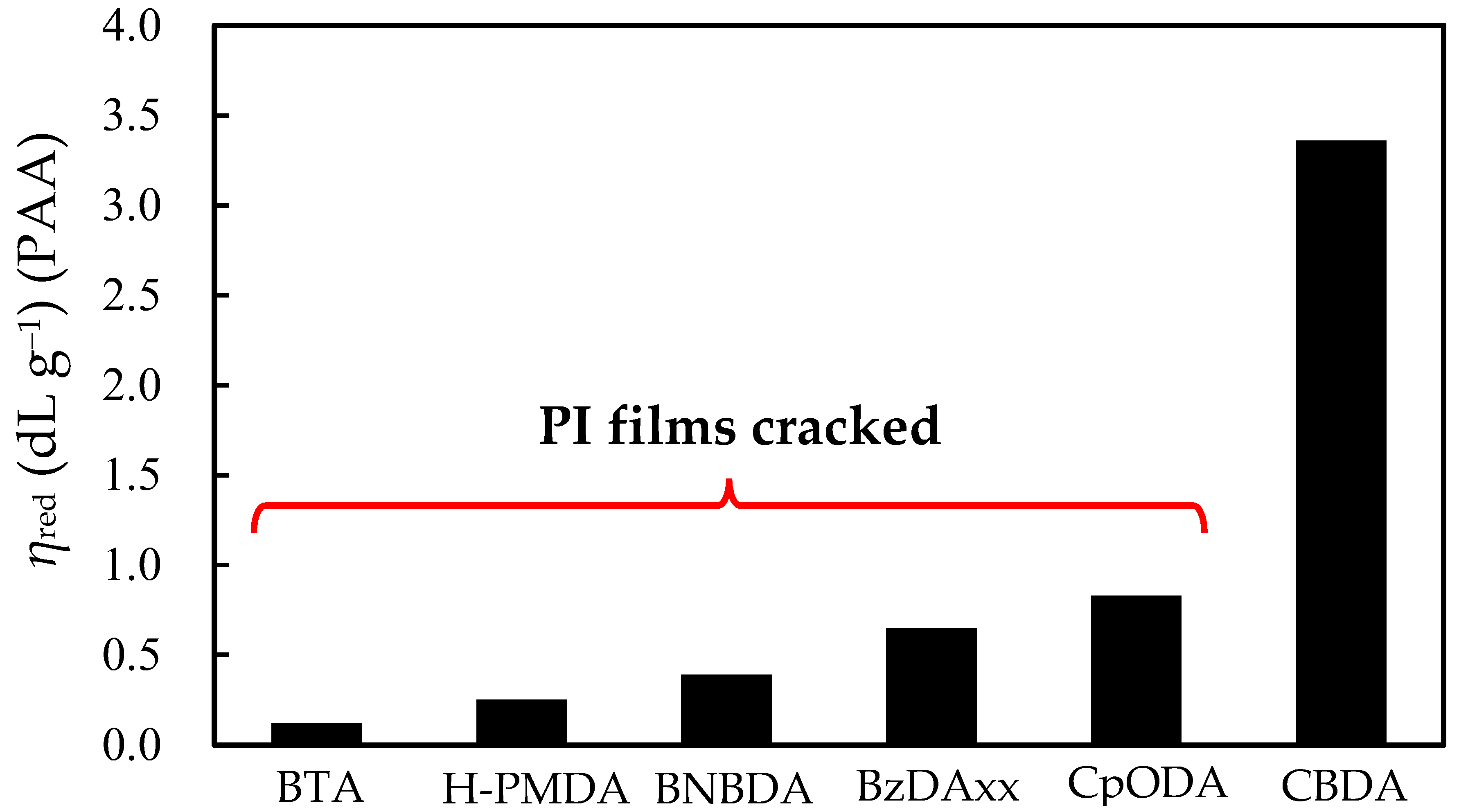
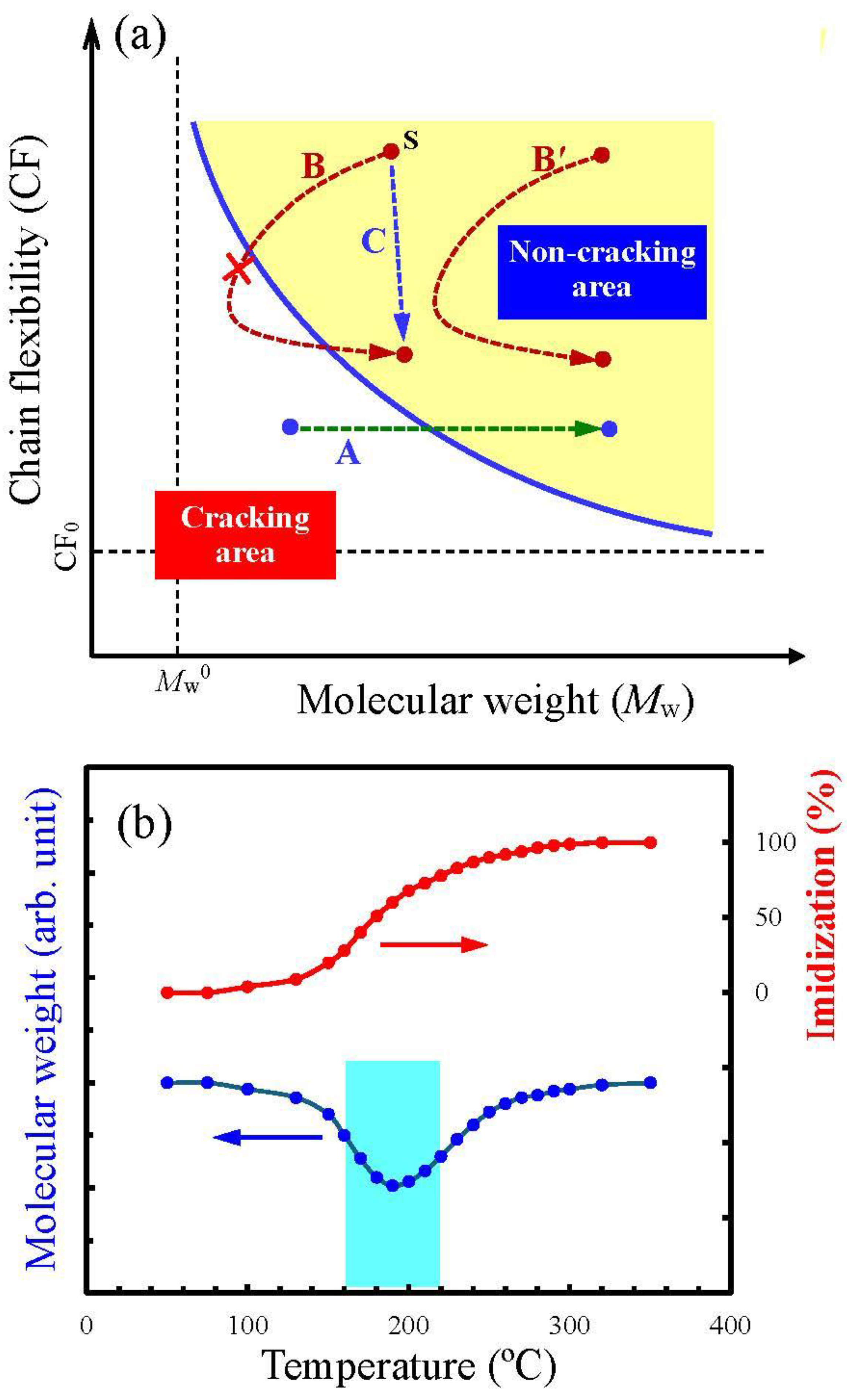
3.3.2. Factors for Film Cracking
3.4. Role of Modified One-Pot Polymerization Process
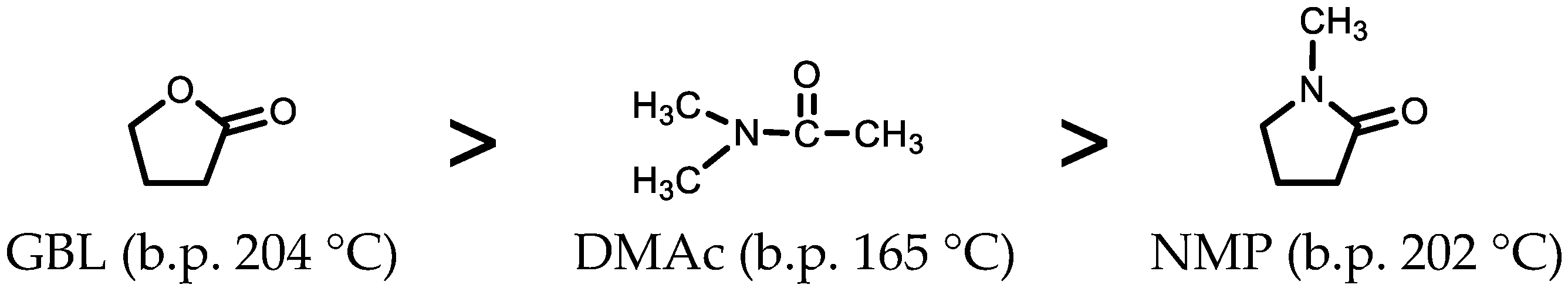
3.4.1. Refinement of One-Pot Polymerization Conditions
- (1)
- Solvents
- (2)
- Azeotropic agents
- (3)
- Temperature-rising profile
- (4)
- Reaction apparatus
- (5)
- Catalysts
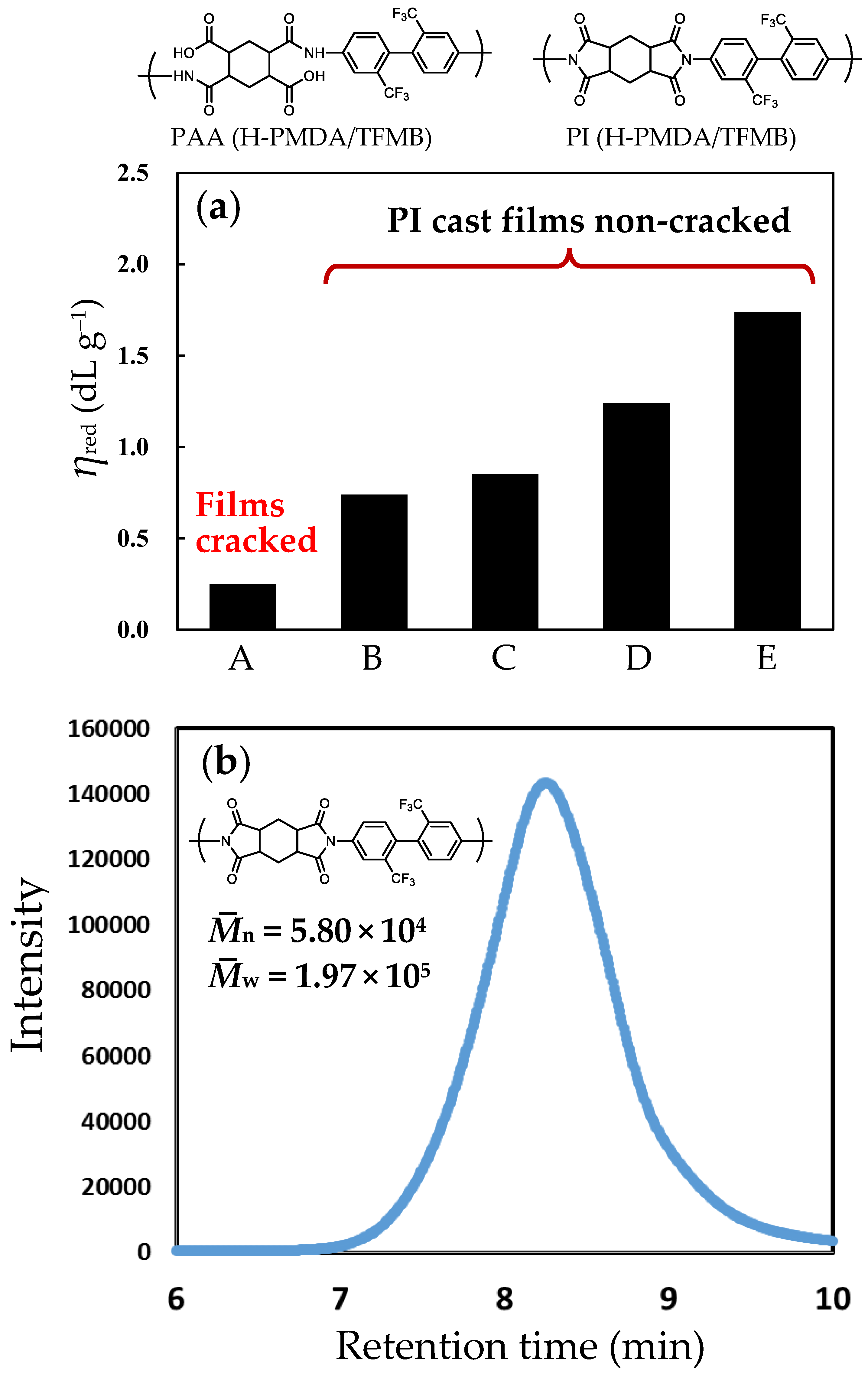
3.4.2. Impact of Imidization-Accelerating Catalyst on Mw Enhancement in the H-PMDA/TFMB System
3.4.3. Presumed Mechanism for the Effect of 1-EP/BA Combined Catalyst
3.4.4. Relationship Between Catalyst-Induced Imidization Acceleration and Molecular Weight Enhancement
- (1)
- A concomitant rapid decrease in the AA concentration ([AA]);
- (2)
- A resultant rightward shift of the equilibrium of the addition reaction (acid anhydride + amine ⇄ AA).
