The Impact of PEO and PVP Additives on the Structure and Properties of Silk Fibroin Adsorption Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Methods
3. Results
3.1. Dynamic Surface Elasticity and Dynamic Surface Tension
3.2. Compression Isotherms
3.3. Lissajous Plots
3.4. Ellipsometry
3.5. Atomic Force Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.u.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning Nanofiber Scaffolds for Soft and Hard Tissue Regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Biswal, B.; Dan, A.K.; Sengupta, A.; Das, M.; Bindhani, B.K.; Das, D.; Parhi, P.K. Extraction of Silk Fibroin with Several Sericin Removal Processes and Its Importance in Tissue Engineering: A Review. J. Polym. Environ. 2022, 30, 2222–2253. [Google Scholar] [CrossRef]
- O’Connell, G.; Garcia, J.; Amir, J. 3D Bioprinting: New Directions in Articular Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 2657–2668. [Google Scholar] [CrossRef] [PubMed]
- Von Der Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale Engineering of Biomimetic Surfaces: Cues from the Extracellular Matrix. Cell Tissue Res. 2010, 339, 131–153. [Google Scholar] [CrossRef]
- Yazawa, K.; Hidaka, K.; Negishi, J. Cell Adhesion Behaviors on Spider Silk Fibers, Films, and Nanofibers. Langmuir 2022, 38, 7766–7774. [Google Scholar] [CrossRef]
- Jiang, Q.; Hasan, S.M.K. Phase Separated Fibrous Structures: Mechanism Study and Applications. ACS Symp. Ser. 2014, 1175, 127–141. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.Y.; Huh, M.; Yun, S. Il Porous Poly(3-Hydroxybutyrate) Scaffolds Prepared by Non-Solvent-Induced Phase Separation for Tissue Engineering. Macromol. Res. 2020, 28, 835–843. [Google Scholar] [CrossRef]
- Ariga, K.; Jia, X.; Song, J.; Hill, J.P.; Leong, D.T.; Jia, Y.; Li, J. Nanoarchitectonics beyond Self-Assembly: Challenges to Create Bio-Like Hierarchic Organization. Angew. Chem. Int. Ed. Engl. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Hense, D.; Büngeler, A.; Kollmann, F.; Hanke, M.; Orive, A.; Keller, A.; Grundmeier, G.; Huber, K.; Strube, O.I. Self-Assembled Fibrinogen Hydro- And Aerogels with Fibrin-like 3D Structures. Biomacromolecules 2021, 22, 4084–4094. [Google Scholar] [CrossRef]
