Exploring the Potential of Cellulose Nanocrystals Originated from Ramie (Boehmeria nivea L. Gaud) in Formation of Microspheres for Enhanced Solubility of Furosemide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
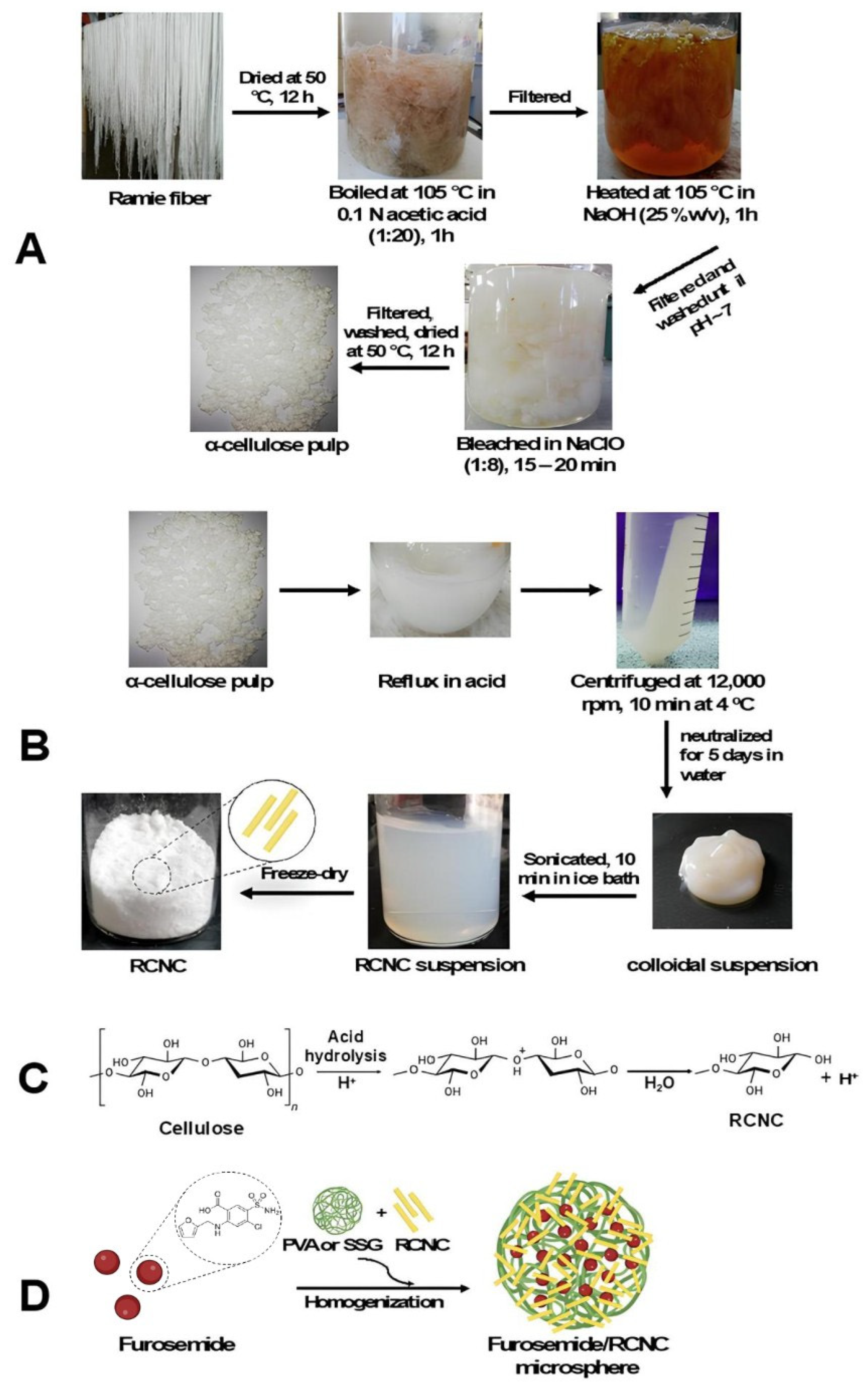
2.2. Preparation of RNCC
2.3. Preparation of Furosemide/RNCC Microsphere
2.4. Characterization
2.4.1. Particle Size Analyzer
2.4.2. Conductometric Titration
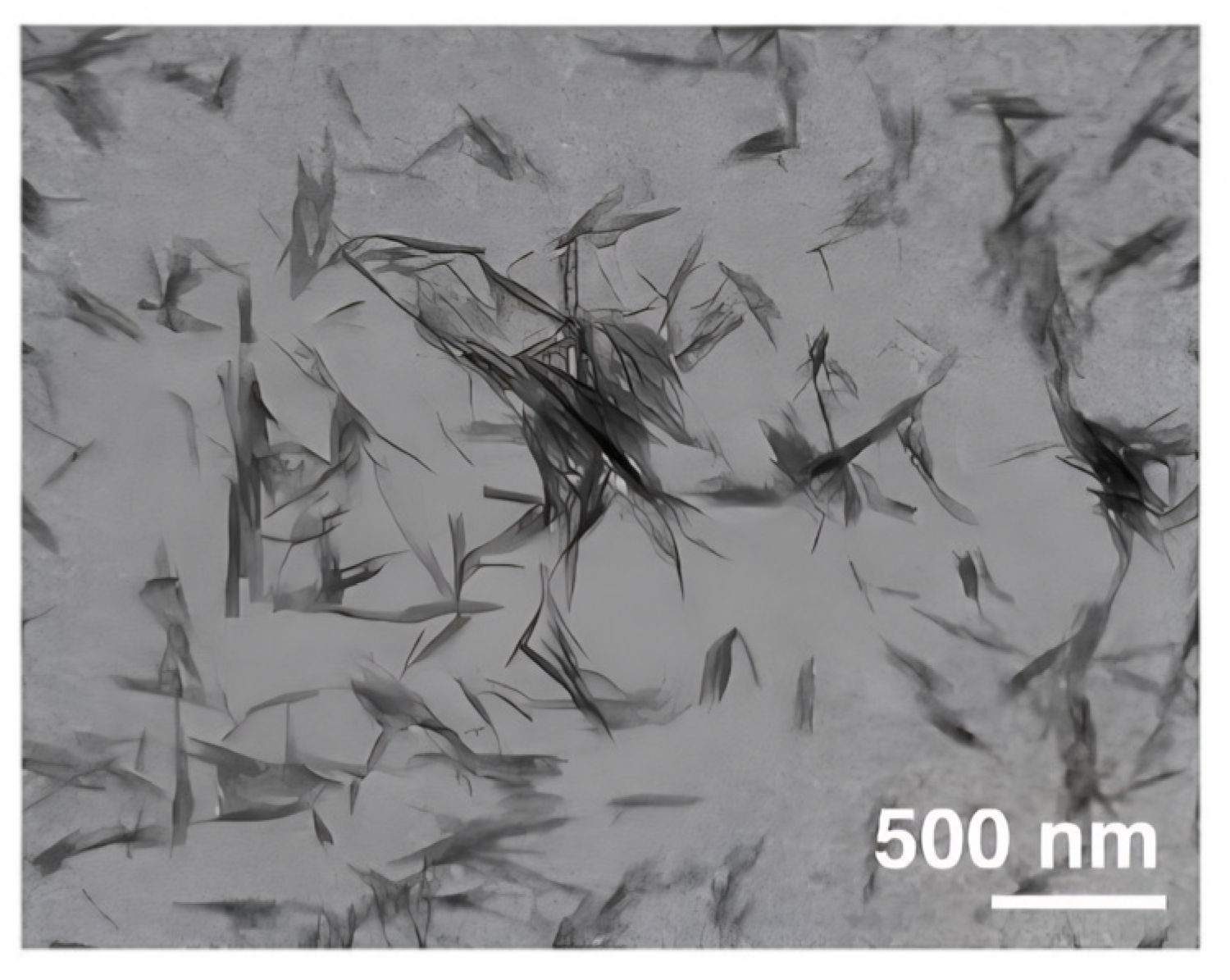
2.4.3. Transmission Electron Microscopy (TEM)
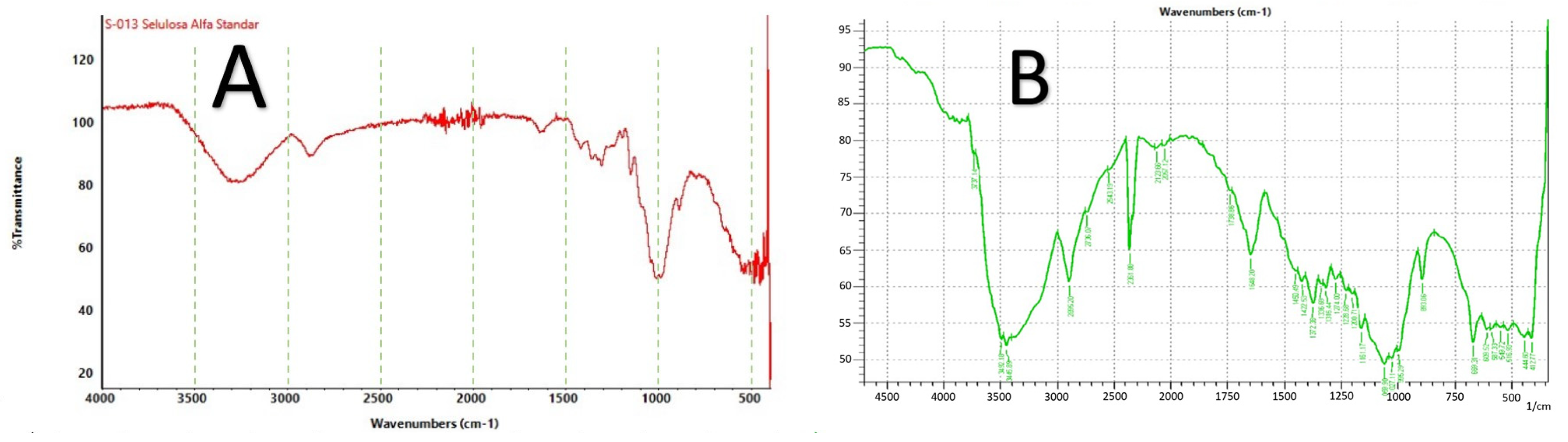
2.4.4. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.5. X-Ray Diffractometry (XRD)
2.4.6. Thermal Analysis Using Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.5. Drug Loading and Encapsulation Efficiency
2.6. In Vitro Furosemide Dissolution in RNCC Microsphere
3. Results
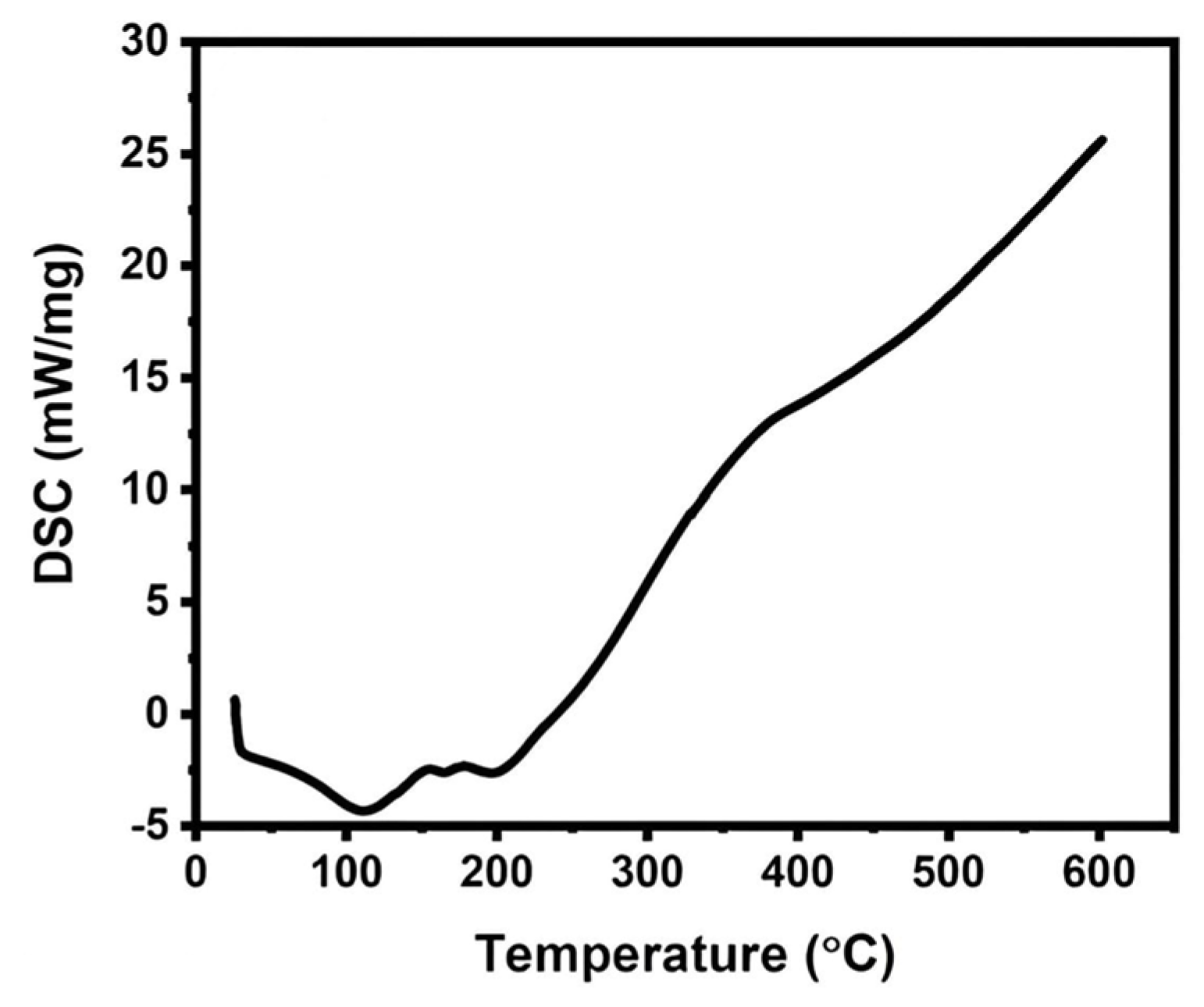
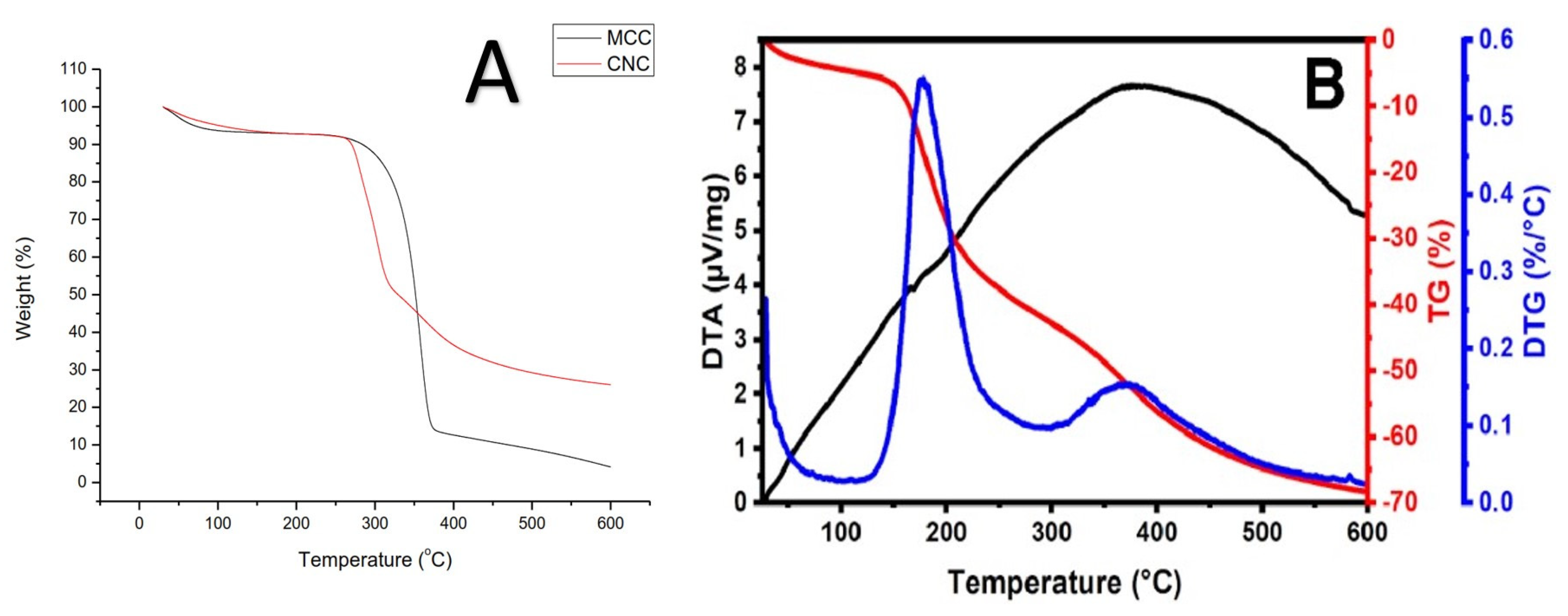
3.1. Characterization of RNCC
3.2. Characterization of Furosemide/RNCC Microsphere
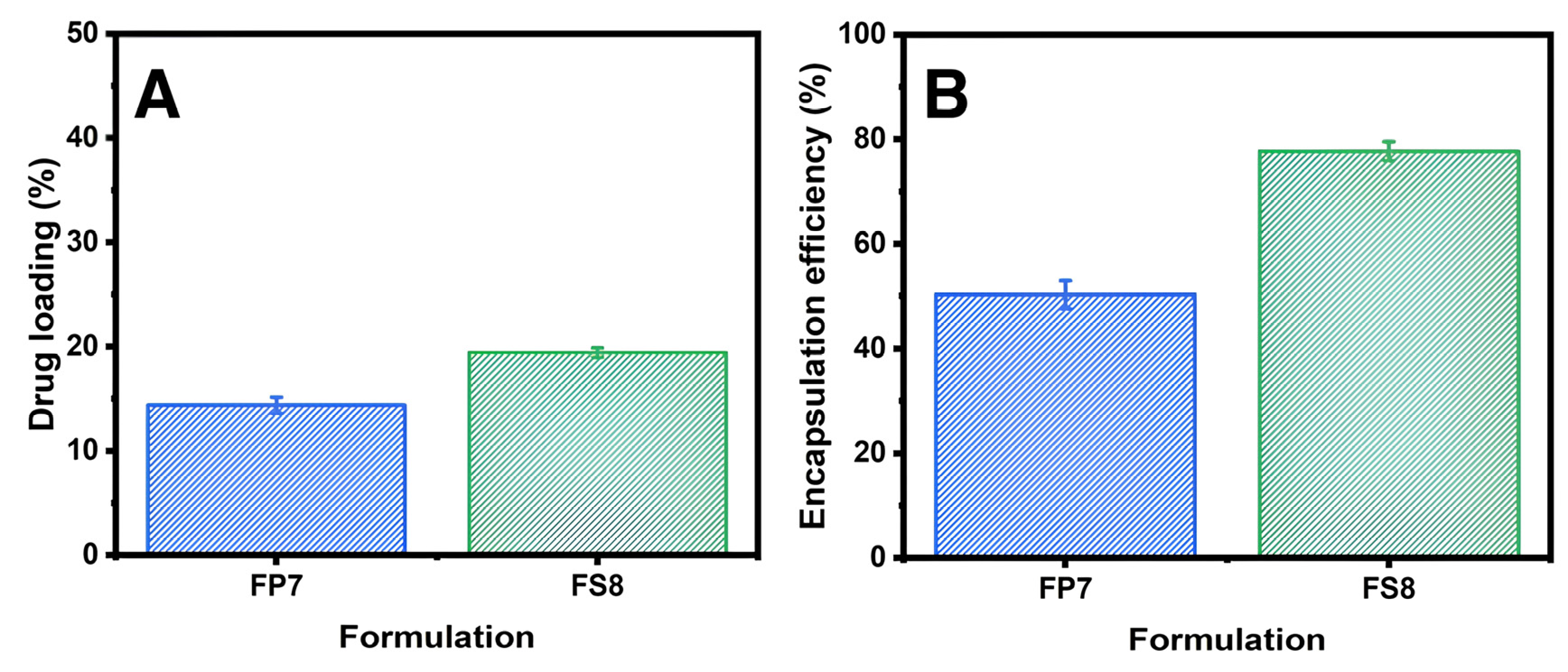
3.3. Drug Loading and Encapsulation Efficiency of Furosemide
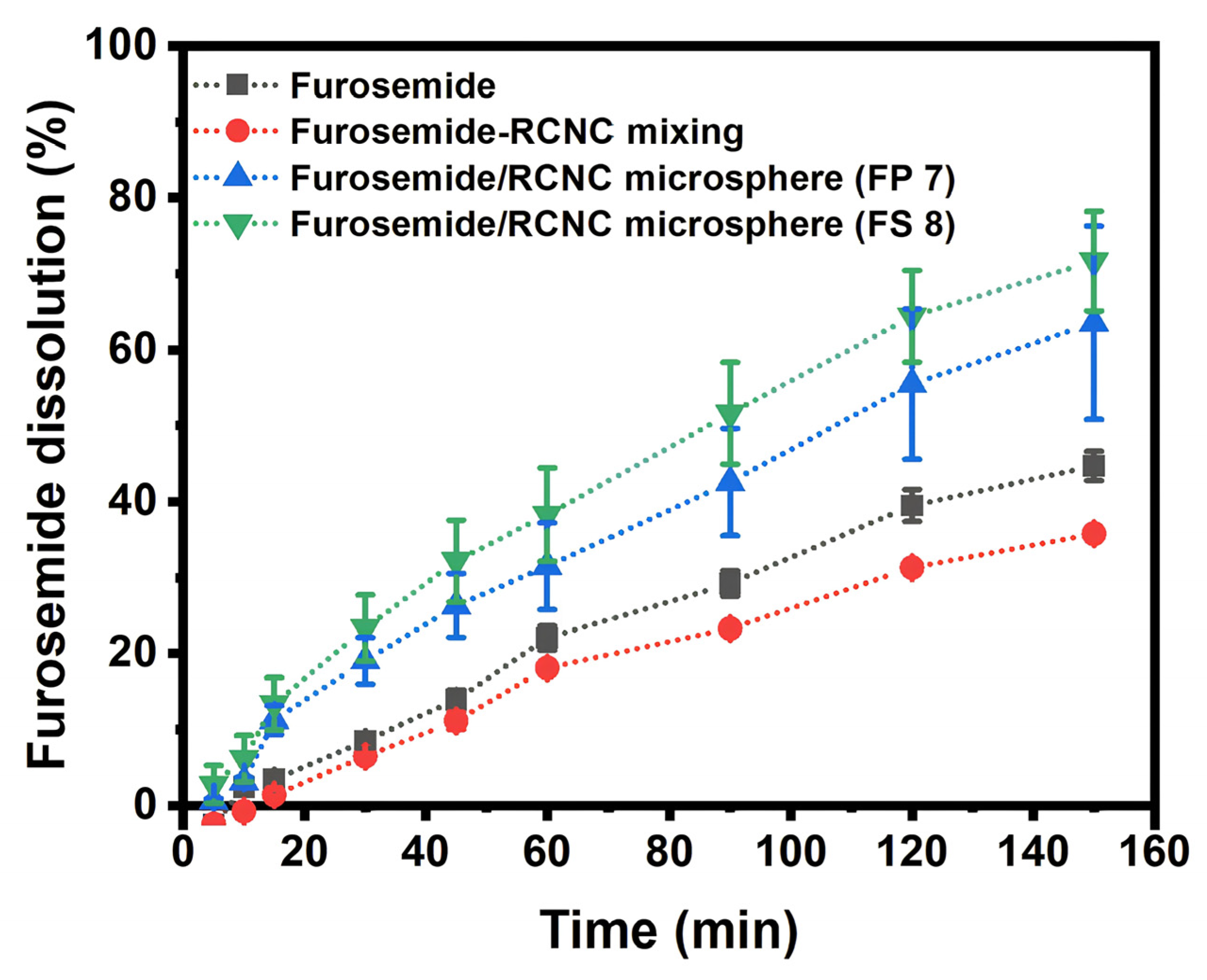
3.4. In Vitro Dissolution of Furosemide in RNCC Microspheres
4. Discussion
4.1. Characterization of RNCC
4.2. Characterization of Furosemide/RNCC Microsphere
4.3. Drug Loading and Encapsulation Efficiency of Furosemide
4.4. In Vitro Dissolution of Furosemide in RNCC Microspheres
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RNCC | Ramie Cellulose Nanocrystals |
| BCS | Biopharmaceutical Classification System |
| MCC | Microcrystalline Cellulose |
| NCC | Nanocrystalline Cellulose |
| H2SO4 | Sulfuric Acid |
| HCl | Hydrochloric Acid |
| PVA | Polyvinyl Alcohol |
| NaOH | Sodium Hydroxide |
| NaClO | Sodium Hypochlorite |
| SSG | Sodium Starch Glycolate |
| TEM | Transmission Electron Microscopy |
| FT-IR | Fourier Transform Infrared |
| XRD | X-Ray Diffractometry |
| DSC | Differential Scanning Calorimetry |
| TGA | Thermogravimetric Analysis |
References
- Lomba, L.; Polo, A.; Alejandre, J.; Martínez, N.; Giner, B. Solubility Enhancement of Caffeine and Furosemide Using Deep Eutectic Solvents Formed by Choline Chloride and Xylitol, Citric Acid, Sorbitol or Glucose. J. Drug Deliv. Sci. Technol. 2023, 79, 104010. [Google Scholar] [CrossRef]