- (3)
- Chain extension of PAA (= increase in Mw of PAA)= increase in the Mw of the PI.
3.5. Polymerization Reactivity of CpODA and BzDAxx with TFMB in Modified One-Pot Process
3.6. Properties of CpODA-Based PIs
3.6.1. CpODA/4,4′-ODA System

3.6.2. CpODA/TFMB System
3.6.3. Attempts to Improve the Film Toughness of CpODA/TFMB Using Common Flexible Diamines
3.7. Properties of BzDAxx-Based PIs
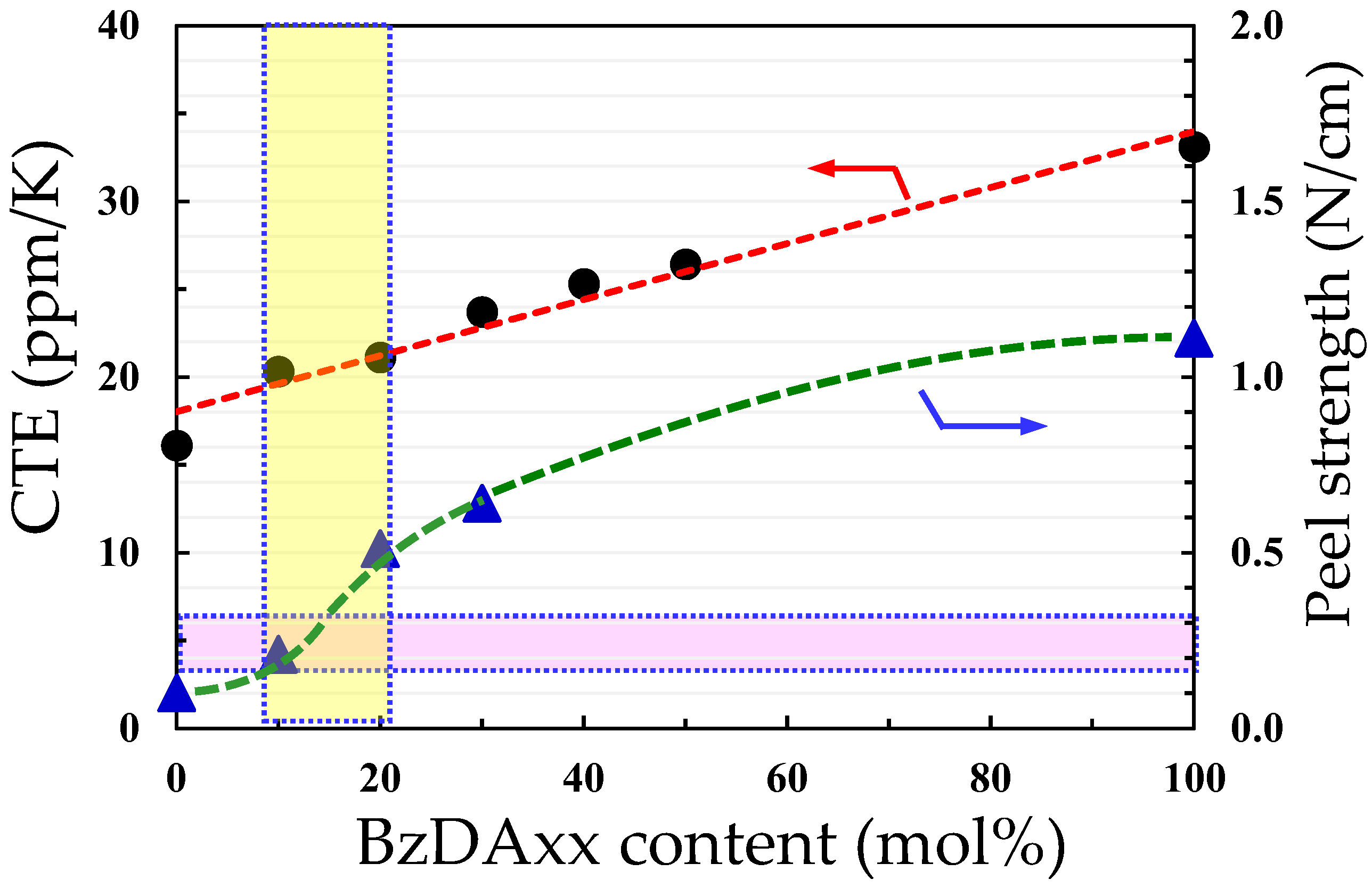
3.8. Modification of CpODA/TFMB Using BzDAxx
3.9. Compatibility with the Controlled Soft Adhesion/Easy Removal Process
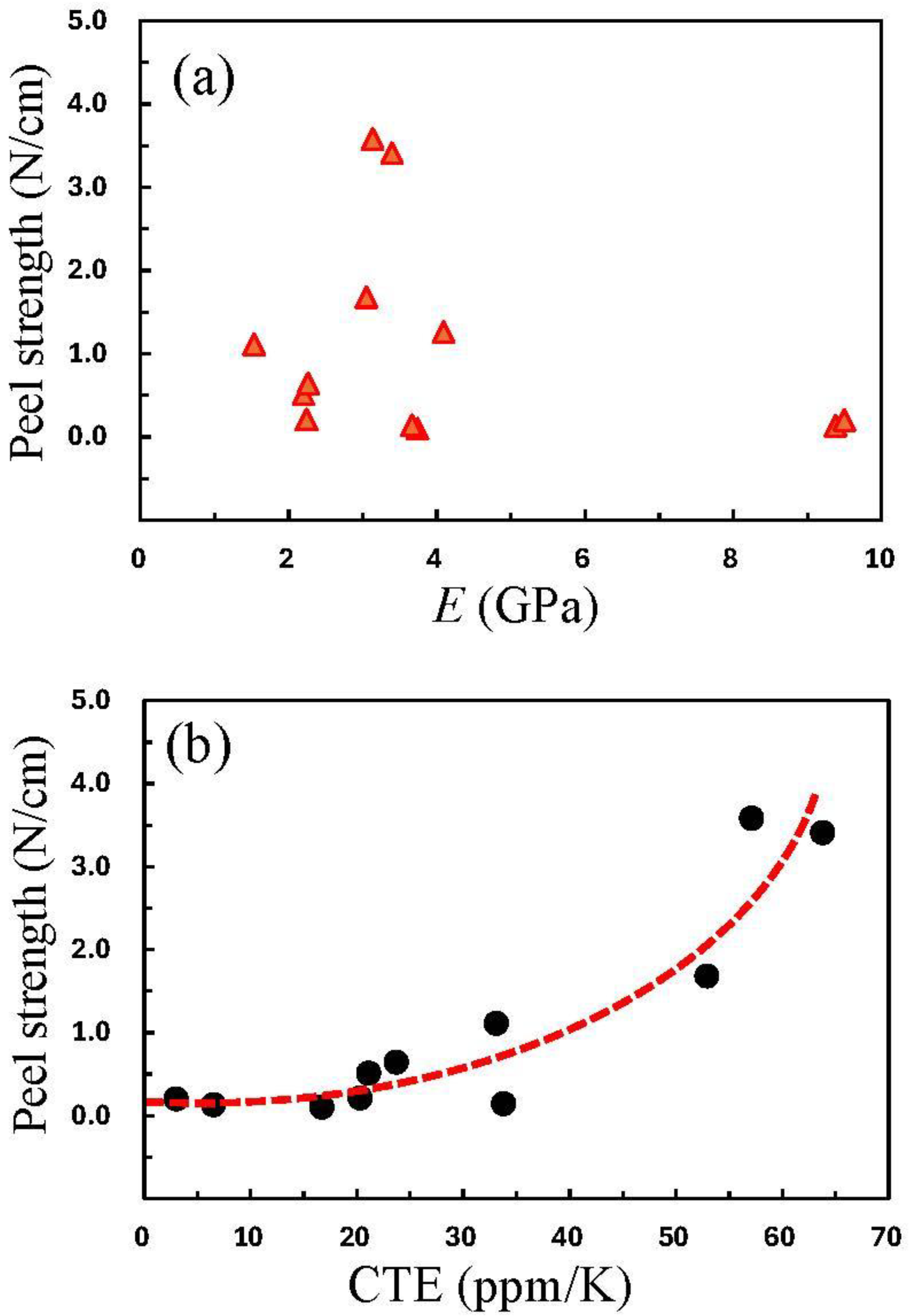
3.9.1. Impact of PI Structure on the Adhesion Strength

3.9.2. A Possible Hidden Parameter Dominating the Adhesion Strength
3.9.3. Attempt of Simultaneous Control of CTE, Film Toughness, and Adhesion Strength
3.10. Performance Balance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Bocko, P.L. The challenges of higher-generation glass. Inf. Disp. 2003, 19, 12–15. [Google Scholar]
- MacDonald, W.A.; Looney, M.K.; MacKerron, D.; Eveson, R.; Adam, R.; Hashimoto, K.; Rakos, K. Latest advances in substrates for flexible electronics. J. Soc. Inf. Disp. 2007, 15/12, 1075–1083. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, K.; Liu, Y.; Guo, Y.; Liu, Y. Intrinsically flexible displays: Key materials and devices. Natl. Sci. Rev. 2022, 9, nwac090. [Google Scholar] [CrossRef]
- SUMIKAEXCELTM PES Data Sheet. Available online: http://www.sumitomo-chem.co.jp/sep/english/products/pes/index.html (accessed on 1 June 2025).
