Gamma Irradiation-Induced Synthesis of Nano Au-PNiPAAm/PVA Bi-Layered Photo-Thermo-Responsive Hydrogel Actuators with a Switchable Bending Motion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gold Nanospheres and Gold Nanorods
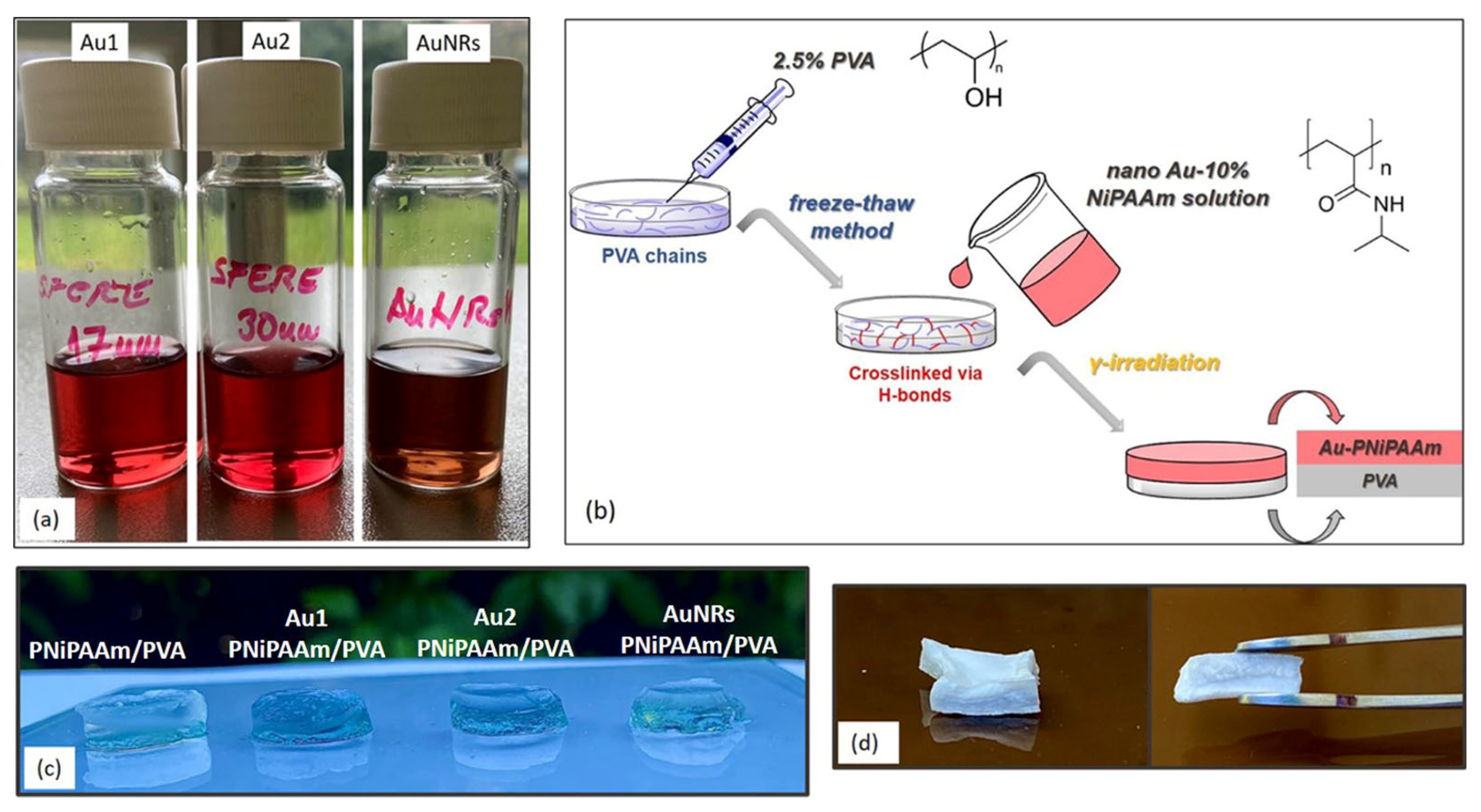
2.3. Synthesis of Bi-Layered Nano Au-PNiPAAm/PVA Hydrogel Nanocomposites
2.4. Methods of Characterization
3. Results and Discussion
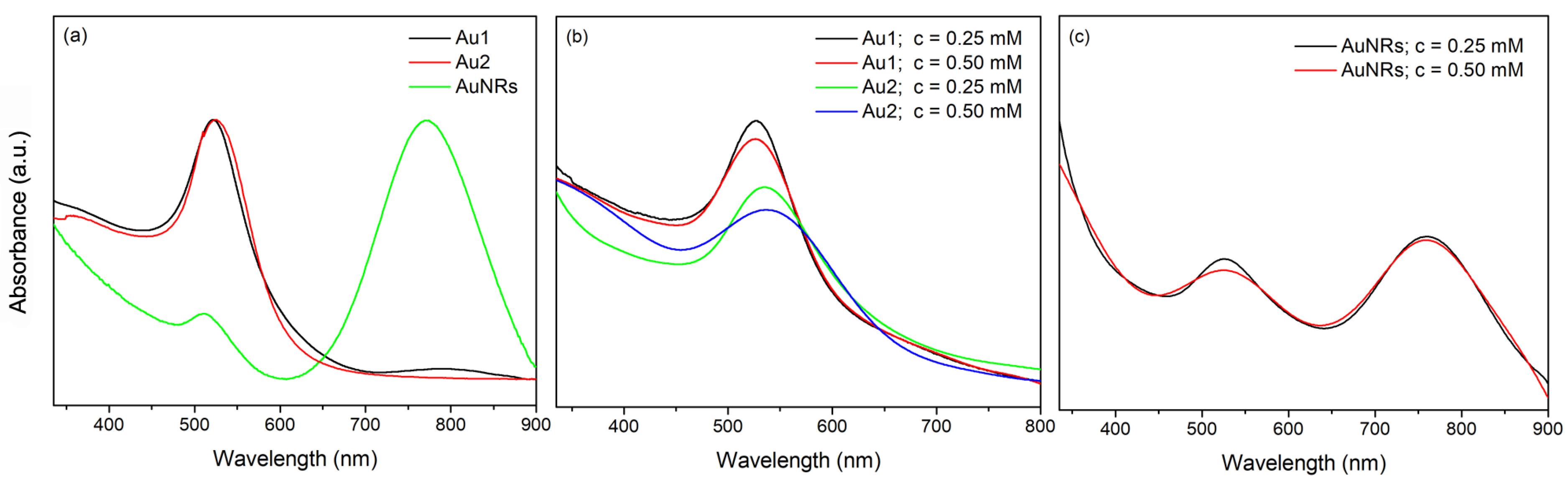
3.1. Optical Properties of AuNPs and Bi-Layered Nano Au-PNiPAAm/PVA Hydrogel Nanocomposites
3.2. The Effectiveness of the Chosen Synthesis Methodology
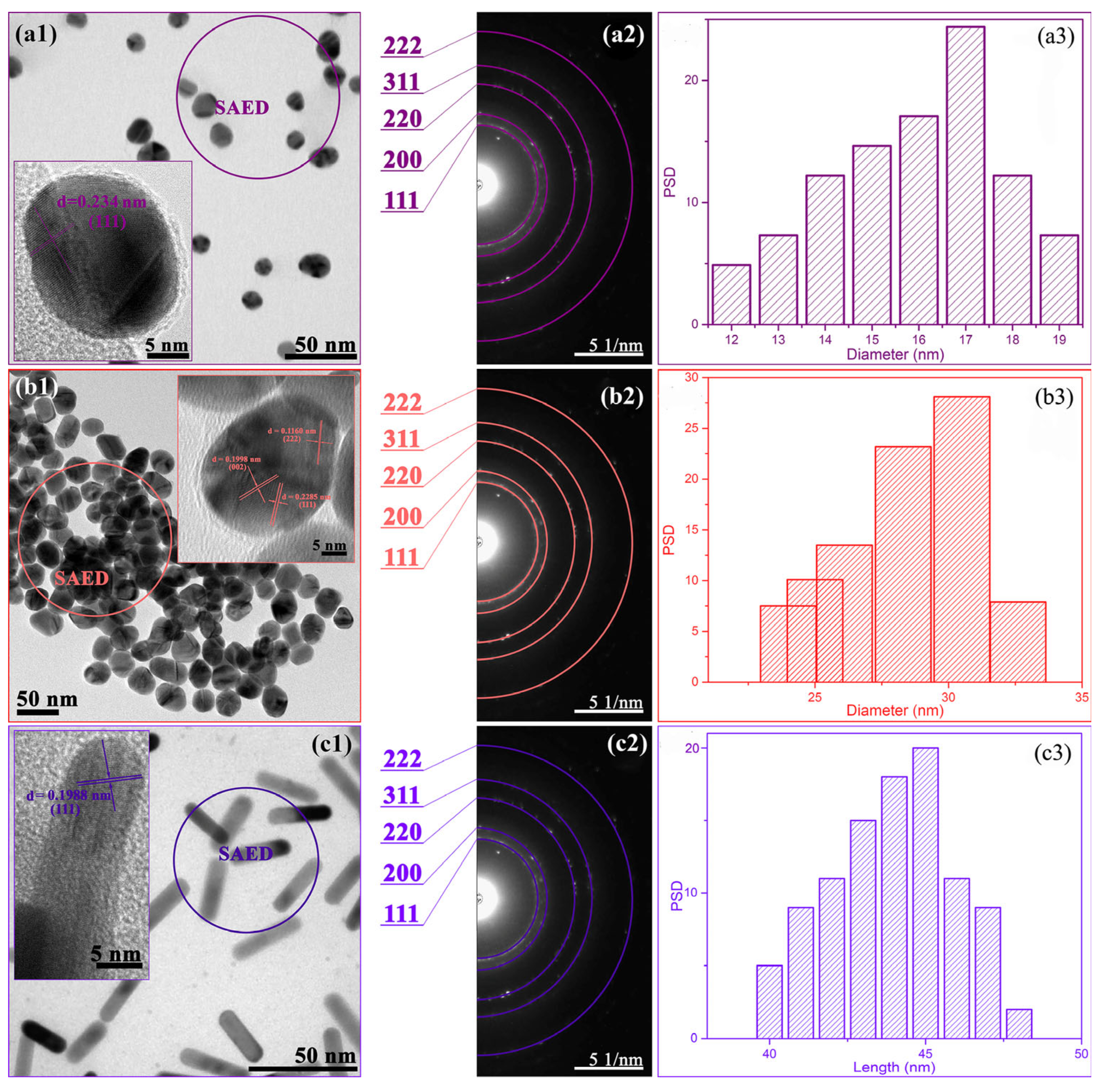
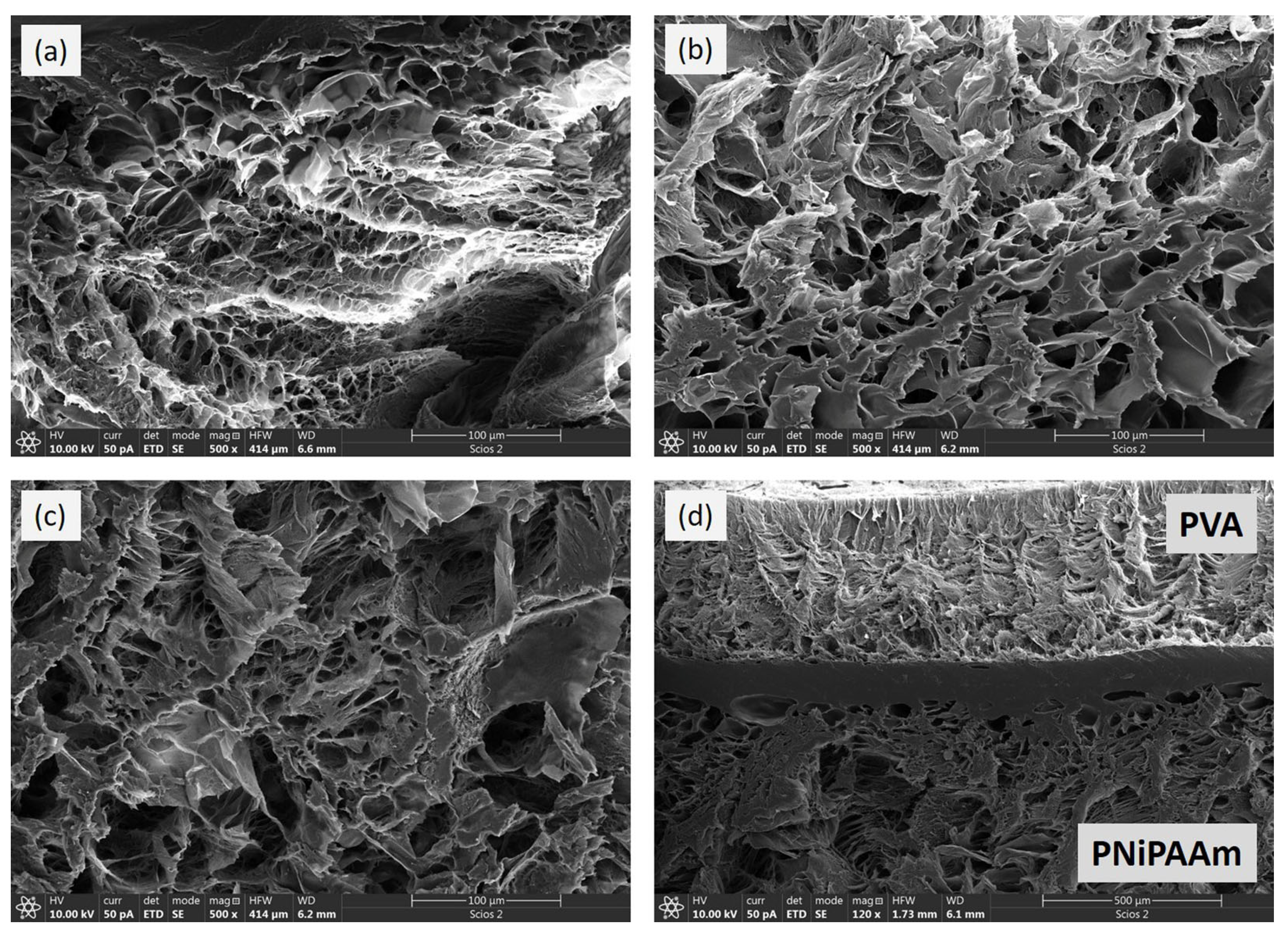
3.3. Morphological Properties of the Nanoparticles and Polymer Networks
3.4. Physicochemical Investigation of Hydrogel Nanocomposites
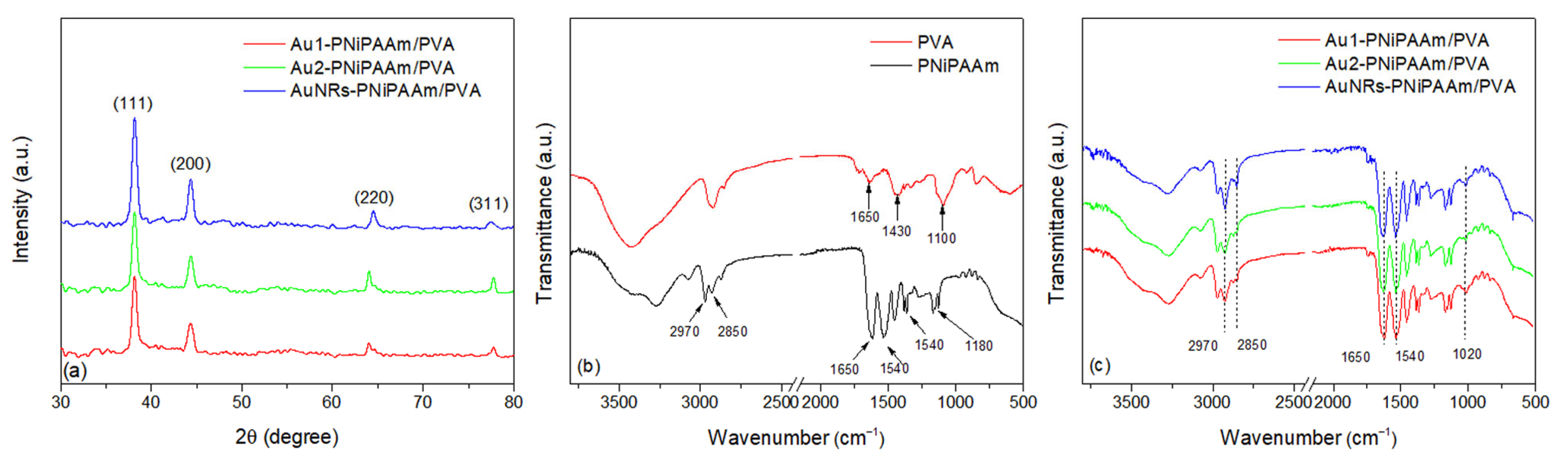
3.5. Structural Characterization of AuNPs Embedded into the Polymer Network
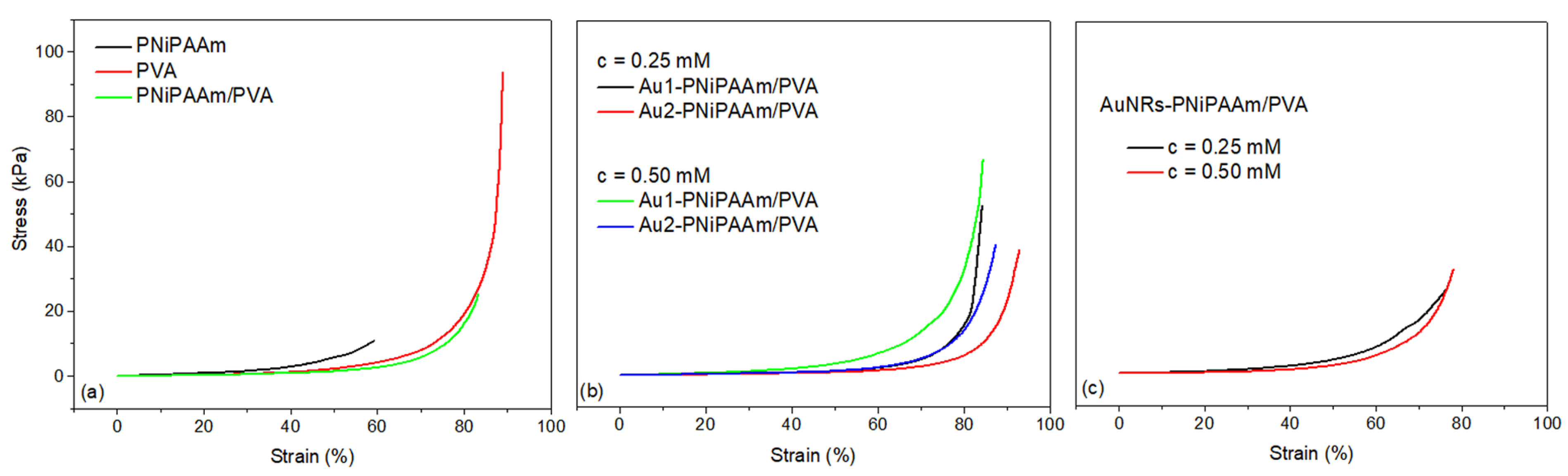
3.6. Mechanical Properties of Hydrogels
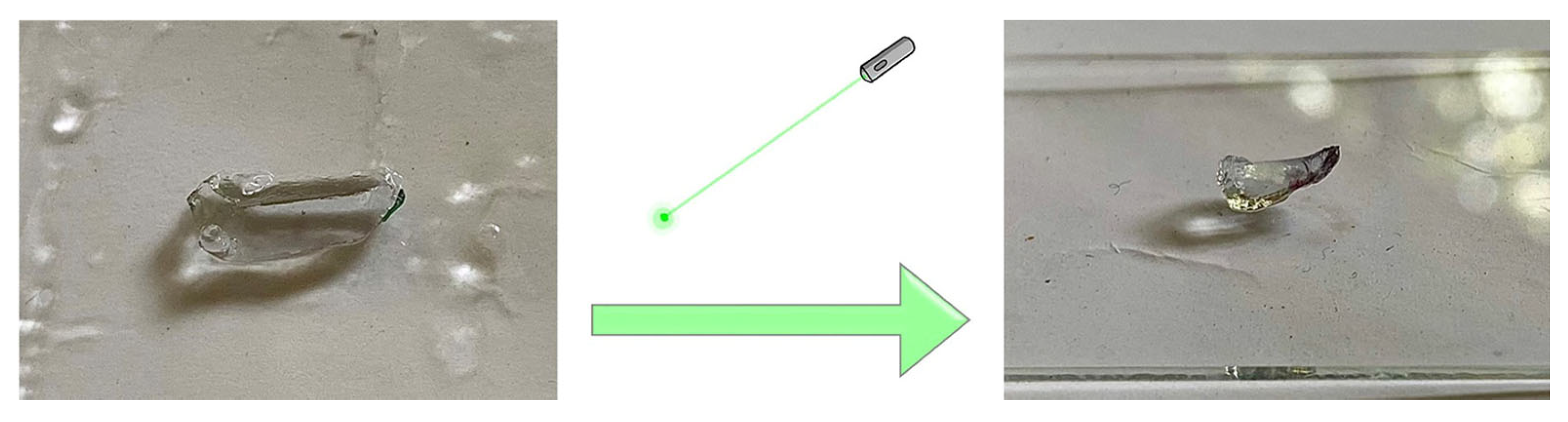
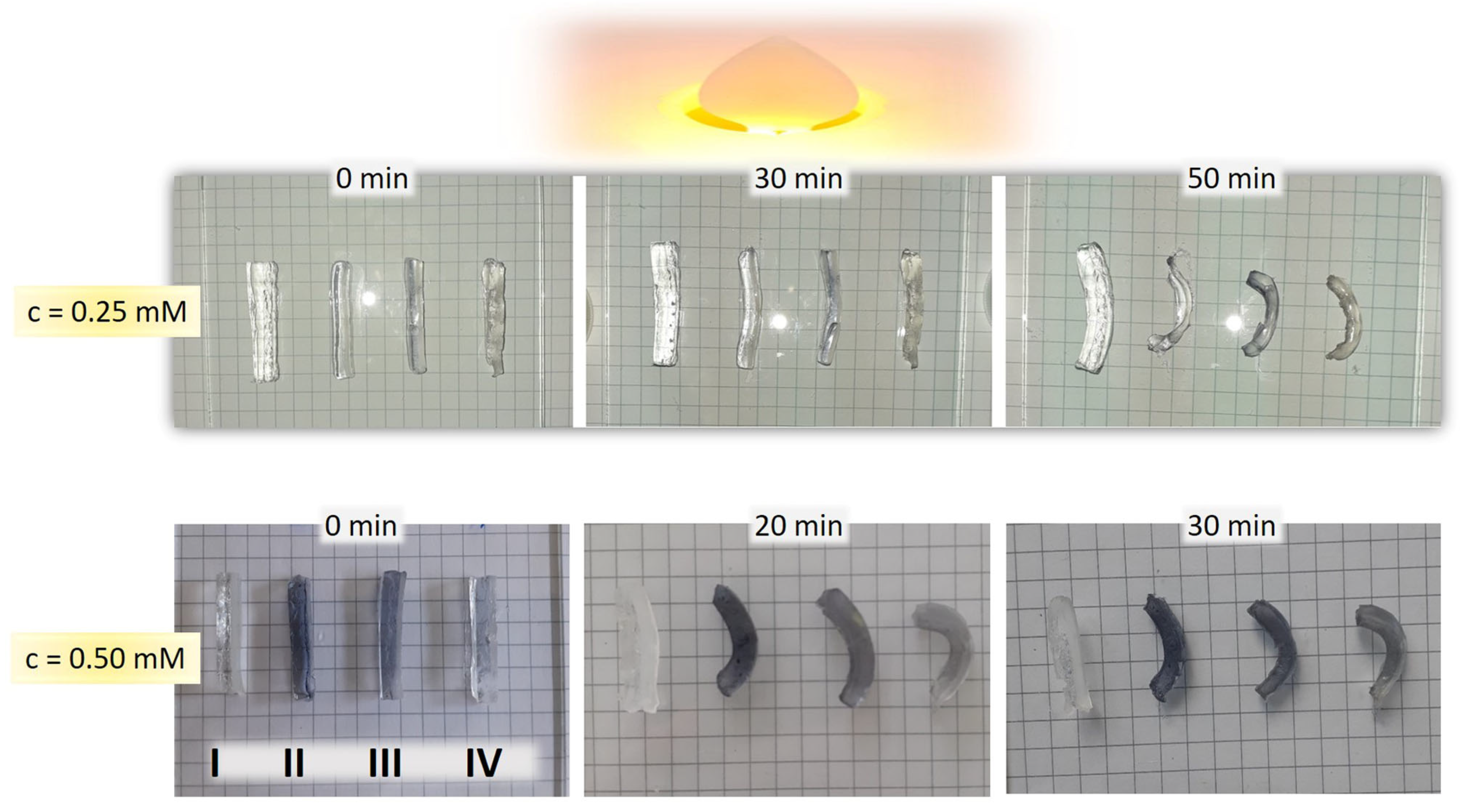
3.7. Photo–Thermal Effect of Bi-Layered Nano Au-PNiPAAm/PVA Hydrogel Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basak, S.; Bandyopadhyay, A. Next-gen biomimetic actuators: Bilayer hydrogel evolution in the 21st century and its advancements from a post-2020 perspective. RSC Appl. Polym. 2024, 2, 583. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X. Bio-Inspired Double-Layered Hydrogel Robot with Fast Response via Thermo-Responsive Effect. Materials 2024, 17, 3679. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, H.; Hakobyan, K.; Jiang, Z.; Liang, K.; Xu, J. Robust Hydrogel Actuators Functioning in Multi-Environments Enabled by Thermo-Responsive Polymer Nanoparticle Coatings on Hydrogel Surfaces. Small 2024, 20, 2400534. [Google Scholar] [CrossRef]
- Banerjee, H.; Suhail, M.; Ren, H. Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges. Biomimetics 2018, 3, 15. [Google Scholar] [CrossRef]
