Assessment of the Influence of Antisolvent 3D Printing Conditions on the Mechanical and Biological Properties of Poly(lactic-co-glycolic) Acid Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Three-Dimensional Printing
2.3. Mechanical Testing
2.4. Molecular Weight Distribution of PLGA
2.5. Microscopy
2.6. Cell Culture
2.7. Biocompatibility
2.8. Statistics
3. Results and Discussion
3.1. Formation of Three-Dimensional PLGA Scaffolds
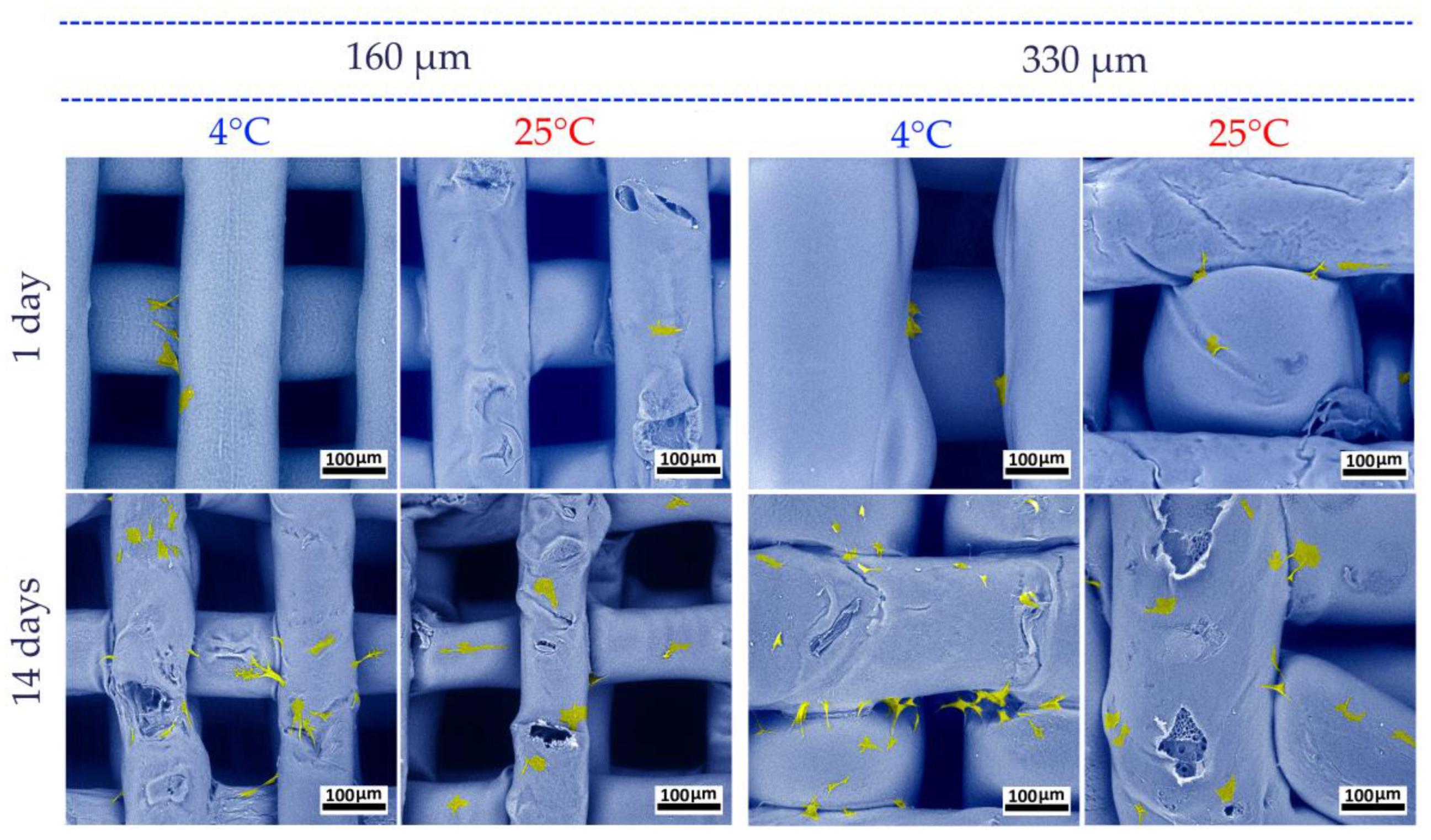
3.2. Study of the PLGA Scaffold Microstructure
3.3. Study of the Mechanical Properties of PLGA Scaffolds
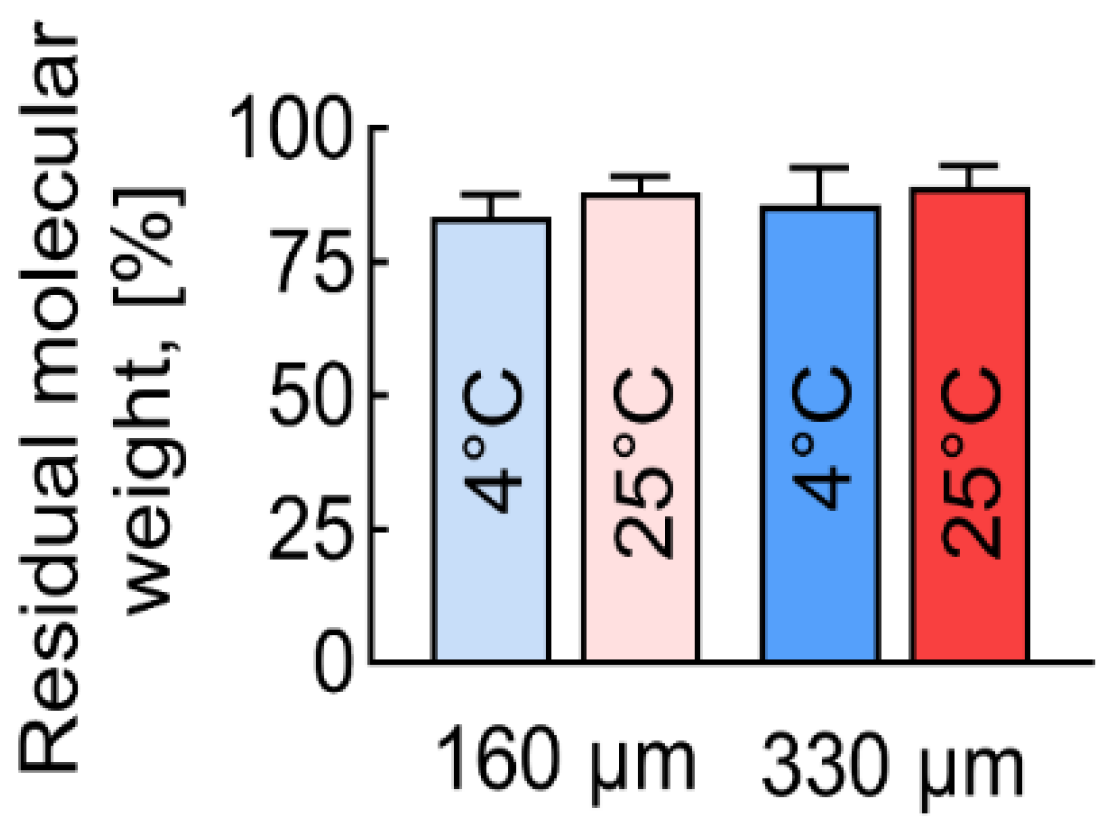
3.4. Molecular Mass Distribution of PLGA Scaffolds
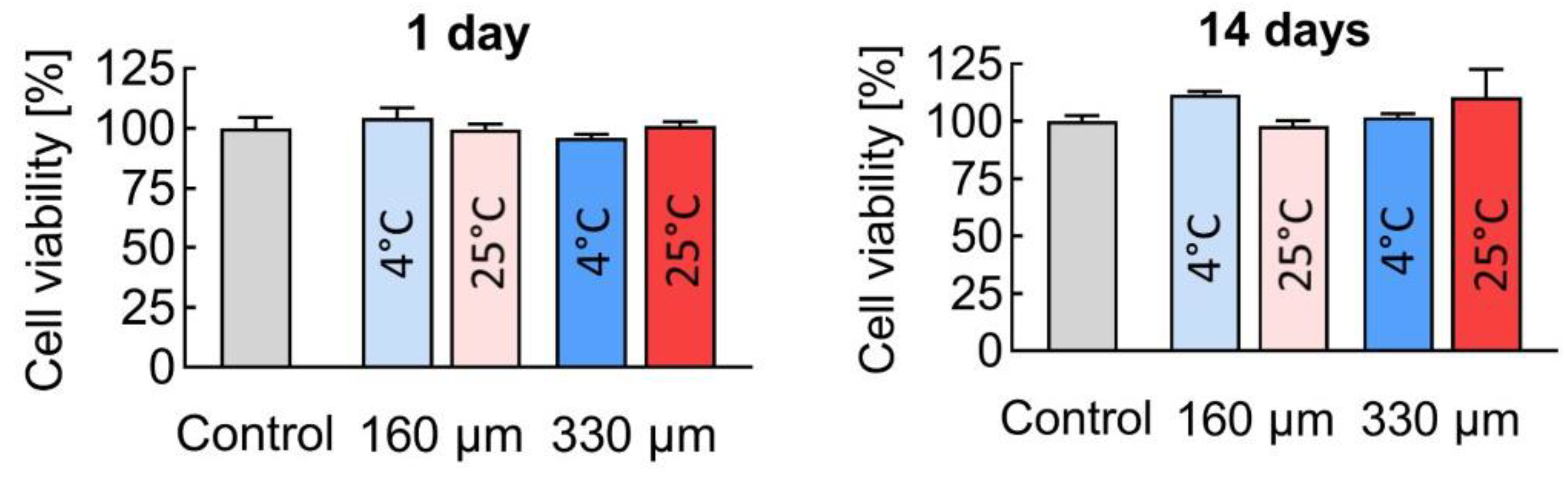
3.5. Biocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kulakov, A.A.; Gvetadze, R.S.; Brailovskaya, T.V.; Khar’kova, A.A.; Dzikovitskaya, L.S. Modern Approaches to Dental Implants Placement in Deficient Alveolar Bone. Stomatologiya 2017, 96, 43. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, P.W.; Al-Nawas, B. Bone Reconstruction of Extensive Maxillomandibular Defects in Adults. Periodontol 2023, 93, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.