- Panda, P.K.; Hsieh, C.-Y.; Shen, Y.-T.; Tsai, Y.-H.; Tsai, H.-W.; Yao, C.-L.; Chen, Y.; Yang, P.-C. Synthesis and Physicochemical Properties of Doxorubicin-Loaded PEGA Containing Amphiphilic Block Polymeric Micelles. J. Polym. Res. 2024, 31, 306. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Li, Y.; Pan, W.; Tan, G. Silk Fibroin Combined with Electrospinning as a Promising Strategy for Tissue Regeneration. Macromol. Biosci. 2023, 23, e2200380. [Google Scholar] [CrossRef] [PubMed]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of Natural Polymers for the Production of Nanofibres for Wound Healing Applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Ewaldz, E.; Brettmann, B. Molecular Interactions in Electrospinning: From Polymer Mixtures to Supramolecular Assemblies. ACS Appl. Polym. Mater. 2019, 1, 298–308. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent Advances in Electrospinning of Nanofibers from Bio-Based Carbohydrate Polymers and Their Applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Kim, K.; Kloxin, C.J.; Saven, J.G.; Pochan, D.J. Nanofibers Produced by Electrospinning of Ultrarigid Polymer Rods Made from Designed Peptide Bundlemers. ACS Appl. Mater. Interfaces 2021, 13, 26339–26351. [Google Scholar] [CrossRef]
- Bavatharani, C.; Muthusankar, E.; Wabaidur, S.M.; Alothman, Z.A.; Alsheetan, K.M.; AL-Anazy, M.m.; Ragupathy, D. Electrospinning Technique for Production of Polyaniline Nanocomposites/Nanofibres for Multi-Functional Applications: A Review. Synth. Met. 2021, 271, 116609. [Google Scholar] [CrossRef]
- Yang, Q.; Zhenyu, L.I.; Hong, Y.; Zhao, Y.; Qiu, S.; Wang, C.E.; Wei, Y. Influence of Solvents on the Formation of Ultrathin Uniform Poly(Vinyl Pyrrolidone) Nanofibers with Electrospinning. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Vallejo-Martinez, M.; Puerta, M.; Restrepo-Osorio, A. Electrospun Silk Fibroin Using Aqueous and Formic Acid Solutions. MRS Adv. 2021, 6, 975–979. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Xiao, Z.; Zhang, W.; Li, L.; Fan, Y. Electrospun Polymeric Fibers Decorated with Silk Microcapsules via Encapsulation and Surface Immobilization for Drug Delivery. Macromol. Biosci. 2023, 23, e2300190. [Google Scholar] [CrossRef]
- Kopp, A.; Smeets, R.; Gosau, M.; Kröger, N.; Fuest, S.; Köpf, M.; Kruse, M.; Krieger, J.; Rutkowski, R.; Henningsen, A.; et al. Effect of Process Parameters on Additive-Free Electrospinning of Regenerated Silk Fibroin Nonwovens. Bioact. Mater. 2020, 5, 241–252. [Google Scholar] [CrossRef]
