New Alternatives in the Valorisation of Eucalyptus globulus By-Products for the Textile Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Fourier Transform Infrared Spectroscopy (FTIR) Assay
2.3. Scanning Electron Microscopy
2.4. Chemical Characterisation of (BLE) Particles
2.4.1. Phenol Content
2.4.2. Sugar Content
2.4.3. Protein Content
2.4.4. Extraction Yield
2.5. Coating of Cotton Fabrics with (BLE) Particles
2.6. Coated Fabrics Characterisation
2.6.1. Colour and Colour Fastness Evaluation
2.6.2. Surface Wettability
2.6.3. Differential Scanning Calorimetry (DSC)
2.6.4. Simultaneous Thermal Analysis (STA)
2.6.5. Evaluation of Antioxidant Capacity of the Fabrics
DPPH (2,2-diphenyl-1-picrylhydrazyl) Assay
2.6.6. FRAP Essay
2.7. Statistical Analysis
3. Results and Discussion
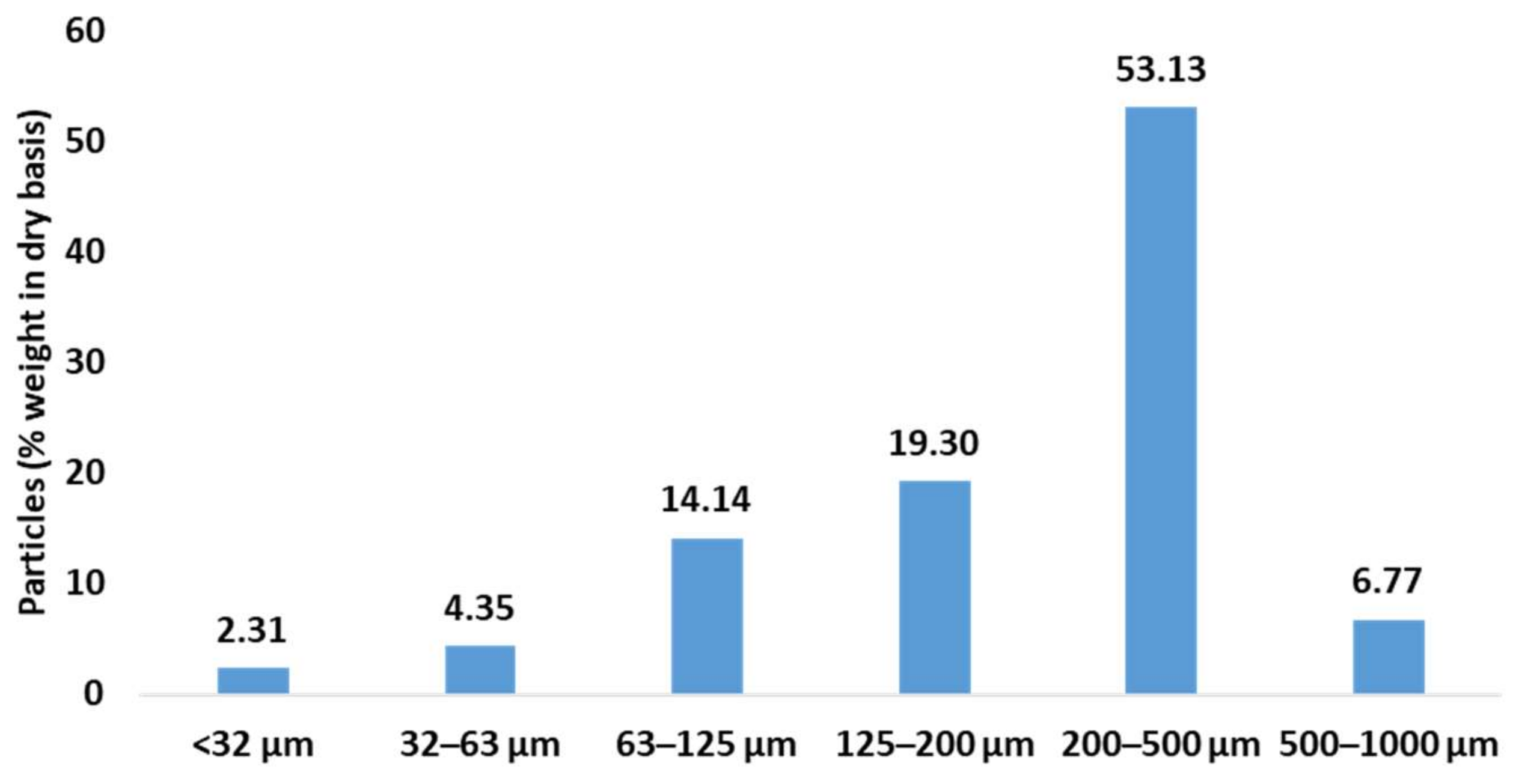
3.1. Characterisation of E. globulus Branches and Leaves
- Thermal characterisation of (BLE) particles by TG
- Chemical characterisation of (BLE) particles and storage time impact
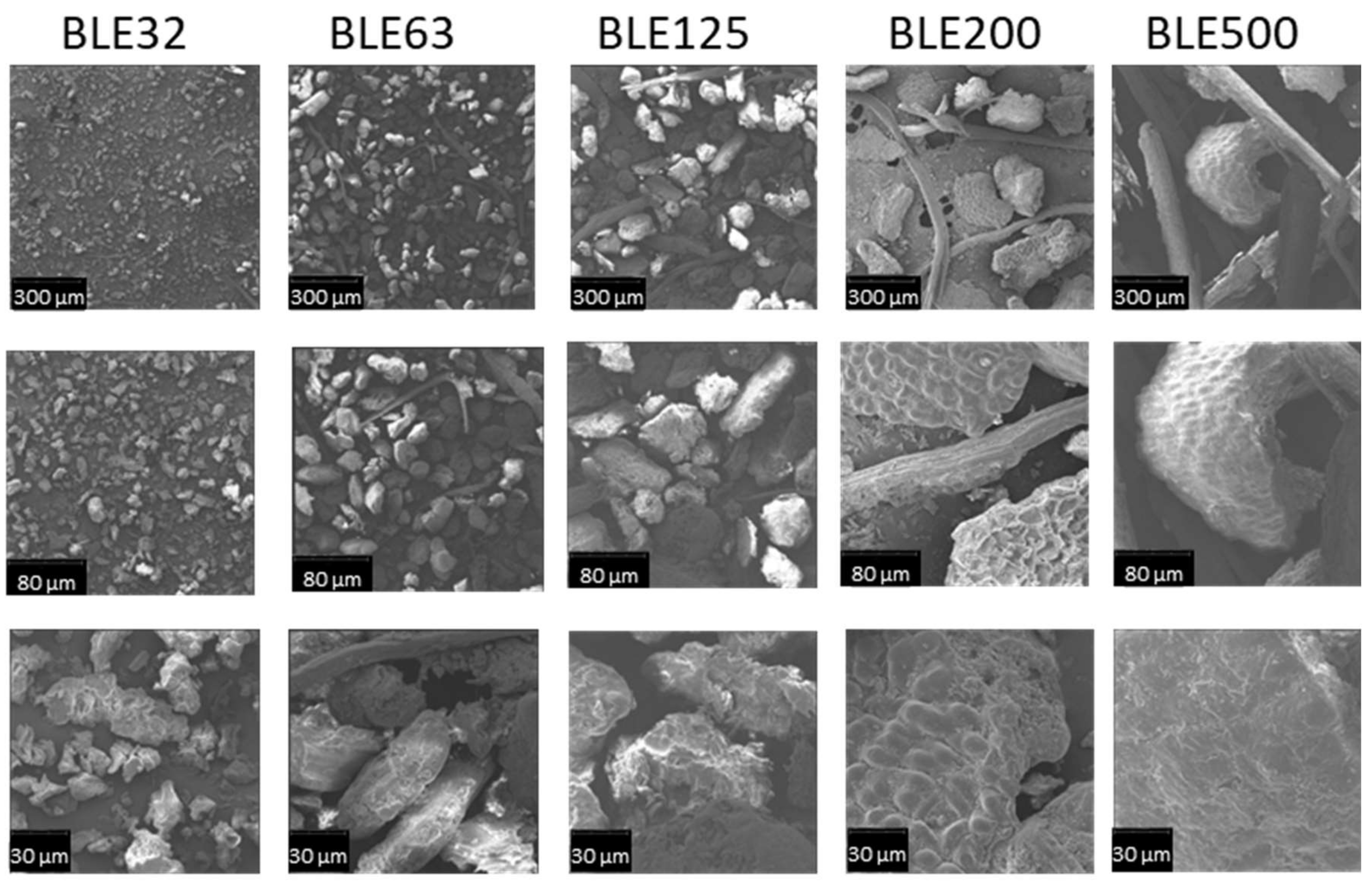
3.1.1. Characterisation of the (BLE) Particles by SEM Microscopy
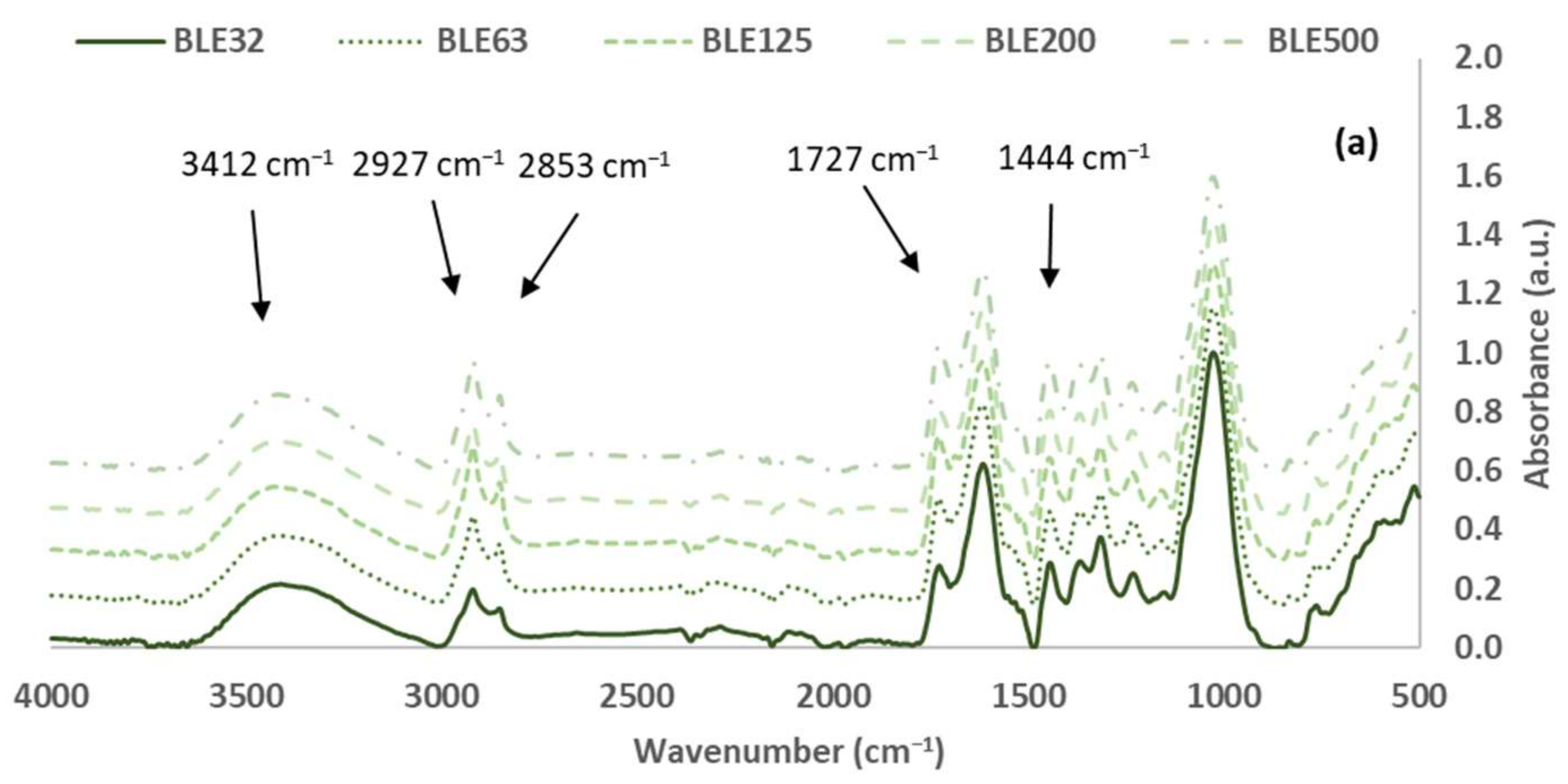
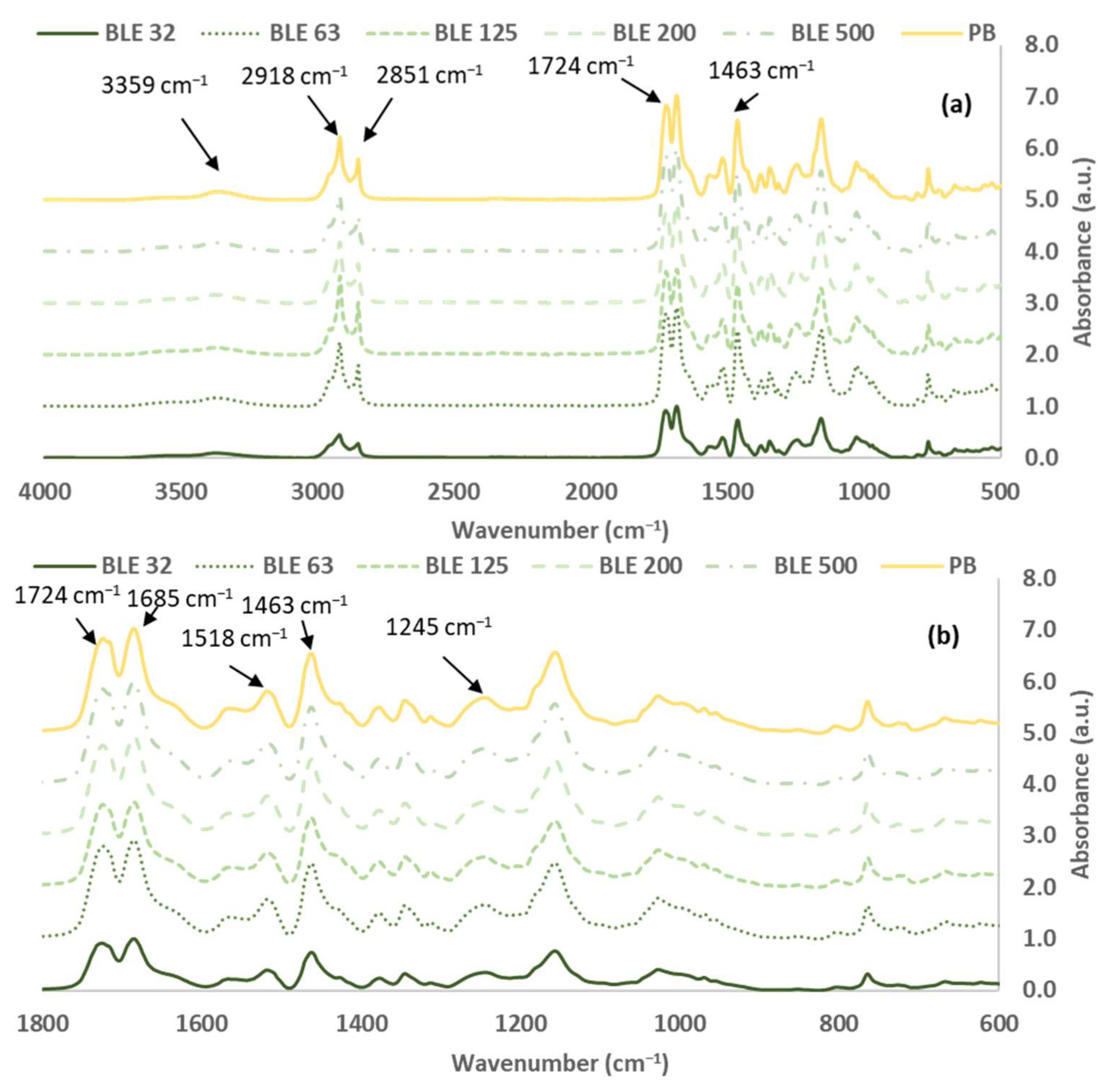
3.1.2. Characterisation of the (BLE) Particles by FTIR-ATR Spectroscopy
3.2. Production and Characterisation of Coated Fabrics with (BLE) Particles
3.2.1. (BLE) Particles Impact on WPU Coating Formulation
3.2.2. Analysis of Fabrics Coated with (BLE) Particles
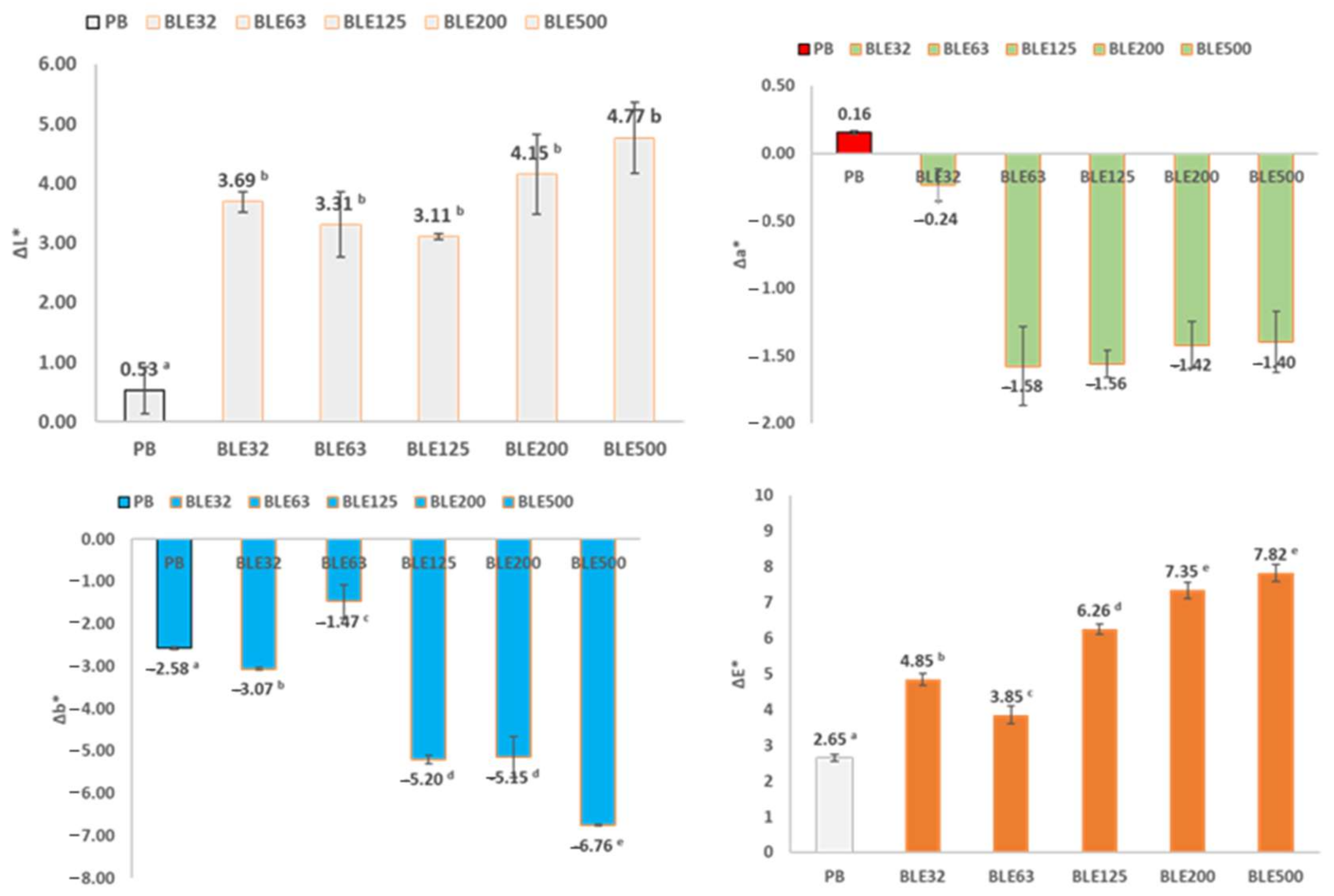
- Analysis of the Effect of (BLE) Particles on the Colour of Coated Fabrics
- Colour Lightfastness Evaluation
- Characterisation of the Samples by SEM Microscopy
- FTIR-ATR Coating Samples Characterisation
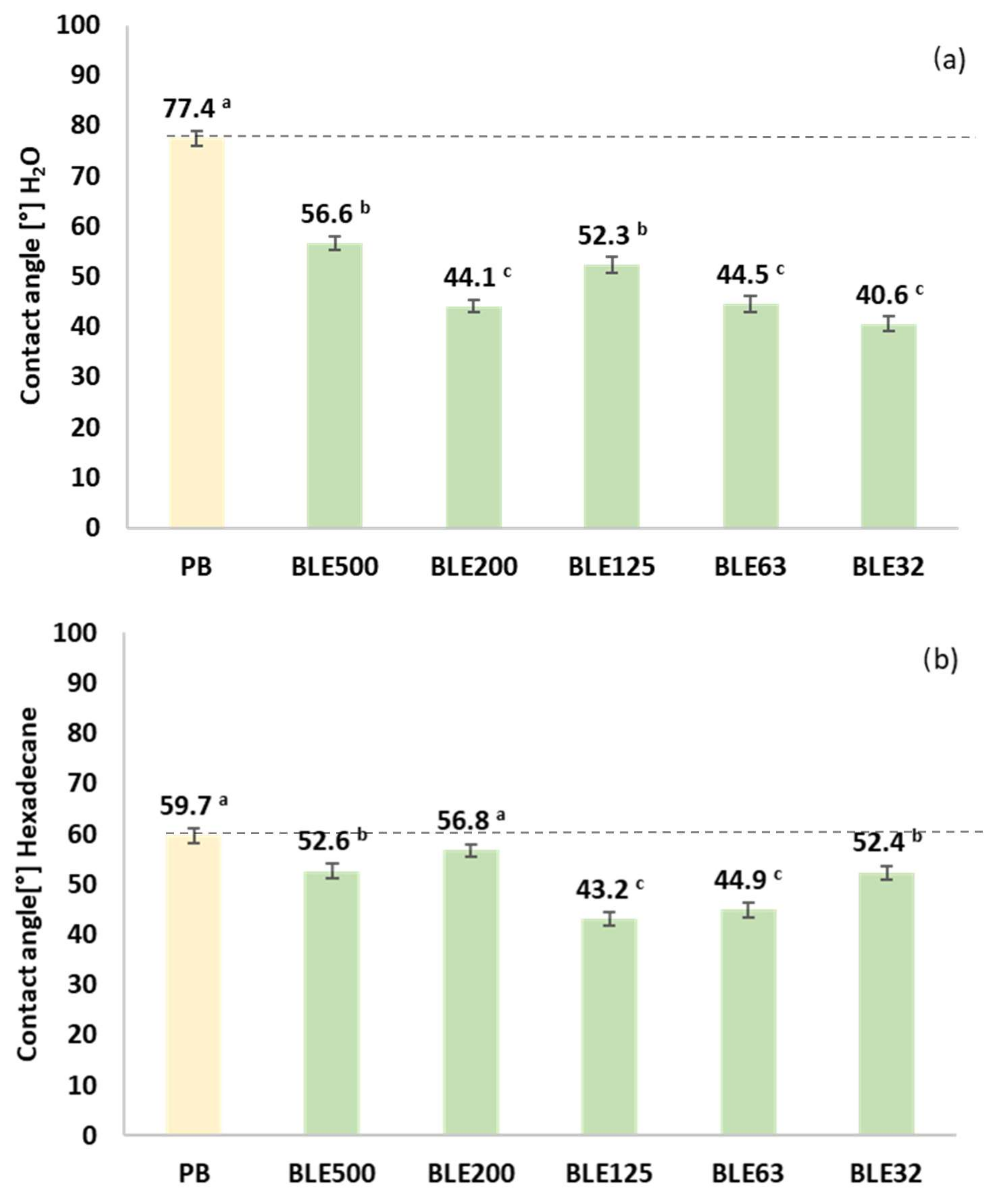
- Hydrophobicity and Oleophobicity of Coating Textile with (BLE)
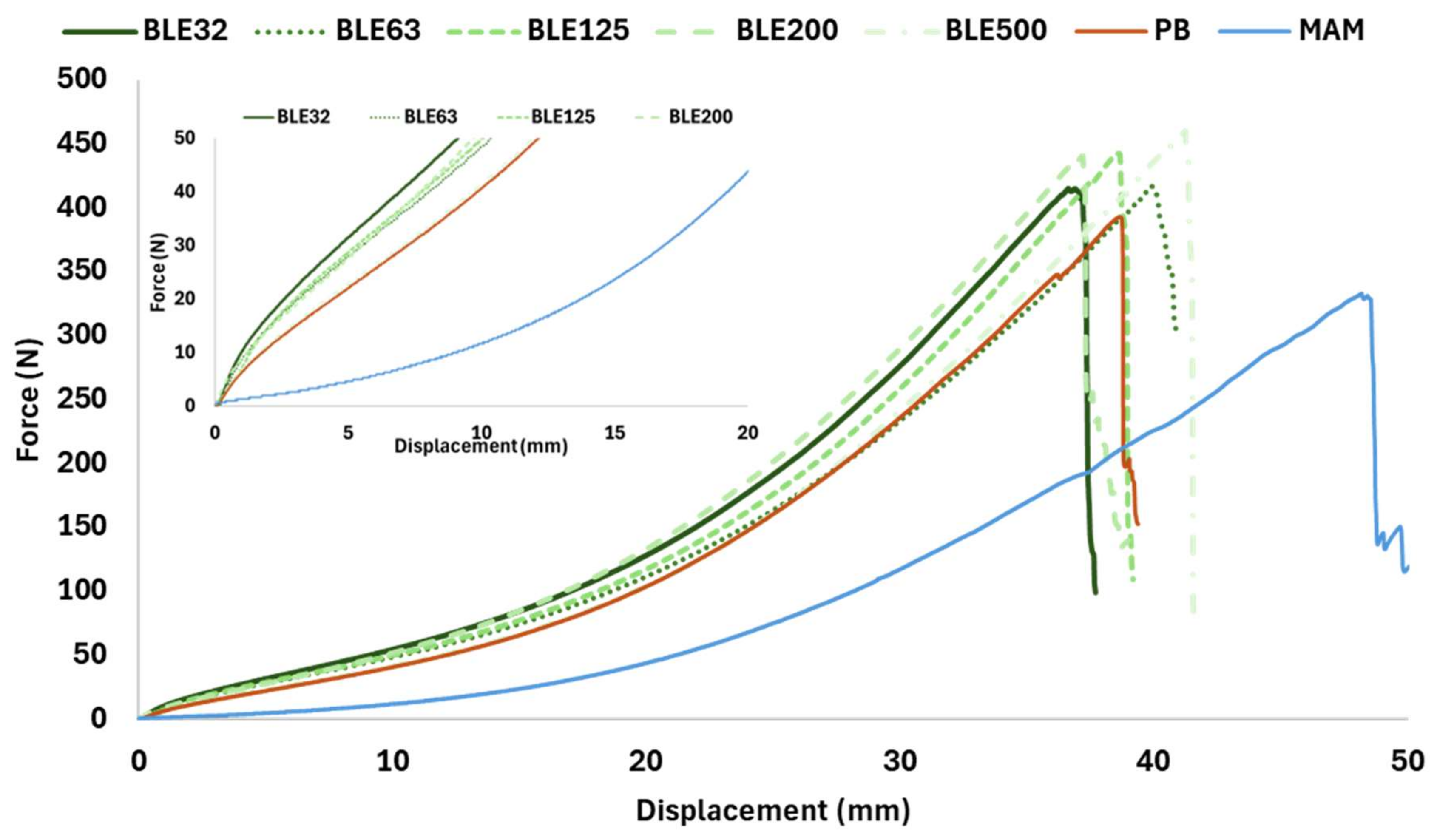
- Coating Textile with (BLE) Particles Performance
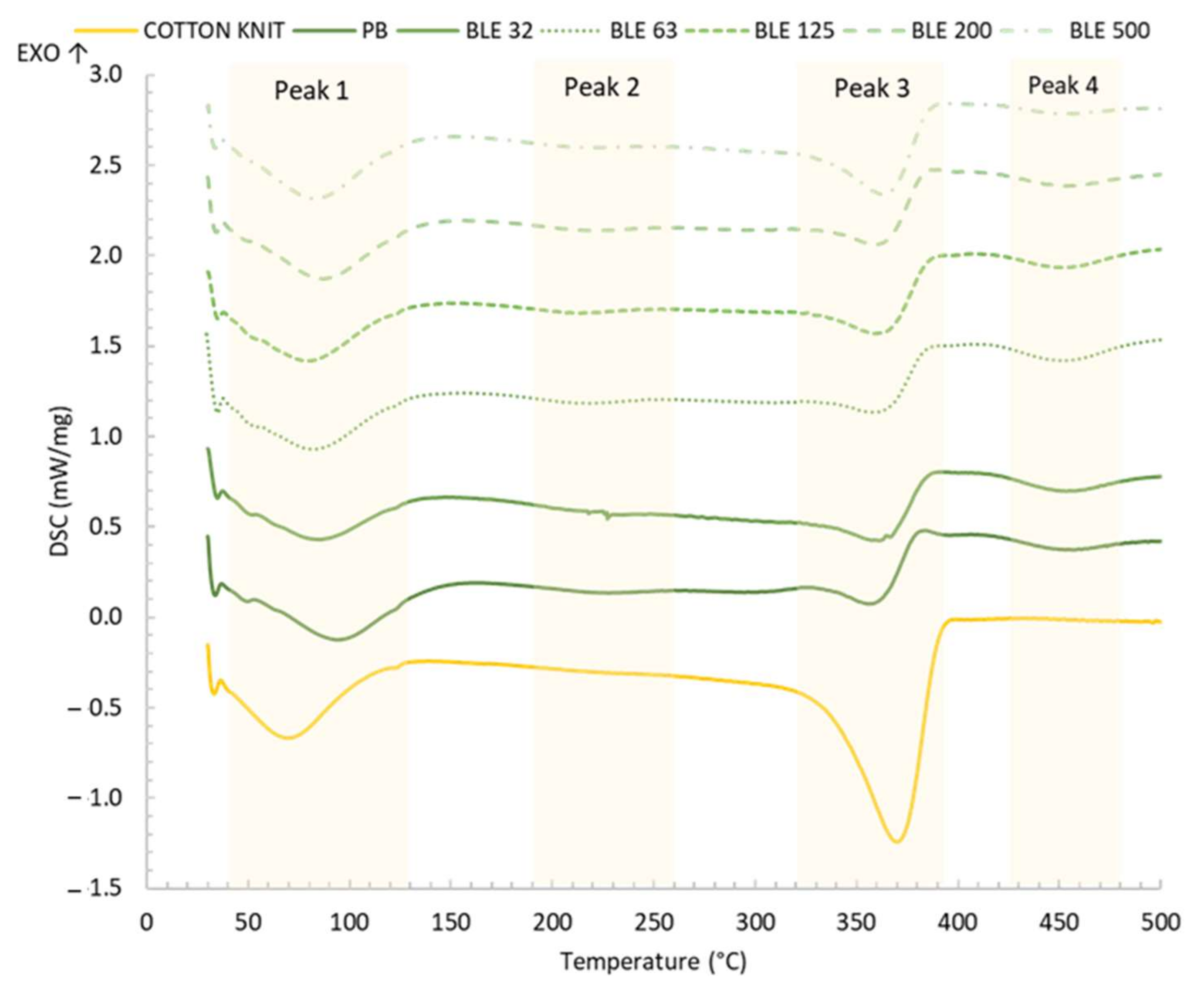
- Coating Textile Products Thermal Behaviour Characterisation
- Simultaneous Thermal Analysis (STA)
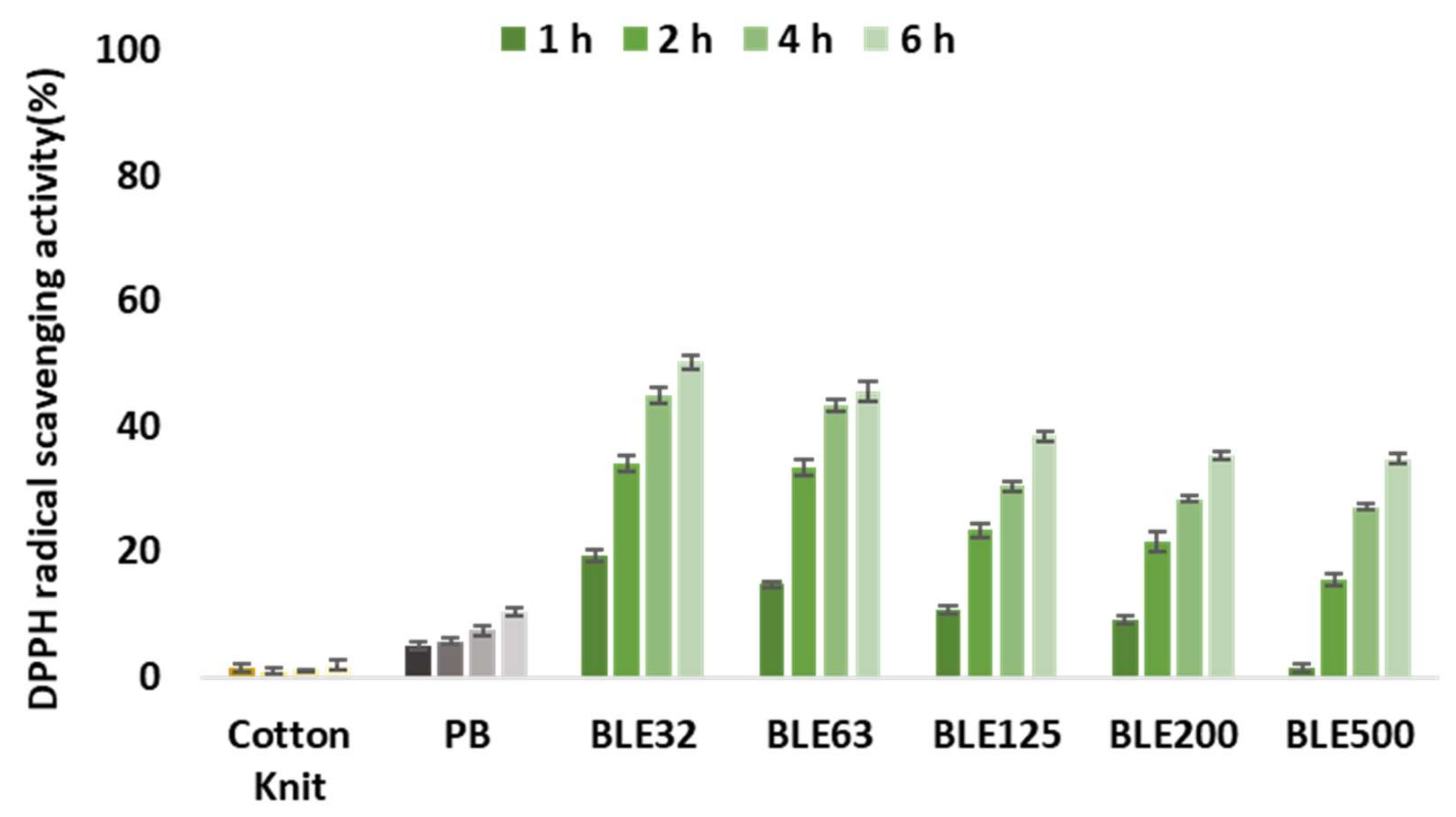
- Antioxidant Activity of Coating Textiles with (BLE)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escobar-Avello, D.; Ferrer, V.; Bravo-Arrepol, G.; Reyes-Contreras, P.; Elissetche, J.P.; Santos, J.; Fuentealba, C.; Cabrera-Barjas, G. Pretreated Eucalyptus Globulus and Pinus Radiata Barks: Potential Substrates to Improve Seed Germination for a Sustainable Horticulture. Forests 2023, 14, 991. [Google Scholar] [CrossRef]
- Ferrer-Villasmil, V.; Fuentealba, C.; Reyes-Contreras, P.; Rubilar, R.; Cabrera-Barjas, G.; Bravo-Arrepol, G.; Escobar-Avello, D. Extracted Eucalyptus Globulus Bark Fiber as a Potential Substrate for Pinus Radiata and Quillaja Saponaria Germination. Plants 2024, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Antorrena, G.; Freire, M.S.; Pizzi, A.; González-Álvarez, J. Environmentally Friendly Wood Adhesives Based on Chestnut (Castanea Sativa) Shell Tannins. Eur. J. Wood Wood Prod. 2017, 75, 89–100. [Google Scholar] [CrossRef]
- Santos, J.; Escobar-Avello, D.; Fuentealba, C.; Cabrera-Barjas, G.; González-Álvarez, J.; Martins, J.M.; Carvalho, L.H. Forest By-Product Valorization: Pilot-Scale Pinus Radiata and Eucalyptus Globulus Bark Mixture Extraction. Forests 2023, 14, 895. [Google Scholar] [CrossRef]