- Tang, L.; Wu, X.; Xu, Y.; Li, Y.; Wu, S.; Gong, L.; Tang, J. Bilayer Hydrogel Actuators with High Mechanical Properties and Programmable Actuation via the Synergy of Double-Network and Synchronized Ultraviolet Polymerization Strategies. Polymers 2024, 16, 840. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.J.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, J.; Li, H.; Hou, W. Synthesis of layered double hydroxide/poly(N isopropylacrylamide) nanocomposite hydrogels with excellent mechanical and thermoresponsive performances. SoftMatter 2018, 14, 1789. [Google Scholar] [CrossRef]
- Radojković, N.; Spasojević, J.; Kačarević-Popović, Z.; Stamenović, U.; Vodnik, V.; Roglić, G.; Radosavljević, A. Thermo-Responsive and Electroconductive Nano Au-PNiPAAm Hydrogel Nanocomposites: Influence of Synthesis Method and Nanoparticle Shape on Physicochemical Properties. Polymers 2024, 16, 3416. [Google Scholar] [CrossRef]
- Promdontree, P.; Ounkaew, A.; Yao, Y.; Zeng, H.; Ummartyotin, S. Temperature-Responsive Injectable Composite Hydrogels Based on Poly(N-Isopropylacrylamide), Chitosan, and Hemp-Derived Cellulose Nanocrystals. Polymers 2024, 16, 2984. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Q.; Che, L.; Li, M.; Leng, X.; Long, Y.; Lu, Y. Multi-Stimuli-Responsive Weldable Bilayer Actuator with Programmable Patterns and 3D Shapes. Adv. Funct. Mater. 2024, 34, 2311398. [Google Scholar] [CrossRef]
- Aspite, I.; Salehi, S.; Ionov, L. Materials for Smart Soft Actuator Systems. Chem. Rev. 2022, 122, 1349–1415. [Google Scholar] [CrossRef]
- Shi, Q.; Xia, H.; Li, P.; Wang, Y.S.; Wang, L.; Li, S.X.; Wang, G.; Lv, C.; Niu, L.G.; Sun, H.B. Photothermal Surface Plasmon Resonance and Interband Transition-Enhanced Nanocomposite Hydrogel Actuators with Hand-Like Dynamic Manipulation. Adv. Opt. Mater. 2017, 5, 1700442. [Google Scholar] [CrossRef]
- Lee, K.S.; El-Sayed, M.A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hea, Y.; Huang, J.; Zhu, J. Synthesis and solar photo-thermal conversion of Au, Ag, and Au-Ag blended plasmonic nanoparticles. Energy Convers. Manag. 2016, 127, 293–300. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Hasan, M.K.; Nam, K.W. A Comprehensive Review of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef]
