Enhancement of Polyvinyl Alcohol-Based Films by Chemically Modified Lignocellulosic Nanofibers Derived from Bamboo Shoot Shells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Modification of LCNF
2.2.1. Preparation of LCNF
2.2.2. Preparation and Characterization of Modified LCNF
2.3. Preparation of the Films
2.4. Characterization of the Films
2.4.1. Microstructure Analysis
2.4.2. Light Transmittance Properties Measurement
2.4.3. X-Ray Diffraction (XRD) Analysis
- I002: (002) crystal peak intensity at 2θ ≈ 22.5°.
- Iam: intensity of amorphous region at 2θ ≈ 18°.
2.4.4. Thermogravimetric Analysis
2.4.5. Tensile Properties Analysis
2.4.6. Swelling and Solubility Testing
2.4.7. Water Vapor and Oxygen Barrier Properties
3. Results
3.1. FTIR Analysis of Modified LCNF
3.2. Microscopic Morphology
3.3. Transparency
3.4. Crystal Structure
3.5. Thermal Stability
3.6. Mechanical Properties
3.7. Swelling and Solubility
3.8. Barrier Performance Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVA | Polyvinyl Alcohol |
| LCNF | Lignocellulosic Nanofibers |
| HCl | Hydrochloric Acid |
| CA | Citric Acid |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| SEM | Scanning Electron Microscope |
| TGA | Thermogravimetric Analysis |
| DTG | Derivative Thermogravimetric |
| XRD | X-Ray Diffraction |
| RH | Relative Humidity |
| WVTR | Water Vapor Transmission Rate |
| OTR | Oxygen Transmission Rate |
| CrI | Crystallinity Index |
| DES | Deep Eutectic Solvent |
| AR | Analytical Reagent |
References
- Yi, T.; Zhao, H.; Mo, Q.; Pan, D.; Liu, Y.; Huang, L.; Xu, H.; Hu, B.; Song, H. From cellulose to cellulose nanofibrils—A comprehensive review of the preparation and modification of cellulose nanofibrils. Materials 2020, 13, 5062. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, T.; Cheng, Y.; Zhao, K.; Meng, Z.; Huang, J.; Cai, W.; Lai, Y. Recent advances in functional cellulose-based materials: Classification, properties, and applications. Adv. Fiber Mater. 2024, 6, 1343–1368. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Wu, M.; Huang, Y. Construction of multiple crosslinked networks for the preparation of high-performance lignin-containing cellulose nanofiber reinforced polyvinyl alcohol films. Int. J. Biol. Macromol. 2024, 259, 129061. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Harussani, M.M.; Ilyas, R.A.; Ashogbon, A.O.; Singh, A.; Trif, M.; Jafari, S.M. Surface modifications of cellulose nanocrystals: Processes, properties, and applications. Food Hydrocoll. 2022, 130, 107689. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, Y.; Zhang, Y.; Huang, Y.; Zhang, C. Electromagnetic interference shielding nanocomposites based on cellulose nanofibers and graphene. ACS Appl. Mater. Interfaces 2023, 15, 13947–13957. [Google Scholar]