- Smith, M.V. Textbook of Rabbit Medicine, 3rd ed.; Elsevier: New York, NY, USA, 2022. [Google Scholar]
- Do, T.T.A.; Grijalvo, S.; Imae, T.; Garcia-Celma, M.J.; Rodríguez-Abreu, C. A Nanocellulose-Based Platform towards Targeted Chemo-Photodynamic/Photothermal Cancer Therapy. Carbohydr. Polym. 2021, 270, 118366. [Google Scholar] [CrossRef]
- Gopinath, V.; Kamath, S.M.; Priyadarshini, S.; Chik, Z.; Alarfaj, A.A.; Hirad, A.H. Multifunctional Applications of Natural Polysaccharide Starch and Cellulose: An Update on Recent Advances. Biomed. Pharmacother. 2022, 146, 112492. [Google Scholar] [CrossRef] [PubMed]
- Lukova, P.; Katsarov, P.; Pilicheva, B. Application of Starch, Cellulose, and Their Derivatives in the Development of Microparticle Drug-Delivery Systems. Polymers 2023, 15, 3615. [Google Scholar] [CrossRef]
- Desai, P.M.; Liew, C.V.; Heng, P.W.S. Review of Disintegrants and the Disintegration Phenomena. J. Pharm. Sci. 2016, 105, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Yohana Chaerunisaa, A.; Sriwidodo, S.; Abdassah, M. Microcrystalline Cellulose as Pharmaceutical Excipient. Pharmaceutical Formulation Design—Recent Practices; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Drug Nanocrystals—Versatile Option for Formulation of Poorly Soluble Materials. Int. J. Pharm. 2018, 537, 73–83. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical Particle Technologies: An Approach to Improve Drug Solubility, Dissolution and Bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Roos, C.; Westergren, J.; Dahlgren, D.; Lennernäs, H.; Sjögren, E. Mechanistic Modelling of Intestinal Drug Absorption—The in Vivo Effects of Nanoparticles, Hydrodynamics, and Colloidal Structures. Eur. J. Pharm. Biopharm. 2018, 133, 70–76. [Google Scholar] [CrossRef]
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A Mini-Review on Types and Use in Drug Delivery Systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Löbmann, K.; Wohlert, J.; Müllertz, A.; Wågberg, L.; Svagan, A.J. Cellulose Nanopaper and Nanofoam for Patient-Tailored Drug Delivery. Adv. Mater. Interfaces 2017, 4, 1600655. [Google Scholar] [CrossRef]