- Spasojević, J.; Radosavljević, A.; Krstić, J.; Jovanović, D.; Spasojević, V.; Kalagasidis-Krušić, M.; Kačarević-Popović, Z. Dual responsive antibacterial Ag-poly(N-isopropylacrylamide/itaconic acid) hydrogel nanocomposites synthesized by gamma irradiation. Eur. Polym. J. 2015, 69, 168–185. [Google Scholar] [CrossRef]
- Bogdanović, U.; Vodnik, V.; Ahrenkiel, S.; Stoiljković, M.; Ćirić-Marjanović, G.; Nedeljković, J. Interfacial synthesis and characterization of gold/polyaniline nanocomposites. Synthetic Met. 2014, 195, 122–131. [Google Scholar] [CrossRef]
- Milikic, J.; Stamenovic, U.; Vodnik, V.; Ahrenkiel, S.; Sljukic, B. Gold nanorod-polyaniline composites: Synthesis and evaluation as anode electrocatalysts for direct borohydride fuel cells. Electrochim. Acta 2019, 328, 135115. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Krajczewski, J.; Koła, K.; Kudelski, A. Plasmonic nanoparticles in chemical analysis. RSC Adv. 2017, 7, 17559. [Google Scholar] [CrossRef]
- López-Muñoz, G.A.; Pescador-Rojas, J.A.; Ortega-Lopez, J.; Salazar, J.S.; Balderas-López, J.A. Thermal diffusivity measurement of spherical gold nanofluids of different sizes/concentrations. Nanoscale Res. Lett. 2012, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Eid, M.; El-Arnaouty, M.B.; Salah, M.; Soliman, E.S.; Hegazy, E.S.A. Radiation synthesis and characterization of poly(vinyl alcohol)/poly(N-vinyl-2-pyrrolidone) based hydrogels containing silver nanoparticles. J. Polym. Res. 2012, 19, 9835. [Google Scholar] [CrossRef]
- Zheng, J.; Clogston, J.; Patri, A.; Dobrovolskaia, M.; McNeil, S. Sterilization of Silver Nanoparticles Using Standard Gamma Irradiation Procedure Affects Particle Integrity and Biocompatibility. J. Nanomed. Nanotechnol. 2011, 2011 (Suppl. S5), 001. [Google Scholar] [CrossRef]
- Zheng, J.; Clogston, J.D.; Patri, A.K.; McNeil, S.E.; Dobrovolskaia, M.A. Sterilization Case Study 2: Effects of Sterilization Techniques on Silver Nanoparticles. In Handbook of Immunological Properties of Engineered Nanomaterials, 2nd ed.; Dobrovolskaia, M.A., McNeil, S.E., Eds.; World Scientific Publishing Co., Inc.: Hackensack, NJ, USA, 2016; Chapter 5; pp. 93–108. [Google Scholar] [CrossRef]