- Yi, B.; Zhang, H.; Yu, Z.; Yuan, H.; Wang, X.; Zhang, Y. Fabrication of High Performance Silk Fibroin Fibers: Via Stable Jet Electrospinning for Potential Use in Anisotropic Tissue Regeneration. J. Mater. Chem. B 2018, 6, 3934–3945. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, C.; Mu, X.; Kaplan, D.L. Engineering Silk Materials: From Natural Spinning to Artificial Processing. Appl. Phys. Rev. 2020, 7, 011313. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Murab, S.; Karmakar, S.; Chowdhury, P.K.; Ghosh, S. Modulation of Self-Assembly Process of Fibroin: An Insight for Regulating the Conformation of Silk Biomaterials. Biomacromolecules 2015, 16, 3936–3944. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.Y.; Kim, I.S.; Zhang, K.Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H.J.; Kaplan, D.L.; Rutledge, G.C. Mechanical Properties of Electrospun Silk Fibers. Macromolecules 2004, 37, 6856–6864. [Google Scholar] [CrossRef]
- Mitropoulos, A.N.; Marelli, B.; Ghezzi, C.E.; Applegate, M.B.; Partlow, B.P.; Kaplan, D.L.; Omenetto, F.G. Transparent, Nanostructured Silk Fibroin Hydrogels with Tunable Mechanical Properties. ACS Biomater. Sci. Eng. 2015, 1, 964–970. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Z.; Yang, Y.; Fang, G.; Yao, J.; Shao, Z.; Chen, X. Robust Protein Hydrogels from Silkworm Silk. ACS Sustain. Chem. Eng. 2016, 4, 1500–1506. [Google Scholar] [CrossRef]
- Su, D.; Yao, M.; Liu, J.; Zhong, Y.; Chen, X.; Shao, Z. Enhancing Mechanical Properties of Silk Fibroin Hydrogel through Restricting the Growth of β-Sheet Domains. ACS Appl. Mater. Interfaces 2017, 9, 17489–17498. [Google Scholar] [CrossRef]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement Controls Stiffness, Strength and Mechanical Toughness of Β-Sheet Crystals in Silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [CrossRef]
- Farokhi, M.; Aleemardani, M.; Solouk, A.; Mirzadeh, H.; Teuschl, A.H.; Redl, H. Crosslinking Strategies for Silk Fibroin Hydrogels: Promising Biomedical Materials. Biomed. Mater. 2021, 16, 022004. [Google Scholar] [CrossRef]
- Koh, L.D.; Cheng, Y.; Teng, C.P.; Khin, Y.W.; Loh, X.J.; Tee, S.Y.; Low, M.; Ye, E.; Yu, H.D.; Zhang, Y.W.; et al. Structures, Mechanical Properties and Applications of Silk Fibroin Materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Li, C.; Vepari, C.; Jin, H.J.; Kim, H.J.; Kaplan, D.L. Electrospun Silk-BMP-2 Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Morikawa, H.; Yamanaka, S.; Tamada, Y. Electrospinning of Silk Fibroin from All Aqueous Solution at Low Concentration. Mater. Sci. Eng. C 2017, 73, 498–506. [Google Scholar] [CrossRef]