- Casas-Ledón, Y.; Daza Salgado, K.; Cea, J.; Arteaga-Pérez, L.E.; Fuentealba, C. Life Cycle Assessment of Innovative Insulation Panels Based on Eucalyptus Bark Fibers. J. Clean. Prod. 2020, 249, 119356. [Google Scholar] [CrossRef]
- Santos, J.; Freitas, B.; Fernandes, R.A.; Escobar-Avello, D.; Gomes, T.; Magalhães, P.; Magalhães, F.D.; Martins, J.M.; Carvalho, L.H. Ultrasonic-Assisted Water Extraction from Eucalyptus Globulus Leaves and Branches, to Obtain Natural Textile Dyes with Antioxidant Properties. Ind. Crops Prod. 2025, 228, 120885. [Google Scholar] [CrossRef]
- Meyer, M.; Dietrich, S.; Schulz, H.; Mondschein, A. Comparison of the Technical Performance of Leather, Artificial Leather, and Trendy Alternatives. Coatings 2021, 11, 226. [Google Scholar] [CrossRef]
- Silva, A.; Vilaça, H.; Antunes, J.; Rocha, A.; Silva, C. Textile Bio-Based and Bioactive Coatings Using Vegetal Waste and by-Products. Base Diseño E Innovación 2022, 7, 57–70. [Google Scholar] [CrossRef]
- Coelho, L.; Isabel Magalhães, A.; Fernandes, S.; Batista, P.; Pintado, M.; Faria, P.; Costa, C.; Moura, B.; Marinho, A.; Maria, R.; et al. Innovation of Textiles through Natural By-Products and Wastes. In Waste in Textile and Leather Sectors; InTechOpen: London, UK, 2020. [Google Scholar]
- Nolasco, A.; Esposito, F.; Cirillo, T.; Silva, A.; Silva, C. Coffee Silverskin: Unveiling a Versatile Agri-Food By-Product for Ethical Textile Coatings. In Advanced Research in Technologies, Information, Innovation and Sustainability; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Castro, J.A.; Balbo, R.; Silva, C.J.; Fernández, C.F.; Carrasco, F.A. Material Textile Design as a Trigger for Transdisciplinary Collaboration: Coating Bio-Based Textiles Using Waste from the Wood Industry. Coatings 2025, 15, 216. [Google Scholar] [CrossRef]
- Ghazal, H.; Saad, F.; Hegazy, B.M.; Sedik, A.; Hassabo, A.G. Polyurethane and Its Application in Textile Industry. Lett. Appl. NanoBioScience 2024, 13. [Google Scholar] [CrossRef]
- Neiva, D.M.; Araújo, S.; Gominho, J.; de Cássia Carneiro, A.; Pereira, H. Potential of Eucalyptus Globulus Industrial Bark as a Biorefinery Feedstock: Chemical and Fuel Characterization. Ind. Crops Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Khoddami, A.; Alihosseini, F.; Jing, S.; Ribeiro, A.; Cavaco-Paulo, A.; Silva, C. Antioxidant Cosmetotextiles: Cotton Coating with Nanoparticles Containing Vitamin E. Process Biochem. 2017, 59, 46–51. [Google Scholar] [CrossRef]
- Das, P.; Sherazee, M.; Marvi, P.K.; Ahmed, S.R.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Waste-Derived Sustainable Fluorescent Nanocarbon-Coated Breathable Functional Fabric for Antioxidant and Antimicrobial Applications. ACS Appl. Mater. Interfaces 2023, 15, 29425–29439. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, F. Investigating the Usage of Eucalyptus Leaves in Antibacterial Finishing of Textiles against Gram-Positive and Gram-Negative Bacteria. J. Text. Inst. 2021, 112, 341–345. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Olmo-Cunillera, A.; Lozano-Castellón, J.; Marhuenda-Muñoz, M.; Vallverdú-Queralt, A. A Targeted Approach by High Resolution Mass Spectrometry to Reveal New Compounds in Raisins. Molecules 2020, 25, 1281. [Google Scholar] [CrossRef]