- Asnag, G.M.; Oraby, A.H.; Abdelghany, A.M. Effect of gamma-irradiation on the structural, optical and electrical properties of PEO/starch blend containing different concentrations of gold nanoparticles. Radiat. Eff. Defects. Solids. 2019, 174, 579–595. [Google Scholar] [CrossRef]
- Nikolic, N.; Spasojevic, J.; Radosavljevic, A.; Milosevic, M.; Barudzija, T.; Rakocevic, L.; Kacarevic-Popovic, Z. Influence of poly(vinyl alcohol)/poly(N-vinyl-2-pyrrolidone) polymer matrix composition on the bonding environment and characteristics of Ag nanoparticles produced by gamma irradiation. Radiat. Phys. Chem. 2023, 202, 110564. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, P.; Le, X.; Lu, W.; Théato, P.; Ma, C.; Du, B.; Zhang, J.; Huang, Y.; Chen, T. Mimosa Inspired Bilayer Hydrogel Actuator Functioning in Multi-Environment. J. Mater. Chem. C 2018, 6, 1320–1327. [Google Scholar] [CrossRef]
- Hoffman, A. Hydrogels for biomedical applications. Adv. Drug Deliver. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Zhang, C.L.; Cao, F.H.; Wang, J.L.; Yu, Z.L.; Ge, J.; Lu, Y.; Wang, Z.H.; Yu, S.H. A Highly Stimuli-Responsive Au Nanorods/Poly(N isopropylacrylamide) (PNIPAM) Composite Hydrogel for Smart Switch. ACS Appl. Mater. Interfaces. 2017, 9, 24857–24863. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; He, S.; Zhang, J.; Shao, W. Recent progress in PNIPAM-based multi-responsive actuators: A mini-review. Chem. Eng. J. 2022, 433, 133496. [Google Scholar] [CrossRef]
- Awasthi, S.; Singhal, R. Mathematical modeling for the prediction of the overall swelling profile from poly (AM-co-AA-co-HEA) hydrogels: Effect of glycidyl methacrylate and ammonium persulphate. Int. J. Plast. Technol. 2015, 19, 241–262. [Google Scholar] [CrossRef]
- Spasojević, J.; Milošević, M.; Vidičević-Novaković, S.; Tasić, J.; Milovanović, P.; Djurić, M.; Ranković, D.; Kačarević-Popović, Z.; Radosavljević, A. Multifunctional Ag-Poly(N-isopropylacrylamide/itaconic Acid) Hydrogel Nanocomposites Prepared by Gamma Irradiation for Potential Application as Topical Treatment Dressings. Polymers 2024, 16, 3211. [Google Scholar] [CrossRef]
- Mullarney, M.P.; Seery, T.A.P.; Weiss, R.A. Drug diffusion in hydrophobically modified N,N-dimethylacrylamide hydrogels. Polymer 2006, 47, 3845–3855. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef]