- Mantas, A.; Mihranyan, A. Immediate-Release Nifedipine Binary Dry Powder Mixtures with Nanocellulose Featuring Enhanced Solubility and Dissolution Rate. Pharmaceutics 2019, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Mantas, A.; Labbe, V.; Loryan, I.; Mihranyan, A. Amorphisation of Free Acid Ibuprofen and Other Profens in Mixtures with Nanocellulose: Dry Powder Formulation Strategy for Enhanced Solubility. Pharmaceutics 2019, 11, 68. [Google Scholar] [CrossRef]
- Lin, Y.J.; Shatkin, J.A.; Kong, F. Evaluating Mucoadhesion Properties of Three Types of Nanocellulose in the Gastrointestinal Tract in Vitro and Ex Vivo. Carbohydr. Polym. 2019, 210, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Gurtovenko, A.A.; Mukhamadiarov, E.I.; Kostritskii, A.Y.; Karttunen, M. Phospholipid-Cellulose Interactions: Insight from Atomistic Computer Simulations for Understanding the Impact of Cellulose-Based Materials on Plasma Membranes. J. Phys. Chem. B 2018, 122, 9973–9981. [Google Scholar] [CrossRef]
- Raza, M.; Abu-Jdayil, B.; Banat, F.; Al-Marzouqi, A.H. Isolation and Characterization of Cellulose Nanocrystals from Date Palm Waste. ACS Omega 2022, 7, 25366–25379. [Google Scholar] [CrossRef]
- Verma, C.; Chhajed, M.; Gupta, P.; Roy, S.; Maji, P.K. Isolation of Cellulose Nanocrystals from Different Waste Bio-Mass Collating Their Liquid Crystal Ordering with Morphological Exploration. Int. J. Biol. Macromol. 2021, 175, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Trilokesh, C.; Uppuluri, K.B. Isolation and Characterization of Cellulose Nanocrystals from Jackfruit Peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef]
- Sai Prasanna, N.; Mitra, J. Isolation and Characterization of Cellulose Nanocrystals from Cucumis Sativus Peels. Carbohydr. Polym. 2020, 247, 116706. [Google Scholar] [CrossRef]
- Doh, H.; Lee, M.H.; Whiteside, W.S. Physicochemical Characteristics of Cellulose Nanocrystals Isolated from Seaweed Biomass. Food Hydrocoll. 2020, 102, 105542. [Google Scholar] [CrossRef]