- Lam, N.T.; Kim, D.; Park, S.; Lee, H. Nanocellulose-based packaging: Sustainable solution with enhanced oxygen barrier and mechanical properties. Polymers 2023, 15, 1456. [Google Scholar]
- Pei, Y.; Zhang, L.; Xu, M.; Wang, X.; Chen, H. Applications of cellulose nanofibers in food packaging and preservation. Nanomaterials 2022, 12, 1394. [Google Scholar]
- Jorfi, M.; Foster, E.J. Biomedical applications of cellulose nanofibers: A review. Nanomaterials 2020, 10, 65. [Google Scholar]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Appl. Sci. 2021, 11, 1682. [Google Scholar] [CrossRef]
- Raghav, N.; Kumar, R.; Chatterjee, S. Recent developments in cellulose-based materials for cosmetics and personal care. Polymers 2022, 14, 894. [Google Scholar]
- Dutta, S.D.; Patel, D.K.; Lim, K.T. Cellulose and its derivatives in pharmaceutical and biomedical applications. Pharmaceutics 2020, 12, 576. [Google Scholar]
- Yao, C.; Li, F.; Chen, T.; Tang, Y. Green preparation of cellulose nanofibers via high-pressure homogenization and their film-forming properties. Ind. Crops Prod. 2023, 206, 117575. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Li, L.; Coseri, S. Cellulose nanofiber extraction from unbleached kraft pulp for paper strengthening. Cellulose 2023, 30, 3219–3235. [Google Scholar] [CrossRef]
- Mahamude, A.S.F.; Wan Harun, W.S.; Kadirgama, K.; Farhana, K.; Ramasamy, D. A short review of nano-cellulose preparation from textile spinning waste cotton. In Proceedings of the 2021 International Congress of Advanced Technology and Engineering (ICOTEN), Taiz, Yemen, 4–5 July 2021; pp. 1–7. [Google Scholar]
- Zhu, C.; Wang, H.; Cheng, H.; Zhou, H.; Chen, Z. High-surface-area lignin-containing cellulose nanofibrils prepared via deep eutectic solvent pretreatment. Ind. Crops Prod. 2022, 187, 115335. [Google Scholar]
- Gao, B.; Sun, C.; Yang, Q.; Wen, Q.; You, S.; Yang, Q.; Cheng, H.; Zhou, H.; Chen, Z. High-solid lignocellulose processing enabled by natural deep eutectic solvent for lignin extraction and industrially relevant production of renewable chemicals. ACS Sustain. Chem. Eng. 2023, 8, 12205–12216. [Google Scholar]