- Członka, S.; Strakowska, A.; Pospiech, P.; Strzelec, K. Effects of Chemically Treated Eucalyptus Fibers on Mechanical, Thermal and Insulating Properties of Polyurethane Composite Foams. Materials 2020, 13, 1781. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.Y.; Son, Y.G.; Kang, S.D.; Lee, S.W.; Kim, K.D.; Kim, J.Y. Characterization of Chemical Composition and Antioxidant Activity of Eucalyptus Globulus Leaves under Different Extraction Conditions. Appl. Sci. 2023, 13, 9984. [Google Scholar] [CrossRef]
- Singh, S.; Raghav, S.; Kalyanaraman, K.; Saifi, A. Partial Purification and De- Staining Property of Protease from Leaves and Seeds of Eucalyptus Globulus and Fruit of Azadirachta Indica. Int. J. Pharma Bio Sci. 2019, 10. [Google Scholar] [CrossRef]
- García, C.; Montero, G.; Coronado, M.A.; Valdez, B.; Stoytcheva, M.; Rosas, N.; Torres, R.; Sagaste, C.A. Valorization of Eucalyptus Leaves by Essential Oil Extraction as an Added Value Product in Mexico. Waste Biomass Valorization 2017, 8, 1187–1197. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Soares, C.P.B.; Fehrmann, L.; Jacovine, L.A.G.; von Gadow, K. Biomassa Acima e Abaixo Do Solo e Estimativas de Carbono Para Um Plantio Clonal de Eucalipto No Sudeste Do Brasil. Rev. Arvore 2015, 39, 353–363. [Google Scholar] [CrossRef]
- Wendler, R.; Carvalho, P.O.; Pereira, J.S.; Millard, P. Role of Nitrogen Remobilization from Old Leaves for New Leaf Growth of Eucalyptus Globulus Seedlings. Tree Physiol. 1995, 15, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Grasel, F.D.S.; Ferrão, M.F.; Wolf, C.R. Development of Methodology for Identification the Nature of the Polyphenolic Extracts by FTIR Associated with Multivariate Analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Yemele, M.C.N.; Koubaa, A.; Cloutier, A.; Soulounganga, P.; Wolcott, M. Effect of Bark Fiber Content and Size on the Mechanical Properties of Bark/HDPE Composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 131–137. [Google Scholar] [CrossRef]
- Kačík, F.; Ďurkovič, J.; Kačíková, D. Chemical Profiles of Wood Components of Poplar Clones for Their Energy Utilization. Energies 2012, 5, 5243–5256. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative Visualization of Lignocellulose Components in Transverse Sections of Moso Bamboo Based on FTIR Macro- and Micro-Spectroscopy Coupled with Chemometrics. Biotechnol. Biofuels 2018, 11, 263. [Google Scholar] [CrossRef]
- Santos, J.; Delgado, N.; Fuentes, J.; Fuentealba, C.; Vega-Lara, J.; García, D.E. Exterior Grade Plywood Adhesives Based on Pine Bark Polyphenols and Hexamine. Ind. Crops Prod. 2018, 122, 340–348. [Google Scholar] [CrossRef]
- Santos, J.; Pereira, J.; Escobar-Avello, D.; Ferreira, I.; Vieira, C.; Magalhães, F.D.; Martins, J.M.; Carvalho, L.H. Grape Canes (Vitis vinifera L.) Applications on Packaging and Particleboard Industry: New Bioadhesive Based on Grape Extracts and Citric Acid. Polymers 2022, 14, 1137. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi Júnior, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Santos, J.; Escobar-Avello, D.; Magalhães, P.; Magalhães, F.D.; Martins, J.M.; González-Álvarez, J.; de Carvalho, L.H. High-Value Compounds Obtained from Grape Canes (Vitis vinifera L.) by Steam Pressure Alkali Extraction. Food Bioprod. Process. 2022, 133, 153–167. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and Quantitative Analysis of Lignocellulosic Biomass Using Infrared Techniques: A Mini-Review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Nahar, K.; Arju, S.N.; Ferdush, J.; Islam, M.; Akter, T. Colorimetric Analysis and Fastness Properties of Jute Fabric Dyed with Eucalyptus Leaves. Tekstilec 2020, 63, 195–202. [Google Scholar] [CrossRef]