- Soleimani, S.; Heydari, A.; Fattahi, M. Isolation and Characterization of Cellulose Nanocrystals from Waste Cotton Fibers Using Sulfuric Acid Hydrolysis. Starch/Staerke 2022, 74, 2200159. [Google Scholar] [CrossRef]
- Kusmono; Affan, M.N. Isolation and Characterization of Nanocrystalline Cellulose from Ramie Fibers via Phosphoric Acid Hydrolysis. J. Nat. Fibers 2022, 19, 2744–2755. [Google Scholar] [CrossRef]
- Habibie, S.; Suhendra, N.; Setiawan, B.A.; Hamzah, M.; Aisah, N.; Fitriani, D.A.; Tasomara, R.; Anggaravidya, M. Prospect of Ramie Fiber Development in Indonesia and Manufacturing of Ramie Fiber Textile-Based Composites for Industrial Needs, an Overview. Int. J. Compos. Mater. 2021, 11, 43–53. [Google Scholar]
- Kusmono; Listyanda, R.F.; Wildan, M.W.; Ilman, M.N. Preparation and Characterization of Cellulose Nanocrystal Extracted from Ramie Fibers by Sulfuric Acid Hydrolysis. Heliyon 2020, 6, e05486. [Google Scholar] [CrossRef]
- Raquez, J.M.; Murena, Y.; Goffin, A.L.; Habibi, Y.; Ruelle, B.; DeBuyl, F.; Dubois, P. Surface-Modification of Cellulose Nanowhiskers and Their Use as Nanoreinforcers into Polylactide: A Sustainably-Integrated Approach. Compos. Sci. Technol. 2012, 72, 544–549. [Google Scholar] [CrossRef]
- Lu, Y.; Weng, L.; Cao, X. Morphological, Thermal and Mechanical Properties of Ramie Crystallites—Reinforced Plasticized Starch Biocomposites. Carbohydr. Polym. 2006, 63, 198–204. [Google Scholar] [CrossRef]
- Alloin, F.; D’Aprea, A.; El Kissi, N.; Dufresne, A.; Bossard, F. Nanocomposite Polymer Electrolyte Based on Whisker or Microfibrils Polyoxyethylene Nanocomposites. Electrochim. Acta 2010, 55, 5186–5194. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Kyrychenko, A.; Pasko, D.A.; Kalugin, O.N. Poly(Vinyl Alcohol) as a Water Protecting Agent for Silver Nanoparticles: The Role of Polymer Size and Structure. Phys. Chem. Chem. Phys. 2017, 19, 8742–8756. [Google Scholar] [CrossRef]
- Putra, O.N.; Musfiroh, I.; Elisa, S.; Musa, M.; Ikram, E.H.K.; Chaidir, C.; Muchtaridi, M. Sodium Starch Glycolate (SSG) from Sago Starch (Metroxylon Sago) as a Superdisintegrant: Synthesis and Characterization. Molecules 2024, 29, 151. [Google Scholar] [CrossRef]