- Cassidy, P.E. Thermally Stable Polymers: Syntheses and Properties; Marcel Dekker: New York, NY, USA, 1980. [Google Scholar]
- Polyimides: Synthesis, Characterization, and Applications; Mittal, K.L., Ed.; Plenum Press: New York, NY, USA, 1984; Volumes 1 and 2. [Google Scholar]
- Polyimides: Thermally Stable Polymers; Bessonov, M.I., Koton, M.M., Kudryavtsev, V.V., Laius, L.A., Eds.; Plenum: New York, NY, USA, 1987. [Google Scholar]
- Polyimides: Materials, Chemistry and Characterization; Feger, C., Khojasteh, M.M., McGrath, J.E., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Polyimides and Other High-Temperature Polymers; Abadie, M.J.M., Sillion, B., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Polyamic Acid and Polyimides: Synthesis, Transformation and Structure; Bessonov, M.I., Zubkov, V.A., Eds.; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Advances in Polyimide Science and Technology; Feger, C., Khojasteh, M.M., Htoo, M.S., Eds.; Technomic Publishing: Lancaster, UK, 1993. [Google Scholar]
- Polyimides: Fundamentals and Applications; Ghosh, M.K., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Advances in Polyimides and Low Dielectric Polymers; Sachdev, H.S., Khojasteh, M.M., Feger, C., Eds.; Society of Plastic Engineers: New York, NY, USA, 1997. [Google Scholar]
- Imai, Y.; Taoka, I.; Uno, K.; Iwakura, Y. Polybenzoxazoles and polybenzothiazoles. Die Makromol. Chem. 1965, 83, 167–178. [Google Scholar] [CrossRef]
- Kitagawa, T.; Murase, H.; Yabuki, K. Morphological study on poly-p-phenylenebenzobisoxazole (PBO) fiber. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 39–48. [Google Scholar] [CrossRef]
- Makabe, H.; Banba, T.; Hirano, T. A novel positive working photosensitive polymer for semiconductor surface coating. J. Photopolym. Sci. Technol. 1997, 10, 307–312. [Google Scholar] [CrossRef]
- Sroog, C.E. Polyimide. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Progress in Polyimide Chemistry I & II, Advances in Polymer Science; Kricheldorf, H.R., Ed.; Springer: New York, NY, USA, 1999; pp. 140–141. [Google Scholar]
- Hergenrother, P.M. The Use, design, synthesis, and properties of high performance/high temperature polymers: An overview. High Perform. Polym. 2003, 15, 3–45. [Google Scholar] [CrossRef]
- Ree, M. High performance polyimides for applications in microelectronics and flat panel displays. Macromol. Res. 2006, 14, 1–33. [Google Scholar] [CrossRef]
- The Latest Polyimides: Fundamentals and Applications, 2nd ed.; Ando, S., Ueda, M., Kakimoto, M., Kochi, M., Takeichi, T., Hasegawa, M., Yokota, R., Eds.; NTS: Tokyo, Japan, 2010. [Google Scholar]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Advanced Polyimide Materials: Synthesis, Characterization, and Applications; Yang, S.Y., Ed.; Chemical Industry Press, Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Polyimide for Electronic and Electrical Engineering Applications; Diaham, S., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- KAPTON® H Data Sheet. Available online: https://www.td-net.co.jp/kapton/data/download/documents/kapton2007.pdf (accessed on 1 June 2025).
- UPILEX®-S data sheet. Available online: https://www.ube.com/upilex/catalog/pdf/upilex_s.pdf (accessed on 1 June 2025).
- Matsuura, T.; Hasuda, Y.; Nishi, S.; Yamada, N. Polyimide derived from 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl. 1. Synthesis and characterization of polyimides prepared with 2,2′-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride or pyromellitic dianhydride. Macromolecules 1991, 24, 5001–5005. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishigami, T.; Ishii, J.; Sugiura, K.; Fujii, M. Solution-processable transparent polyimides with low coefficient of thermal expansion and self-orientation behavior induced by solution casting. Eur. Polym. J. 2013, 49, 3657–3672. [Google Scholar] [CrossRef]
- Volksen, W.; Cha, H.J.; Sanchez, M.I.; Yoon, D.Y. Polyimides derived from nonaromatic monomers: Synthesis, characterization and potential applications. React. Funct. Polym. 1996, 30, 61–69. [Google Scholar] [CrossRef]
- Seino, H.; Sasaki, T.; Mochizuki, A.; Ueda, M. Synthesis of fully aliphatic polyimides. High Perform. Polym. 1999, 11, 255–262. [Google Scholar] [CrossRef]
- Mathews, A.S.; Kim, I.; Ha, C.S. Synthesis, characterization, and properties of fully aliphatic polyimides and their derivatives for microelectronics and optoelectronics applications. Macromol. Res. 2007, 15, 114–128. [Google Scholar] [CrossRef]
- Tsuda, Y.; Etou, K.; Hiyoshi, N.; Nishikawa, M.; Matsuki, Y.; Bessho, N. Soluble copolyimides based on 2,3,5-tricarboxycyclopentyl acetic dianhydride and conventional aromatic tetracarboxylic dianhydrides. Polym. J. 1998, 30, 222–228. [Google Scholar] [CrossRef]