-T.J.; Ferreira, N.; Simpson, A.H.R.W. The Reconstruction of Critical Bone Loss: The Holy Grail of Orthopaedics. Bone Jt. Res. 2022, 11, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Sun, X.; Wang, H.; Li, C.; Zhao, Y.; Tian, J.; Lin, Y. Application of 3D-Printed, PLGA-Based Scaffolds in Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 5831. [Google Scholar] [CrossRef]
- Patrick, C.W.; Mikos, A.G.; McIntire, L.V. (Eds.) Frontiers in Tissue Engineering, 1st ed.; Pergamon: Oxford, UK; New York, NY, USA, 1998; ISBN 978-0-08-042689-1. [Google Scholar]
- Vert, M.; Li, S.M.; Spenlehauer, G.; Guerin, P. Bioresorbability and Biocompatibility of Aliphatic Polyesters. J. Mater. Sci. Mater. Med. 1992, 3, 432–446. [Google Scholar] [CrossRef]
- Wang, L.; Venkatraman, S.; Gan, L.H.; Kleiner, L. Structure Formation in Injectable Poly(Lactide- Co -glycolide) Depots. II. Nature of the Gel. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72B, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, X.; Ma, P.X. Induction of Osteoblast Differentiation Phenotype on Poly(l-Lactic Acid) Nanofibrous Matrix. Biomaterials 2008, 29, 3815–3821. [Google Scholar] [CrossRef]