- Singhal, R.; Awasthi, S. Hydrogels: Mathematical Approaches. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Taylor and Francis: New York, NY, USA, 2015; pp. 3929–3950. [Google Scholar] [CrossRef]
- Peppas, N.A.; Reinhart, C.T. Solute diffusion in swollen membranes. Part I. A new theory. J. Membr. Sci. 1983, 15, 275–287. [Google Scholar] [CrossRef]
- Reinhart, C.T.; Peppas, N.A. Solute diffusion in swollen membranes. Part II. Influence of crosslinking on diffusive properties. J. Membr. Sci. 1984, 18, 227–239. [Google Scholar] [CrossRef]
- Korsmeyer, R.W. Diffusion controlled systems: Hydrogels. In Polymers for Controlled Drug Delivery; CRC Press: Boca Raton, FL, USA, 1990; pp. 16–34. [Google Scholar]
- Zhao, Z.X.; Li, Z.; Xia, Q.B.; Bajalis, E.; Xi, H.X.; Lin, Y.S. Swelling/deswelling kinetics of PNIPAAm hydrogels synthesized by microwave irradiation. Chem. Eng. J. 2008, 142, 263–270. [Google Scholar] [CrossRef]
- Li, M.; Bae, J. Programmable Dual-Responsive Actuation of Single-Hydrogel-Based Bilayer Actuators by Photothermal and Skin Layer Effects with Graphene Oxides. Adv. Mater. Interfaces 2023, 10, 2300169. [Google Scholar] [CrossRef]
- Holder, C.; Schaak, R. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Radinović, K.; Milikić, J.; Stamenović, U.; Vodnik, V.; Otoničar, M.; Škapin, S.; Šljukić, B. Tailoring gold-conducting polymer nanocomposites for sensors applications: Proof of concept for As(III) sensing in aqueous media. Synth. Met. 2021, 278, 116834. [Google Scholar] [CrossRef]
- Sant, D.; Gujarathi, T.; Harne, S.; Ghosh, S.; Kitture, R.; Kale, S.; Chopade, B.; Pardesi, K. Adiantum philippense L. Frond Assisted Rapid Green Synthesis of Gold and Silver Nanoparticles. J. Nanoparticles 2013, 2013, 182320. [Google Scholar] [CrossRef]
- Sathiyaraj, S.; Suriyakala, G.; Gandhi, A.D.; Babujanarthanam, R.; Almaary, K.S.; Chen, T.W.; Kaviyarasu, K. Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. J. Infect. Public Health 2021, 14, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mohdy, H.L.; Aly, H.M. Characterization, Properties and Antimicrobial Activity of Radiation Induced Phosphorus-Containing PVA Hydrogels. Arab. J. Sci. Eng. 2023, 48, 341–351. [Google Scholar] [CrossRef]