- Ni, H.; Liu, J.; Wang, Z.; Yang, S. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Suzuki, H.; Abe, T.; Takaishi, K.; Narita, M.; Hamada, F. The synthesis and X-ray structure of 1,2,3,4-cyclobutanetetracarboxylic dianhydride and the preparation of a new type of polyimide showing excellent transparency and heat resistance. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 108–116. [Google Scholar] [CrossRef]
- Matsumoto, T. Nonaromatic polyimides derived from cycloaliphatic monomers. Macromolecules 1999, 32, 4933–4939. [Google Scholar] [CrossRef]
- Li, J.; Kato, J.; Kudo, K.; Shiraishi, S. Synthesis and properties of novel soluble polyimides having an unsymmetric spiro tricyclic dianhydride unit. Macromol. Chem. Phys. 2000, 201, 2289–2297. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kasamatsu, K.; Koseki, K. Colorless poly(ester imide)s derived from hydrogenated trimellitic anhydride. Eur. Polym. J. 2012, 48, 483–498. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride with controlled steric structure. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horiuchi, M.; Kumakura, K.; Koyama, J. Colorless polyimides with low coefficient of thermal expansion derived from alkyl-substituted cyclobutanetetracarboxylic dianhydrides. Polym. Int. 2014, 63, 486–500. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujii, M.; Ishii, J.; Yamaguchi, S.; Takezawa, E.; Kagayama, T.; Ishikawa, A. Colorless polyimides derived from 1S,2S,4R,5R-cyclohexanetetracarboxylic dianhydride, self-orientation behavior during solution casting, and their optoelectronic applications. Polymer 2014, 55, 4693–4708. [Google Scholar] [CrossRef]
- Shiotani, A.; Shimazaki, H.; Matsuo, M. Preparation of transparent polyimides derived from cis- and trans-dicyclohexyl-3,3′,4,4′-tetracarboxylic dianhydrides. Macromol. Mater. Eng. 2001, 286, 434–441. [Google Scholar] [CrossRef]
- Guo, Y.; Song, H.; Zhai, L.; Liu, J.; Yang, S. Synthesis and characterization of novel semi-alicyclic polyimides from methyl-substituted tetralin dianhydride and aromatic diamines. Polym. J. 2012, 44, 718–723. [Google Scholar] [CrossRef]
- Fang, X.; Yang, Z.; Zhang, S.; Gao, L.; Ding, M. Synthesis and properties of polyimides derived from cis- and trans-1,2,3,4-cyclohexanetetracarboxylic dianhydrides. Polymer 2004, 45, 2539–2549. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, D.; Du, H.; Wu, X.; Zhang, Y.; Tan, Y.; Wu, L.; Liu, J.; Zhang, X. Reduced coefficients of linear thermal expansion of colorless and transparent semi-alicyclic polyimide films via incorporation of rigid-rod amide moiety: Preparation and properties. Polymers 2020, 12, 413. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Hu, X.; Yan, J.; Wang, Y.; Mu, H.; Wang, Z.; Cheng, H.; Zhao, F.; Wang, Z. Colorless polyimides derived from 2R,5R,7S,10S-naphthanetetracarboxylic dianhydride. Polym. Chem. 2017, 8, 6165–6172. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ozawa, H.; Ishiguro, E.; Komatsu, S. Properties of alicyclic polyimides with bis-spironorbornane structure prepared in various solvents. J. Photopolym. Sci. Technol. 2016, 29, 237–242. [Google Scholar] [CrossRef]
- Ozawa, H.; Ishiguro, E.; Kyoya, Y.; Kikuchi, Y.; Matsumoto, T. Colorless polyimides derived from an alicyclic tetracarboxylic dianhydride, CpODA. Polymers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Liu, H.; Zhai, L.; Bai, L.; He, M.; Wang, C.; Mo, S.; Fan, L. Synthesis and characterization of optically transparent semi-aromatic polyimide films with low fluorine content. Polymer 2019, 163, 106–114. [Google Scholar] [CrossRef]
- Hu, X.; Mu, H.; Wang, Y.; Wang, Z.; Yan, J. Colorless polyimides derived from isomeric dicyclohexyl-tetracarboxylic dianhydrides for optoelectronic applications. Polymer 2018, 134, 8–19. [Google Scholar] [CrossRef]
- Hasegawa, M.; Watanabe, Y.; Tsukuda, S.; Ishii, J. Solution-processable colorless polyimides with ultralow coefficients of thermal expansion for optoelectronic applications. Polym. Int. 2016, 65, 1063–1073. [Google Scholar] [CrossRef]
- Hasegawa, M.; Takahashi, S.; Tsukuda, S.; Hirai, T.; Ishii, J.; Yamashina, Y.; Kawamura, Y. Symmetric and asymmetric spiro-type colorless poly(ester imide)s with low coefficients of thermal expansion, high glass transition temperatures, and excellent solution-processability. Polymer 2019, 169, 167–184. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ichikawa, K.; Takahashi, S.; Ishii, J. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride: Strategies to reduce the coefficients of thermal expansion by maximizing the spontaneous chain orientation behavior during solution casting. Polymers 2022, 14, 1131. [Google Scholar] [CrossRef]