- Cilurzo, F.; Gennari, C.G.M.; Selmin, F.; Marotta, L.A.; Minghetti, P.; Montanari, L. An Investigation into Silk Fibroin Conformation in Composite Materials Intended for Drug Delivery. Int. J. Pharm. 2011, 414, 218–224. [Google Scholar] [CrossRef]
- Tao, C.; Zhang, Y.; Li, B.; Chen, L. Hierarchical Micro/Submicrometer-Scale Structured Scaffolds Prepared: Via Coaxial Electrospinning for Bone Regeneration. J. Mater. Chem. B 2017, 5, 9219–9228. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Chen, Y.; Bayat, A.; Yuan, X.F. Rheology and Electrospinning of Regenerated Bombyx Mori Silk Fibroin Aqueous Solutions. Biomacromolecules 2014, 15, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Firouzi, M.; Hassannejad, Z.; Haririan, I.; Zahedi, P. Fabrication and Characterization of Electrospun Laminin-Functionalized Silk Fibroin/Poly(Ethylene Oxide) Nanofibrous Scaffolds for Peripheral Nerve Regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1595–1604. [Google Scholar] [CrossRef]
- Jin, H.J.; Fridrikh, S.V.; Rutledge, G.C.; Kaplan, D.L. Electrospinning Bombyx Mori Silk with Poly(Ethylene Oxide). Biomacromolecules 2002, 43, 743–744. [Google Scholar] [CrossRef]
- Wang, M.; Yu, J.H.; Kaplan, D.L.; Rutledge, G.C. Production of Submicron Diameter Silk Fibers under Benign Processing Conditions by Two-Fluid Electrospinning. Macromolecules 2006, 39, 1102–1107. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Shao, H.; Hu, X. Electrospinning and Rheology of Regenerated Bombyx Mori Silk Fibroin Aqueous Solutions: The Effects of PH and Concentration. Polymer 2008, 49, 2880–2885. [Google Scholar] [CrossRef]
- Lovett, M.; Cannizzaro, C.; Daheron, L.; Messmer, B.; Vunjak-Novakovic, G.; Kaplan, D.L. Silk Fibroin Microtubes for Blood Vessel Engineering. Biomaterials 2007, 28, 5271–5279. [Google Scholar] [CrossRef]
- Jin, H.J.; Park, J.; Valluzzi, R.; Cebe, P.; Kaplan, D.L. Biomaterial Films of Bombyx Mori Silk Fibroin with Poly(Ethylene Oxide). Biomacromolecules 2004, 5, 711–717. [Google Scholar] [CrossRef]
- Ling, S.; Qi, Z.; Watts, B.; Shao, Z.; Chen, X. Structural Determination of Protein-Based Polymer Blends with a Promising Tool: Combination of FTIR and STXM Spectroscopic Imaging. Phys. Chem. Chem. Phys. 2014, 16, 7741–7748. [Google Scholar] [CrossRef] [PubMed]
