Long-Term Physical and Chemical Stability and Energy Recovery Potential Assessment of a New Chelating Resin Used in Brine Treatment for Chlor-Alkali Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. ATR-FTIR Technique
2.2. SEM-EDX Technique
2.3. DVS Technique
2.4. Thermal Analysis
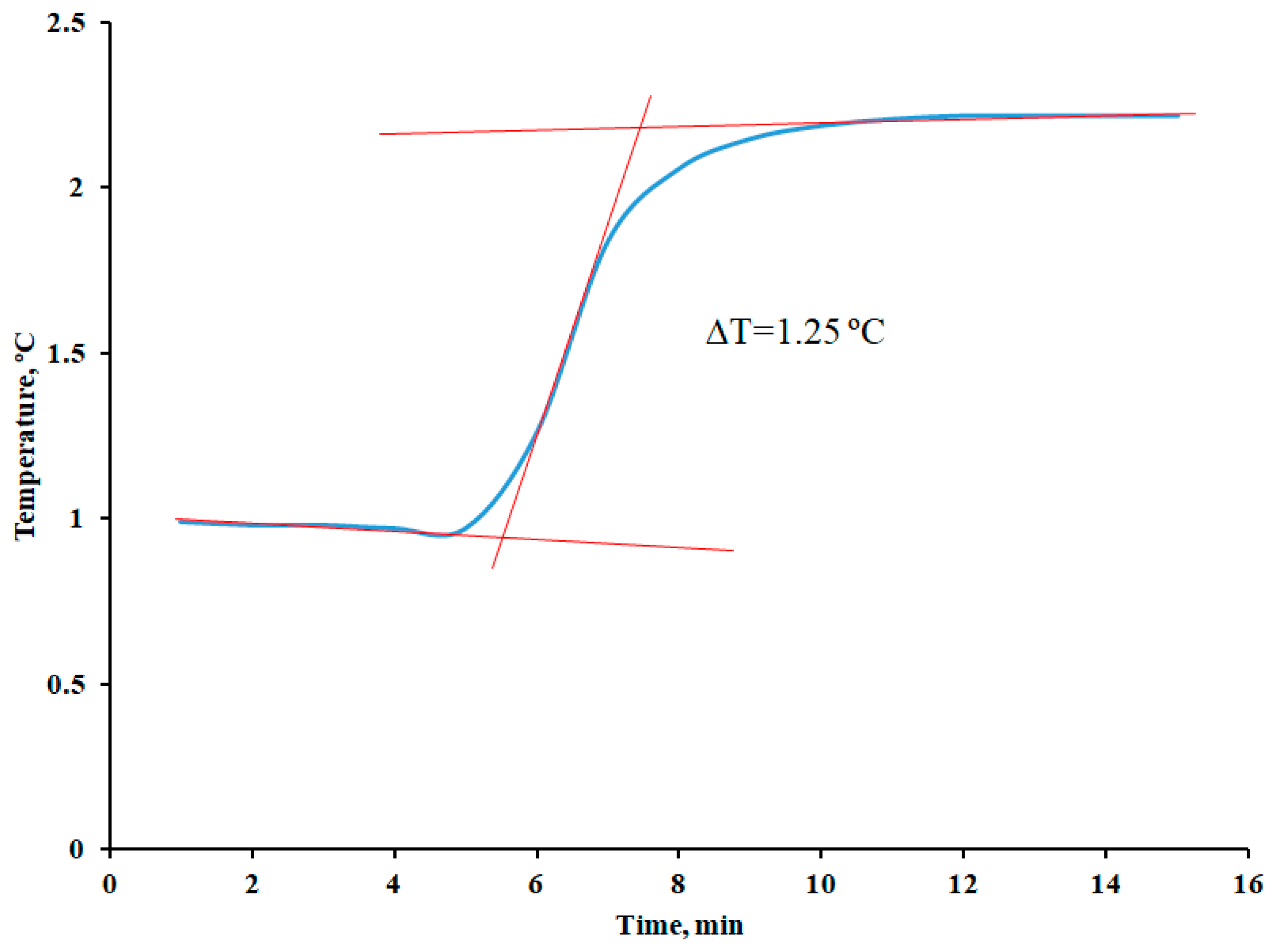
2.5. Determination of Combustion Enthalpy Using Bomb Calorimetry
3. Results
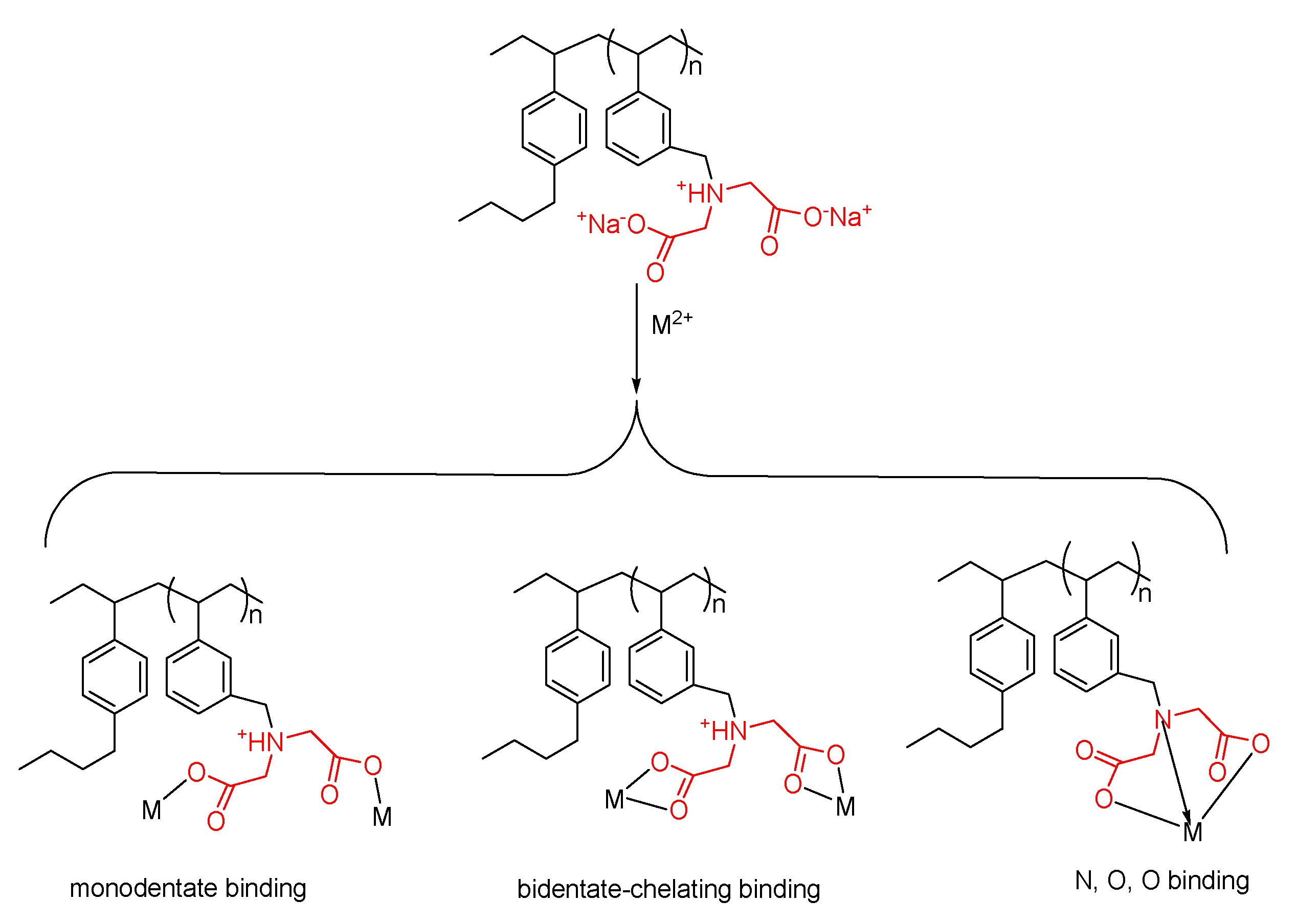
3.1. Analysis by Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
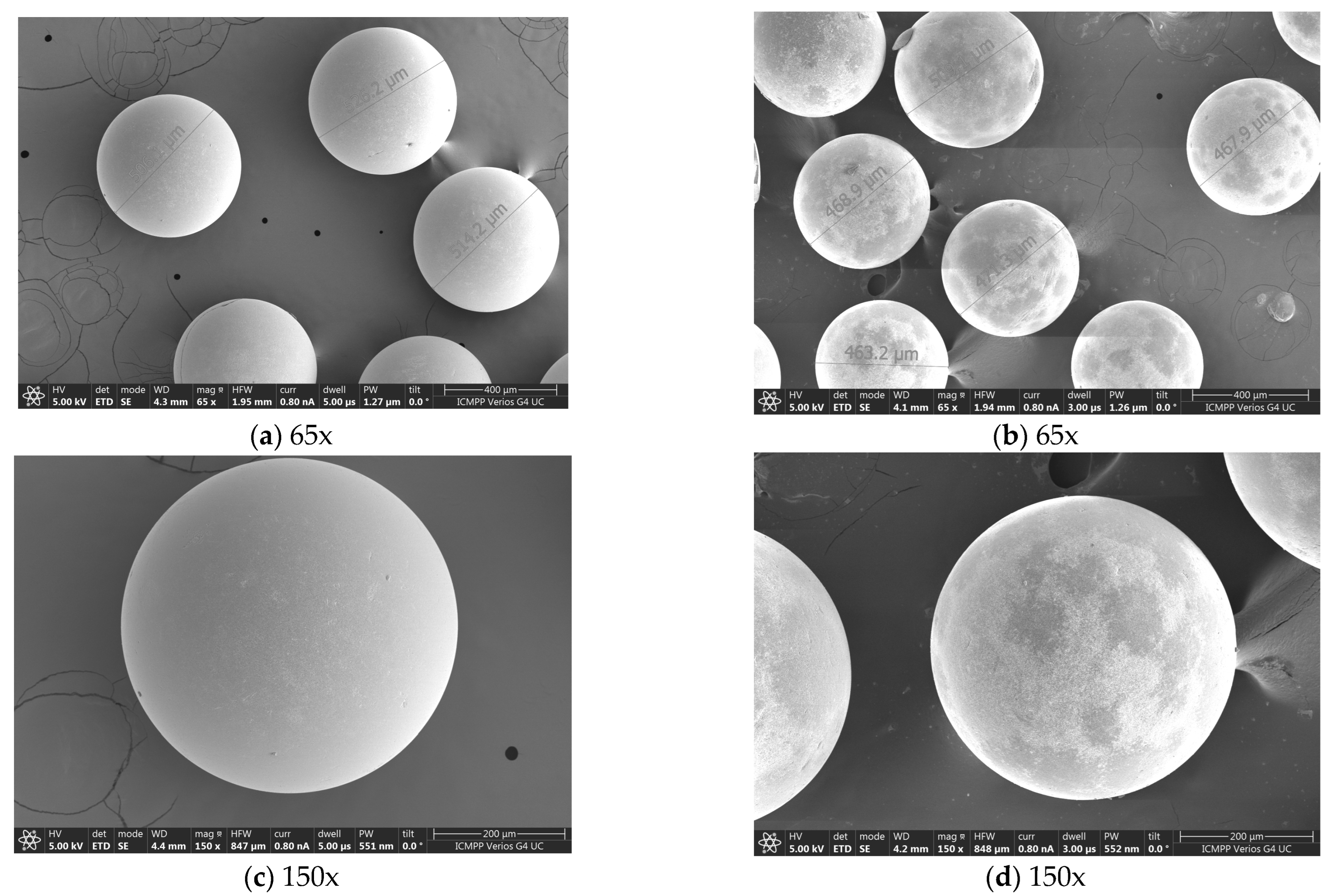