- Yu, H.C.; Kumar, S.V.; Lee, J.H.; Oh, S.Y.; Chung, C.M. Preparation of robust, flexible, transparent films from partially aliphatic copolyimide. Macromol. Res. 2015, 23, 566–573. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sato, H.; Hoshino, K.; Arao, Y.; Ishii, J. Colorless polyimides derived from octahydro-2,3,6,7-anthracenetetracarboxylic dianhydride. Macromol 2023, 3, 175–199. [Google Scholar] [CrossRef]
- Tang, Y.; Li, L.; Ma, K.; Chen, G.; Wang, W.; Fang, X. Transparent and organosoluble cardo polyimides with different trans/cis ratios of 1,4-diaminocyclohexane via aromatic nucleophilic substitution polymerization. Polym. Int. 2018, 67, 598–605. [Google Scholar] [CrossRef]
- Miao, J.; Hu, X.; Wang, X.; Meng, X.; Wang, Z.; Yan, J. Colorless polyimides derived from adamantane-containing diamine. Polym. Chem. 2021, 11, 6009–6016. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Ma, X.; Ghanem, B.S.; Pinnau, I. Synthesis and characterization of organo-soluble polyimides derived from alicyclic dianhydrides and a dihydroxyl-functionalized spirobisindane diamine. ACS Appl. Polym. Mater. 2019, 1, 63–69. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent trends on transparent colorless polyimides with balanced thermal and optical properties: Design and synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wu, D. Synthetic strategies for highly transparent and colorless polyimide film. J. Appl. Polym. Sci. 2022, 139, e52604. [Google Scholar] [CrossRef]
- Hasegawa, M.; Miyama, T.; Ishii, J.; Watanabe, D.; Uchida, A. Colorless polyimides derived from 5,5′-bis(2,3-norbornanedicarboxylic anhydride): Strategies to reduce the linear coefficients of thermal expansion and improve the film toughness. Polymers 2023, 15, 3838. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujii, M.; Wada, Y. Approaches to improve the film ductility of colorless cycloaliphatic polyimides. Polym. Adv. Technol. 2018, 29, 921–933. [Google Scholar] [CrossRef]
- Maeda, S. A new thermally stable polyimide film for advanced microelectronics packaging. J. Photopolym. Sci. Technol. 2008, 21, 95–99. [Google Scholar] [CrossRef]
- Okuyama, T.; Kobayashi, I.; Watanabe, N.; Tsuchiya, T.; Nakamura, M. Low Coefficient of Linear Thermal Expansion Polyimide Film for TFT Device Substrate. In Proceedings of the 20th International Display Workshops (IDW 2013), Sapporo, Japan, 3–6 December 2013; pp. 1542–1545. [Google Scholar]
- Maeda, S. Laminated Body, Manufacturing Method thereof, and Laminated Circuit Board. PCT International Application WOA2010-071145, 24 June 2010. [Google Scholar]
- Delmdahl, R.; Fricke, M.; Fechner, B. Laser lift-off systems for flexible-display production. J. Inf. Disp. 2014, 15, 1–4. [Google Scholar] [CrossRef]
- Nakagawa, K. Optical anisotropy of polyimide. J. Appl. Polym. Sci. 1990, 41, 2049–2058. [Google Scholar] [CrossRef]
- Boese, D.; Lee, H.; Yoon, D.Y.; Swalen, J.D.; Rabolt, J.F. Chain orientation and anisotropies in optical and dielectric properties in thin films of stiff polyimides. J. Polym. Sci. Part B Polym. Phys. 1992, 30, 1321–1327. [Google Scholar] [CrossRef]
- Numata, S.; Oohara, S.; Fujisaki, K.; Imaizumi, J.; Kinjo, N. Thermal expansion behavior of various aromatic polyimides. J. Appl. Polym. Sci. 1986, 31, 101–110. [Google Scholar] [CrossRef]
- Numata, S.; Fujisaki, K.; Kinjyo, N. Re-examination of the relationship between packing coefficient and thermal expansion coefficient for aromatic polyimides. Polymer 1987, 28, 2282–2288. [Google Scholar] [CrossRef]
- Hasegawa, M.; Takeuchi, Y.; Saito, T. Poly(ester imide)s with low linear coefficients of thermal expansion and low water uptake (VIII): Structure–flame retardancy relationship. Polymers 2024, 16, 1967. [Google Scholar] [CrossRef]
- Kimura, R.; Matsumoto, T. Colorless and thermally stable polymer—An alicyclic polyimide with cyclopentanone bis-spironorbornane structure. Kobunshi Ronbunshu 2011, 68, 127–131. [Google Scholar] [CrossRef]
- James, D.E.; Stille, J.K. The palladium(II) catalyzed olefin carbonylation reaction. Mechanisms and synthetic utility. J. Am. Chem. Soc. 1976, 98, 1810–1823. [Google Scholar] [CrossRef]
- James, D.E.; Stille, J.K. The palladium(II)-catalyzed olefin carbonylation reaction. IV. Carbon-13 nuclear magnetic resonance analysis of the reaction products. J. Org. Chem. 1976, 41, 1504–1511. [Google Scholar] [CrossRef]