- Ivanova, T.A.; Golubeva, E.N. Aliphatic Polyesters for Biomedical Purposes: Design and Kinetic Regularities of Degradation in Vitro. Russ. J. Phys. Chem. B 2022, 16, 426–444. [Google Scholar] [CrossRef]
- Ding, D.; Zhu, Q. Recent Advances of PLGA Micro/Nanoparticles for the Delivery of Biomacromolecular Therapeutics. Mater. Sci. Eng. C 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Devi, M.G.; Amutheesan, M.; Govindhan, R.; Karthikeyan, B. A Review of Three-Dimensional Printing for Biomedical and Tissue Engineering Applications. Open Biotechnol. J. 2018, 12, 241–255. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current Trends in the Design of Scaffolds for Computer-Aided Tissue Engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef]
- Abueidda, D.W.; Bakir, M.; Abu Al-Rub, R.K.; Bergström, J.S.; Sobh, N.A.; Jasiuk, I. Mechanical Properties of 3D Printed Polymeric Cellular Materials with Triply Periodic Minimal Surface Architectures. Mater. Des. 2017, 122, 255–267. [Google Scholar] [CrossRef]
- Goldstein, A.S.; Zhu, G.; Morris, G.E.; Meszlenyi, R.K.; Mikos, A.G. Effect of Osteoblastic Culture Conditions on the Structure of Poly(DL-Lactic-Co-Glycolic Acid) Foam Scaffolds. Tissue Eng. 1999, 5, 421–433. [Google Scholar] [CrossRef]
- Algebraistova, P.Y.; Basko, A.V.; Ilyasova, A.N.; Lebedeva, T.N.; Mironov, A.V.; Pochivalov, K.V.; Popov, V.K. Phase Equilibria and Structure Formation in the Polylactic-Co-Glycolic Acid/Tetraglycol/Water Ternary System. Polymers 2023, 15, 1281. [Google Scholar] [CrossRef] [PubMed]
- Moriya, A.; Maruyama, T.; Ohmukai, Y.; Sotani, T.; Matsuyama, H. Preparation of Poly(Lactic Acid) Hollow Fiber Membranes via Phase Separation Methods. J. Membr. Sci. 2009, 342, 307–312. [Google Scholar] [CrossRef]
- Krebs, M.D.; Sutter, K.A.; Lin, A.S.P.; Guldberg, R.E.; Alsberg, E. Injectable Poly(Lactic-Co-Glycolic) Acid Scaffolds with in Situ Pore Formation for Tissue Engineering. Acta Biomater. 2009, 5, 2847–2859. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.V.; Grigoryev, A.M.; Krotova, L.I.; Skaletsky, N.N.; Popov, V.K.; Sevastianov, V.I. 3D Printing of PLGA Scaffolds for Tissue Engineering. J. Biomed. Mater. Res. A 2017, 105, 104–109. [Google Scholar] [CrossRef]