- Mandal, S.; Dasmahapatra, A.K. Effect of aging on the microstructure and physical properties of Poly (vinyl alcohol) hydrogel. J. Polym. Res. 2021, 28, 269. [Google Scholar] [CrossRef]
- Krstić, J.; Spasojević, J.; Radosavljević, A.; Perić-Grujić, A.; Djurić, M.; Kačarević-Popović, Z.; Popović, S. In Vitro Silver Ion Release Kinetics from Nanosilver/Poly(vinyl alcohol) Hydrogels Synthesized by Gamma Irradiation. J. Appl. Polym. Sci. 2014, 131, 40321. [Google Scholar] [CrossRef]
- Shah, L.A.; Farooqi, Z.H.; Naeem, H.; Shah, S.M.; Siddiq, M. Synthesis and Characterization of Poly(N-isopropylacrylamide) Hybrid Microgels with different Cross-linker Contents. J. Chem. Soc. Pak. 2013, 35, 6. [Google Scholar]
- Khan, A. Preparation and characterization of N-isopropylacrylamide/acrylic acid copolymer core-shell microgel particles. J. Colloid. Interf. Sci. 2007, 313, 697–704. [Google Scholar] [CrossRef]
- Safo, I.A.; Werheid, M.; Dosche, C.; Oezaslan, M. The role of polyvinylpyrrolidone (PVP) as a capping and structure-directing agent in the formation of Pt nanocubes. Nanoscale Adv. 2019, 1, 3095–3106. [Google Scholar] [CrossRef]
- Dueramae, I.; Tanaka, F.; Shinyashiki, N.; Yagihara, S.; Kita, R. UV-Crosslinked Poly(N-isopropylacrylamide) Interpenetrated into Chitosan Structure with Enhancement of Mechanical Properties Implemented as Anti-Fouling Materials. Gels 2024, 10, 20. [Google Scholar] [CrossRef]
- Maheswari, B.; Jagadeesh Babu, P.E.; Agarwal, M. Role of N-vinyl-2-pyrrolidinone on the thermoresponsive behavior of PNIPAm hydrogel and its release kinetics using dye and vitamin-B12 as model drug. J. Biomat. Sci.-Polym. E 2014, 25, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Puleo, G.L.; Zulli, F.; Piovanelli, M.; Giordano, M.; Mazzolai, B.; Beccaia, L.; Andreozzi, L. Mechanical and rheological behavior of pNIPAAM crosslinked macrohydrogel. React. Funct. Polym. 2013, 73, 1306–1318. [Google Scholar] [CrossRef]
- Ugrinovic, V.; Markovic, M.; Bozic, B.; Panic, V.; Veljovic, D. Physically Crosslinked Poly(methacrylic acid)/Gelatin Hydrogels with Excellent Fatigue Resistance and Shape Memory Properties. Gels 2024, 10, 444. [Google Scholar] [CrossRef]