- Xie, J.; Luo, Y.; Liu, Y.; Ma, Y.; Yue, P.; Yang, M. Novel Redispersible Nanosuspensions Stabilized by Co-Processed Nanocrystalline Cellulose–Sodium Carboxymethyl Starch for Enhancing Dissolution and Oral Bioavailability of Baicalin. Int. J. Nanomed. 2019, 14, 353–369. [Google Scholar] [CrossRef]
- Dong, S.; Bortner, M.J.; Roman, M. Analysis of the Sulfuric Acid Hydrolysis of Wood Pulp for Cellulose Nanocrystal Production: A Central Composite Design Study. Ind. Crops Prod. 2016, 93, 76–87. [Google Scholar] [CrossRef]
- Nomura, S.; Kugo, Y.; Erata, T. 13C NMR and XRD Studies on the Enhancement of Cellulose II Crystallinity with Low Concentration NaOH Post-Treatments. Cellulose 2020, 27, 3553–3563. [Google Scholar] [CrossRef]
- Mangal, G.; Dhobale, S.M. Development of UV spectrophotometric methods and validation for estimation of furosemide in bulk and tablet dosage form by absorbance maxima and area under the curve method. World J. Pharm. Pharm. Sci. SJIF Impact Factor. 2017, 5, 160–170. [Google Scholar]
- Goud, N.R.; Gangavaram, S.; Suresh, K.; Pal, S.; Manjunatha, S.G.; Nambiar, S.; Nangia, A. Novel Furosemide Cocrystals and Selection of High Solubility Drug Forms. J. Pharm. Sci. 2012, 101, 664–680. [Google Scholar] [CrossRef]
- Liu, W.; Pan, H.; Zhang, C.; Zhao, L.; Zhao, R.; Zhu, Y.; Pan, W. Developments in Methods for Measuring the Intestinal Absorption of Nanoparticle-Bound Drugs. Int. J. Mol. Sci. 2016, 17, 1171. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile Extraction of Thermally Stable Cellulose Nanocrystals with a High Yield of 93% through Hydrochloric Acid Hydrolysis under Hydrothermal Conditions. J. Mater. Chem. A Mater. 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Speight, J.G.; Speight, J.G. Chapter Three—Industrial Inorganic Chemistry. In Environmental Inorganic Chemistry for Engineers; Elsevier: New York, NY, USA, 2017. [Google Scholar]