- Tian, R.; Wang, C.; Jiang, W.; Janaswamy, S.; Yang, G.; Ji, X.; Lyu, G. Biodegradable, strong, and hydrophobic regenerated cellulose films enriched with esterified lignin nanoparticles. Small 2024, 20, 2309651. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Lin, Y.; Wang, X.; Zhang, L.; Chen, L. Surface and interface engineering for nanocellulosic advanced materials. Adv. Mater. 2021, 33, 2002264. [Google Scholar] [CrossRef]
- Ouyang, C.; Zhang, H.; Zhu, Y.; Zhao, J.; Ren, H.; Zhai, H. Lignin-containing cellulose nanocrystals enhanced electrospun polylactic acid-based nanofibrous mats: Strengthen and toughen. Int. J. Biol. Macromol. 2024, 235, 135617. [Google Scholar] [CrossRef]
- Trifol, J.; Moriana, R. Barrier packaging solutions from residual biomass: Synergetic properties of CNF and LCNF in films. Ind. Crops Prod. 2022, 177, 114493. [Google Scholar] [CrossRef]
- Wang, S.; Pei, L.; Wei, J.; Xie, J.; Ji, X.; Wang, Y.; Jia, P.; Jiao, Y. Preparation of environmentally friendly oil- and water-resistant paper using holo-lignocellulosic nanofibril (LCNF)-based composite coating. Polymers 2024, 16, 1078. [Google Scholar] [CrossRef]
- Elgharbawy, A.S.; El Demerdash, A.M.; Sadik, W.A.; Kasaby, M.A.; Lotfy, A.H.; Osman, A.I. Enhancing the biodegradability, water solubility, and thermal properties of polyvinyl alcohol through natural polymer blending: An approach toward sustainable polymer applications. Polymers 2024, 16, 2141. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Das, S.N.; Das, A. Mechanical, thermal, moisture absorption and biodegradation behaviour of date palm leaf reinforced PVA/starch hybrid composites. Mater. Today Proc. 2021, 41, 376–381. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, Y.; Li, J.; Wang, S. Improving thermal and moisture resistance of PVA films by crosslinking with natural agents. Carbohydr. Polym. 2021, 252, 117152. [Google Scholar]
- Huang, J.; Guo, Q.; Zhu, R.; Liu, Y.; Xu, F.; Zhang, X. Facile fabrication of transparent lignin sphere/PVA nanocomposite films with excellent UV-shielding and high strength performance. Int. J. Biol. Macromol. 2021, 189, 635–640. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Shi, X.; Zhang, R.; He, Y.; Zhang, H.; Wang, W. Application of polyvinyl alcohol/chitosan copolymer hydrogels in biomedicine: A review. Int. J. Biol. Macromol. 2023, 242, 125192. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Gao, D.; Wu, M.; Huang, Y. Preparation of ultrafine hydrophobic lignin-contained cellulose nanofiber via mechanochemical method and its films. Ind. Crops Prod. 2024, 222, 120064. [Google Scholar] [CrossRef]