- Hoflich, W. The Use of Polyester Fibres and Natural Dyes in Automotive Textiles. Polyest.-Und Naturfasern Im Automob. 1998, 41. [Google Scholar]
- Freeman, H.S.; Berthelon, N.; Edwards, L.C. Studies Towards Lightfast Automotive Dyes for Polyester. Stud. Ir. Q. Rev. 2004, 3, 1–14. [Google Scholar]
- Morales-Cerrada, R.; Tavernier, R.; Caillol, S. Fully Bio-Based Thermosetting Polyurethanes from Bio-Based Polyols and Isocyanates. Polymers 2021, 13, 1255. [Google Scholar] [CrossRef]
- Zuliani, A.; Rapisarda, M.; Chelazzi, D.; Baglioni, P.; Rizzarelli, P. Synthesis, Characterization, and Soil Burial Degradation of Biobased Polyurethanes. Polymers 2022, 14, 4948. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Asefnejad, A.; Khorasani, M.T.; Behnamghader, A.; Farsadzadeh, B.; Bonakdar, S. Manufacturing of Biodegradable Polyurethane Scaffolds Based on Polycaprolactone Using a Phase Separation Method: Physical Properties and in Vitro Assay. Int. J. Nanomed. 2011, 6, 2375–2384. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, K.; Tang, X.; Zhang, Z.; Dai, J.; Long, R.; Liao, W. Preparation and Characterization of Polyurethane (PU)/Polyvinylidene Fluoride (PVDF) Blending Membrane. Desalination Water Treat. 2016, 57, 3405–3413. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Adamopoulos, S.; Echart, A.S.; Eceiza, A. Polyurethane Films Prepared with Isophorone Diisocyanate Functionalized Wheat Starch. Eur. Polym. J. 2021, 161, 110826. [Google Scholar] [CrossRef]
- Meng, Q.B.; Lee, S.-I.; Nah, C.; Lee, Y.S. Preparation of Waterborne Polyurethanes Using an Amphiphilic Diol for Breathable Waterproof Textile Coatings. Prog. Org. Coat. 2009, 66, 382–386. [Google Scholar] [CrossRef]
- Costa, F.; Mendanha, D.; Gomes, J.M.; Oliveira, J.A.S.A.; Ribeiro, C.; Miranda, A.F.; Barbosa, J.R.M.; Soares, O.S.G.P.; Pereira, M.F.R.; Santos, J.; et al. A Comprehensive Study on the Physicochemical Characterisation of Plant-Based By-Products. Materials 2025, 18, 2054. [Google Scholar] [CrossRef]
- Katović, D.; Grgac, S.F.; Bischof-Vukušić, S.; Katović, A. Formaldehyde Free Binding System for Flame Retardant Finishing of Cotton Fabrics. Fibres Text. East. Eur. 2012, 90, 94–98. [Google Scholar]
- Son, T.W.; Lee, D.W.; Lim, S.K. Thermal and Phase Behavior of Polyurethane Based on Chain Extender, 2,2-Bis-[4-(2-Hydroxyethoxy)Phenyl]Propane. Polym. J. 1999, 31, 563–568. [Google Scholar] [CrossRef]
- Noureddine, B.; Zitouni, S.; Achraf, B.; Houssém, C.; Jannick, D.R.; Jean-François, G. Development and Characterization of Tailored Polyurethane Foams for Shock Absorption. Appl. Sci. 2022, 12, 2206. [Google Scholar] [CrossRef]
- Bakhshi, H.; Yeganeh, H.; Yari, A.; Nezhad, S.K. Castor Oil-Based Polyurethane Coatings Containing Benzyl Triethanol Ammonium Chloride: Synthesis, Characterization, and Biological Properties. J. Mater. Sci. 2014, 49, 5365–5377. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma(FRAP)as a Measure of “Antioxidan Power”:The FRAP Assay Analytical Biochemistry. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Allehyani, E.S. Surface Functionalization of Polyester Textiles for Antibacterial and Antioxidant Properties. Polymers 2022, 14, 5512. [Google Scholar] [CrossRef]