- Valluzzi, R.; Gido, S.P.; Muller, W.; Kaplan, D.L. Orientation of Silk III at the Air-Water Interface. Int. J. Biol. Macromol. 1999, 24, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dicko, C.; Bain, C.D.; Gong, Z.; Jacobs, R.M.J.; Shao, Z.; Terry, A.E.; Vollrath, F. Behavior of Silk Protein at the Air-Water Interface. Soft Matter 2012, 8, 9705–9712. [Google Scholar] [CrossRef]
- Kim, T.; Kim, B.J.; Bonacchini, G.E.; Ostrovsky-Snider, N.A.; Omenetto, F.G. Silk Fibroin as a Surfactant for Water-Based Nanofabrication. Nat. Nanotechnol. 2024, 19, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, H.; Lin, Z.; Lin, Y.; van Esch, J.H.; Liu, X.Y. Programing Performance of Silk Fibroin Materials by Controlled Nucleation. Adv. Funct. Mater. 2016, 26, 8978–8990. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Schauer, C.L. A Review: Electrospinning of Biopolymer Nanofibers and Their Applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Meng, L.; Shao, C.; Cui, C.; Xu, F.; Lei, J.; Yang, J. Autonomous Self-Healing Silk Fibroin Injectable Hydrogels Formed via Surfactant-Free Hydrophobic Association. ACS Appl. Mater. Interfaces 2020, 12, 1628–1639. [Google Scholar] [CrossRef]
- Jayawardane, D.; Pan, F.; Lu, J.R.; Zhao, X. Interfacial Adsorption of Silk Fibroin Peptides and Their Interaction with Surfactants at the Solid-Water Interface. Langmuir 2016, 32, 8202–8211. [Google Scholar] [CrossRef]
- Qiao, X.; Miller, R.; Sun, K. Interfacial Adsorption, Viscoelasticity and Recovery of Silk Fibroin Layers at Different Oil/Water Interface. Colloids Surf. A Physicochem. Eng. Asp. 2017, 519, 179–186. [Google Scholar] [CrossRef]
- Qiao, X.; Miller, R.; Schneck, E.; Sun, K. Influence of PH on the Surface and Foaming Properties of Aqueous Silk Fibroin Solutions. Soft Matter 2020, 16, 3695–3704. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Miller, R.; Schneck, E.; Sun, K. Influence of Surfactant Charge and Concentration on the Surface and Foaming Properties of Biocompatible Silk Fibroin. Mater. Chem. Phys. 2022, 281, 125920. [Google Scholar] [CrossRef]
- Milyaeva, O.Y.; Akentiev, A.V.; Bykov, A.G.; Miller, R.; Rafikova, A.R.; Rotanova, K.Y.; Noskov, B.A. Silk Fibroin Self-Assembly at the Air—Water Interface. Colloids Interfaces 2024, 8, 35. [Google Scholar] [CrossRef]