- Gallardo-Sánchez, M.A.; Diaz-Vidal, T.; Navarro-Hermosillo, A.B.; Figueroa-Ochoa, E.B.; Casillas, R.R.; Hernández, J.A.; Rosales-Rivera, L.C.; Martínez, J.F.A.S.; Enríquez, S.G.; Macías-Balleza, E.R. Optimization of the Obtaining of Cellulose Nanocrystals from Agave Tequilana Weber Var. Azul Bagasse by Acid Hydrolysis. Nanomaterials 2021, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Chieng, B.W.; Lee, S.H.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Isolation and Characterization of Cellulose Nanocrystals from Oil Palm Mesocarp Fiber. Polymers 2017, 9, 355. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Osorio, D.A.; Cranston, E.D. Optimization of Cellulose Nanocrystal Length and Surface Charge Density through Phosphoric Acid Hydrolysis. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170041. [Google Scholar] [CrossRef] [PubMed]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose Nanocrystal Based Composites: A Review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Pourmoazzen, Z.; Sadeghifar, H.; Chen, J.; Yang, G.; Zhang, K.; Lucia, L. The Morphology, Self-Assembly, and Host-Guest Properties of Cellulose Nanocrystals Surface Grafted with Cholesterol. Carbohydr. Polym. 2020, 233, 115840. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Agblevor, F.A.; El-Zawawy, W.K. Isolation and Characterization of Cellulose and Lignin from Steam-Exploded Lignocellulosic Biomass. Bioresources 2010, 5, 397–418. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous Cellulose—Structure and Characterization. Cellul. Chem. Technol. 2011, 45, 13. [Google Scholar]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of Nanocellulose Fibrils from Lignocellulosic Fibres: A Novel Approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, X.F.; Zhao, H.; Sun, R.C. Isolation and Characterization of Cellulose from Sugarcane Bagasse. Polym. Degrad. Stab. 2004, 84, 331–339. [Google Scholar] [CrossRef]
- Mern, C.K.; Ting, S.S.; Lin, O.H. Comparative Properties Analysis between Microcrystalline Cellulose and Cellulose Nanocrystals Extracted From Rice Straw. Malays. J. Microsc. 2019, 15, 146–154. [Google Scholar]
- Al-Sahaf, Z.; Raimi-Abraham, B.; Licciardi, M.; de Mohac, L.M. Influence of Polyvinyl Alcohol (PVA) on PVA-Poly-N-Hydroxyethyl-Aspartamide (PVA-PHEA) Microcrystalline Solid Dispersion Films. AAPS PharmSciTech 2020, 21, 267. [Google Scholar] [CrossRef]