| Sample | Polyurethane % (wt/wt Dry Basis) | Isocyanate % (wt/wt Dry Basis) | (BLE) % (wt/wt Dry Basis) | Additives % (wt/wt Dry Basis) |
|---|---|---|---|---|
| PB | 62.0 | 29.5 | 0.0 | 8.6 |
| BLE32 | 55.8 | 26.5 | 10.0 | 7.7 |
| BLE63 | 55.8 | 26.5 | 10.0 | 7.7 |
| BLE125 | 55.8 | 26.5 | 10.0 | 7.7 |
| BLE200 | 55.8 | 26.5 | 10.0 | 7.7 |
| BLE500 | 55.8 | 26.5 | 10.0 | 7.7 |
| Samples | Particle Size (µm) | Fiber | Amorphous Particle | |
|---|---|---|---|---|
| Length (µm) | Width (µm) | Length (µm) | ||
| BLE32 | >32 | 247.0 ± 41.0 | 15.0 ± 2.1 | 28.6 ± 11.9 |
| BLE63 | 32–63 | 225.9 ± 83.2 | 18.7 ± 5.3 | 51.2 ± 14.4 |
| BLE125 | 63–125 | 535.8 ± 180.2 | 45.0 ± 4.2 | 112.5 ± 39.9 |
| BLE200 | 125–200 | 176.6 ± 43.2 | 49.3 ± 7.3 | 176.6 ± 43.2 |
| BLE500 | 200–500 | 912.5 ± 20.1 | 136.5 ± 7.8 | 398.0 ± 70.7 |
| Samples | Fiber | Amorphous Particle | ||||
|---|---|---|---|---|---|---|
| C (%) | O (%) | N (%) | C (%) | O (%) | N (%) | |
| BLE32 | 74.7 ± 2.9 a | 21.8 ± 2.5 a | 3.6 ± 1.4 a | 66.0 ± 6.9 a | 26.5 ± 6.4 a | 7.5 ± 2.6 a |
| BLE63 | 66.0 ± 5.5 b | 27.9 ± 4.8 b | 6.1 ± 0.8 b | 52.2 ± 5.9 a | 32.9 ± 8.5 a | 14.9 ± 3.6 b |
| BLE125 | 65.7± 6.2 b | 28.9 ± 4.9 b | 6.2 ± 0.6 b | 60.6 ± 7.8 a | 31.5 ± 7.6 a | 7.8 ± 1.6 a |
| BLE200 | 48.3 ± 0.9 c | 42.3 ± 2.8 c | 10.8 ± 2.0 b | 62.1 ± 3.1 a | 30.8 ± 3.5 a | 7.0 ± 2.2 a |
| BLE500 | 50.7 ± 5.6 c | 40.5 ± 4.1 c | 10.0 ± 3.6 b | 47.2 ± 5.8 b | 44.5 ± 6.4 b | 8.2 ± 4.0 a |
| BLE32 | BLE63 | BLE125 | BLE200 | BLE500 | |||
|---|---|---|---|---|---|---|---|
| cm−1 | Intensity | Intensity | Intensity | Intensity | Intensity | Group | Range |
| 3412 | 21.7 ± 0.1 | 23.3 ± 0.2 | 24.6 ± 0.2 | 25.2 ± 0.7 | 26.0 ± 0.7 | -OH stretch | 3300–3400 |
| 2920 | 19.9 ± 0.3 | 29.9 ± 2.9 | 39.0 ± 3.3 | 30.8 ± 3.3 | 37.7 ± 7.0 | -CH2- asymmetric stretch | 2916–2936 |
| 2853 | 13.5 ± 0.2 | 20.4 ± 2.0 | 26.5 ± 2.3 | 20.7 ± 2.3 | 25.7 ± 4.9 | -CH2- symmetric stretch | 2843–2863 |
| 1727 | 27.9 ± 0.7 | 35.8 ± 1.9 | 42.9 ± 3.4 | 36.6 ± 2.1 | 42.5 ± 3.6 | C=O stretch in unconjugated ketones, carbonyls and in ester groups (hemicellulose) | 1738 |
| 1615 | 62.3 ± 1.6 | 67.6 ± 0.5 | 67.2 ± 1.8 | 69.8 ± 0.6 | 68.5 ± 4.8 | Aromatic skeletal vibration and C=O stretch (lignin) | 1595 |
| 1551 | 17.7 ± 0.4 | 19.8 ± 0.0 | 17.9 ± 1.0 | 17.5 ± 0.9 | 17.6 ± 0.8 | CAR = CAR (Pp cd.) | 1500–1600 |
| 1454 | 23.7 ± 0.8 | 27.0 ± 1.2 | 30.5 ± 1.4 | 31.1 ± 1.5 | 33.6 ± 3.2 | C=C and C-H bond O-H in plane deformation (lignin and hemicellulose) | 1450–1453 |
| 1444 | 28.8 ± 0.2 | 31.3 ± 0.4 | 34.7 ± 0.6 | 35.4 ± 1.0 | 37.0 ± 3.8 | CH- deformation; asymmetric in -CH3 and -CH2- (cellulose) | 1430–1485 |
| 1367 | 29.4 ± 0.2 | 31.5 ± 0.2 | 34.1 ± 0.7 | 34.4 ± 0.7 | 36.1 ± 2.6 | CH deformation (cellulose and hemicellulose) | 1372 |
| 1315 | 37.5 ± 0.6 | 37.4 ± 0.3 | 38.3 ± 0.4 | 40.0 ± 0.6 | 39.7 ± 2.9 | Ph-CHR-OH deformation | 1260–1350 |
| 1232 | 25.3 ± 0.1 | 28.2 ± 0.2 | 29.9 ± 1.4 | 28.6 ± 1.5 | 29.9 ± 2.1 | Syringyl ring and C=C stretch in lignin and xylan | 1235 |
| 1153 | 18.9 ± 0.0 | 21.5 ± 0.9 | 24.0 ± 2.2 | 21.1 ± 0.8 | 23.2 ± 1.8 | Involves C-O stretching of C-OH/C-O-C (cellulose) | 1160 |
| 1027 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.1 ± 0.1 | 100.0 ± 0.1 | C-O, C-C, and C-C-O stretch (cellulose, hemicellulose, and lignin) | 1025–1035 |