- Iglesias, M.C.; Gomez-Maldonado, D.; Davis, V.A.; Peresin, M.S. A review on lignocellulose chemistry, nanostructure, and their impact on interfacial interactions for sustainable products development. J. Mater. Sci. 2023, 58, 685–706. [Google Scholar] [CrossRef]
- Cao, X.; Li, X.; Wu, R.; Liu, B.; Lin, W. Enhancing the performance of biodegradable lignin nanoparticle/PVA composite films via phenolation pretreatment of lignin using a novel ternary deep eutectic solvent. Coatings 2024, 14, 1544. [Google Scholar] [CrossRef]
- Du, J.; Yang, S.; Zhu, Q.; Wu, Y.; Guo, J.; Jiang, J. Preparation and characterization of thermoplastic starch/bamboo shoot processing by-product microcrystalline cellulose composites. Biomass Convers. Biorefin. 2021, 13, 12105. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Chen, J. Bamboo shoot processing in China: Generation and characteristics of shell waste. Ind. Crops Prod. 2021, 170, 113798. [Google Scholar]
- Nuruddin, M.; Chowdhury, R.A.; Lopez-Perez, N.; Montes, F.J.; Youngblood, J.P.; Howarter, J.A. Structure–property relationship of cellulose nanocrystal–polyvinyl alcohol thin films for high barrier coating applications. ACS Appl. Mater. Interfaces 2021, 13, 12472–12484. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, S.; Ye, H.; Zhang, Q.; Wang, X. Revitalizing Bamboo Shoot Industry in Ninghai Mountainous Areas: Challenges and Strategic Practices. Tree Genet. Mol. Breed. 2023, 1, 114103. [Google Scholar]
- Yu, Y.; Wang, Y. Liquefaction of Bamboo Shoot Shell for the Production of Polyols. BioResources 2010, 5, 2371–2380. [Google Scholar]
- Chen, H.; Liu, Z.; Zhang, W. Compositional characterization and cellulose extraction from bamboo shoot shells. Carbohydr. Polym. 2020, 248, 116790. [Google Scholar]
- Wang, X.; Li, D.; Sun, Y. Industrial-scale bamboo shoot processing and byproduct management: Challenges and opportunities. J. Clean. Prod. 2023, 398, 136540. [Google Scholar]
- Khin, M.N.; Ahammed, S.; Aziz, T.; Al-Alsmari, F.; Sameeh, M.Y.; Cui, H.; Lin, L. Characterization of composite film containing polyvinyl alcohol cross-linked with dialdehyde cellulose using citric acid as a catalyst for sustainable packaging. Packaging Technol. Sci. 2024, 37, 1–13. [Google Scholar] [CrossRef]
- Pawcenis, D.; Leśniak, M.; Szumera, M.; Sitarz, M.; Profic-Paczkowska, J. Effect of hydrolysis time, pH and surfactant type on stability of hydrochloric acid hydrolyzed nanocellulose. Int. J. Biol. Macromol. 2022, 222, 1996–2005. [Google Scholar] [CrossRef]