- Matsumoto, T.; Komatsu, S. Norbornane-2-spiro-α-cycloalkanone-α′-spiro-2″-norbornane-5,5″,6,6″-tetracarboxylic dianhydride, norbornane-2-spiro-α-cyclopentanone-α′-spiro-2″-norbornane-5,5″,6,6″-tetracarboxylic acid and ester thereof, method for producing norbornane-2-spiro-α-cycloalkanone-α′-spiro-2″-norbornane-5,5″,6,6″-tetracarboxylic dianhydride, polyimide obtained using same, and method for producing polyimide. International patent publication WO2011-099518, 18 August 2011. [Google Scholar]
- Watanabe, D.; Hasegawa, T.; Kyobu, A. Tetracarboxylic dianhydride, Carbonyl Compound, Polyimide Precursor Resin, and Polyimide. International patent publication WO2019-172460, 28 November 2019. [Google Scholar]
- Nielsen, L.E. Mechanical Properties of Polymers and Composites; Marcel Dekker: New York, NY, USA, 1975. [Google Scholar]
- Volksen, W.; Cotts, P.; Yoon, D.Y. Molecular weight dependence of mechanical properties of poly(p,p′-oxydiphenylene pyromellitimide) films. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 2487–2495. [Google Scholar] [CrossRef]
- Sensui, N.; Ishii, J.; Takata, A.; Oami, Y.; Hasegawa, M.; Yokota, R. Ultra-low CTE and improved toughness of PMDA/PDA polyimide-based molecular composites containing asymmetric BPDA-type polyimides. High Perform. Polym. 2009, 21, 709–728. [Google Scholar] [CrossRef]
- Fukumaru, T.; Fujigaya, T.; Nakashima, N. Extremely high thermal resistive poly(p-phenylene benezobisoxazole) with desired shape and form from a newly synthesized soluble precursor. Macromolecules 2012, 45, 4247–4253. [Google Scholar] [CrossRef]
- Young, P.R.; Davis, J.R.J.; Chang, A.C.; Richardson, J.N. Characterization of a thermally imidized polyimide film. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 3107–3122. [Google Scholar] [CrossRef]
- Pryde, C.A. IR studies of polyimides. I. Effects of chemical and physical changes during cure J. Polym. Sci. Part A Polym. Chem. 1989, 27, 711–724. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sensui, N.; Shindo, Y.; Yokota, R. Structure and properties of novel asymmetric biphenyl type polyimides. Homo- and copolymers and blends. Macromolecules 1999, 32, 387–396. [Google Scholar] [CrossRef]
- Shi, Z.; Hasegawa, M.; Shindo, Y.; Yokota, R.; He, F.; Yamaguchi, H.; Ozawa, H. Thermo-processable polyimides with high thermo-oxidative stability as derived from oxydiphthalic anhydride (ODPA) and bisphenol A type dianhydride. High Perform. Polym. 2000, 12, 377–393. [Google Scholar] [CrossRef]
- Inoue, H.; Sasaki, Y.; Ogawa, T. Comparison of one-pot and two-step polymerization of polyimide from BPDA/ODA. J. Appl. Polym. Sci. 1996, 60, 123–131. [Google Scholar] [CrossRef]
- Harris, F.W.; Hsu, S.L.-C. Synthesis and characterization of polyimides based on 3,6-diphenylpyromellitic dianhydride. High Perform. Polym. 1989, 1, 3–16. [Google Scholar] [CrossRef]
- Kuznetsov, A.A. One-pot polyimide synthesis in carboxylic acid medium. High Perform. Polym. 2000, 12, 445–460. [Google Scholar] [CrossRef]
- Brekner, M.J.; Feger, C. Curing studies of a polyimide precursor. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 2005–2020. [Google Scholar] [CrossRef]
- Brekner, M.J.; Feger, C. Curing studies of a polyimide precursor. II. Polyamic acid. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 2479–2491. [Google Scholar] [CrossRef]
- Brady, J.E.; Humiston, G.E. General Chemistry: Principles and Structure, 4th ed.; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Polymer Handbook, 4th ed.; Brandrup, J., Immergut, E.H., Grulke, E.A., Eds.; John Wiley: New York, NY, USA, 1999. [Google Scholar]
- Hasegawa, M.; Matano, T.; Shindo, Y.; Sugimura, T. Spontaneous molecular orientation of polyimides induced by thermal imidization (2). In-plane orientation. Macromolecules 1996, 29, 7897–7909. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horii, S. Low-CTE Polyimides derived from 2,3,6,7-Naphthalenetetracarboxylic dianhydride. Polym. J. 2007, 39, 610–621. [Google Scholar] [CrossRef]
- XENOMAX® Data Sheet. Available online: https://xenomax.jp/pdf/Xenomax_data2024.pdf (accessed on 1 June 2025).
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; Khan, F.U.; Ullah, R.; Iqbal, M.; Ali, A.; Ullah, B. Recent progress in silane coupling agent with its emerging applications. J. Polym. Environ. 2021, 29, 3427–3443. [Google Scholar] [CrossRef]
- Wu, S.; Liang, J.; Shi, Y.; Huang, M.; Bi, X.; Wang, Z.; Jin, J. Design of interchain hydrogen bond in polyimide membrane for improved gas selectivity and membrane stability. J. Membr. Sci. 2021, 618, 118659. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tsujimura, Y.; Koseki, K.; Miyazaki, T. Poly(ester imide)s possessing low CTE and low water absorption (II). Effect of substituents. Polym. J. 2008, 40, 56–67. [Google Scholar]