| 832 | 2.3 ± 0.1 | 2.6 ± 0.1 | 3.0 ± 0.1 | 3.4 ± 0.3 | 3.6 ± 0.4 | C-O-C aromatic ethers, symmetric stretch | 810–850 |
| 763 | 14.4 ± 0.3 | 13.6 ± 0.0 | 12.7 ± 0.6 | 12.4 ± 1.3 | 12.9 ± 0.7 | C-C Alkanes skeletal vibrations | 720–750 |
| BLE32 | BLE63 | BLE125 | BLE200 | BLE500 | Group | Integration Range | |
|---|---|---|---|---|---|---|---|
| cm−1 | % Area | % Area | % Area | % Area | % Area | ||
| 1444 | 3.12 ± 0.05 a | 3.09 ± 0.01 a | 3.40 ± 0.00 a | 3.47 ± 0.00 b | 3.53 ± 0.00 b | CH2 deformation medium-weak | 1400–1485 |
| 1367 | 2.18 ± 0.02 a | 2.16 ± 0.02 a | 2.24 ± 0.00 a | 2.32 ± 0.02 b | 2.35 ± 0.05 b | CH deformation | 1390–1350 |
| 1315 | 3.62 ± 0.05 | 3.36 ± 0.01 | 3.29 ± 0.01 | 3.54 ± 0.09 | 3.35 ± 0.12 | CH2 deformation medium-weak | 1340–1280 |
| 1153 | 2.15 ± 0.04 | 2.20 ± 0.13 | 2.26 ± 0.23 | 2.09 ± 0.06 | 2.20 ± 0.01 | C-O stretch of C-OH/C-O-C medium | 1195–1130 |
| 1100 | 2.72 ± 0.16 | 2.51 ± 0.17 | 2.44 ± 0.17 | 2.52 ± 0.14 | 2.49 ± 0.15 | C-O-C stretch medium | 1130–1090 |
| 1027 | 13.55 ± 0.09 a | 12.52 ± 0.03 a | 12.05 ± 0.20 b | 12.60 ± 0.06 a | 12.40 ± 0.62 a | CO stretch medium-strong | 1068–990 |
| ∑ Area | 27.16 ± 0.21 a | 25.75 ± 0.31 b | 25.59 ± 0.14 b | 26.45 ± 0.16 a | 26.29 ± 0.61 a |
| BLE32 | BLE63 | BLE125 | BLE200 | BLE500 | Group | Integration Range | |
|---|---|---|---|---|---|---|---|
| cm−1 | % Area | % Area | % Area | % Area | % Area | ||
| 1688 | 1.72 ± 0.32 | 1.74 ± 0.09 | 1.86 ± 0.23 | 1.68 ± 0.14 | 1.60 ± 0.23 | C=O stretch | 1670–1700 |
| 1616 | 7.61 ± 0.51 | 7.87 ± 0.30 | 7.60 ± 0.36 | 7.70 ± 0.52 | 7.33 ± 0.71 | Aryl ring stretch, asymmetric | 1560–1640 |
| 1516 | 0.62 ± 0.03 a | 0.69 ± 0.01 b | 0.58 ± 0.04 a | 0.57 ± 0.01 a | 0.57 ± 0.01 a | Aryl ring stretch, asymmetric | 1488–1525 |
| 1446 | 3.39 ± 0.13 | 3.50 ± 0.06 | 3.60 ± 0.02 | 3.24 ± 0.14 | 3.43 ± 0.71 | OH deformation, asymmetric, OCH3 CH deformation, asymmetric, S-mode | 1400–1485 |
| 1315 | 3.21 ± 0.26 | 3.08 ± 0.10 | 2.99 ± 0.26 | 3.08 ± 0.28 | 2.74 ± 0.58 | Aryl ring breathing mode; CO stretch; S-mode. | 1290–1340 |
| 1234 | 3.13 ± 0.14 | 3.09 ± 0.19 | 3.18 ± 0.31 | 3.07 ± 0.35 | 3.20 ± 0.36 | Syringyl ring and C=C stretch in lignin and Xylan | 1195–1265 |
| 1160 | 2.08 ± 0.11 | 2.16 ± 0.09 | 2.23 ± 0.03 | 2.11 ± 0.07 | 2.14 ± 0.29 | C-H stretch in G-ring | 1135–1190 |
| ∑ Area | 20.89 ± 0.14 a | 21.32 ± 0.28 a | 22.50 ± 0.47 a,b | 20.82 ± 0.88 a | 19.86 ± 0.57 a,a |
| BLE32 | BLE63 | BLE125 | BLE200 | BLE500 | Group | Integration Range | |
|---|---|---|---|---|---|---|---|
| cm−1 | % Area | % Area | % Area | % Area | % Area | ||
| 2920 | 4.15 ± 0.16 a | 5.98 ± 0.19 b | 6.35 ± 0.26 b | 6.74 ± 0.05 b,b | 5.70 ± 0.36 b,c | -CH2- asymmetric stretch | 3000–2800 |
| 2852 | -CH2- symmetric stretch | ||||||
| 1467 | 0.41 ± 0.01 a | 0.52 ± 0.02 b | 0.53 ± 0.03 b | 0.53 ± 0.03 b | 0.54 ± 0.03 b | CH- deformation; asymmetric in -CH2 | 1480–1460 |
| 1315 | 3.40 ± 0.04 | 3.11 ± 0.07 | 3.10 ± 0.02 | 3.22 ± 0.22 | 3.24 ± 0.02 | CH2 deformatio nmedium-weak | 1340–1285 |
| 721 | 0.55 ± 0.01 | 0.58 ± 0.06 | 0.52 ± 0.04 | 0.53 ± 0.01 | 0.55 ± 0.01 | C-C Alkanes skeletal vibrations | 730–710 |
| ∑ Area | 8.50 ± 0.22 a | 10.35 ± 0.39 b | 11.02 ± 0.66 b | 11.22 ± 0.39 b | 10.03 ± 0.40 b |
| Sample | µ (mPa⋅s) | Foam Density (g/L) | Solid Content (%) |
|---|---|---|---|
| PB | 8.6 | 218.1 | 39.6 |
| BLE32 | 54.05 | 263.6 | 42.5 |
| BLE63 | 63.41 | 275.6 | 42.8 |
| BLE125 | 70.41 | 217.1 | 42.4 |