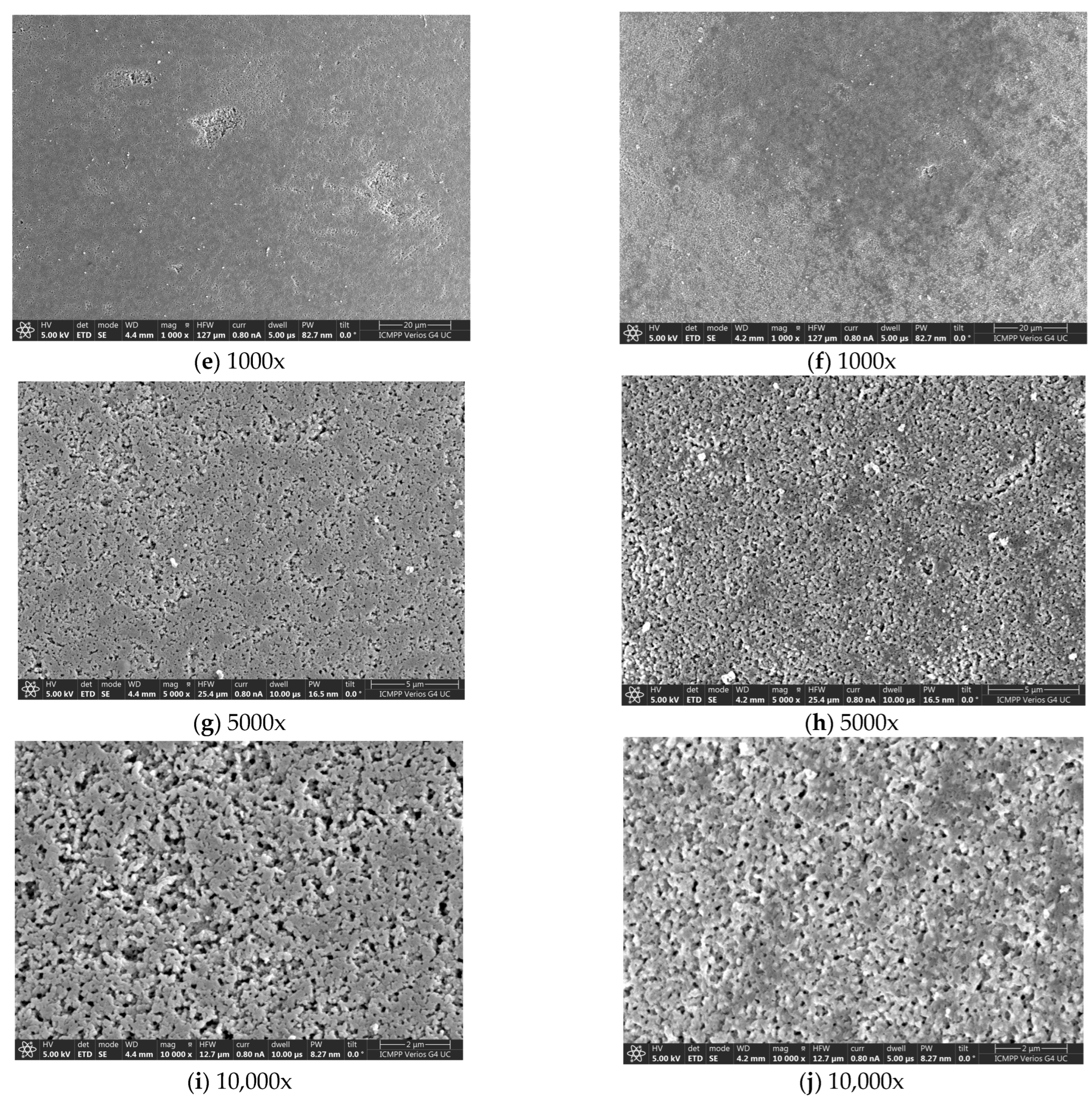
3.2. SEM-EDX Characterizations
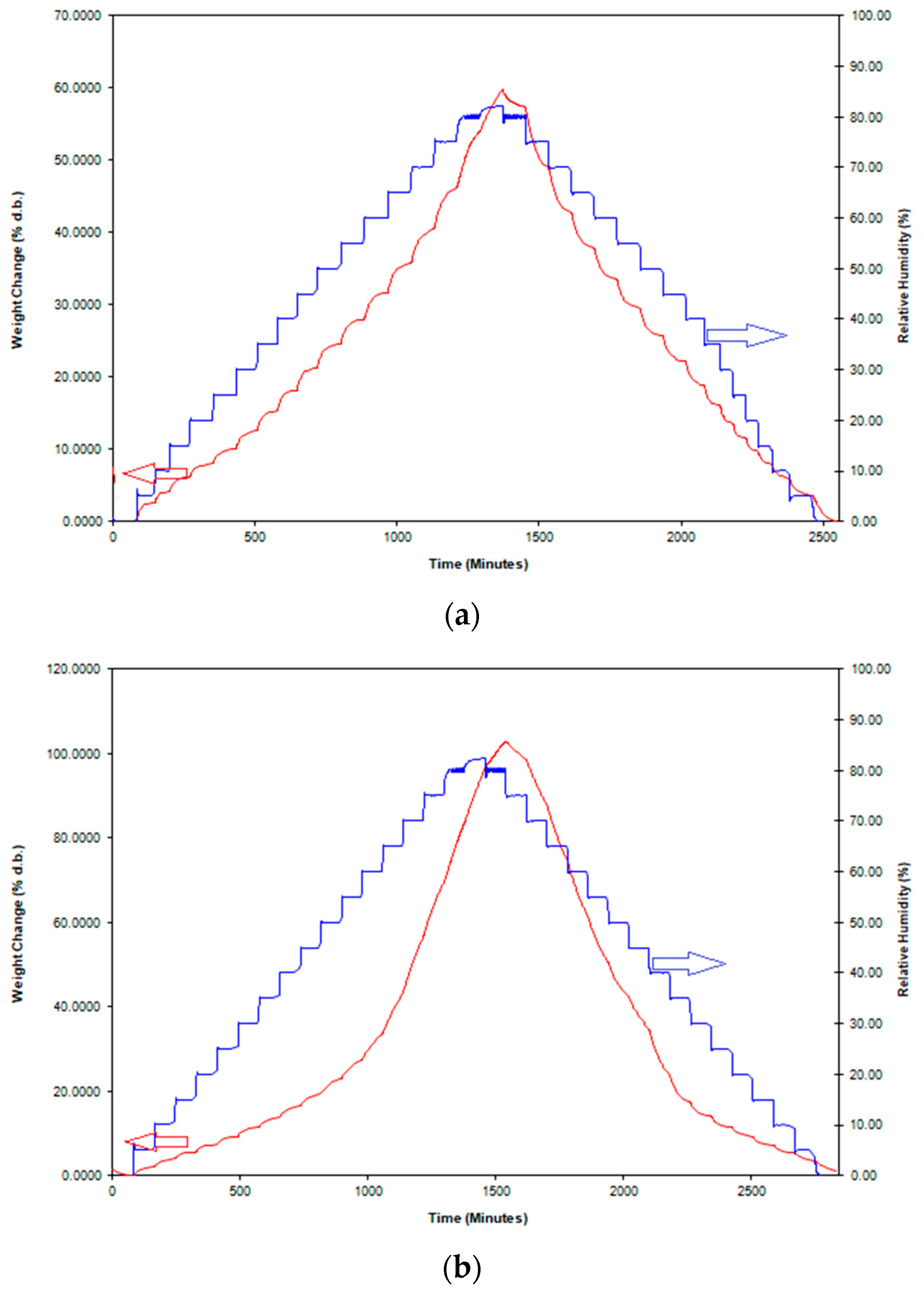
3.3. Water Adsorption Isotherms
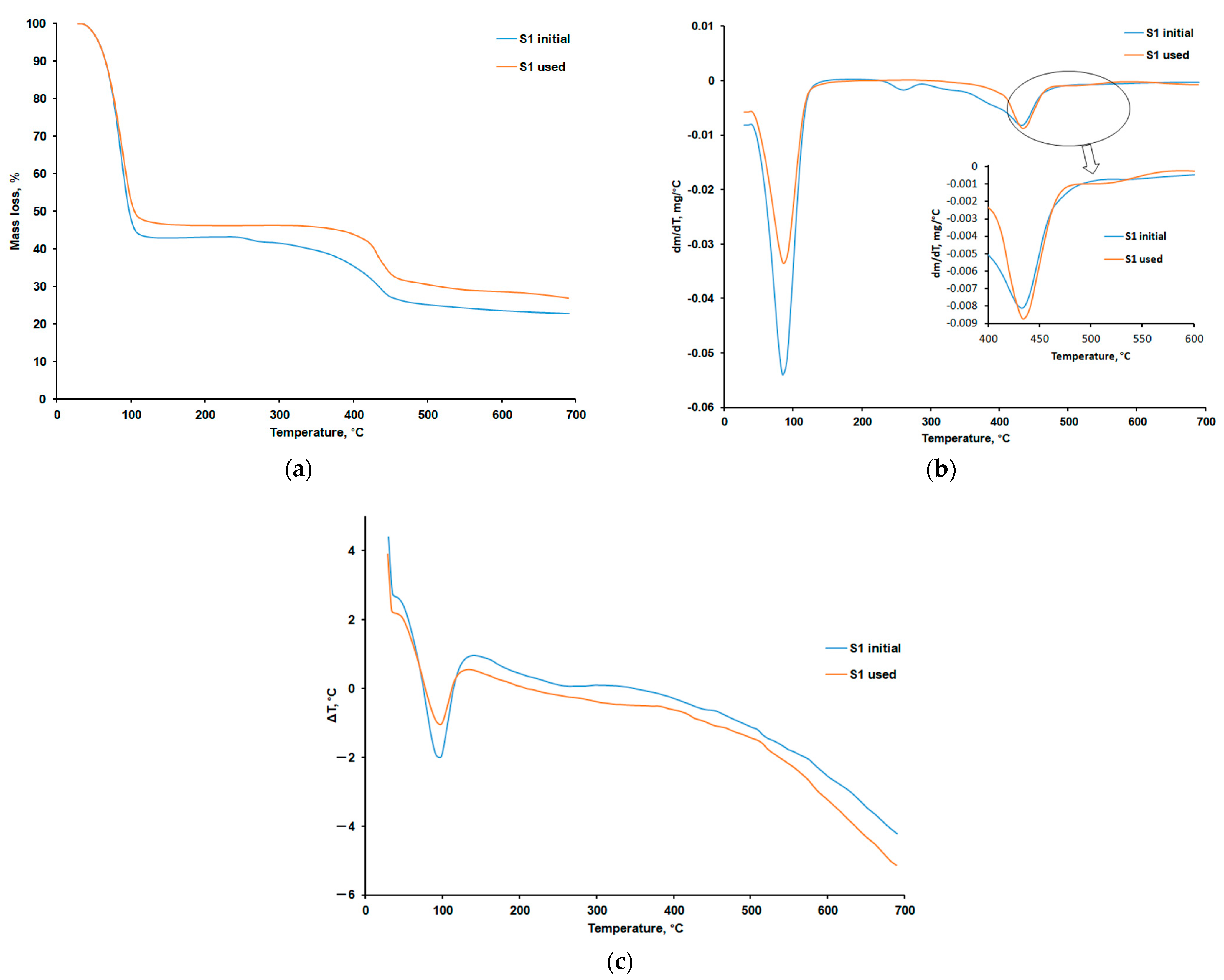
3.4. Evaluation of the Thermal Decomposition Process
3.4.1. Thermogravimetric Analysis in Air
3.4.2. Thermogravimetric Analysis in Nitrogen
3.5. Evaluation of the Energy Recovery Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kermeli, K.; Worrell, E. Energy Efficiency and Cost-Saving Opportunities for the Chlor-Alkali Industry, An ENERGY STAR® Guide for Energy and Plant Managers, 2025, EPA U.S. Environmental Protection Agency, Document No 430-R-24008. Available online: https://www.energystar.gov/sites/default/files/2025-01/EPA_ES_Chlor-Alkali_Guide_20250114.pdf (accessed on 29 April 2025).