- Qiao, X.; Miller, R.; Schneck, E.; Sun, K. Foaming Properties and the Dynamics of Adsorption and Surface Rheology of Silk Fibroin at the Air/Water Interface. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124553. [Google Scholar] [CrossRef]
- Qiao, X.; Miller, R.; Schneck, E.; Sun, K. Influence of Salt Addition on the Surface and Foaming Properties of Silk Fibroin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125621. [Google Scholar] [CrossRef]
- Chen, P.; Kim, H.S.; Park, C.Y.; Kim, H.S.; Chin, I.J.; Jin, H.J. PH-Triggered Transition of Silk Fibroin from Spherical Micelles to Nanofibrils in Water. Macromol. Res. 2008, 16, 539–543. [Google Scholar] [CrossRef]
- Valluzzi, R.; Gido, S.P. The Crystal Structure of Bombyx Mori Silk Fibroin at the Air-Water Interface. Biopolymers 1997, 42, 705–717. [Google Scholar] [CrossRef]
- Noskov, B.A.; Bykov, A.G. Dilational Rheology of Monolayers of Nano- and Micropaticles at the Liquid-Fluid Interfaces. Curr. Opin. Colloid Interface Sci. 2018, 37, 1–12. [Google Scholar] [CrossRef]
- Milyaeva, O.Y.; Miller, R.; Loglio, G.; Rafikova, A.R.; Wan, Z.; Noskov, B.A. Impact of Surfactants on Silk Fibroin Self-Assembly at the Air–Water Interface. Polymers 2025, 17, 529. [Google Scholar] [CrossRef]
- Wang, L.; Xie, H.; Qiao, X.; Goffin, A.; Hodgkinson, T.; Yuan, X.; Sun, K.; Fuller, G.G. Interfacial Rheology of Natural Silk Fibroin at Air/Water and Oil/Water Interfaces. Langmuir 2012, 28, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials Fabrication from Bombyx Mori Silk Fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Mikhailovskaya, A.A.; Noskov, B.A.; Lin, S.-Y.; Loglio, G.; Miller, R. Formation of Protein/Surfactant Adsorption Layer at the Air/Water Interface as Studied by Dilational Surface Rheology. J. Phys. Chem. B 2011, 115, 9971–9979. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, D.; Tessier, A.; Leblanc, R.M.R.M. Null Ellipsometer for the Studies of Thin Films at Gas-Water Interface. Rev. Sci. Instrum. 1987, 58, 571–578. [Google Scholar] [CrossRef]
- Motschmann, H.; Teppner, R. Ellipsometry in Interface Science. Stud. Interface Sci. 2001, 11, 1–42. [Google Scholar] [CrossRef]
- Noskov, B.A.; Akentiev, A.V.; Bilibin, A.Y.; Grigoriev, D.O.; Loglio, G.; Zorin, I.M.; Miller, R. Dynamic Surface Properties of Poly(N-Isopropylacrylamide) Solutions. Langmuir 2004, 20, 9669–9676. [Google Scholar] [CrossRef]
- Noskov, B.A.; Akentiev, A.V.; Miller, R. Dynamic Surface Properties of Poly(Vinylpyrrolidone) Solutions. J. Colloid Interface Sci. 2002, 255, 417–424. [Google Scholar] [CrossRef]