| BLE200 | 65.01 | 222.6 | 42.2 |
| BLE500 | 41.25 | 200.6 | 43.0 |
| Samples/Coatings | L * | a * | b * | ΔL * | Δa * | Δb * | ΔE * |
|---|---|---|---|---|---|---|---|
| PB | 87.7 ± 0.2 a | 0.12 ± 0.10 a | 9.2 ± 0.3 a | 0 | 0 | 0 | 0 |
| BLE32 | 51.3 ± 1.6 b | 8.10 ± 0.48 b | 33.6 ± 1.0 b | −22.3 | 5.1 | 23.1 | 32.5 |
| BLE63 | 46.8 ± 0.9 c | 10.73 ± 0.18 c | 30.5 ± 0.9 c | −26.2 | 6.4 | 25.3 | 37.0 |
| BLE125 | 57.1 ± 0.4 d | 7.65 ± 0.15 b | 34.9 ± 0.3 b | −30.6 | 7.5 | 25.7 | 40.7 |
| BLE200 | 61.5 ± 1.0 e | 6.54 ± 0.30 d | 34.6 ± 0.6 b | −40.9 | 10.6 | 21.3 | 47.3 |
| BLE500 | 65.4 ± 1.3 f | 5.22 ± 0.12 e | 32.3 ± 0.4 c | −36.4 | 8.0 | 24.3 | 44.5 |
| PB-BLE32 | PB-BLE63 | PB-BLE125 | PB-BLE200 | PB-BLE500 | PB | Group | Integration Range (cm−1) | |
|---|---|---|---|---|---|---|---|---|
| cm−1 | Area (%) | Area (%) | Area (%) | Area (%) | Area (%) | Area (%) | ||
| 3520 | 2.16 ± 0.06 a | 2.05 ± 0.16 a | 1.87 ± 0.08 b | 2.02 ± 0.08 a | 2.13 ± 0.04 a | 1.22 ± 0.01 c | -OH stretch | 3450–3700 |
| 3383 | 4.13 ± 0.09 a | 3.66 ± 0.18 b | 3.02 ± 0.36 c | 3.63 ± 0.09 b | 3.75 ± 0.07 b | 3.42 ± 0.06 b | -N-H stretch | 3200–3450 |
| 2917 | 7.96 ± 0.26 a | 8.84 ± 0.17 a | 10.1 ± 0.9 b | 8.6 ± 0.1 a | 8.7 ± 0.5 a | 9.1 ± 0.1 b | -CH2- asymmetric stretch | 2875–3020 |
| 2851 | 2.50 ± 0.10 a | 2.96 ± 0.06 a | 3.66 ± 0.47 b | 2.91 ± 0.04 a | 2.88 ± 0.23 a | 3.05 ± 0.03 a | -CH2- symmetric stretch | 2800–2875 |
| -- | -- | -- | -- | -- | -- | -- | NCO isocyanate groups stretch | 2260–2270 |
| 1724 | 8.37 ± 0.01 a | 8.11 ± 0.00 b | 7.55 ± 0.05 c | 8.03 ± 0.00 b | 8.30 ± 0.00 a | 8.47 ± 0.00 a | Urethane carbonyl groups non-hydrogen bonded [38] | 1745–1705 |
| 1685 | 9.07 ± 0.10 a | 8.74 ± 0.02 a | 8.08 ± 0.35 b | 8.90 ± 0.04 a | 9.01 ± 0.14 a | 9.19 ± 0.02 a | Urethane carbonyl groups hydrogen bonded [38] | 1660–1705 |
| 1518 | 3.55 ± 0.02 a | 3.48 ± 0.01 a | 3.27 ± 0.04 b | 3.47 ± 0.01 a | 3.58 ± 0.01 a | 3.82 ± 0.00 c | –NH and –C–N vibrations of the urethane linkages [37] | 1490–1540 |
| 1463 | 5.75 ± 0.00 a | 5.80 ± 0.00 a | 5.81 ± 0.01 a | 5.98 ± 0.00 b | 5.87 ± 0.01 a | 6.28 ± 0.00 c | -CH2, -CH3 bending vibrations [37,39] | 1440–1490 |
| 1245 | 4.84 ± 0.00 a | 4.62 ± 0.00 b | 4.41 ± 0.01 b | 4.70 ± 0.00 a | 4.81 ± 0.02 a | 4.82 ± 0.00 a | Deformation vibrations of the N-H bond and of the O-C-N bonds | 1220–1285 |
| 1180 | 3.67 ± 0.00 a | 3.61 ± 0.00 a | 3.37 ± 0.03 b | 3.64 ± 0.00 a | 3.74 ± 0.00 a | 3.80 ± 0.00 a | Coupled C-N and C-O stretching vibrations | 1165–1195 |
| 1065 | 0.97 ± 0.00 a | 0.91 ± 0.00 b | 0.91 ± 0.00 b | 0.85 ± 0.00 c | 0.83 ± 0.00 c | 0.76 ± 0.00 d | C–H stretching vibration [40] | 1080–1055 |
| 1027 | 3.42 ± 0.01 a | 3.34 ± 0.01 a | 3.26 ± 0.00 a | 3.21 ± 0.00 b | 3.14 ± 0.00 b | 3.11 ± 0.00 b | C-O, C-C, and C-C-O stretch | 1005–1040 |
| 764 | 1.85 ± 0.00 a | 1.82 ± 0.00 a | 1.71 ± 0.01 b | 1.85 ± 0.00 a | 1.84 ± 0.01 a | 1.67 ± 0.00 b | N-H out of plane bending | 740–785 |
| 717 | 0.69 ± 0.00 a | 0.74 ± 0.00 b | 0.80 ± 0.00 c | 0.75 ± 0.00 b | 0.74 ± 0.0 b | 0.62 ± 0.00 d | C-C Alkanes skeletal vibrations | 705–735 |
| Samples/Coatings | Peak 1 | Peak 2 | Peak 3 | Peak 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | J/g | Temperature (°C) | J/g | Temperature (°C) | J/g | Temperature (°C) | J/g | |
| Cotton Knit | 36.9–125.6 (70.2) | −87.8 | -- | -- | 326.7–398.0 (369.7) | −220.3 | -- | -- |