- Brinkmann, T.; Santonja, G.G.; Schorcht, F.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for the Production of Chlor-Alkali, Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); Publications Office of the European Union: Luxembourg, 2014. [Google Scholar] [CrossRef]
- Backer, S.N.; Bouaziz, I.; Kallayi, N.; Thomas, R.T.; Preethikumar, G.; Takriff, M.S.; Laoui, T.; Atieh, M.A. Review: Brine solution: Current status, future management and technology development. Sustainability 2022, 14, 6752. [Google Scholar] [CrossRef]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Kazemi, V. Treatment of saturated brine in chlor-alkali process using membranes. Sep. Purif. Technol. 2008, 61, 68–74. [Google Scholar] [CrossRef]
- Lazar, L.; Bandrabur, B.; Tataru-Fărmuş, R.-E.; Drobotă, M.; Bulgariu, L.; Gutt, G. FTIR analysis of ion exchange resins with application in permanent hard water softening. Environ. Eng. Manag. J. 2014, 13, 2145–2152. [Google Scholar] [CrossRef]
- Bandrabur, B.; Tataru-Fărmuş, R.-E.; Lazăr, L.; Bulgariu, L.; Gutt, G. Use of strong acid resin Purolite C100E for removing permanent hardness of water—Factors affecting cationic exchange capacity. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2012, 13, 295–304. [Google Scholar]
- Bandrabur, B.; Tataru-Fărmuş, R.-E.; Lazăr, L.; Gutt, G. Application of a strong acid resin as ion exchange material for water softening equilibrium and thermodynamic analysis. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2012, 13, 361–370. [Google Scholar]
- Atkinson, S. Ion exchangers make brine treatment using chlor-alkali electrolysis more efficient. Membr. Technol. 2019, 2019, 8. [Google Scholar] [CrossRef]
- Miraflores, A.H.; Gómez, K.H.; Muro, C.; Hernández, M.C.D.; Blancas, V.D.; Álvarez Sánchez, J.; Isordia, G.E.D. Ultrapure water production by a saline industrial effluent treatment. Membranes 2025, 15, 116. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Velizarov, S. New insights into the definition of membrane cleaning strategies to diminish the fouling impact in ion exchange membrane separation processes. Sep. Purif. Technol. 2021, 277, 119445. [Google Scholar] [CrossRef]
- Mika, L.; Sztekler, K.; Bujok, T.; Boruta, P.; Radomska, E. Seawater treatment technologies for hydrogen production by electrolysis—A review. Energies 2024, 17, 6255. [Google Scholar] [CrossRef]
- Aziz, H.A.; Ghazali, M.F.; Yusoff, M.S.; Hung, Y.-T. Waste Treatment and Management in Chlor-Alkali Industries. In Handbook of Advanced Industrial and Hazardous Wastes Management, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 100–143. [Google Scholar]