- He, X.; Luzi, F.; Yang, W.; Xiao, Z.; Torre, L.; Xie, Y.; Puglia, D. Citric acid as green modifier for tuned hydrophilicity of surface modified cellulose and lignin nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 9966–9978. [Google Scholar] [CrossRef]
- Guo, J.; Du, J.; Yang, S.; Zhu, Q.; Gu, J.; Guo, J. Preparation of lignocellulosic nanofibrils from bamboo shoot shells by the 3-deep eutectic solvents method and performance studies. Food Ind. Sci. Technol. 2025, 46, 1–10. (In Chinese) [Google Scholar]
- Yan, M.; Wu, T.; Ma, J.; Lu, H.; Zhou, X. A systematic study of lignocellulose nanofibrils (LCNF) prepared from wheat straw by varied acid pretreatments. Ind. Crops Prod. 2022, 185, 115126. [Google Scholar] [CrossRef]
- Wu, X.; Yan, X.; Zhang, J.; Wu, X.; Luan, M.; Zhang, Q. Preparation and characterization of pH-sensitive intelligent packaging films based on cassava starch/polyvinyl alcohol matrices containing Aronia melanocarpa anthocyanins. LWT 2024, 194, 115818. [Google Scholar] [CrossRef]
- Du, J.; Zhu, Q.; Guo, J.; Gu, J.; Guo, J.; Wu, Y.; Ren, L.; Yang, S.; Jiang, J. Preparation and characterization of edible films from gelatin and hydroxypropyl methyl cellulose/sodium carboxymethyl cellulose. Heliyon 2025, 11, e41613. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, J.; Zhang, M.; Xu, T.; Zheng, C.; Yuan, Z.; Si, C. Cellulose-based aerogels, films, and fibers for advanced biomedical applications. Chem. Eng. J. 2024, 497, 154434. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Z.; Niu, Z.; Chen, F.; Liu, C. Glutaraldehyde crosslinked high content of amylose/polyvinyl alcohol blend films with potent tensile strength and Young’s modulus. Polymers 2022, 14, 5550. [Google Scholar] [CrossRef] [PubMed]
- Amri, N.; Ghemati, D.; Bouguettaya, N.; Aliouche, D. Swelling kinetics and rheological behavior of chitosan-PVA/montmorillonite hybrid polymers. Period. Polytech. Chem. Eng. 2019, 63, 179–189. [Google Scholar] [CrossRef]
- Oviedo-Padilla, M.; Enciso, J.C.; Jiménez-Gómez, R.; Martínez-Hernández, A.L.; Sosa-Hernández, J.E.; Torres-Rodríguez, J.M. Evaluation of Physical, Mechanical and Antibacterial Properties of Pinto Bean Starch-Polyvinyl Alcohol Biodegradable Films Reinforced with Cinnamon Essential Oil. Polymers 2021, 13, 1404. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, S.; Lee, B.-K. PVA/CNC/TiO2 nanocomposite for food-packaging: Improved mechanical, UV/water vapor barrier, and antimicrobial properties. Carbohydr. Polym. 