- Cao, Y.; Li, W.; Quan, F.; Xia, Y.; Xiong, Z. Green–Light–Driven Poly(N-isopropylacrylamide-acrylamide)/Fe3O4 Nanocomposite Hydrogel Actuators. Front. Mater. Sec. Biomater. 2022, 9, 827608. [Google Scholar] [CrossRef]
- Scanone, A.C.; Pérez, M.E.; Vallina, P.A.; Durantini, E.N.; Arenas, G.F.; Hoppe, C.E. Soft hydrogels obtained by photothermal curing of poly(vinylpyrrolidone)/gold nanoparticles dispersions showing anisotropic swelling and photo-activated antimicrobial properties. Eur. Polym. J. 2024, 220, 113491. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.S.; Yoon, J. Highly bendable bilayer-type photo-actuators comprising of reduced graphene oxide dispersed in hydrogels. Sci. Rep. 2016, 6, 20921. [Google Scholar] [CrossRef]
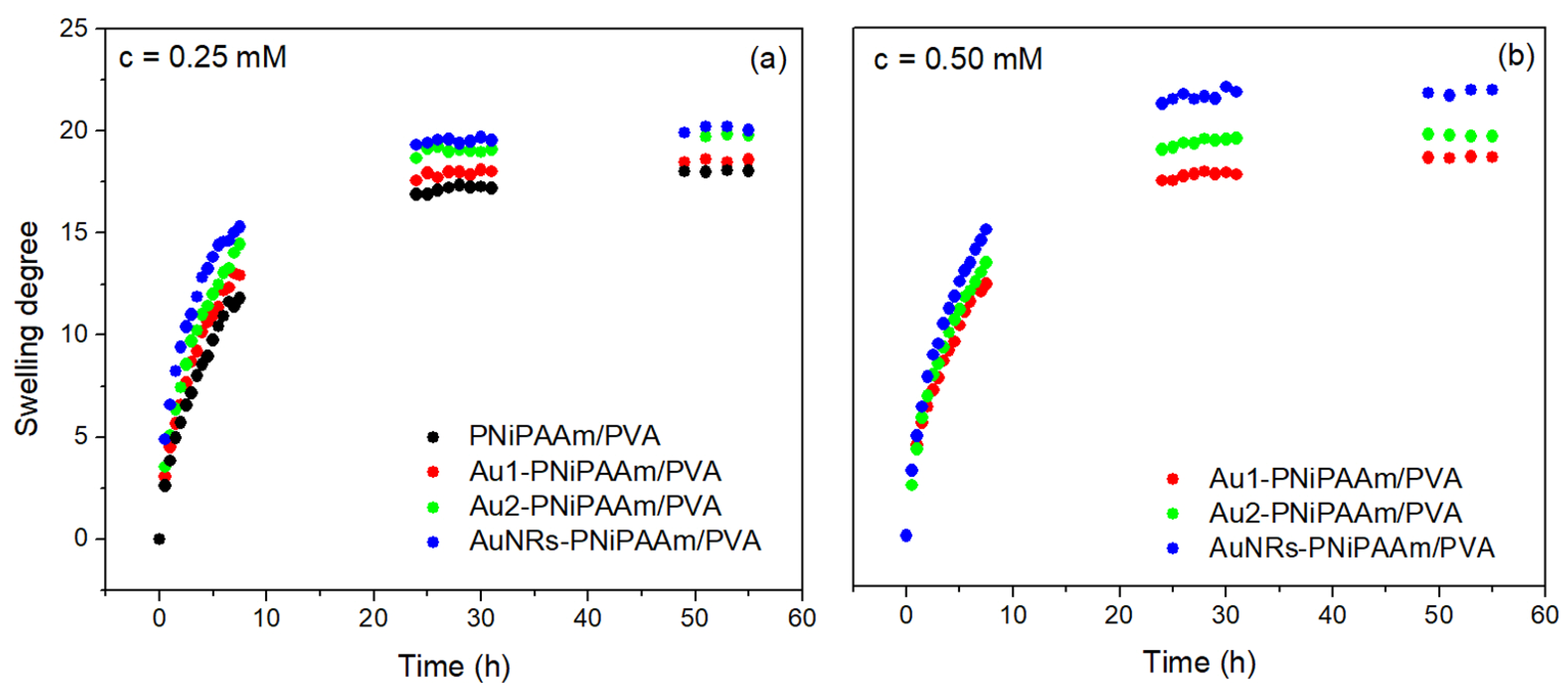
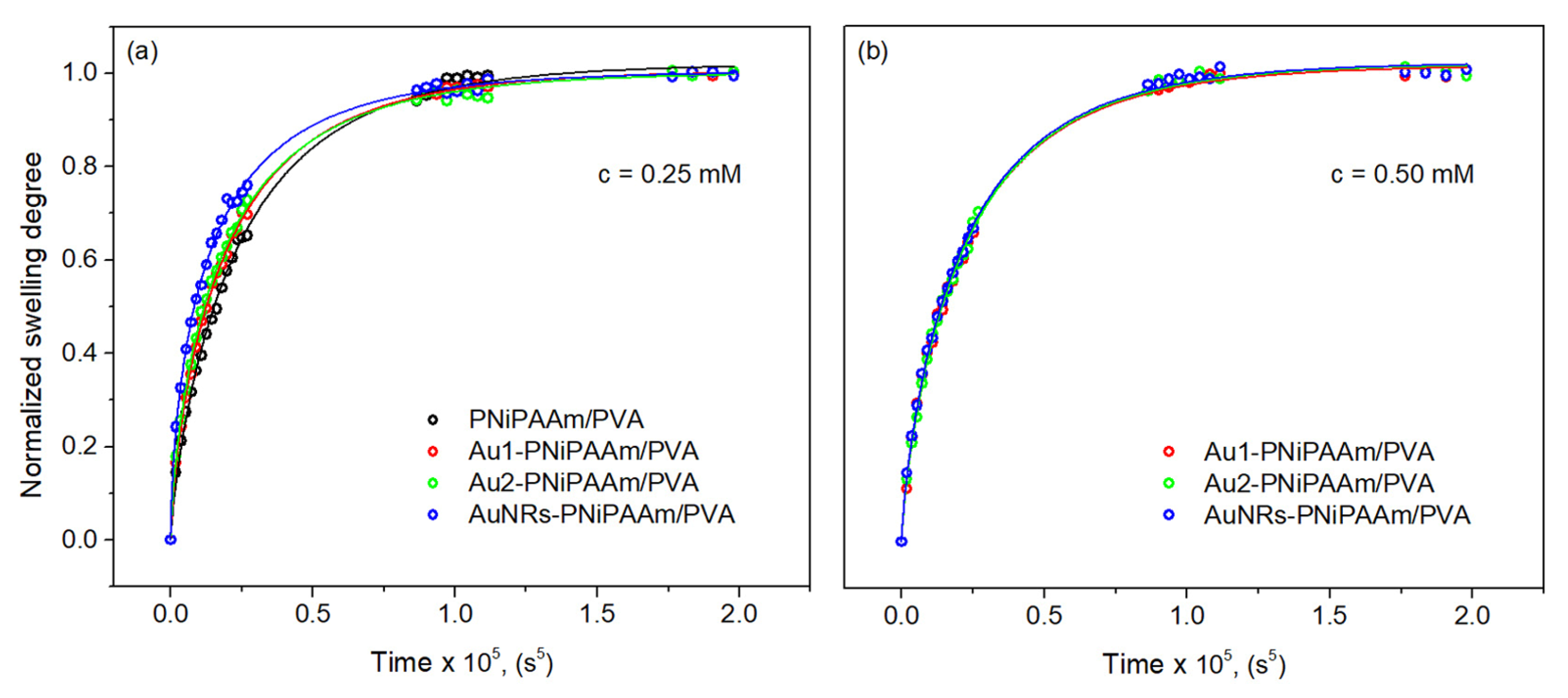
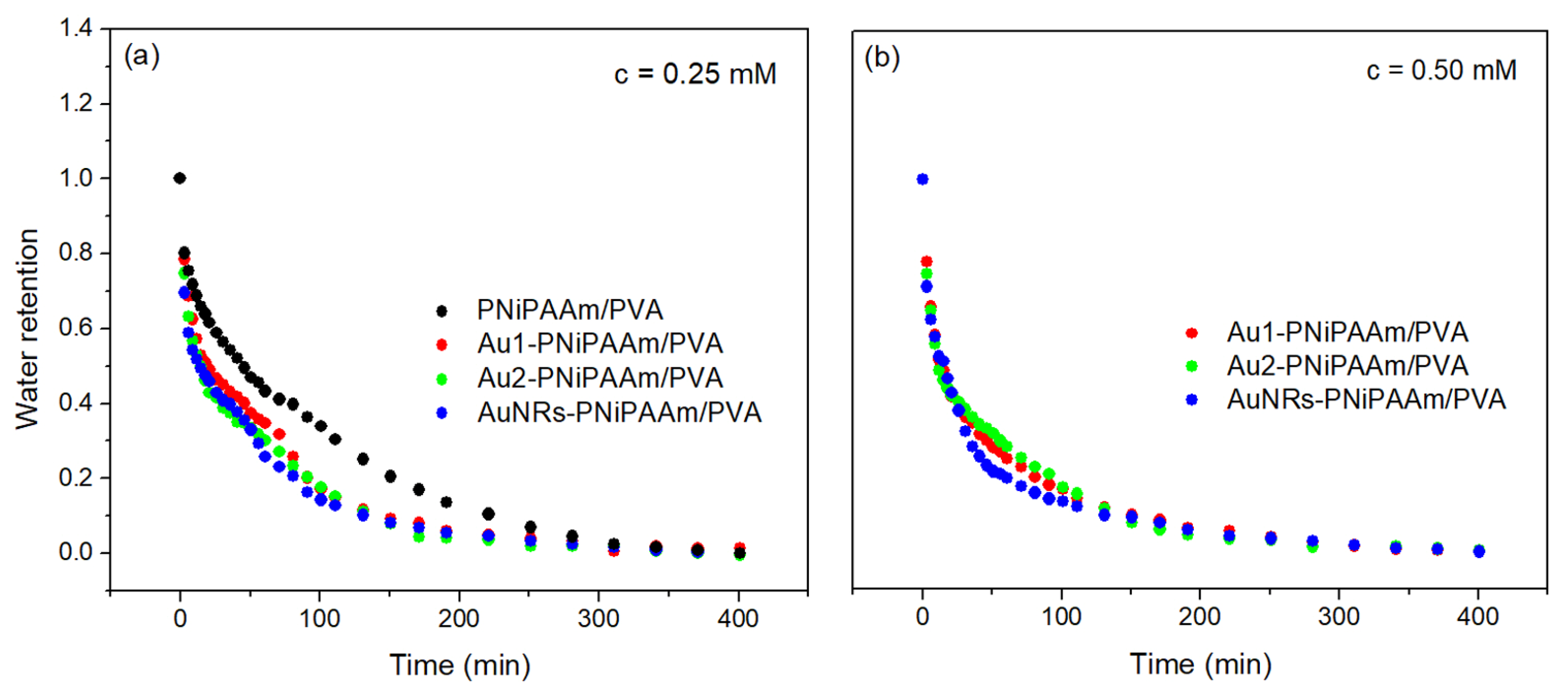
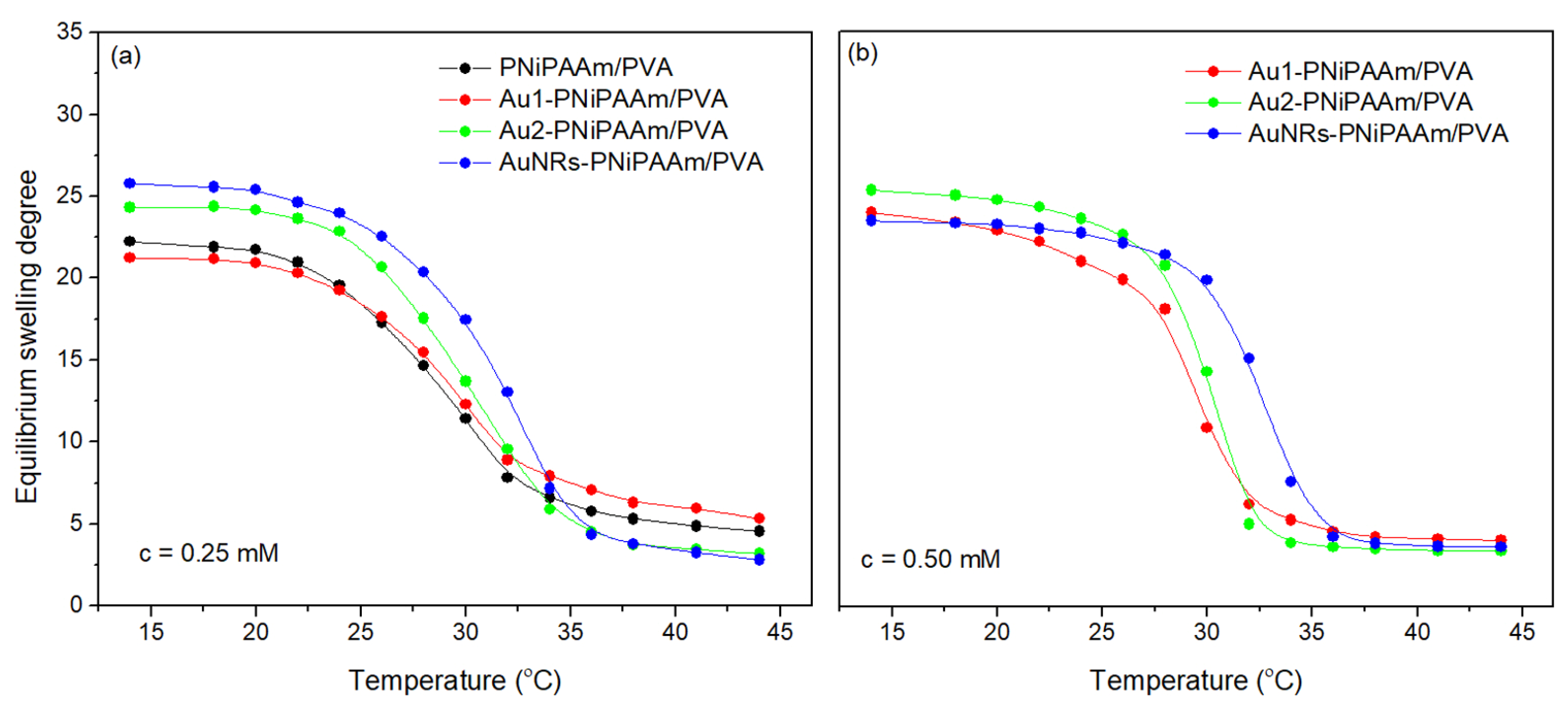
| Sample | c(Au) (mM) | dav (nm) | SDeq | n | Kd × 102 (1/min) | VPTT (°C) |
|---|---|---|---|---|---|---|
| PNiPAAm/PVA | 0 | 18.1 ± 0.2 | 0.57 ± 0.01 | 1.96 ± 0.08 | 29.5 ± 0.5 | |
| AuNSs-PNiPAAm/PVA | 0.25 | 17 | 18.5 ± 0.3 | 0.57 ± 0.02 | 2.18 ± 0.07 | 29.6 ± 0.6 |
| 30 | 19.3 ± 0.4 | 0.55 ± 0.02 | 2.52 ± 0.08 | 30.1 ± 0.5 | ||
| 0.50 | 17 | 19.5 ± 0.5 | 0.62 ± 0.03 | 2.33 ± 0.08 | 29.7 ± 0.4 | |
| 30 | 19.6 ± 0.3 | 0.61 ± 0.01 | 2.49 ± 0.09 | 30.0 ± 0.5 | ||
| AuNRs-PNiPAAm/PVA | 0.25 | 20.1 ± 0.5 | 0.56 ± 0.01 | 4.10 ± 0.11 | 32.0 ± 0.7 | |
| 0.50 | 21.8 ± 0.6 | 0.58 ± 0.02 | 4.30 ± 0.12 | 32.3 ± 0.6 |
| Sample | c(Au) (mM) | dav (nm) | Dearly × 108 (cm2/s) | R2 | Dlate × 108 (cm2/s) | R2 | DEtters × 108 (cm2/s) | R2 |
|---|---|---|---|---|---|---|---|---|
| PNiPAAm/PVA | 0 | 2.50 ± 0.11 | 0.99 | 3.40 ± 0.16 | 0.98 | 3.35 ± 0.09 | 0.98 | |
| AuNSs-PNiPAAm/PVA | 0.25 | 17 | 3.12 ± 0.15 | 0.98 | 3.81 ± 0.19 | 0.97 | 3.63 ± 0.11 | 0.99 |
| 30 | 3.37 ± 0.17 | 0.99 | 3.97 ± 0.20 | 0.98 | 3.85 ± 0.12 | 0.99 | ||
| 0.50 | 17 | 3.25 ± 0.16 | 0.99 | 5.12 ± 0.23 | 0.97 | 3.55 ± 0.10 | 0.99 | |
| 30 | 3.89 ± 0.28 | 0.97 | 3.56 ± 0.18 | 0.99 | 3.61 ± 0.14 | 0.99 | ||