| PB | 55.0–125.1 (93.9) | −46.8 | 175.1–257.0 (227.3) | −7.8 | 330.7–376.2 (355.9) | −42.2 | 142.9–494.4 (457.1) | −16.8 |
| BLE32 | 54.3–138.8 (85.2) | −45.9 | 180.7–274.6 (227.1) | −7.3 | 321.5–387.3 (361.6) | −53.0 | 406.8–499.5 (453.3) | −24.2 |
| BLE63 | 57.4–124.6 (82.5) | −39.0 | 170.8–251.0 (218.4) | −8.2 | 325.1–387.8 (358.8) | −44.7 | 417.3–499.3 (451.4) | −26.4 |
| BLE125 | 57.0–120.9 (78.8) | −35.2 | 162.1–246.9 (211.8) | −8.5 | 327.7–389.2 (359.2) | −56.1 | 415.7–497.9 (451.2) | −21.3 |
| BLE200 | 52.2–125.6 (87.0) | −53.4 | 162.1–249.7 (221.0) | −7.7 | 325.6–383.5 (359.7) | −52.5 | 413.9–498.8 (453.1) | −17.7 |
| BLE500 | 53.9–122.4 (82.6) | −53.4 | 162.6–254.8 (220.0) | −8.2 | 320.3–389.0 (362.5) | −80.8 | 419.5–494.7 (452.6) | −9.7 |
| Cotton Knit | PB | BLE32 | BLE63 | BLE125 | BLE200 | BLE500 | |
|---|---|---|---|---|---|---|---|
| Peak 1 (°C) | 355.7 | 335.9 | 340.5 | 336.1 | 330.5 | 330.5 | 335.0 |
| Peak 2 (°C) | -- | 471.0 | 464.3 | -- | 468.2 | 464.3 | 456.6 |
| Peak 3 (°C) | -- | 484.1 | -- | -- | -- | -- | -- |
| Temperature (Td 5%) | 285.7 | 225.9 | 221.5 | 243.3 | 230.1 | 246.6 | 269.0 |
| Temperature (Td 20%) | 325.3 | 315.5 | 317.8 | 318.3 | 313.2 | 316.8 | 318.8 |
| Temperature (Td 50%) | 345.0 | 340.6 | 340.9 | 337.2 | 327.8 | 331.5 | 333.9 |
| Temperature (Td 75%) | 357.5 | 370.5 | 379.9 | 420.6 | 330.8 | 381.3 | 342.4 |
| Residual mass 500 °C (%) | 2.4 | 2.9 | 7.4 | 10.9 | 2.5 | 3.5 | 4.7 |
| Samples/Coatings | Peak 1 | Peak 2 | Peak 3 | |||
|---|---|---|---|---|---|---|
| Temperature (°C) | J/g | Temperature (°C) | J/g | Temperature (°C) | J/g | |
| PB | 283.2–357.6 (355.6) | 1157 | 429.2–474.6 (472.9) | 597.5 | 474.6–487.0 (483.4) | 267.3 |
| BLE32 | 279.9–352.4 (350.2) | 1028 | 403.7–483.5 (465.2) | 380.8 | -- | -- |
| BLE63 | 279.6–347.8 (346.0) | 687.9 | 404.2–495.2 (473.9) | 395.9 | -- | -- |
| BLE125 | 298.6–342.0 (338.2) | 2145 | 427.0–498.9 (465.0) | 288.7 | -- | -- |
| BLE200 | 281.3–349.6 (339.6) | 1004 | 427.6–470.5 (469.5) | 1147 | -- | -- |
| BLE500 | 305.1–345.9 (341.0) | 935.4 | 439.2–463.8 (461.6) | 821.2 | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.; Silva, C.; Fernandes, R.A.; Ferreira, N.; Escobar-Avello, D.; Magalhães, P.; Magalhães, F.D.; Martins, J.M.; Carvalho, L.H. New Alternatives in the Valorisation of Eucalyptus globulus By-Products for the Textile Industry. Polymers 2025, 17, 1619. https://doi.org/10.3390/polym17121619
Santos J, Silva C, Fernandes RA, Ferreira N, Escobar-Avello D, Magalhães P, Magalhães FD, Martins JM, Carvalho LH. New Alternatives in the Valorisation of Eucalyptus globulus By-Products for the Textile Industry. Polymers. 2025; 17(12):1619. https://doi.org/10.3390/polym17121619
Chicago/Turabian StyleSantos, Jorge, Carlos Silva, Raquel A. Fernandes, Nuno Ferreira, Danilo Escobar-Avello, Pedro Magalhães, Fernão D. Magalhães, Jorge M. Martins, and Luisa H. Carvalho. 2025. "New Alternatives in the Valorisation of Eucalyptus globulus By-Products for the Textile Industry" Polymers 17, no. 12: 1619. https://doi.org/10.3390/polym17121619
APA StyleSantos, J., Silva, C., Fernandes, R. A., Ferreira, N., Escobar-Avello, D., Magalhães, P., Magalhães, F. D., Martins, J. M., & Carvalho, L. H. (2025). New Alternatives in the Valorisation of Eucalyptus globulus By-Products for the Textile Industry. Polymers, 17(12), 1619. https://doi.org/10.3390/polym17121619