2022, 298, 120064. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Shah, A.A.; Chandio, A.D.; Jumah, M.N. UV blocking and oxygen barrier coatings based on polyvinyl alcohol and zinc oxide nanoparticles for packaging applications. Coatings 2022, 12, 897. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Ma, L.; Zhang, L.; Fan, Y.; Wang, Z. Contribution of lignin in esterified lignocellulose nanofibers (LCNFs) prepared by deep eutectic solvent treatment to the interface compatibility of LCNF/PLA composites. Ind. Crops Prod. 2021, 166, 113460. [Google Scholar] [CrossRef]
- Ewulonu, C.M.; Wang, H.; Liu, X.; Wu, M.; Huang, Y. Spectra and crystallographic analysis of combined ultrasonic and mild acid hydrolysis structural effects on lignin-containing cellulose nanofibrils (LCNFs) and cellulose nanofibrils (CNFs). J. Wood Chem. Technol. 2022, 42, 125–135. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Wang, J.; Chen, L. Mechanical Properties of Polylactic Acid Nanofiber Films Reinforced by Modified Cellulose Nanocrystals. Food Bioeng. 2024, 6, 100–110. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Liu, X. Transparency and barrier performance of biodegradable PLA-based packaging films. J. Food Packag. Sci. 2023, 45, 112–119. [Google Scholar]
- Nguyen, T.H.; Tran, M.D.; Chen, Y. Optical and mechanical properties of starch-based films reinforced with cellulose nanocrystals. Carbohydr. Polym. 2022, 291, 119564. [Google Scholar]
- Li, X.; Wei, L.; Wang, S.; Zhang, Y.; Liu, J.; Chen, M.; Huang, T.; Zhao, Q.; Feng, Y.; Yang, R.; et al. Enhancing cellulose nanocrystal reinforcement via amorphous cellulose hydrolysis and epoxy-based composite interface design. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar]
- Zhang, Y.; Li, J.; Chen, X.; Wu, H.; Liu, Y.; Ma, Q.; Zhou, Z.; Tang, W.; Sun, B.; Zhao, X.; et al. Fiber crowding effects on crystallinity reduction and dispersion limitations in cellulose nanocomposites: A combined XRD and SEM study. Compos. Sci. Technol. 2021, 215, 109003. [Google Scholar]
- Almashhadani, N.J.H.; Ricci, L.; Bouyacoub, N.; Belbachir, M.; Bertoldo, M. Thermal characterization by DSC and TGA analyses of PVA hydrogels with organic and sodium MMT. Polym. Bull. 2021, 77, 929–948. [Google Scholar]
- Sathishkumar, G.K.; Mohamed Ibrahim, M.; Mohamed Akheel, G.; Rajkumar, B.; Gopinath, R.; Karpagam, P.; Karthik, M.; Martin Charles, G.; Gautham, G.; Gowri Shankar, G. Synthesis and mechanical properties of natural fiber reinforced epoxy/polyester/polypropylene composites: A review. J. Nat. Fibers 2022, 19, 3718–3741. [Google Scholar] [CrossRef]