- Ikasari, E.D.; Cahyani, I.M.; Collusy, D.M. Optimization of croscarmellose and sodium starch glycolate on orally disintegrating metoclopramide HCL tablets. J. Farm. Sains Dan. Prakt. 2022, 8, 261–267. [Google Scholar] [CrossRef]
- Zahra, F.T.; Quick, Q.; Mu, R. Electrospun PVA Fibers for Drug Delivery: A Review. Polymers 2023, 15, 3837. [Google Scholar] [CrossRef]
- Kádár, R.; Spirk, S.; Nypelö, T. Cellulose Nanocrystal Liquid Crystal Phases: Progress and Challenges in Characterization Using Rheology Coupled to Optics, Scattering, and Spectroscopy. ACS Nano 2021, 15, 7931–7945. [Google Scholar] [CrossRef]
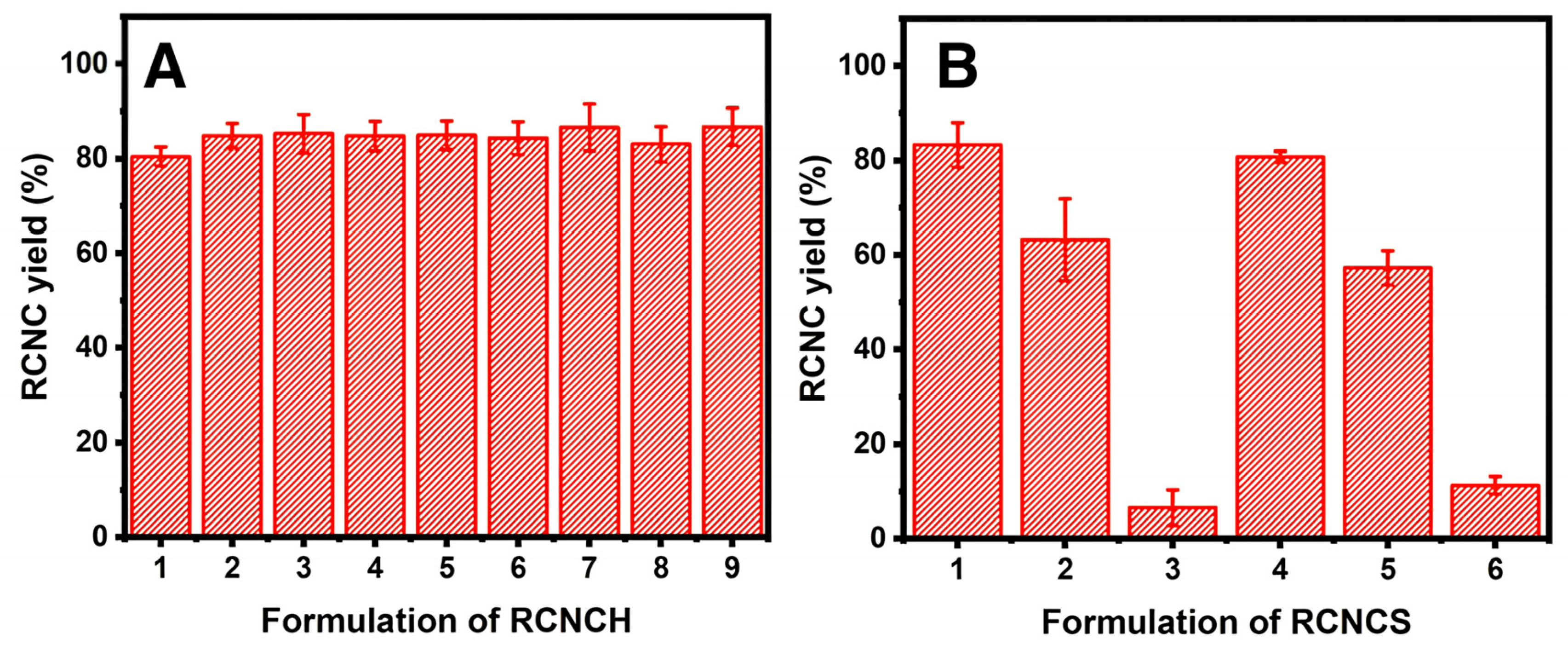


| Formulation | Acid | Concentration (%) | Temperature (°C) | Time (min) |
|---|---|---|---|---|
| RNCCH 1 | HCl | 15 | 60 | 120 |
| RNCCH 2 | HCl | 20 | 60 | 120 |
| RNCCH 3 | HCl | 25 | 60 | 120 |
| RNCCH 4 | HCl | 15 | 60 | 180 |
| RNCCH 5 | HCl | 20 | 60 | 180 |
| RNCCH 6 | HCl | 25 | 60 | 180 |
| RNCCH 7 | HCl | 15 | 60 | 360 |
| RNCCH 8 | HCl | 20 | 60 | 360 |
| RNCCH 9 | HCl | 25 | 60 | 360 |
| RNCCS 1 | H2SO4 | 45 | 45 | 45 |
| RNCCS 2 | H2SO4 | 50 | 45 | 45 |
| RNCCS 3 | H2SO4 | 55 | 45 | 45 |
| RNCCS 4 | H2SO4 | 45 | 45 | 60 |
| RNCCS 5 | H2SO4 | 50 | 45 | 60 |
| RNCCS 6 | H2SO4 | 55 | 45 | 60 |
| Materials | FP1 (g) | FP2 (g) | FP3 (g) | FP4 (g) | FP5 (g) | FP6 (g) | FP7 (g) | FP8 (g) | FP9 (g) |
|---|---|---|---|---|---|---|---|---|---|
| Furosemide | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| RNCC | 0.5 | 1 | 2 | 0.5 | 1 | 2 | 0.5 | 1 | 2 |
| PVA | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 2 | 2 | 2 |
| FS1 (g) | FS2 (g) | FS3 (g) | FS4 (g) | FS5 (g) | FS6 (g) | FS7 (g) | FS8 (g) | FS9 (g) | |
| Furosemide | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| RNCC | 0.5 | 1 | 2 | 0.5 | 1 | 2 | 0.5 | 1 | 2 |
| SSG | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1 |
| Sample | Particle Size (nm) | pH | Sulfate Density (mmol/kg) |
|---|---|---|---|
| RNCCH 1 | 1500 ± 0.56 | 6.76 ± 0.30 | - |
| RNCCH 2 | 1410 ± 0.59 | 6.72 ± 0.20 | - |
| RNCCH 3 | 1410 ± 0.60 | 6.25 ± 0.14 | - |
| RNCCH 4 | 1420 ± 0.59 | 6.11 ± 0.05 | - |
| RNCCH 5 | 1410 ± 0.58 | 6.13 ± 0.03 | - |
| RNCCH 6 | 1500 ± 0.57 | 6.18 ± 0.03 | - |