- Noskov, B.A.; Akentiev, A.V.; Loglio, G.; Miller, R. Dynamic Surface Properties of Solutions of Poly(Ethylene Oxide) and Polyethylene Glycols. J. Phys. Chem. B 2000, 104, 7923–7931. [Google Scholar] [CrossRef]
- Noskov, B.A. Protein Conformational Transitions at the Liquid-Gas Interface as Studied by Dilational Surface Rheology. Adv. Colloid Interface Sci. 2014, 206, 222–238. [Google Scholar] [CrossRef]
- Huang, S.; Peng, J.; Zi, Y.; Zheng, Y.; Xu, J.; Gong, H.; Kan, G.; Wang, X.; Zhong, J. Regenerated Silk Fibroin for the Stabilization of Fish Oil-Loaded Pickering Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132523. [Google Scholar] [CrossRef]
- Blocher McTigue, W.C.; Perry, S.L. Protein Encapsulation Using Complex Coacervates: What Nature Has to Teach Us. Small 2020, 16, 1907671. [Google Scholar] [CrossRef]
- Comert, F.; Dubin, P.L. Liquid–Liquid to Liquid–Solid Phase Separation: A Transition from Rain to Snow. Adv. Colloid Interface Sci. 2017, 239, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Blocher, W.C.; Perry, S.L. Complex Coacervate-Based Materials for Biomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2017, 9, 76–78. [Google Scholar] [CrossRef]
- Milyaeva, O.Y.; Akentiev, A.V.; Chirkov, N.S.; Lin, S.-Y.; Tseng, W.-C.; Vlasov, P.S.; Miller, R.; Noskov, B.A. Surface Properties of Protein–Polyelectrolyte Solutions. Impact of Polyelectrolyte Hydrophobicity. Langmuir 2023, 39, 8424–8434. [Google Scholar] [CrossRef]
- Milyaeva, O.Y.; Noskov, B.A.; Lin, S.Y.; Loglio, G.; Miller, R. Influence of Polyelectrolyte on Dynamic Surface Properties of BSA Solutions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 63–68. [Google Scholar] [CrossRef]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P.G. Protein-Surface Interactions in the Presence of Polyethylene Oxide. I. Simplified Theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Yuan, H.; Qian, B.; Zhang, W.; Lan, M. Protein Adsorption Resistance of PVP-Modified Polyurethane Film Prepared by Surface-Initiated Atom Transfer Radical Polymerization. Appl. Surf. Sci. 2016, 363, 483–489. [Google Scholar] [CrossRef]
- Nie, S.; Qin, H.; Li, L.; Zhang, C.; Yan, W.; Liu, Y.; Luo, J.; Chen, P. Influence of Brush Length of PVP Chains Immobilized on Silicon Wafers on Their Blood Compatibility. Polym. Adv. Technol. 2018, 29, 835–842. [Google Scholar] [CrossRef]