- Huang, H.; Yu, Y.; Zhang, Y. Mechanical and barrier properties of plasticized PVC films for food packaging. J. Appl. Polym. Sci. 2019, 136, 47862. [Google Scholar]
- Hassan, E.A.; Abdelrazek, E.M. Structural, mechanical, and thermal properties of HDPE-based nanocomposites. J. Thermoplast. Compos. Mater. 2021, 34, 183–198. [Google Scholar]
- Lin, Y.; Liu, L.; Cao, J.; Zhang, W.; Zhang, Y. High barrier performance of EVOH-based multilayer films for food packaging applications. J. Appl. Polym. Sci. 2021, 138, 50589. [Google Scholar]




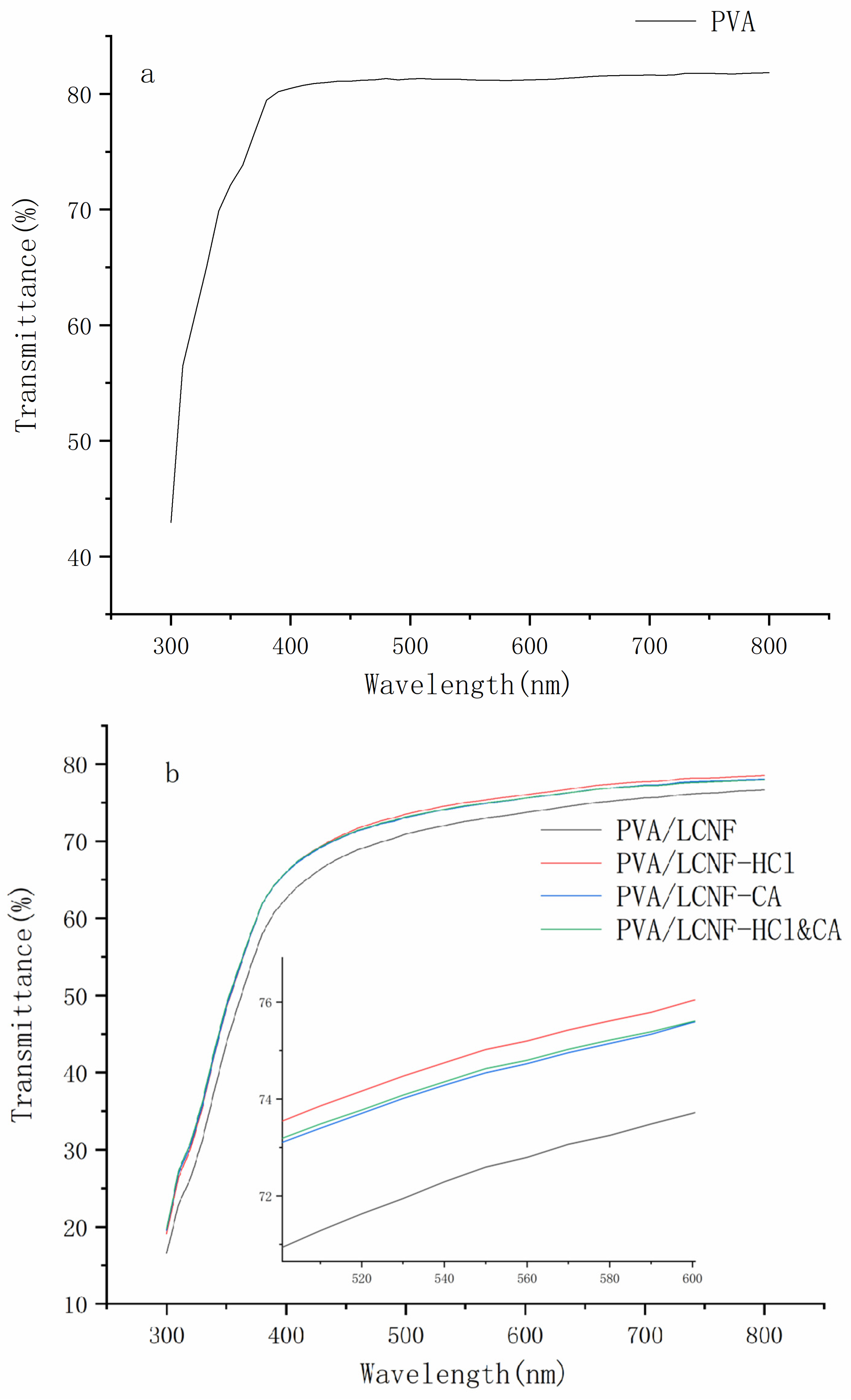

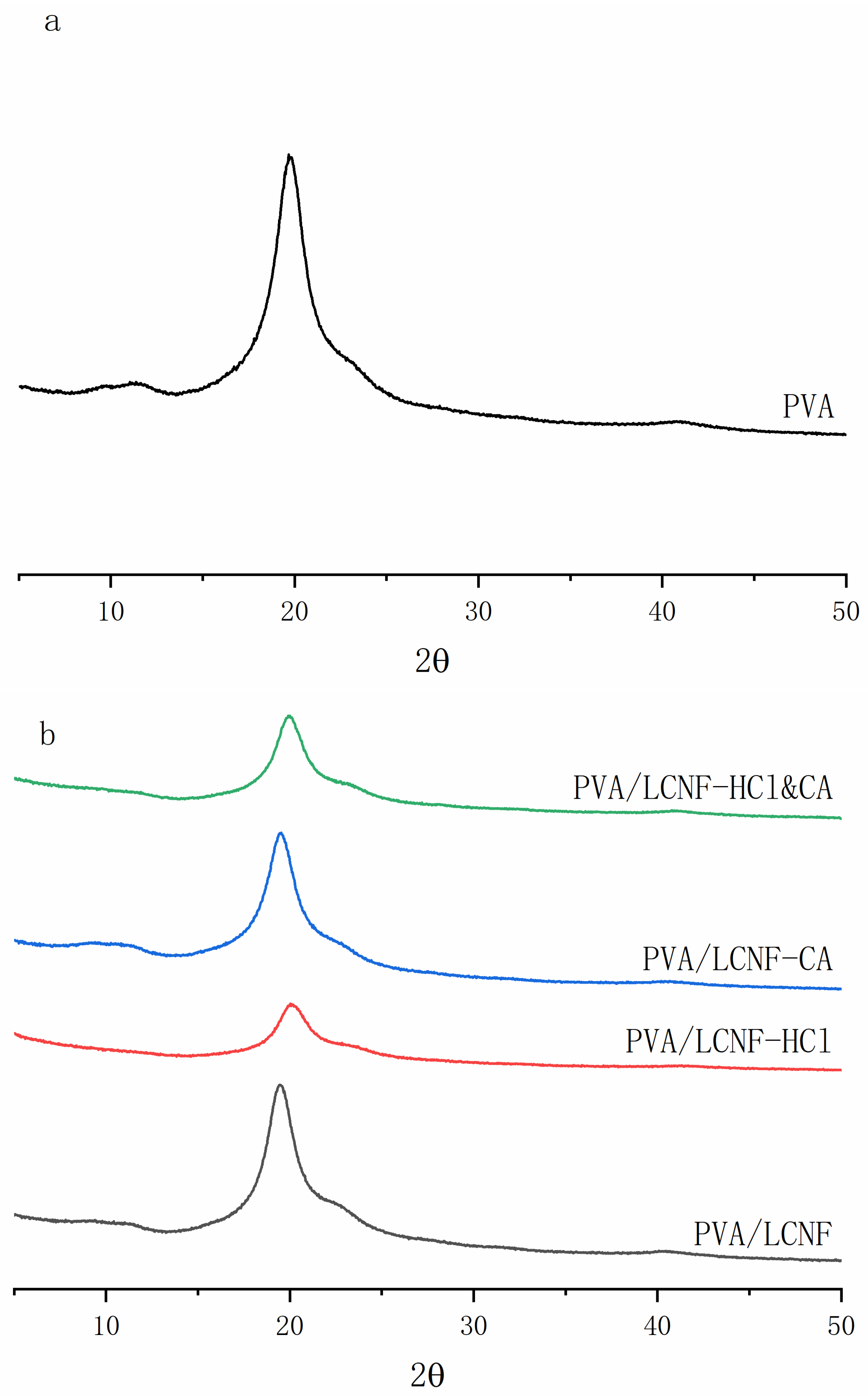



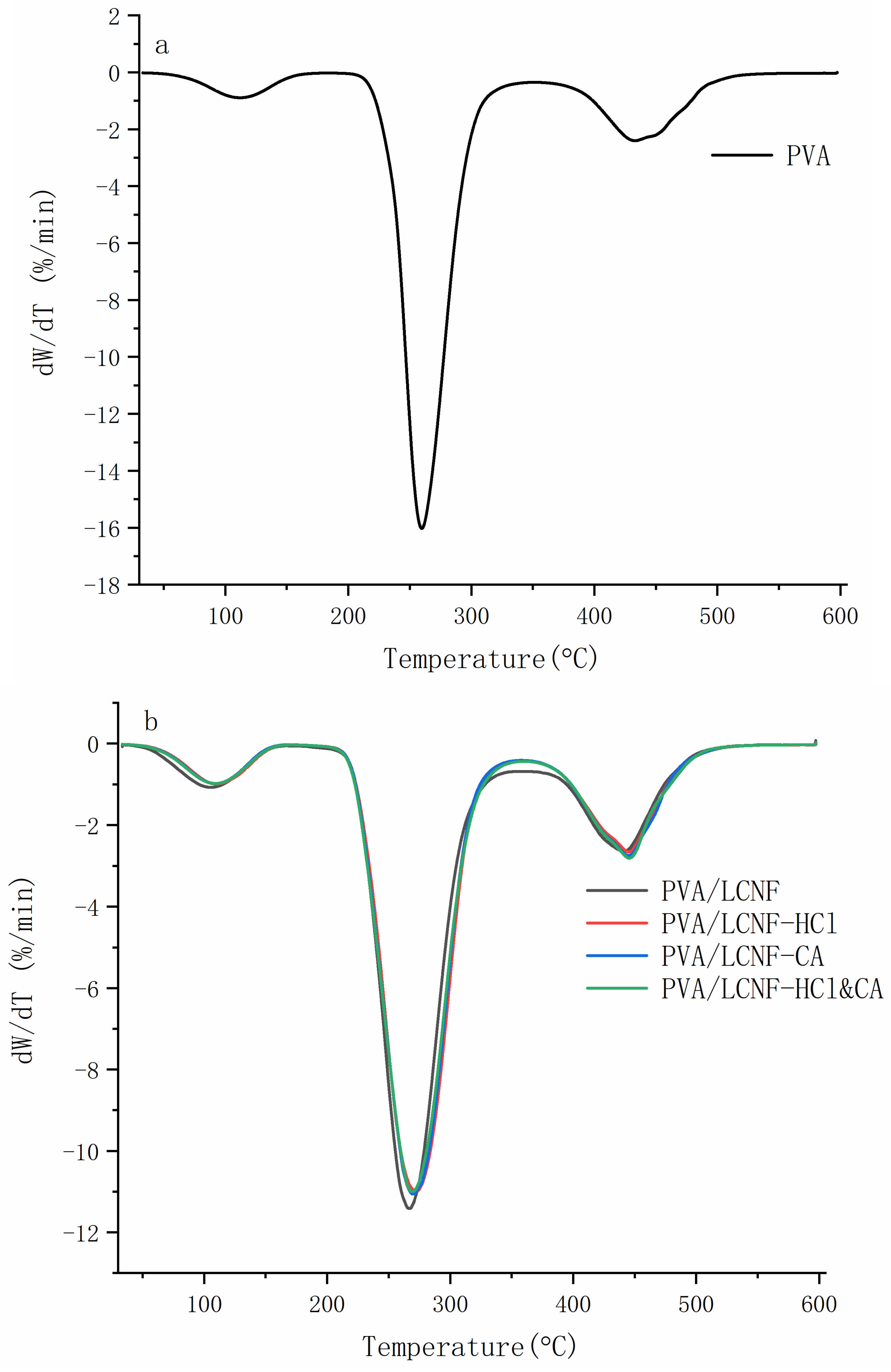

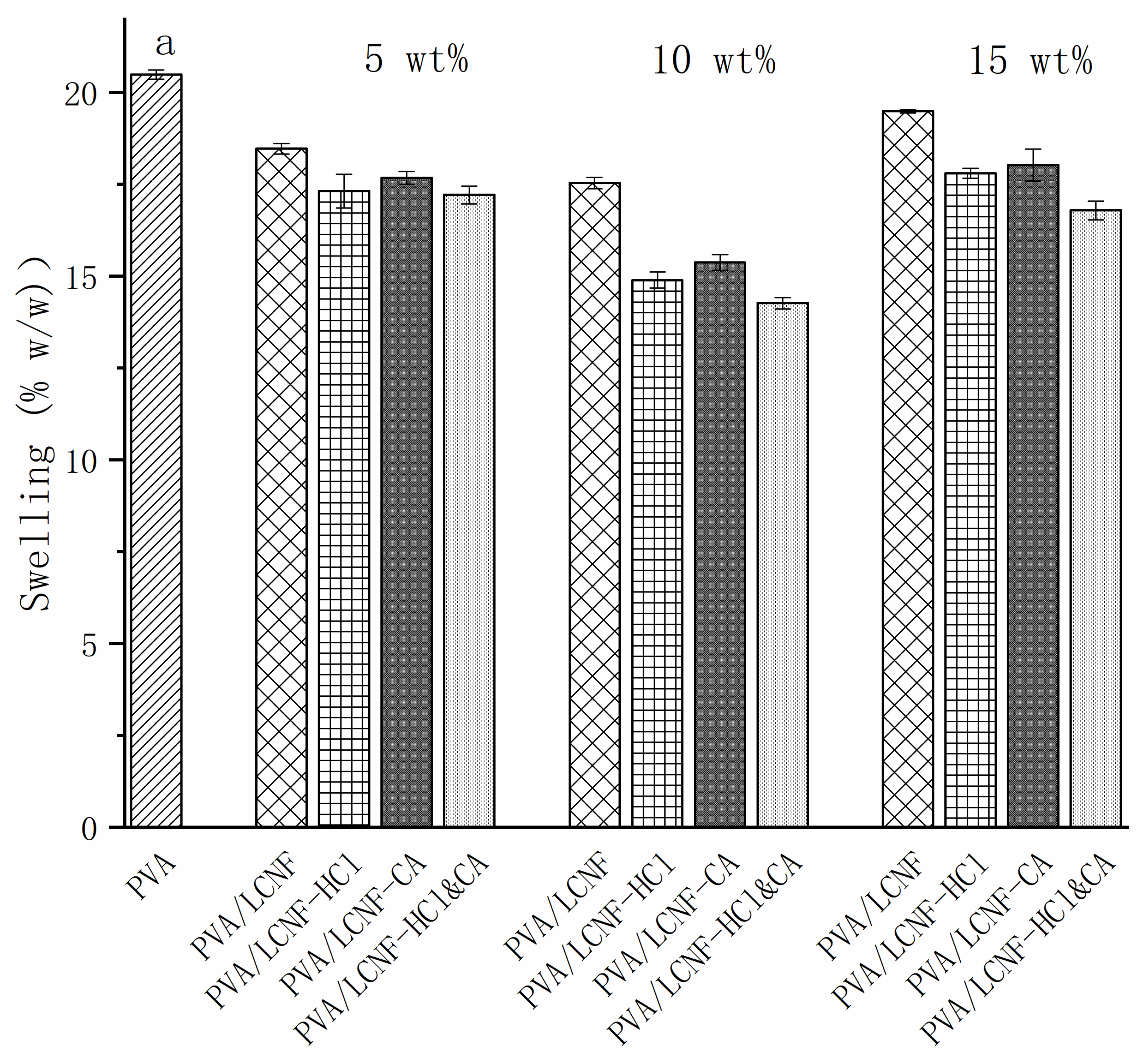
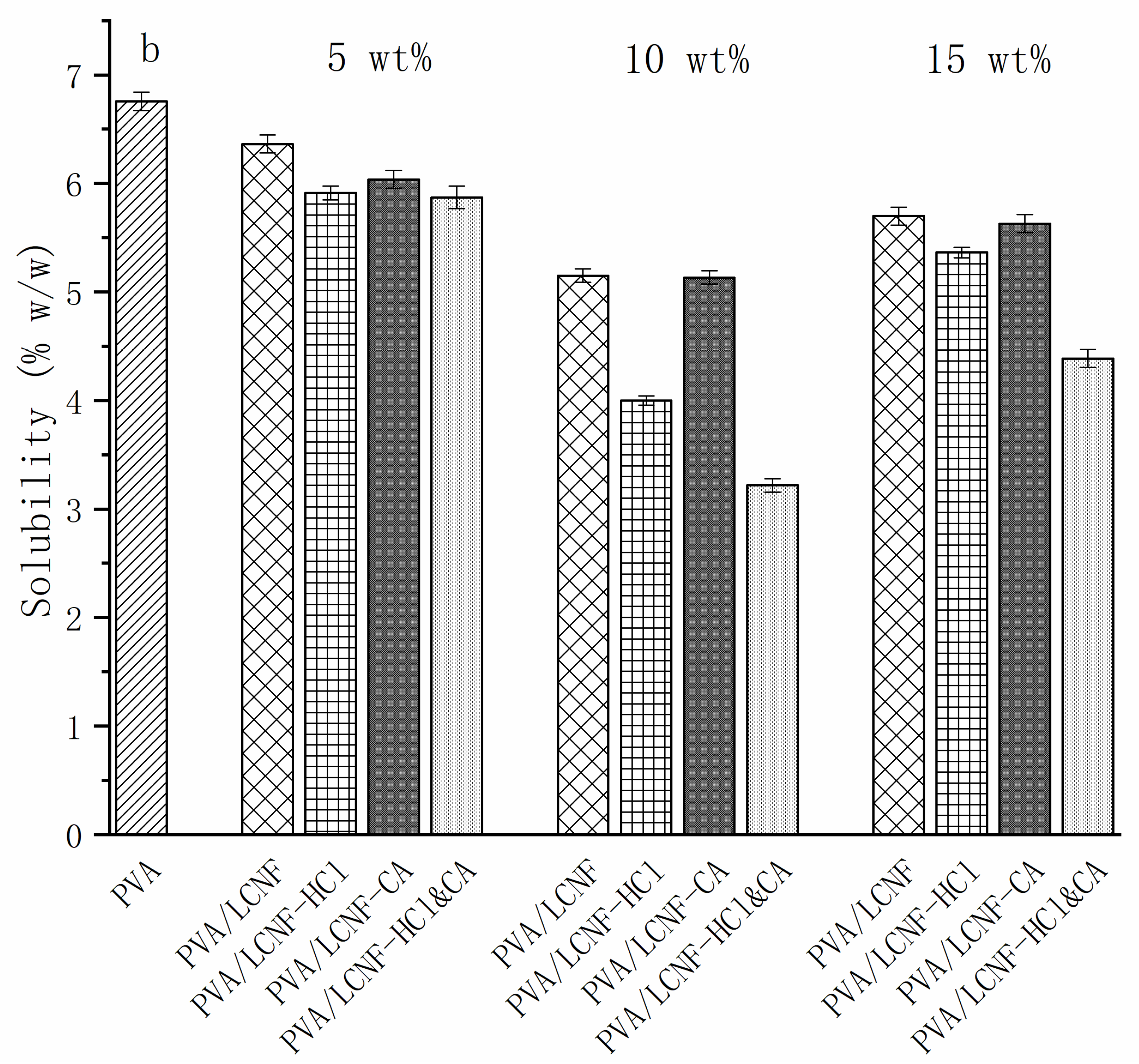
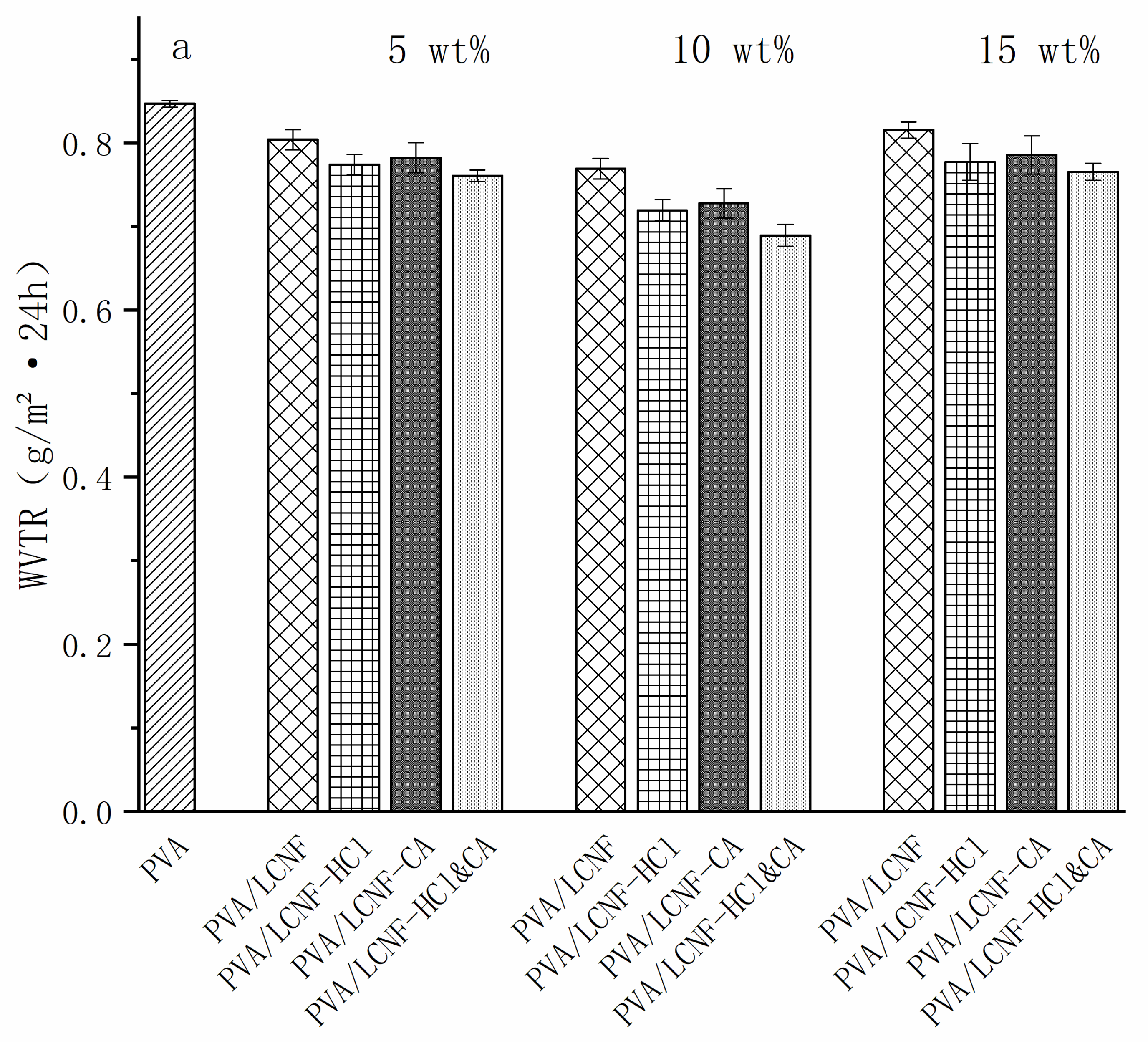
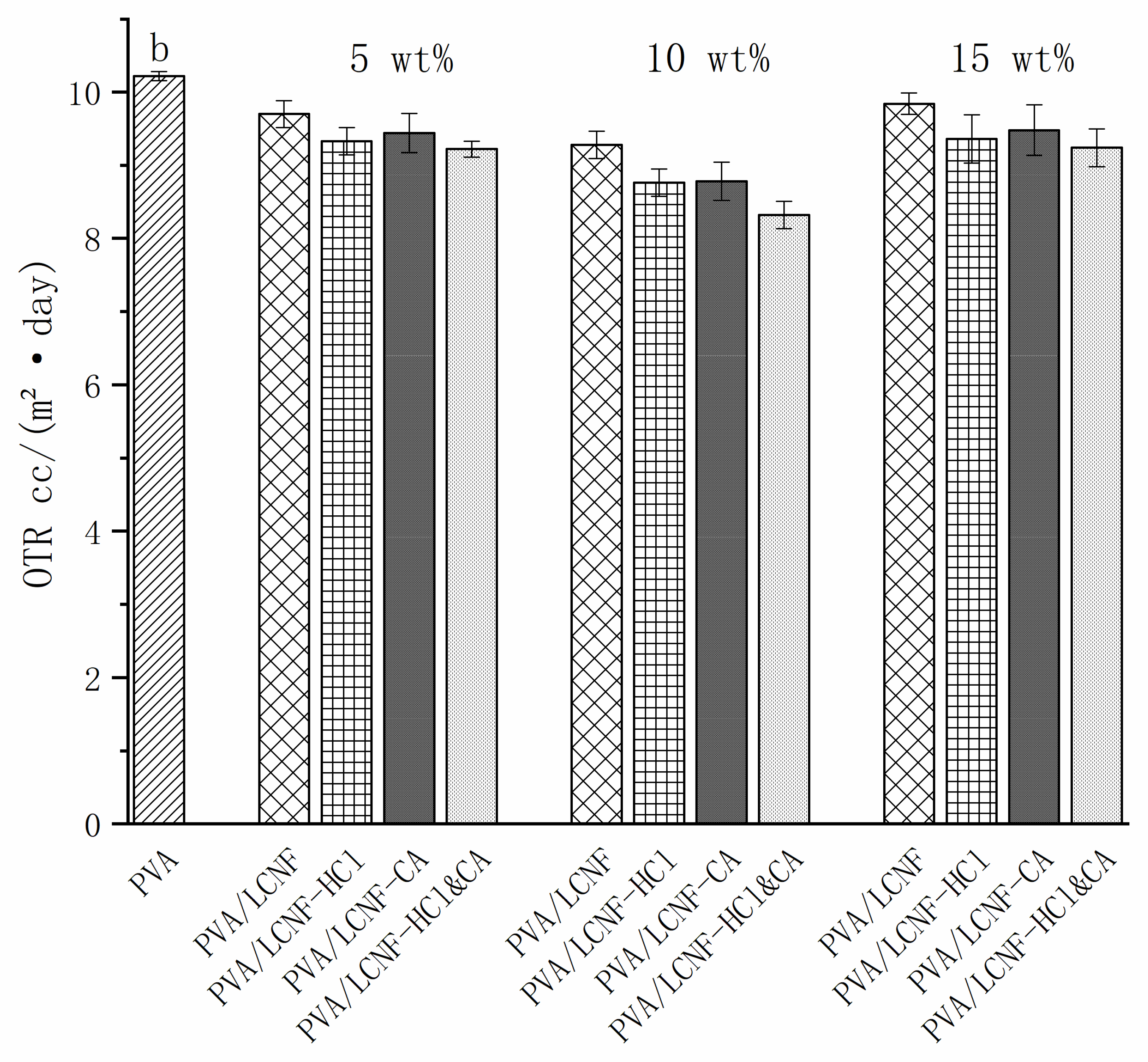
| Sample | 0% | 5 wt% | 10 wt% | 15 wt% |
|---|---|---|---|---|
| PVA | 37.50 ± 0.31 a | - | - | - |
| PVA/LCNF | - | 64.42 ± 0.51 b | 65.41 ± 0.62 c | 62.45 ± 0.48 d |
| PVA/LCNF-HCl | - | 69.23 ± 0.35 e | 79.99 ± 0.81 f | 75.61 ± 0.29 g |
| PVA/LCNF-CA | - | 65.02 ± 0.42 b | 68.20 ± 0.56 h | 64.24 ± 0.57 d |
| PVA/LCNF-HCl&CA | - | 68.50 ± 0.60 e | 77.27 ± 0.72 i | 72.50 ± 0.64 j |
| Filler Content (wt%) | Sample | Tonset (°C) | T50 (°C) | Residue (%) | Max Degradation Rate (%/min) |
|---|---|---|---|---|---|
| 0 | PVA | 265.4 ± 1.23 a | 315.22 ± 1.81 a | 7.91 ± 0.17 a | 16.17 ± 0.51 d |
| 5 | PVA/LCNF | 275.12 ± 1.51 b | 325.23 ± 2.45 b | 8.41 ± 0.28 ab | 11.52 ± 0.45 c |
| PVA/LCNF-HCl | 281.31 ± 1.24 c | 331.63 ± 1.67 c | 8.88 ± 0.23 bc | 10.97 ± 0.56 b | |
| PVA/LCNF-CA | 290.53 ± 1.62 d | 340.83 ± 2.75 d | 8.84 ± 0.31 bc | 10.99 ± 0.54 b | |
| PVA/LCNF-HCl&CA | 292.73 ± 2.03 d | 342.93 ± 1.73 d | 8.98 ± 0.22 c | 10.96 ± 0.61 b | |
| 10 | PVA/LCNF | 280.83 ± 1.89 c | 330.43 ± 1.95 c | 8.45 ± 0.21 ab | 11.41 ± 0.35 c |
| PVA/LCNF-HCl | 285.23 ± 2.06 d | 335.03 ± 2.04 d | 9.05 ± 0.27 cd | 10.93 ± 0.39 b | |
| PVA/LCNF-CA | 286.73 ± 1.73 d | 336.53 ± 1.74 d | 8.63 ± 0.20 bc | 11.38 ± 0.56 c | |
| PVA/LCNF-HCl&CA | 295.43 ± 1.81 e | 345.13 ± 1.88 e | 9.21 ± 0.17 d | 10.81 ± 0.58 a | |
| 15 | PVA/LCNF | 272.53 ± 1.90 b | 320.83 ± 1.65 b | 8.1 ± 0.24 a | 11.10 ± 0.71 c |
| PVA/LCNF-HCl | 278.63 ± 1.42 c | 327.33 ± 1.83 c | 8.65 ± 0.26 bc | 11.39 ± 0.41 c | |
| PVA/LCNF-CA | 283.93 ± 1.23 d | 332.73 ± 1.76 d | 8.29 ± 0.32 ab | 11.46 ± 0.26 c | |