| RNCCH 7 | 1350 ± 0.62 | 6.29 ± 0.01 | - |
| RNCCH 8 | 1380 ± 0.60 | 6.39 ± 0.03 | - |
| RNCCH 9 | 1450 ± 0.58 | 5.43 ± 0.05 | - |
| RNCCS 1 | 1490 ± 0.59 | 3.31 ± 0.08 | 85.44 ± 15.39 |
| RNCCS 2 | 120 ± 0.21 | 3.28 ± 0.03 | 134.69 ± 2.39 |
| RNCCS 3 | 340 ± 0.65 | 3.77 ± 0.07 | - |
| RNCCS 4 | 1400 ± 0.59 | 3.23 ± 0.03 | 66.08 ± 29.90 |
| RNCCS 5 | 120 ± 0.17 | 3.19 ± 0.03 | 154.02 ± 1.85 |
| RNCCS 6 | 120 ± 0.16 | 3.72 ± 0.06 | - |
| Sample | Particle Size (μm) | |
|---|---|---|
| FP * | FS * | |
| 1 | 8.77 ± 8.72 | 36.11 ± 30.09 |
| 2 | 6.47 ± 6.51 | 22.26 ± 15.68 |
| 3 | 14.15 ± 14.94 | 27.73 ± 18.89 |
| 4 | 8.74 ± 9.81 | 31.12 ± 24.70 |
| 5 | 8.74 ± 9.81 | 31.10 ± 23.70 |
| 6 | 9.31 ± 10.52 | 29.80 ± 32.02 |
| 7 | 6.51 ± 7.20 | 23.14 ± 17.53 |
| 8 | 6.66 ± 7.80 | 32.82 ± 19.52 |
| 9 | 6.14 ± 6.39 | 33.36 ± 18.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaerunisaa, A.Y.; Wardhana, Y.W.; Dewi, M.K.; Putri, M.E.; Rahmania, F.J. Exploring the Potential of Cellulose Nanocrystals Originated from Ramie (Boehmeria nivea L. Gaud) in Formation of Microspheres for Enhanced Solubility of Furosemide. Polymers 2025, 17, 1879. https://doi.org/10.3390/polym17131879
Chaerunisaa AY, Wardhana YW, Dewi MK, Putri ME, Rahmania FJ. Exploring the Potential of Cellulose Nanocrystals Originated from Ramie (Boehmeria nivea L. Gaud) in Formation of Microspheres for Enhanced Solubility of Furosemide. Polymers. 2025; 17(13):1879. https://doi.org/10.3390/polym17131879
Chicago/Turabian StyleChaerunisaa, Anis Yohana, Yoga Windhu Wardhana, Mayang Kusuma Dewi, Margaretha Efa Putri, and Fitriani Jati Rahmania. 2025. "Exploring the Potential of Cellulose Nanocrystals Originated from Ramie (Boehmeria nivea L. Gaud) in Formation of Microspheres for Enhanced Solubility of Furosemide" Polymers 17, no. 13: 1879. https://doi.org/10.3390/polym17131879
APA StyleChaerunisaa, A. Y., Wardhana, Y. W., Dewi, M. K., Putri, M. E., & Rahmania, F. J. (2025). Exploring the Potential of Cellulose Nanocrystals Originated from Ramie (Boehmeria nivea L. Gaud) in Formation of Microspheres for Enhanced Solubility of Furosemide. Polymers, 17(13), 1879. https://doi.org/10.3390/polym17131879