- Wojtaszek, M.; Wasielewski, R. The use of waste ion exchange resins as components of the coal charge for the production of metallurgical coke. Fuel 2021, 286, 119249. [Google Scholar] [CrossRef]
- Li, B.; Chen, M. A comprehensive review on treatment technologies of spent ion exchange resins in nuclear power plants. J. Environ. Chem. Eng. 2024, 12, 114116. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Matskevich, A.I.; Markin, N.S.; Palamarchuk, M.S.; Tokar, E.A.; Egorin, A.M. Decontamination of spent ion-exchange resins from the nuclear fuel cycle using chemical decontamination and direct current. J. Clean. Prod. 2024, 449, 141658. [Google Scholar] [CrossRef]
- Lafond, E.; Cau Dit Coumes, C.; Gauffinet, S.; Chartier, D.; Le Bescop, P.; Stefan, L.; Nonat, A. Investigation of the swelling behavior of cationic exchange resins saturated with Na+ ions in a C3S paste. Cem. Concr. Res. 2015, 69, 61–71. [Google Scholar] [CrossRef]
- El-Sayed, Y.S.; Eskander, S.B.; Bayoumi, T.A.; Aly, H.A.; Gaber, M. Incorporation of spent ion exchange resin simulate into cement composites. Appl. Radiat. Isot. 2024, 210, 111357. [Google Scholar] [CrossRef]
- Atkinson, C.M.; Robshaw, T.J.; Walker, G.D.; Rushton, M.J.D.; Middleburgh, S.C.; Lee, W.E.; Ogden, M.D. Optioneering in nuclear ion exchange resin disposal. Prog. Nucl. Energy 2025, 185, 105719. [Google Scholar] [CrossRef]
- Wang, L.; Yi, L.; Wang, G.; Li, L.; Lu, L.; Guo, L. Experimental investigation on gasification of cationic ion exchange resin used in nuclear power plants by supercritical water. J. Hazard. Mater. 2021, 419, 126437. [Google Scholar] [CrossRef]
- Luca, V.; Bianchi, H.L.; Allevatto, F.; Vaccaro, J.O.; Alvarado, A. Low temperature pyrolysis of simulated spent anion exchange resins. J. Environ. Chem. Eng. 2017, 5, 4165–4172. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Z. Treatment and disposal of spent radioactive ion-exchange resins produced in the nuclear industry. Prog. Nucl. Energy 2015, 78, 47–55. [Google Scholar] [CrossRef]
- Galluccio, F.; Santi, A.; Mossini, E.; Magugliani, G.; Fattori, F.; Gatta, G.D.; Lotti, P.; Cori, D.; Macerata, E.; Bilancia, G.; et al. Pre-disposal management of spent ion-exchange resins by Fenton oxidation treatment and conditioning in an alkali-activated matrix. Nucl. Eng. Des. 2024, 429, 113621. [Google Scholar] [CrossRef]
- Leybros, A.; Ruiz, J.C.; D’Halluin, T.; Ferreri, E.; Grandjean, A. Treatment of spent ion exchange resins: Comparison between hydrothermal and acid leaching processes. J. Environ. Chem. Eng. 2020, 8, 103873. [Google Scholar] [CrossRef]