| PVA/LCNF-HCl&CA | 289.23 ± 1.58 e | 338.53 ± 1.47 e | 8.82 ± 0.14 c | 11.09 ± 0.31 c |
| Sample | 0% | 5 wt% | 10 wt% | 15 wt% |
|---|---|---|---|---|
| PVA | 27.50 ± 0.58 d | - | - | - |
| PVA/LCNF | - | 30.12 ± 2.61 c | 35.81 ± 2.47 b | 25.86 ± 1.27 d |
| PVA/LCNF-HCl | - | 33.69 ± 0.47 b | 39.68 ± 1.36 a | 35.91 ± 1.59 b |
| PVA/LCNF-CA | - | 32.35 ± 1.21 b | 36.65 ± 1.24 b | 34.32 ± 1.27 c |
| PVA/LCNF-HCl&CA | - | 37.52 ± 1.45 a | 49.27 ± 1.54 a* | 35.46 ± 1.28 b |
| Sample | 0% | 5 wt% | 10 wt% | 15 wt% |
|---|---|---|---|---|
| PVA | 113.92 ± 1.10 a | - | - | - |
| PVA/LCNF | - | 75.58 ± 2.96 b | 66.71 ± 1.69 c | 56.30 ± 2.41 d |
| PVA/LCNF-HCl | - | 118.32 ± 0.89 a | 120.83 ± 2.58 a | 84.33 ± 3.02 e |
| PVA/LCNF-CA | - | 117.81 ± 2.30 a | 124.03 ± 2.36 f | 82.76 ± 2.41 e |
| PVA/LCNF-HCl&CA | - | 124.18 ± 2.76 f | 131.14 ± 2.93 g | 96.88 ± 2.43 h |
| Sample | 0% | 5 wt% | 10 wt% | 15 wt% |
|---|---|---|---|---|
| PVA | 769.31 ± 5.81 a | - | - | - |
| PVA/LCNF | - | 789.30 ± 26.12 b | 938.02 ± 24.70 c | 791.70 ± 12.75 b |
| PVA/LCNF-HCl | - | 864.30 ± 4.74 d | 1002.65 ± 13.66 e | 971.18 ± 15.91 ef |
| PVA/LCNF-CA | - | 833.33 ± 12.15 d | 997.57 ± 12.49 e | 879.17 ± 13.73 f |
| PVA/LCNF-HCl&CA | - | 944.10 ± 14.56 e | 1024.10 ± 15.43 e | 992.89 ± 12.89 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Guo, J.; Zhu, Q.; Guo, J.; Gu, J.; Wu, Y.; Ren, L.; Yang, S.; Jiang, J. Enhancement of Polyvinyl Alcohol-Based Films by Chemically Modified Lignocellulosic Nanofibers Derived from Bamboo Shoot Shells. Polymers 2025, 17, 1571. https://doi.org/10.3390/polym17111571
Du J, Guo J, Zhu Q, Guo J, Gu J, Wu Y, Ren L, Yang S, Jiang J. Enhancement of Polyvinyl Alcohol-Based Films by Chemically Modified Lignocellulosic Nanofibers Derived from Bamboo Shoot Shells. Polymers. 2025; 17(11):1571. https://doi.org/10.3390/polym17111571
Chicago/Turabian StyleDu, Jingjing, Jianlong Guo, Qian Zhu, Jiagang Guo, Jiayu Gu, Yuhan Wu, Ling Ren, Song Yang, and Jian Jiang. 2025. "Enhancement of Polyvinyl Alcohol-Based Films by Chemically Modified Lignocellulosic Nanofibers Derived from Bamboo Shoot Shells" Polymers 17, no. 11: 1571. https://doi.org/10.3390/polym17111571
APA StyleDu, J., Guo, J., Zhu, Q., Guo, J., Gu, J., Wu, Y., Ren, L., Yang, S., & Jiang, J. (2025). Enhancement of Polyvinyl Alcohol-Based Films by Chemically Modified Lignocellulosic Nanofibers Derived from Bamboo Shoot Shells. Polymers, 17(11), 1571. https://doi.org/10.3390/polym17111571