- Mironov, A.V.; Mironova, O.A.; Syachina, M.A.; Popov, V.K. 3D Printing of Polylactic-Co-Glycolic Acid Fiber Scaffolds Using an Antisolvent Phase Separation Process. Polymer 2019, 182, 121845. [Google Scholar] [CrossRef]
- Pardeshi, S.R.; Nikam, A.; Chandak, P.; Mandale, V.; Naik, J.B.; Giram, P.S. Recent Advances in PLGA Based Nanocarriers for Drug Delivery System: A State of the Art Review. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 49–78. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; Da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.V.; Algebraistova, P.Y.; Komlev, V.S.; Mironova, O.A.; Popov, V.K. An Experimental Device for Studying the 3D Cryoprinting Processes. Instrum. Exp. Tech. 2020, 63, 890–892. [Google Scholar] [CrossRef]
- Khvorostina, M.A.; Mironov, A.V.; Nedorubova, I.A.; Bukharova, T.B.; Vasilyev, A.V.; Goldshtein, D.V.; Komlev, V.S.; Popov, V.K. 3D Printed Gene-Activated Sodium Alginate Hydrogel Scaffolds. Gels 2022, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Lin, Y.-C.; Thirumurugan, S.; Hu, C.-C.; Duann, Y.-F.; Chung, R.-J. Poly(Methyl Methacrylate) in Orthopedics: Strategies, Challenges, and Prospects in Bone Tissue Engineering. Polymers 2024, 16, 367. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Kang, J.-H. Mechanical Properties of Compact Bone Defined by the Stress-Strain Curve Measured Using Uniaxial Tensile Test: A Concise Review and Practical Guide. Materials 2021, 14, 4224. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Callanan, A. Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications. Biomimetics 2023, 8, 205. [Google Scholar] [CrossRef]


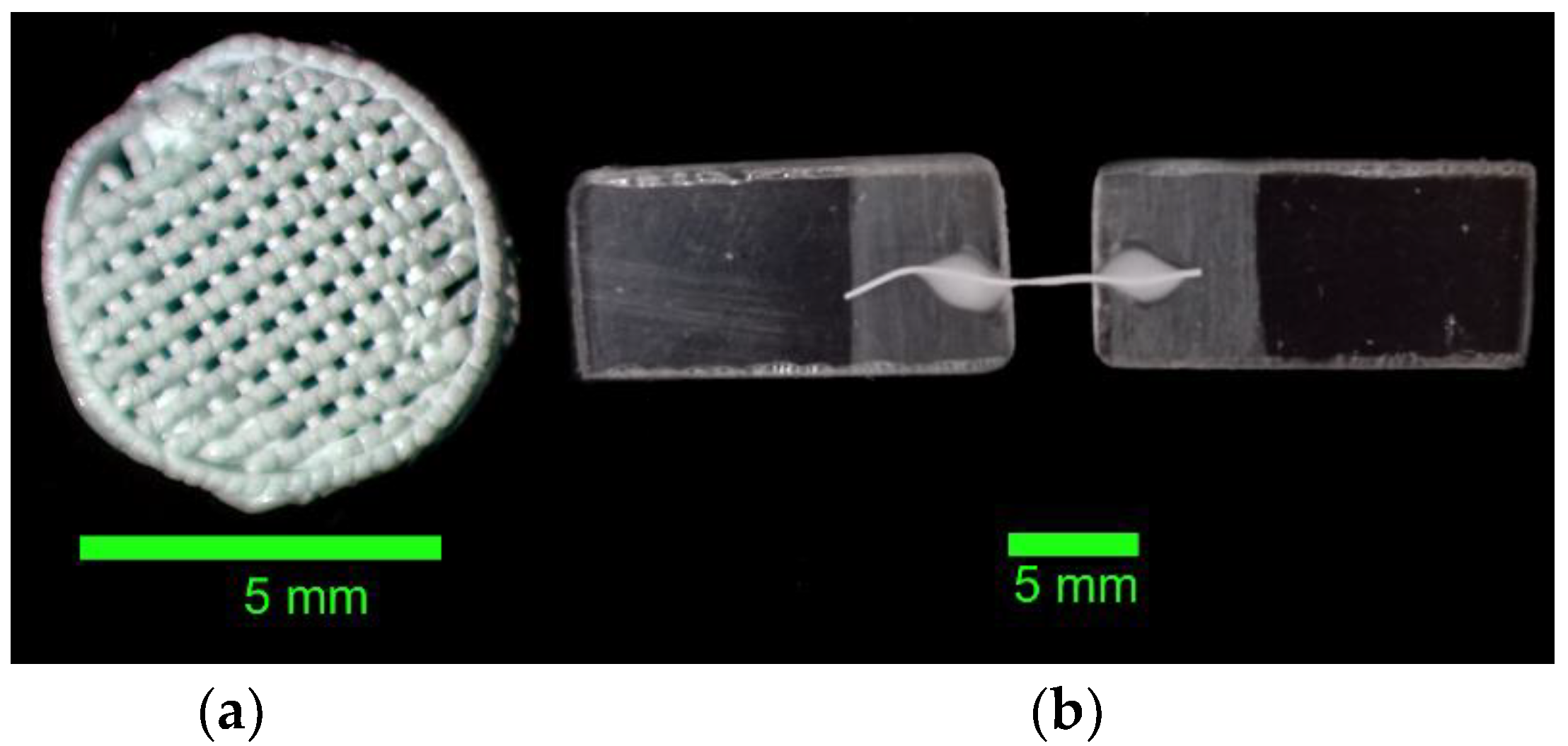
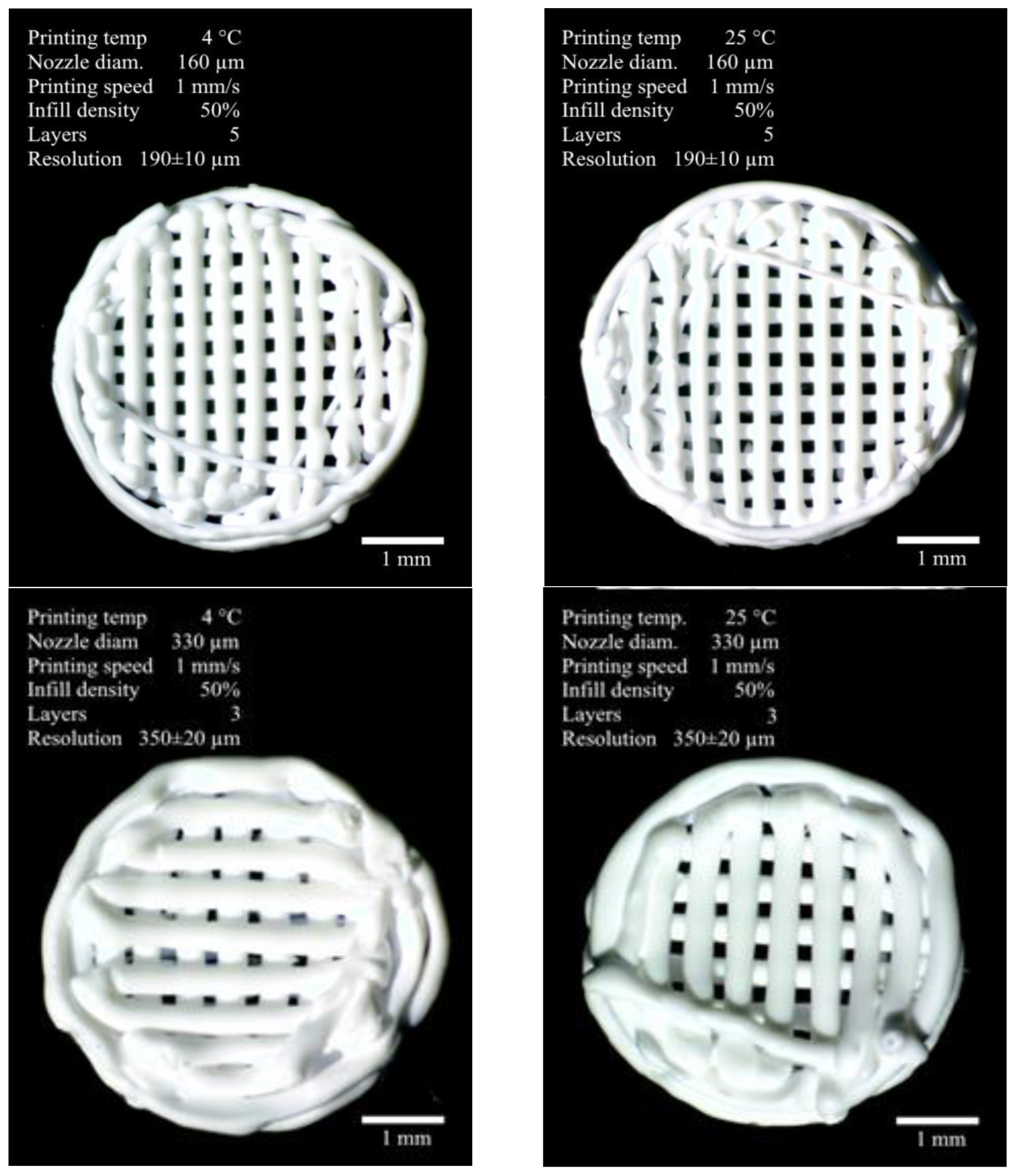
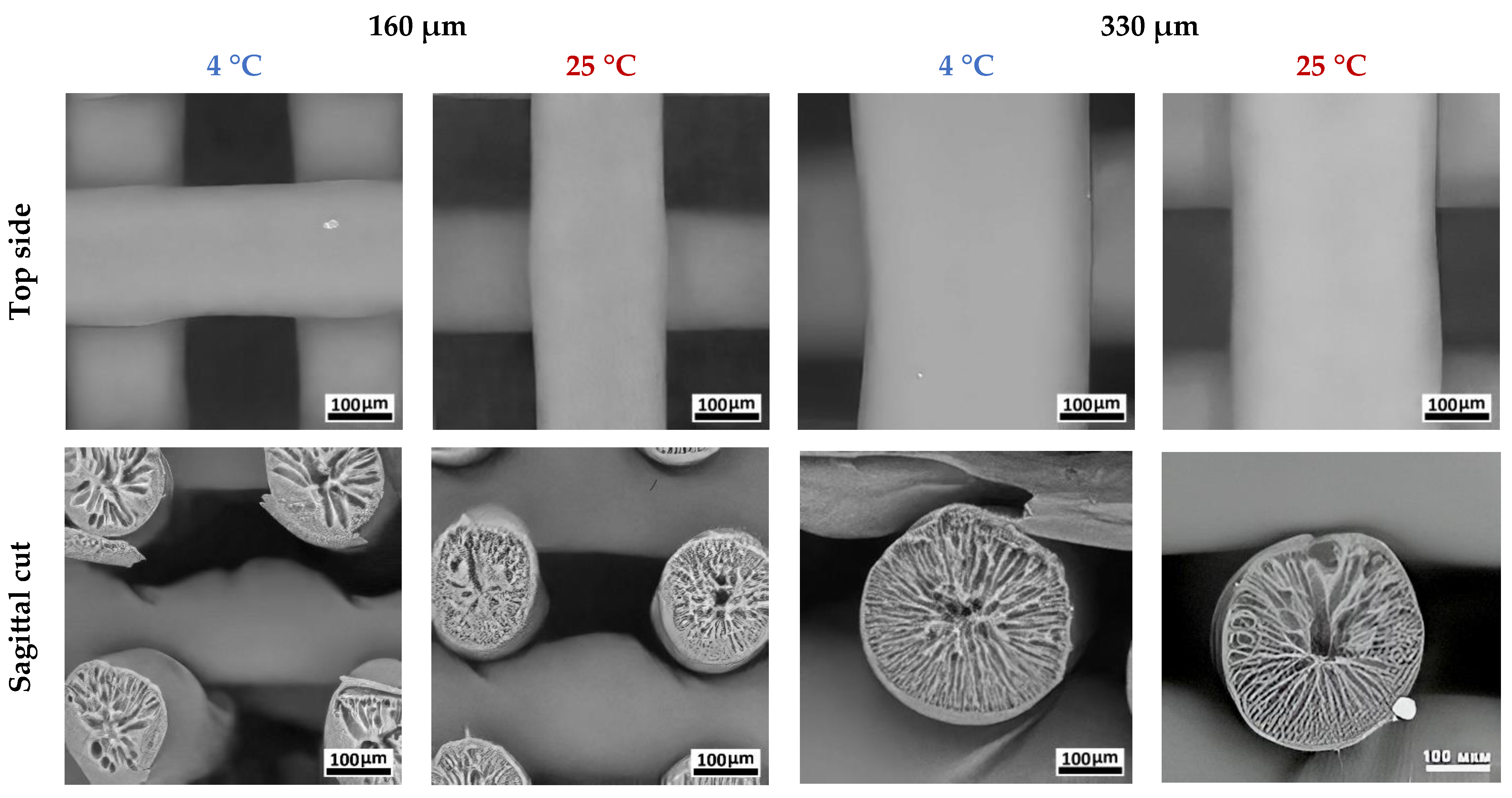
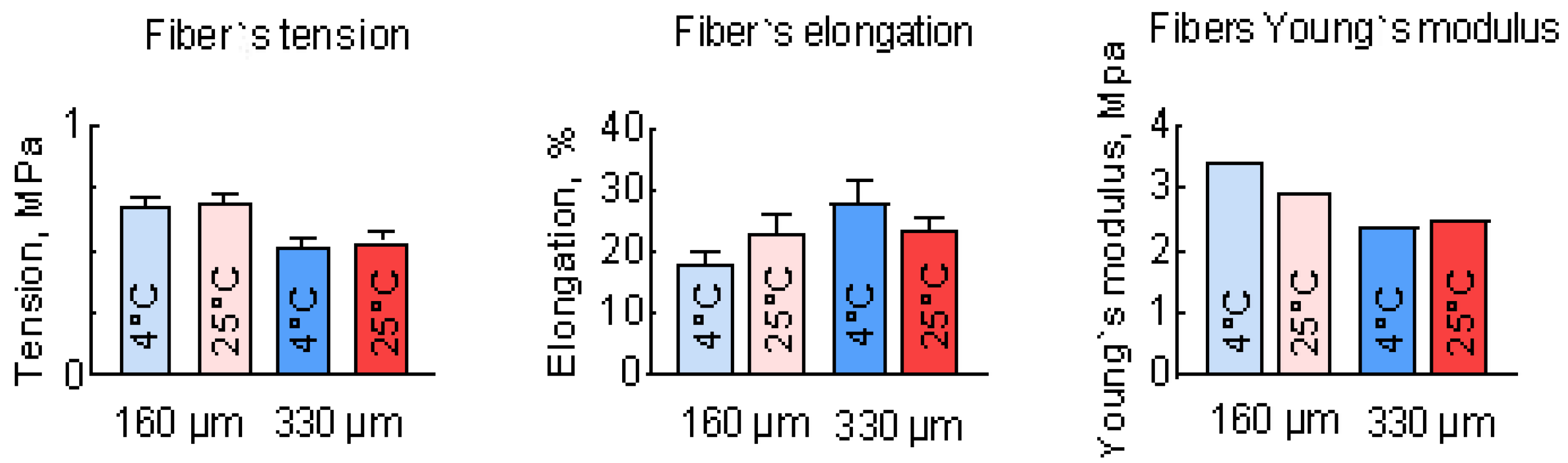


| Sample No.1 | Sample No.2 | Sample No.3 | Sample No.4 | ||
|---|---|---|---|---|---|
| Material | poly(lactic-co-glycolic acid) Purasorb PDLG 7507 | ||||
| Sample geometry * | lattice disc d = 5 mm, h = 1 mm | ||||
| Method | antisolvent 3D printing | ||||