- Tanaka, K.; Takahara, A.; Kajiyama, T. Rheological analysis of surface relaxation process of monodisperse polystyrene films. Macromolecules 2000, 33, 7588–7593. [Google Scholar] [CrossRef]
- Sakai, T.; Ishikawa, K.; Takezoe, H.; Matsuie, N.; Yamamoto, Y.; Ishii, H.; Ouchi, Y.; Oji, H.; Seki, K. Surface orientation of main and side chains of polyimide alignment layer studied by near-edge X-ray absorption fine structure spectroscopy. J. Phys. Chem. B 2001, 105, 9191–9195. [Google Scholar] [CrossRef]

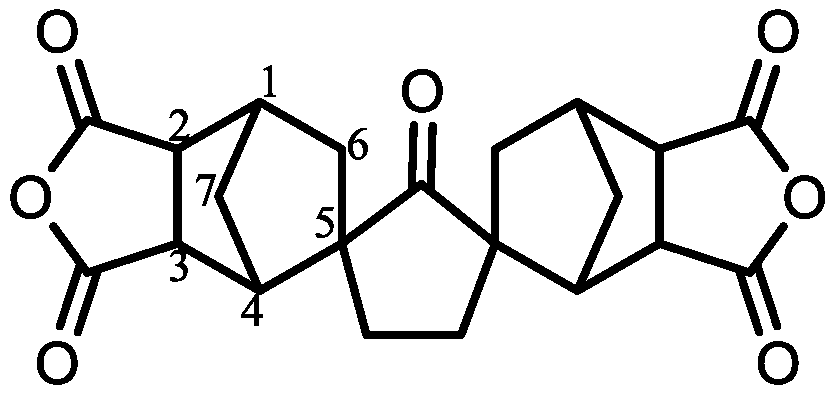
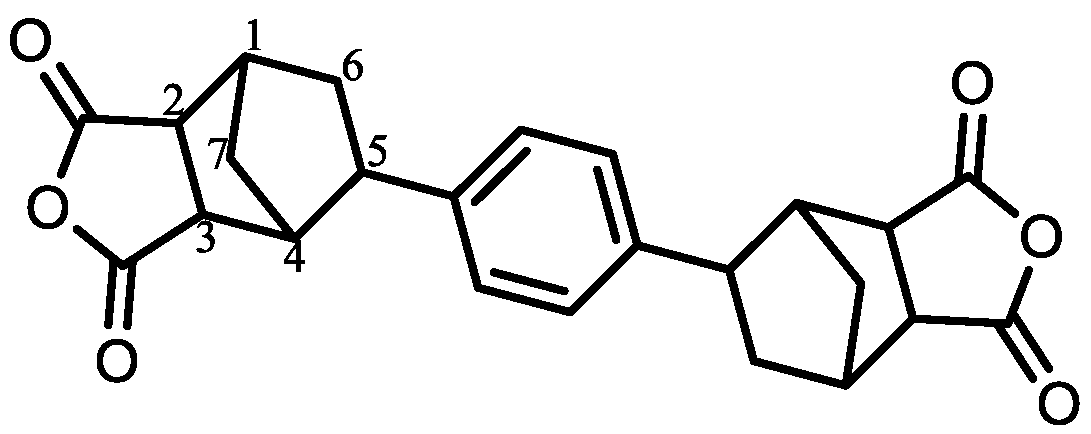


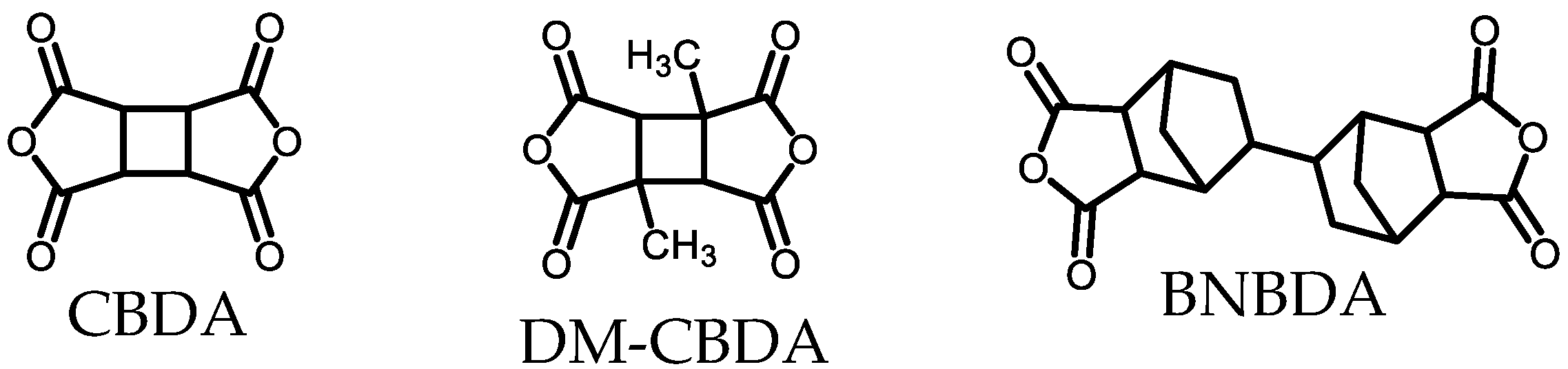


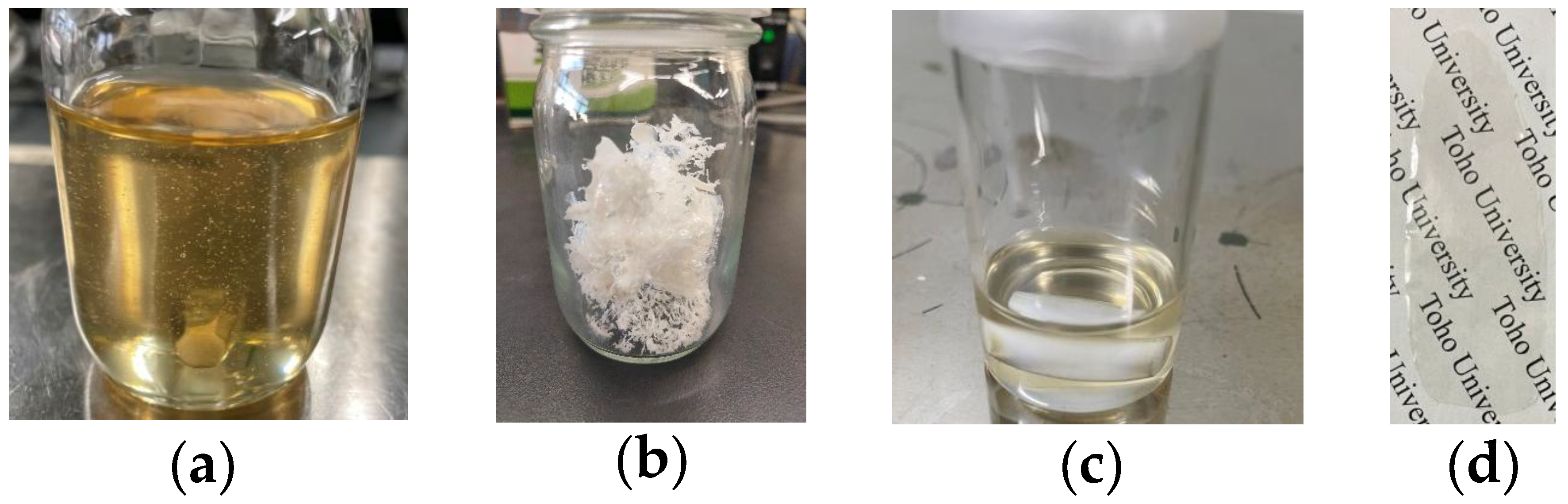


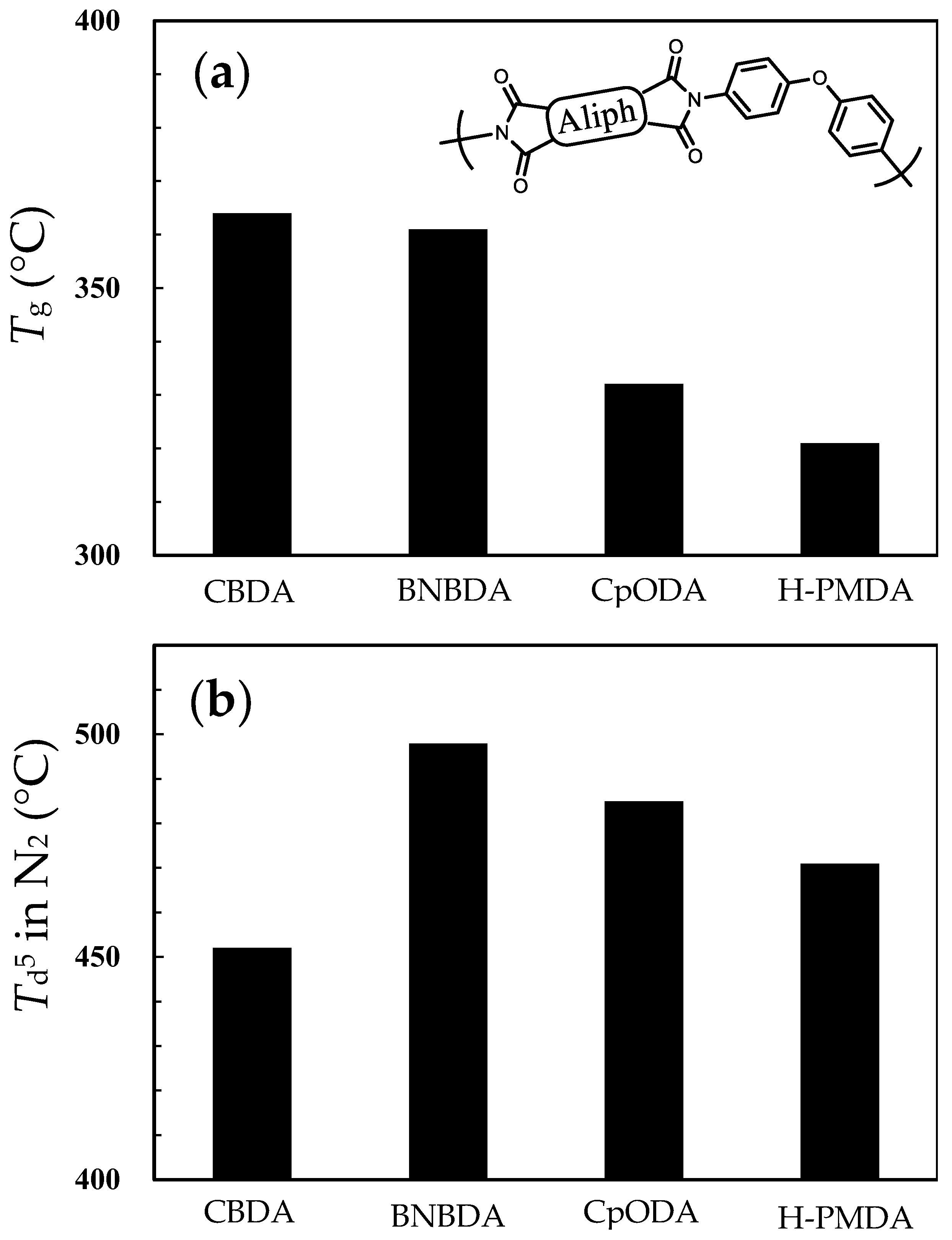
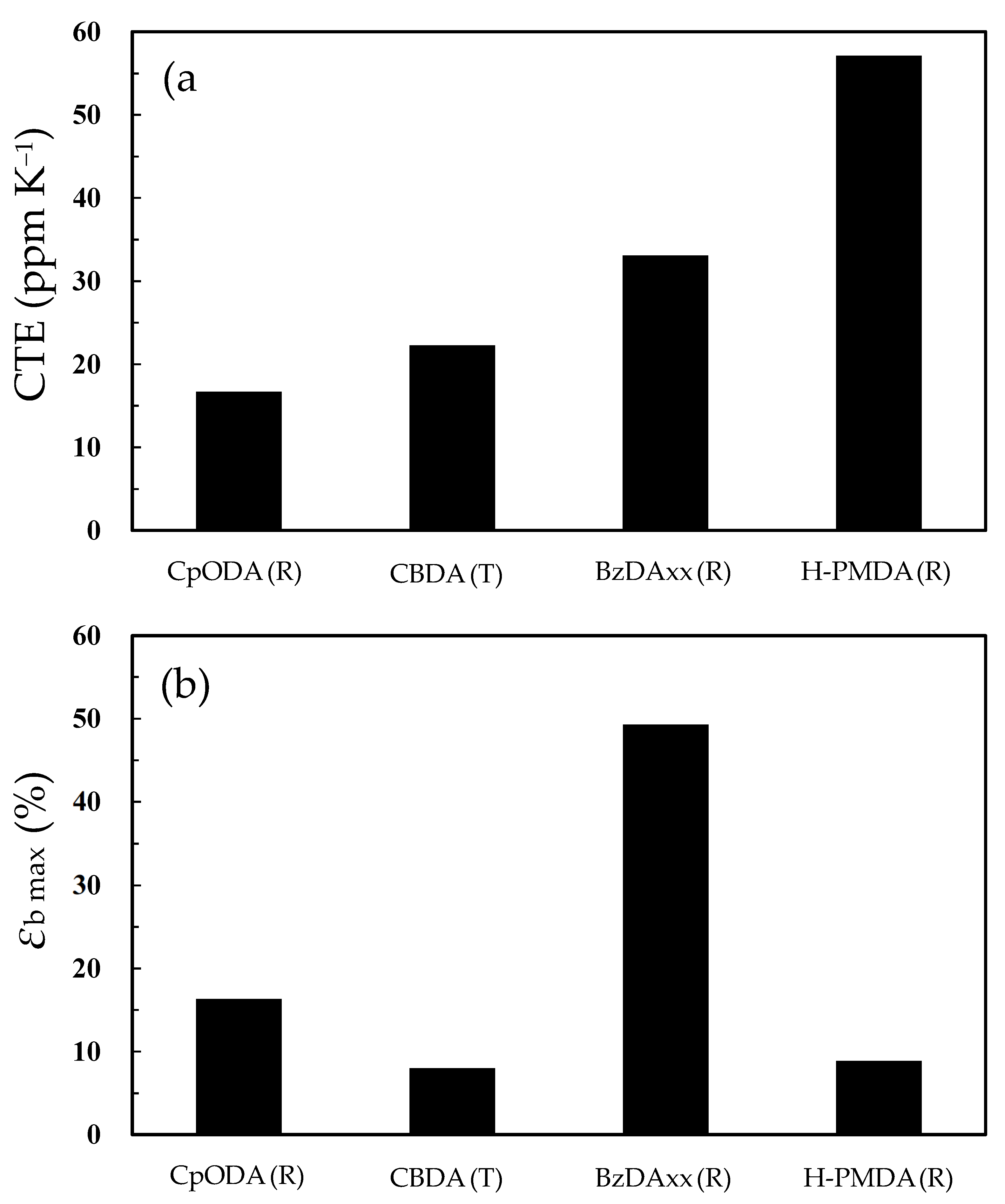
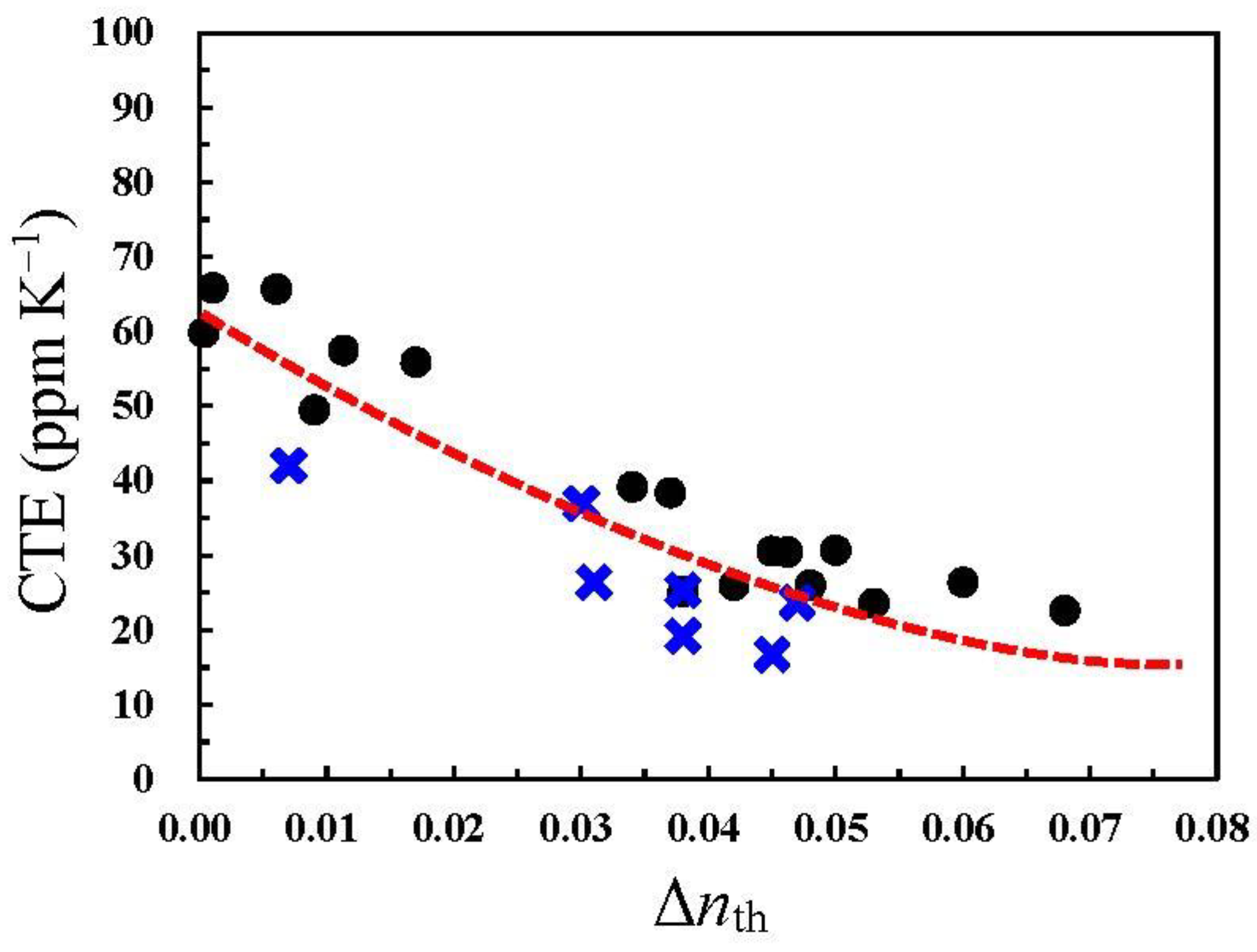
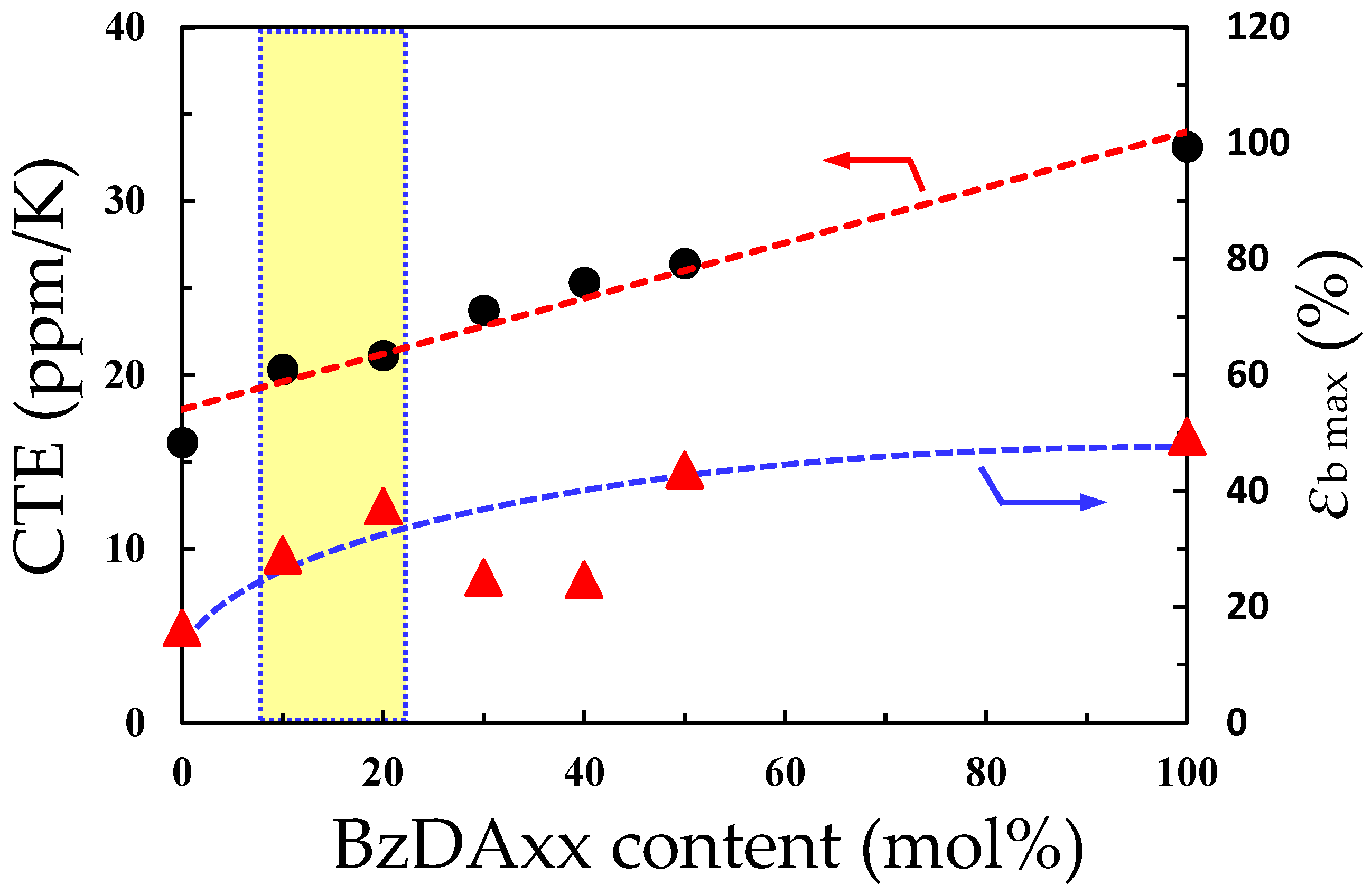
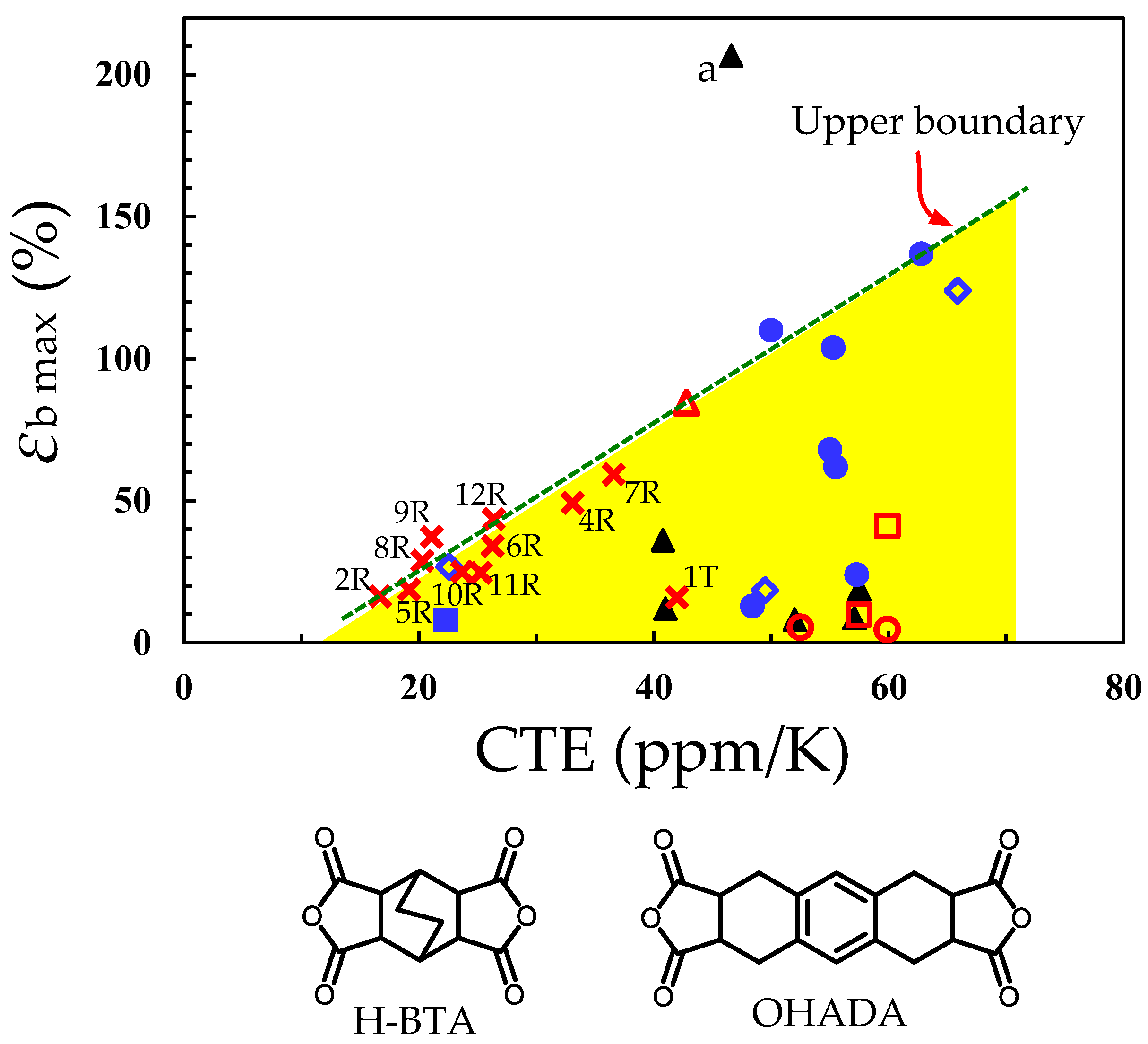

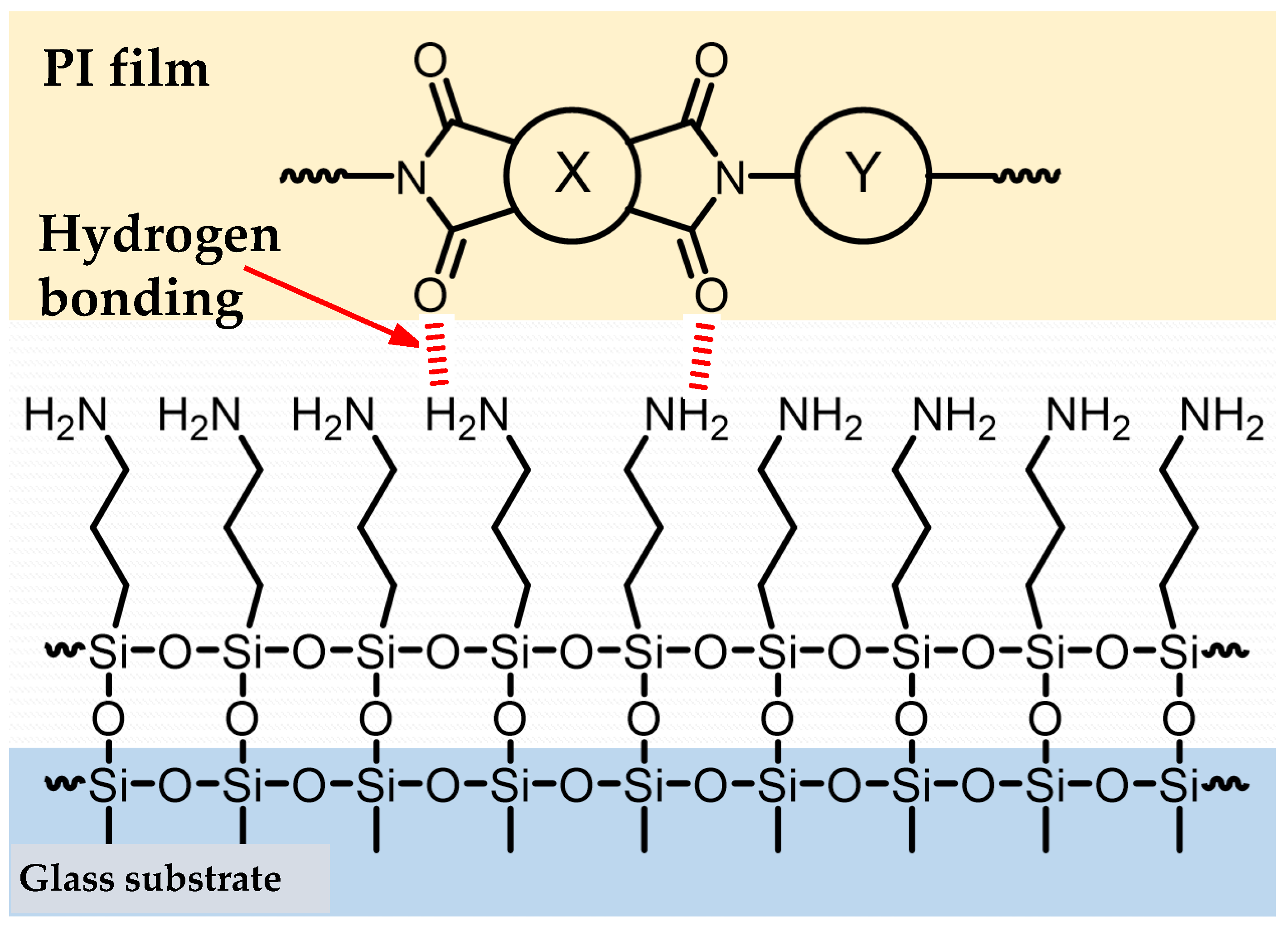
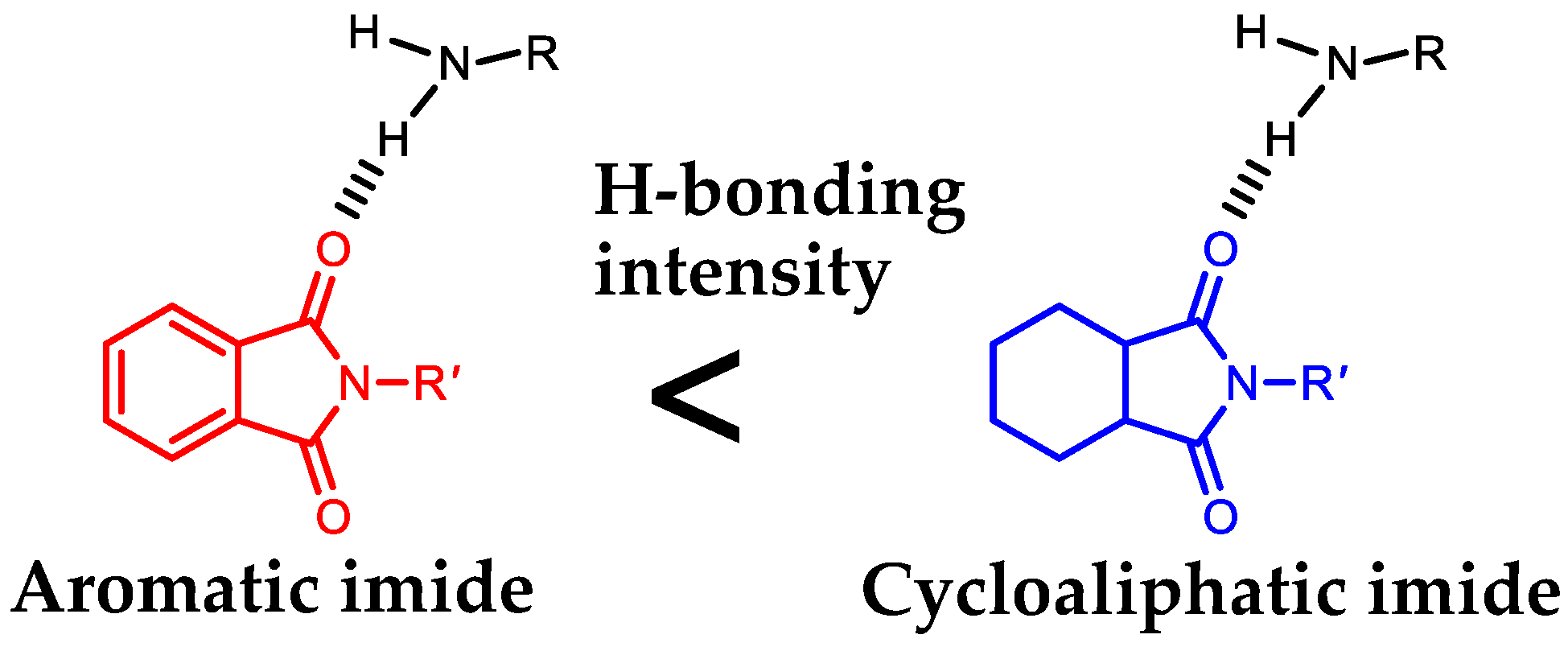

| Monomer | Source | Vacuum-Drying Condition | Melting Point a (°C) |
| Norbornane-2-spiro-α-cyclopentanone-α′-spiro-2″-norbornane-5,5″,6,6″-tetracarboxylic dianhydride (CpODA) | ENEOS | 160 °C/12 h | 333/337 (Double peak) |
| 5,5′-(1,4-Phenylene)-exo-bis(hexahydro-4,7- methanoisobenzofuran-cis-exo-1,3-dione) (BzDAxx) | ENEOS | 160 °C/12 h | 209 (broad) |
| 1,2,3,4-Cyclobutanetetracarboxylic dianhydride (CBDA) | Nissan Chemical Industries | 180 °C/ 12 h | 241 |
| 1-exo,2-exo,4-exo,5-exo-Cyclohexanetetracarboxylic dianhydride (H-PMDA) | Mitsubishi Gas Chemical New Japan Chemical | 160 °C/12 h | 303 |
| 2,2′-Bis(trifluoromethyl)benzidine (TFMB) | Wakayama Seika | 50 °C/12 h | 184 |
| 4,4′-Oxydianiline (4,4′-ODA) | Wako Chemical | 50 °C/24 h | 191 |
| 2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP) | Wakayama Seika | 50 °C/12 h | 130 |
| No. | TCDA | Diamine | Solvent | Solid Content (wt%) Initial → Final | Reaction Period (h) | Appearance of Final Reaction Mixture | ηred (dL g−1) | Appearance of PI Film b |
|---|---|---|---|---|---|---|---|---|
| 1T | CpODA | 4,4′-ODA | NMP | 50 → 29.4 | 72 | Homogeneous | 0.82 (PAA) 0.76 (PAA) a | non-cracked |
| 2T | ibid | TFMB | DMAc | 40 → 30 | 72 | Homogeneous | 0.83 (PAA) | cracked |
| 2C | ibid | ibid | ibid | ibid | ibid | ibid | 0.71 (PAA) 0.99 (PI) | non-cracked but brittle |
| 3T | BzDAxx | 4,4′-ODA | NMP | 50 → 31.6 | 72 | Homogeneous | 0.62 (PAA) | cracked |
| 4T | ibid | TFMB | DMAc | 40 → 30 | 72 | Homogeneous | 0.65 (PAA) | cracked |
| 4C | ibid | ibid | ibid | ibid | 72 | Homogeneous | 0.68 (PAA) 0.90 (PI) | cracked |
| No. | TCDA (mol%) | Diamine (mol%) | Solvent | Monomer Content (wt%) Initial → Final | Appearance of Final Reaction Mixture | ηred (PI) (dL g−1) |
|---|---|---|---|---|---|---|
| 1R | CpODA | 4,4′-ODA | NMP | 50.0 → 5.7 | Precipitation | --- |
| 2R | ibid | TFMB | DMAc | 50.0 → 10.8 | Homogeneous | 4.52 |
| 2R′ | ibid | ibid | GBL | 50.0 → 18.2 | Gelation | 3.29 |
| 5R | ibid | TFMB (90) 4,4′-ODA (10) | NMP | 50.0 → 11.0 | Homogeneous | 3.26 |
| 5R′ | ibid | ibid | DMAc | 50.0 → 8.3 | Slightly turbid | 3.86 |
| 6R | ibid | TFMB (70) BAPP (30) | DMAc | 50.0 → 19.7 | Homogeneous | 2.06 |
| 3R | BzDAxx | 4,4′-ODA | NMP | 50.0 → 4.2 | Precipitation | --- |
| 4R | ibid | TFMB | GBL | 50.0 → 9.9 | Homogeneous | 4.00 |
| 7R | BzDAxx | TFMB (90) 4,4′-ODA (10) | DMAc | 50.0 → 9.0 | Homogeneous | 1.49 |
| 8R | CpODA (90) BzDAxx (10) | TFMB | DMAc | 50.0 → 18.1 | Homogeneous | 1.76 |
| 9R | CpODA (80) BzDAxx (20) | TFMB | DMAc | 50.0 → 15.9 | Homogeneous | 3.07 |
| 10R | CpODA (70) BzDAxx (30) | TFMB | DMAc | 50.0 → 15.4 | Homogeneous | 2.97 |
| 11R | CpODA (60) BzDAxx (40) | TFMB | DMAc | 30.0 → 9.6 | Homogeneous | 4.29 |
| 12R | CpODA (50) BzDAxx (50) | TFMB | DMAc | 50.0 → 8.4 | Homogeneous | 2.35 |
 | ||||||
| No. | TCDA | Diamine (mol%) | ηred (dL g−1) | T400 (%) | YI | λcut (nm) | Ttot (%) | Haze (%) | Δnth | εoptg | Tg (ºC) | CTE (ppm K−1) | E (GPa) | εb ave/max (%) | σb (GPa) | Td5 (N2) (°C) | Td5 (Air) (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1T | CpODA | 4,4′-ODA | 0.82 a | 66.8 | 9.5 | 293 | 86.9 | 0.92 | 0.007 | 2.82 | 332 c 360 d | 42.0 | 2.01 | 11.5/15.9 | 0.064 | 485 | 424 |
| 2C | ibid | TFMB | 0.71 a 0.99 b | 87.0 | 1.7 | 290 | 90.4 | 0.71 | 0.047 | 2.66 | 341 c 336 d | 23.7 | --- e | --- e | --- e | 473 | 421 |
| 2R | ibid | ibid | 4.52 b | 85.0 | 1.8 | 292 | 90.3 | 0.94 | 0.045 | 2.65 | 411 c 410 d | 16.7 | 3.74 | 9.4/16.3 12.5/23.5 f | 0.089 | 478 | 432 |
| 5R | ibid | TFMB (90) 4,4′-ODA (10) | 3.26 b | 77.4 | 6.0 | 294 | 88.9 | 1.29 | 0.038 | 2.66 | 400 c 419 d | 19.2 | 3.32 | 8.7/18.8 | 0.109 | 483 | 425 |