- Noskov, B.A.; Latnikova, A.V.; Lin, S.-Y.; Loglio, G.; Miller, R. Dynamic Surface Elasticity of β-Casein Solutions during Adsorption. J. Phys. Chem. C 2007, 111, 16895–16901. [Google Scholar] [CrossRef]
- Gunning, P.A.; Mackie, A.R.; Gunning, A.P.; Wilde, P.J.; Woodward, N.C.; Morris, V.J. The Effect of Surfactant Type on Protein Displacement from the Air–Water Interface. Food Hydrocoll. 2004, 18, 509–515. [Google Scholar] [CrossRef]

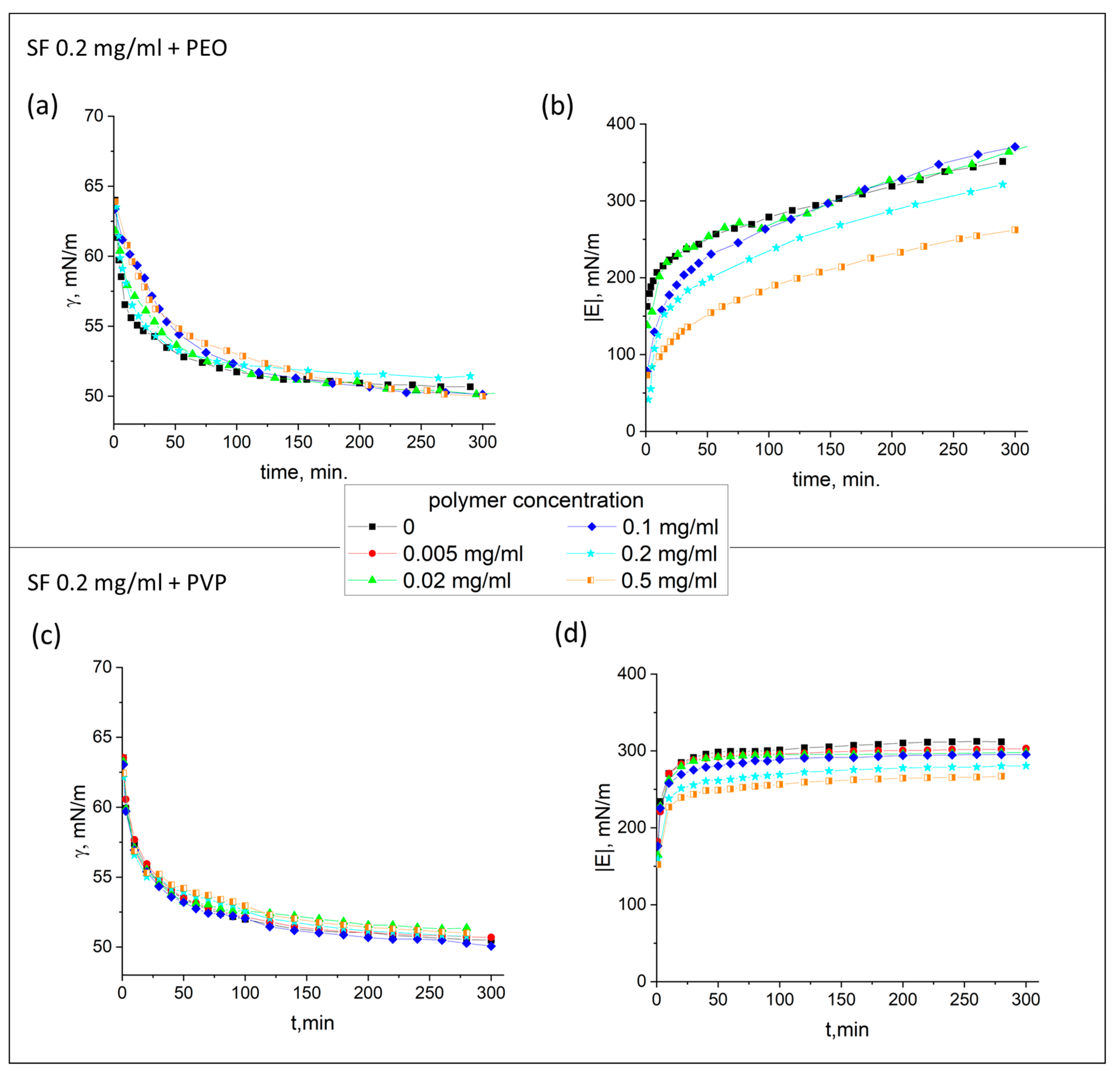



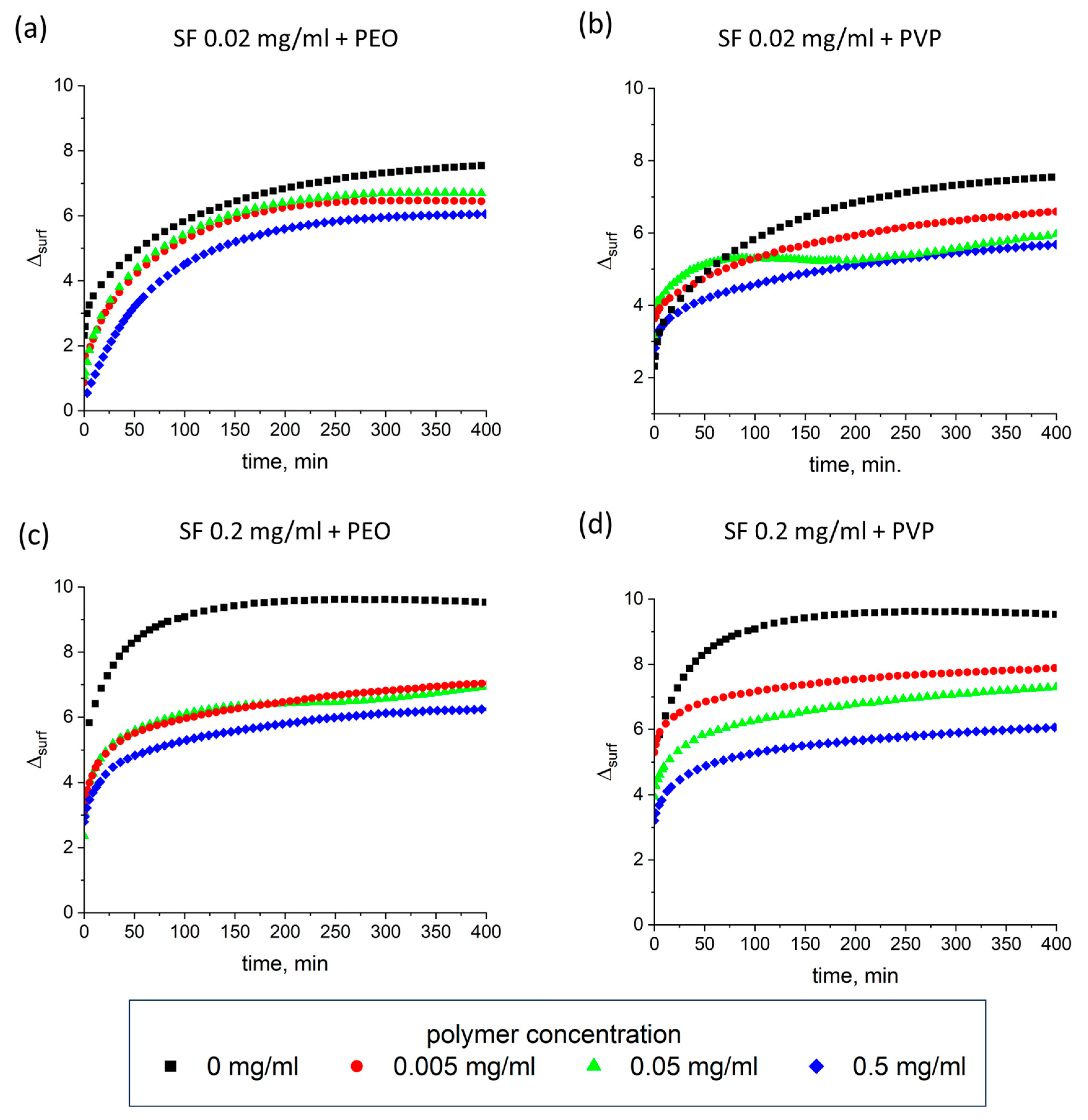

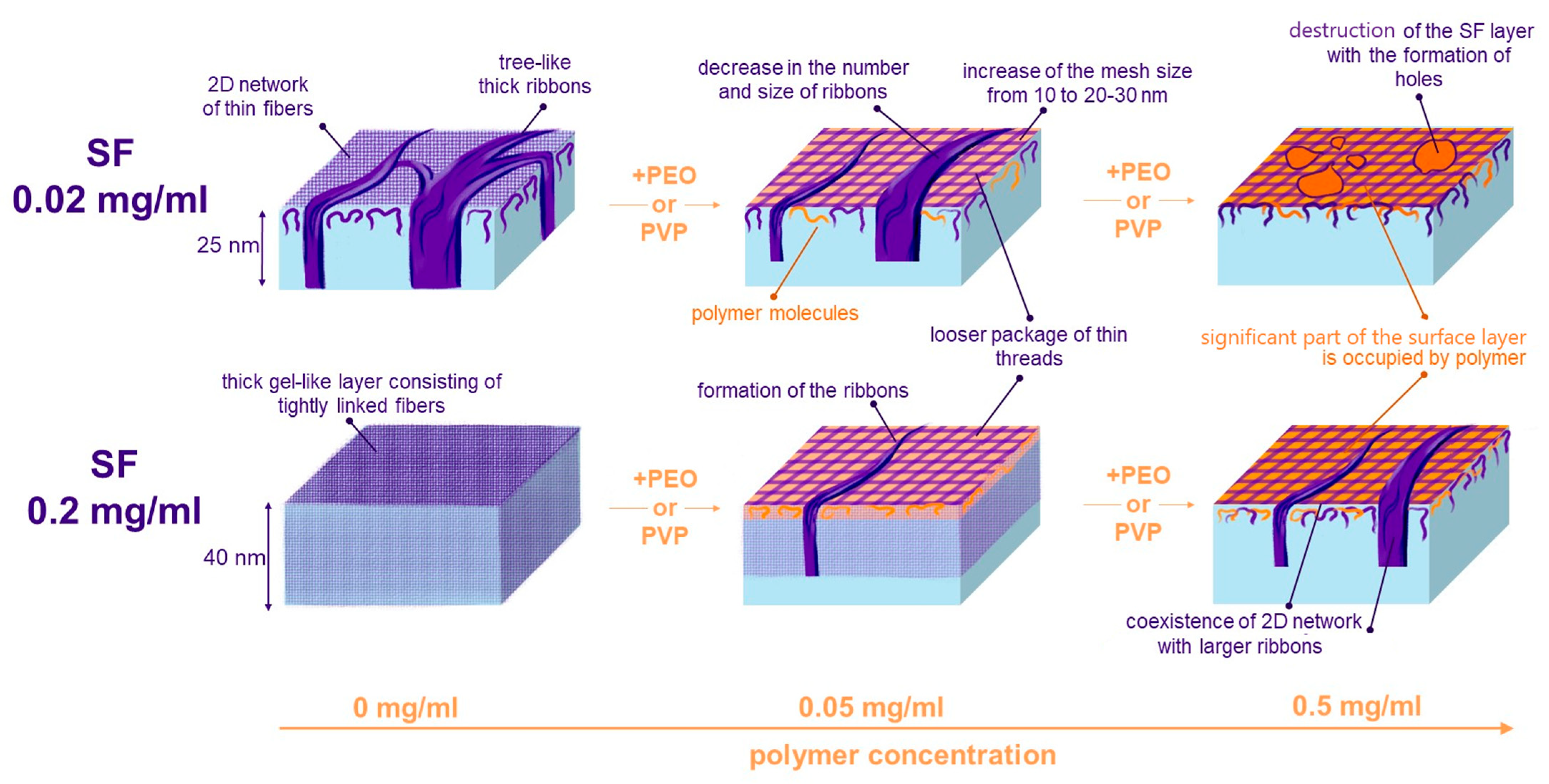
| SF Concentration, mg/mL | PEO Concentration, mg/mL | PVP Concentration, mg/mL | Thickness, nm |
|---|---|---|---|
| 0.02 | -------- | -------- | 25 ± 0.9 |
| 0.02 | 0.005 | -------- | 20 ± 1.8 |
| 0.02 | 0.05 | -------- | 20 ± 2.0 |
| 0.02 | 0.5 | -------- | 15 ± 2.6 |
| 0.02 | -------- | 0.005 | 18 ± 1.5 |
| 0.02 | -------- | 0.05 | 12 ± 1.2 |
| 0.02 | -------- | 0.5 | 8 ± 0.7 |
| 0.2 | -------- | -------- | 40 ± 0.6 |
| 0.2 | 0.005 | -------- | 26 ± 1.1 |
| 0.2 | 0.05 | -------- | 20 ± 1.8 |
| 0.2 | 0.2 | -------- | 18 ± 2.1 |
| 0.2 | 0.5 | -------- | 18 ± 2.4 |
| 0.2 | -------- | 0.005 | 26 ± 1.5 |
| 0.2 | -------- | 0.05 | 22 ± 2.0 |
| 0.2 | --------- | 0.5 | 12 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milyaeva, O.Y.; Rotanova, K.Y.; Rafikova, A.R.; Miller, R.; Loglio, G.; Noskov, B.A. The Impact of PEO and PVP Additives on the Structure and Properties of Silk Fibroin Adsorption Layers. Polymers 2025, 17, 1733. https://doi.org/10.3390/polym17131733
Milyaeva OY, Rotanova KY, Rafikova AR, Miller R, Loglio G, Noskov BA. The Impact of PEO and PVP Additives on the Structure and Properties of Silk Fibroin Adsorption Layers. Polymers. 2025; 17(13):1733. https://doi.org/10.3390/polym17131733
Chicago/Turabian StyleMilyaeva, Olga Yu., Kseniya Yu. Rotanova, Anastasiya R. Rafikova, Reinhard Miller, Giuseppe Loglio, and Boris A. Noskov. 2025. "The Impact of PEO and PVP Additives on the Structure and Properties of Silk Fibroin Adsorption Layers" Polymers 17, no. 13: 1733. https://doi.org/10.3390/polym17131733
APA StyleMilyaeva, O. Y., Rotanova, K. Y., Rafikova, A. R., Miller, R., Loglio, G., & Noskov, B. A. (2025). The Impact of PEO and PVP Additives on the Structure and Properties of Silk Fibroin Adsorption Layers. Polymers, 17(13), 1733. https://doi.org/10.3390/polym17131733