| Nozzle diameter, µm | 160 | 330 | |||
| Printing speed, mm/s ** | 1 | 2 | |||
| Layers number | 5 | 3 | |||
| Flow rate, µL/s | 0.020 | 0.171 | |||
| Printing resolution, µm *** | 190 ± 10 | 350 ± 20 | |||
| Printing temperature, °C | 4 | 25 | 4 | 25 | |
| Control | 190 μm Fiber | 350 μm Fiber | |||
|---|---|---|---|---|---|
| 4 °C | 25 °C | 4 °C | 25 °C | ||
| Mn | 51,721 | 43,143 | 45,541 | 44,275 | 46,057 |
| Mw | 80,292 | 68,879 | 73,271 | 70,127 | 72,652 |
| Pd | 1.54 | 1.6 | 1.59 | 1.58 | 1.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironov, A.V.; Trifanova, E.M.; Bukharova, T.B.; Vasilyev, A.V.; Chernomyrdina, V.O.; Nedorubova, I.A.; Kuznetsova, V.S.; Dunaev, A.G.; Popov, V.K.; Kulakov, A.A.; et al. Assessment of the Influence of Antisolvent 3D Printing Conditions on the Mechanical and Biological Properties of Poly(lactic-co-glycolic) Acid Scaffolds. Polymers 2025, 17, 501. https://doi.org/10.3390/polym17040501
Mironov AV, Trifanova EM, Bukharova TB, Vasilyev AV, Chernomyrdina VO, Nedorubova IA, Kuznetsova VS, Dunaev AG, Popov VK, Kulakov AA, et al. Assessment of the Influence of Antisolvent 3D Printing Conditions on the Mechanical and Biological Properties of Poly(lactic-co-glycolic) Acid Scaffolds. Polymers. 2025; 17(4):501. https://doi.org/10.3390/polym17040501
Chicago/Turabian StyleMironov, Anton V., Ekaterina M. Trifanova, Tatyana B. Bukharova, Andrey V. Vasilyev, Viktoria O. Chernomyrdina, Irina A. Nedorubova, Valeriya S. Kuznetsova, Andrey G. Dunaev, Vladimir K. Popov, Anatoly A. Kulakov, and et al. 2025. "Assessment of the Influence of Antisolvent 3D Printing Conditions on the Mechanical and Biological Properties of Poly(lactic-co-glycolic) Acid Scaffolds" Polymers 17, no. 4: 501. https://doi.org/10.3390/polym17040501
APA StyleMironov, A. V., Trifanova, E. M., Bukharova, T. B., Vasilyev, A. V., Chernomyrdina, V. O., Nedorubova, I. A., Kuznetsova, V. S., Dunaev, A. G., Popov, V. K., Kulakov, A. A., Losev, F. F., & Goldshtein, D. V. (2025). Assessment of the Influence of Antisolvent 3D Printing Conditions on the Mechanical and Biological Properties of Poly(lactic-co-glycolic) Acid Scaffolds. Polymers, 17(4), 501. https://doi.org/10.3390/polym17040501