| 6R | ibid | TFMB (70) BAPP (30) | 2.06 b | 87.5 | 1.3 | 292 | 90.1 | 0.64 | --- | --- | 362 c 360 d | 26.3 | 2.56 | 19.6/34.1 | 0.105 | --- | --- |
| 4R | BzDAxx | TFMB | 4.00 b | 86.5 | 1.4 | 287 | 90.3 | 1.14 | --- | --- | 372 c 374 d | 33.1 | 1.53 | 13.9/49.3 | 0.053 | 472 | 430 |
| 7R | ibid | TFMB (90) 4,4′-ODA (10) | 1.49 b | 80.4 | 4.1 | 289 | 89.9 | 1.20 | 0.029 | 2.69 | 368 c 370 d | 36.6 | 1.98 | 20.5/59.3 | 0.078 | 456 | 414 |
| 8R | CpODA (90) BzDAxx (10) | TFMB | 1.76 b | 87.0 | 1.8 | 295 | 90.4 | 1.79 | --- | --- | 385 d | 20.3 | 2.24 | 13.0/28.9 | 0.081 | 463 | --- |
| 9R | CpODA (80) BzDAxx (20) | ibid | 3.07 b | 86.3 | 1.8 | 294 | 90.4 | 0.92 | --- | --- | 393 d | 21.1 | 2.20 | 20.0/37.3 | 0.098 | 465 | --- |
| 10R | CpODA (70) BzDAxx (30) | ibid | 2.97 b | 87.3 | 1.9 | 294 | 90.6 | 0.82 | --- | --- | 411 d | 23.7 | 2.26 | 11.5/25.0 | 0.085 | 469 | --- |
| 11R | CpODA (60) BzDAxx (40) | ibid | 4.29 b | 85.8 | 1.7 | 291 | 90.4 | 1.00 | 0.038 | 2.65 | 390 c 403 d | 25.3 | 2.13 | 11.8/24.6 | 0.076 | 462 | 408 |
| 12R | CpODA (50) BzDAxx (50) | ibid | 2.35 b | 86.1 | 1.9 | 291 | 90.4 | 1.01 | 0.031 | 2.64 | 383 c 394 d | 26.4 | 2.67 | 17.0/43.6 | 0.107 | 461 | 414 |
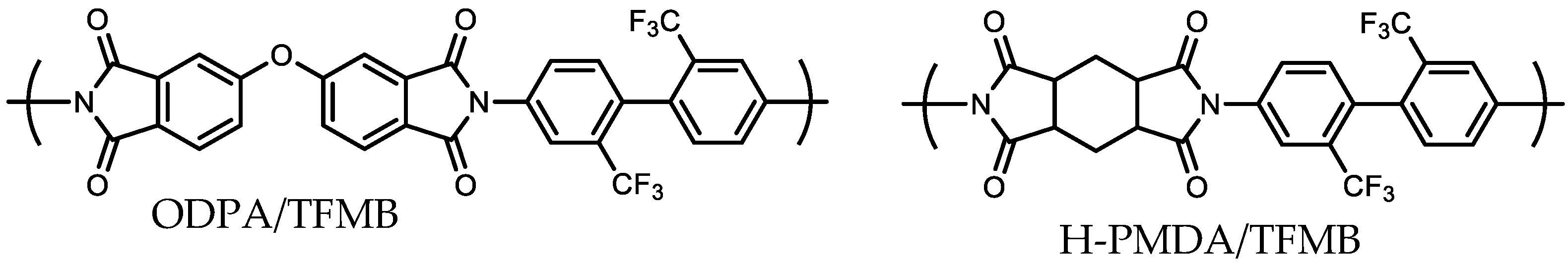
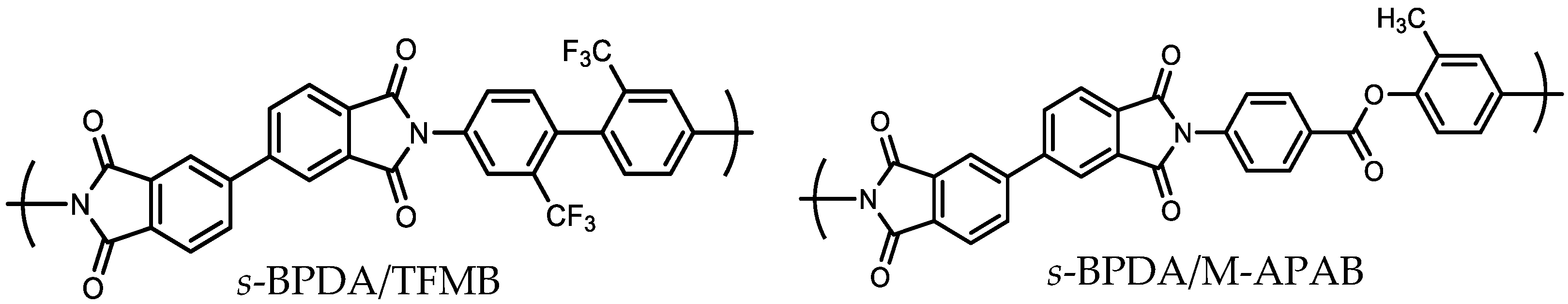
| No. | TCDAs | Diamines | Ci (wt%) | CF (wt%) | E (GPa) | CTE (ppm/K) | σpeel (N/cm) | Process Compatibility Rank (1–5) |
|---|---|---|---|---|---|---|---|---|
| 2R | CpODA | TFMB | 20.95 | 17.05 | 3.74 | 16.7 | 0.10 | 3.5 |
| 8R | CpODA (90) BzDAxx (10) | TFMB | 20.88 | 16.99 | 2.24 | 20.3 | 0.21 | 5 |
| 9R | CpODA (80) BzDAxx (20) | TFMB | 20.81 | 16.94 | 2.20 | 21.1 | 0.51 | 3 |
| 10R | CpODA (70) BzDAxx (30) | TFMB | 20.74 | 16.88 | 2.26 | 23.7 | 0.64 | 3 |
| 4R | BzDAxx | TFMB | 20.28 | 16.50 | 1.53 | 33.1 | 1.11 | 1 |
| 13R | H-PMDA | TFMB | 27.55 | 22.42 | 3.13 a | 57.1 a | 3.58 | 1 |
| 14T | ODPA | TFMB | 23.56 | 19.18 | 3.39 b | 63.8 b | 3.41 | 1 |
| 15C | 6FDA | TFMB | 19.23 | 31.30 | 3.05 c | 52.9 c | 1.68 | 1 |
| 16T | s-BPDA | TFMB | 24.21 | 19.71 | 3.67 d | 33.8 d | 0.14 | 4 |
| 17T | s-BPDA | M-APAB | 27.99 | 0 | 9.38 e | 6.5 e | 0.13 | 4 |
| 18 | XENOMAX® film | --- | --- | 9.5 f | 3.0 g | 0.20 | 5 | |
| Properties | Parameters | Ranking | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Physical heat resistance (HR) | Tg (°C) | ≤ 200 | 220–240 | 250–270 | 280–300 | ≥360 or unclear Tg by DMA |
| Low CTE property (LCTE) | CTE (ppm K−1) | ≥70 | 60–50 | 45–35 | 30–20 | ≤10 |
| Light transmittance (Tr) | T400 (%) | ≤5 | 20–30 | 40–60 | 70–80 | ≥85 |
| Low yellowness (LY) | YI | ≥20 | 10–7 | 6–4 | 3–2 | ≤1 |
| Low haze (LH) | Haze (%) | ≥10 | 4–3 | 3–2 | 2–1 | ≤1 |
| Toughness (To) | εb max (%) | ≤2 or no film-forming ability | 5–10 | 20–30 | 40–60 | ≥80 |
| Solution processability (SP) | Qualitative solubility | Insoluble | Soluble in amide solvents (≤1 wt%) | Soluble in amide solvents (3–5 wt%) | Soluble in amide solvents (>~10 wt%) | Soluble in non-amide solvents (>~10wt%) |
| Controlled soft adhesion/easy removability (S/E) | Peel strength (N/cm) | <0.01 or >1.0 | --- | 0.05–0.08 or 0.40–0.60 | --- | 0.15–0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, M.; Shinoda, T.; Nakadai, K.; Ishii, J.; Okuyama, T.; Tokuda, K.; Wakui, H.; Watanabe, N.; Kitamura, K. Colorless Polyimides with Low Linear Coefficients of Thermal Expansion and Their Controlled Soft Adhesion/Easy Removability on Glass Substrates: Role of Modified One-Pot Polymerization Method. Polymers 2025, 17, 1887. https://doi.org/10.3390/polym17131887
Hasegawa M, Shinoda T, Nakadai K, Ishii J, Okuyama T, Tokuda K, Wakui H, Watanabe N, Kitamura K. Colorless Polyimides with Low Linear Coefficients of Thermal Expansion and Their Controlled Soft Adhesion/Easy Removability on Glass Substrates: Role of Modified One-Pot Polymerization Method. Polymers. 2025; 17(13):1887. https://doi.org/10.3390/polym17131887
Chicago/Turabian StyleHasegawa, Masatoshi, Takehiro Shinoda, Kanata Nakadai, Junichi Ishii, Tetsuo Okuyama, Kaya Tokuda, Hiroyuki Wakui, Naoki Watanabe, and Kota Kitamura. 2025. "Colorless Polyimides with Low Linear Coefficients of Thermal Expansion and Their Controlled Soft Adhesion/Easy Removability on Glass Substrates: Role of Modified One-Pot Polymerization Method" Polymers 17, no. 13: 1887. https://doi.org/10.3390/polym17131887
APA StyleHasegawa, M., Shinoda, T., Nakadai, K., Ishii, J., Okuyama, T., Tokuda, K., Wakui, H., Watanabe, N., & Kitamura, K. (2025). Colorless Polyimides with Low Linear Coefficients of Thermal Expansion and Their Controlled Soft Adhesion/Easy Removability on Glass Substrates: Role of Modified One-Pot Polymerization Method. Polymers, 17(13), 1887. https://doi.org/10.3390/polym17131887