| AuNRs- PNiPAAm/PVA | 0.25 | 3.61 ± 0.17 | 0.96 | 4.88 ± 0.21 | 0.98 | 4.62 ± 0.13 | 0.98 | |
| 0.50 | 3.83 ± 0.20 | 0.98 | 4.91 ± 0.22 | 0.96 | 4.89 ± 0.16 | 0.99 |
| Sample | c(Au) (mM) | dav (nm) | σc (kPa) | Ms (%) | Ec (kPa) |
|---|---|---|---|---|---|
| PNiPAAm | 0 | 10.7 ± 0.4 | 59.0 ± 2.6 | 1.31 ± 0.08 | |
| PVA | 93.8 ± 3.9 | 88.9 ± 3.4 | 2.72 ± 0.15 | ||
| PNiPAAm/PVA | 25.3 ± 1.1 | 83.3 ± 2.9 | 1.90 ± 0.13 | ||
| AuNSs-PNiPAAm/PVA | 0.25 | 17 | 52.7 ± 2.8 | 84.3 ± 3.6 | 3.21 ± 0.19 |
| 30 | 38.9 ± 1.6 | 92.9 ± 3.8 | 1.33 ± 0.11 | ||
| 0.50 | 17 | 67.0 ± 3.2 | 84.4 ± 3.1 | 4.01 ± 0.23 | |
| 30 | 40.5 ± 2.6 | 87.4 ± 3.0 | 2.10 ± 0.14 | ||
| AuNRs-PNiPAAm/PVA | 0.25 | 26.3 ± 1.2 | 76.5 ± 2.5 | 2.14 ± 0.12 | |
| 0.50 | 32.7 ± 2.1 | 78.1 ± 2.8 | 1.81 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radojković, N.; Spasojević, J.; Vukoje, I.; Kačarević-Popović, Z.; Stamenović, U.; Vodnik, V.; Roglić, G.; Radosavljević, A. Gamma Irradiation-Induced Synthesis of Nano Au-PNiPAAm/PVA Bi-Layered Photo-Thermo-Responsive Hydrogel Actuators with a Switchable Bending Motion. Polymers 2025, 17, 1774. https://doi.org/10.3390/polym17131774
Radojković N, Spasojević J, Vukoje I, Kačarević-Popović Z, Stamenović U, Vodnik V, Roglić G, Radosavljević A. Gamma Irradiation-Induced Synthesis of Nano Au-PNiPAAm/PVA Bi-Layered Photo-Thermo-Responsive Hydrogel Actuators with a Switchable Bending Motion. Polymers. 2025; 17(13):1774. https://doi.org/10.3390/polym17131774
Chicago/Turabian StyleRadojković, Nikolina, Jelena Spasojević, Ivana Vukoje, Zorica Kačarević-Popović, Una Stamenović, Vesna Vodnik, Goran Roglić, and Aleksandra Radosavljević. 2025. "Gamma Irradiation-Induced Synthesis of Nano Au-PNiPAAm/PVA Bi-Layered Photo-Thermo-Responsive Hydrogel Actuators with a Switchable Bending Motion" Polymers 17, no. 13: 1774. https://doi.org/10.3390/polym17131774
APA StyleRadojković, N., Spasojević, J., Vukoje, I., Kačarević-Popović, Z., Stamenović, U., Vodnik, V., Roglić, G., & Radosavljević, A. (2025). Gamma Irradiation-Induced Synthesis of Nano Au-PNiPAAm/PVA Bi-Layered Photo-Thermo-Responsive Hydrogel Actuators with a Switchable Bending Motion. Polymers, 17(13), 1774. https://doi.org/10.3390/polym17131774