- Kołodynska, D.; Fila, D.; Hubicki, Z. Static and dynamic studies of lanthanum(III) ion adsorption/desorption from acidic solutions using chelating ion exchangers with different functionalities. Environ. Res. 2020, 191, 110171. [Google Scholar] [CrossRef] [PubMed]
- Maletin, M.; Nikic, J.; Gvoic, V.; Pešic, J.; Cvejic, Ž.; Tubic, A.; Agbaba, J. Optimization and efficiency of novel magnetic-resinbased approaches for enhanced nickel removal from water. Processes 2024, 12, 2287. [Google Scholar] [CrossRef]
- Abeywickrama, J.; Hoth, N.; Grimmer, M.; Haubrich, F.; Drebenstedt, C. Optimizing metal recovery from slag leaching solutions: Advanced ion exchange techniques for sustainable resource extraction. J. Water Process Eng. 2023, 56, 104482. [Google Scholar] [CrossRef]
- Biegun, M.; Kaniewski, M.; Klem-Marciniak, E.; Hoffmann, J. Thermal decomposition characterization and kinetics of copper, iron, manganese and zinc chelates of ethylenediaminetetraacetic acid in air atmosphere. Thermochim. Acta 2022, 716, 179307. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Lisa, G.; Trochimczuk, A.W. Study on metal complexes of chelating resins bearing iminodiacetate groups. Eur. Polym. J. 2009, 45, 2119–2130. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Mucha, I. Freeze dried and thermally dried anion exchanger doped with iron(III) (hydr)oxide—Thermogravimetric studies. Thermochim. Acta 2019, 680, 178359. [Google Scholar] [CrossRef]
- Marin, N.M.; Dolete, G.; Motelica, L.; Trusca, R.; Oprea, O.C.; Ficai, A. Preparation of Eco-Friendly Chelating Resins and Their Applications for Water Treatment. Polymers 2023, 15, 2251. [Google Scholar] [CrossRef]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Geological Institute of Hungary: Budapest, Hungary, 2011; Volume 213, pp. 1–180. [Google Scholar]
- Chambree, D.; Idiţoiu, C.; Segal, E.; Ceśro, A. The study of non-isothermal degradation of acrylic ion-exchange resins. J. Therm. Anal. Calorim. 2005, 82, 803–811. [Google Scholar] [CrossRef]
- McAllister, S.; Chen, J.Y.; Fernandez-Pello, A.C. Fundamentals of Combustion Processes; Springer: New York, NY, USA, 2011; p. 304. [Google Scholar]
- Bejenari, V.; Marcu, A.; Ipate, A.-M.; Rusu, D.; Tudorachi, N.; Anghel, I.; Şofran, I.-E.; Lisa, G. Physicochemical characterization and energy recovery of spent coffee grounds. J. Mater. Res. Technol. 2021, 15, 4437–4451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s. MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
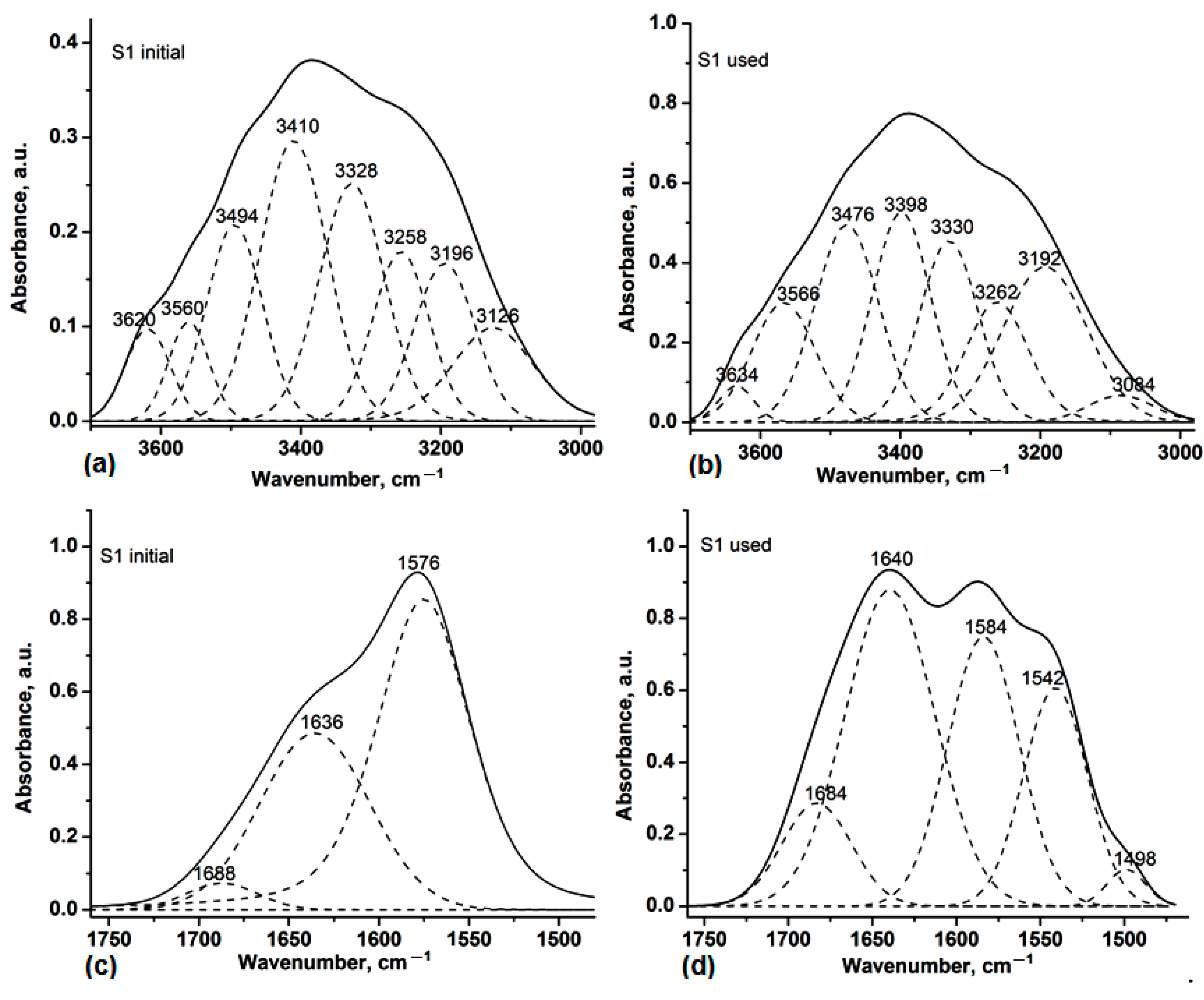
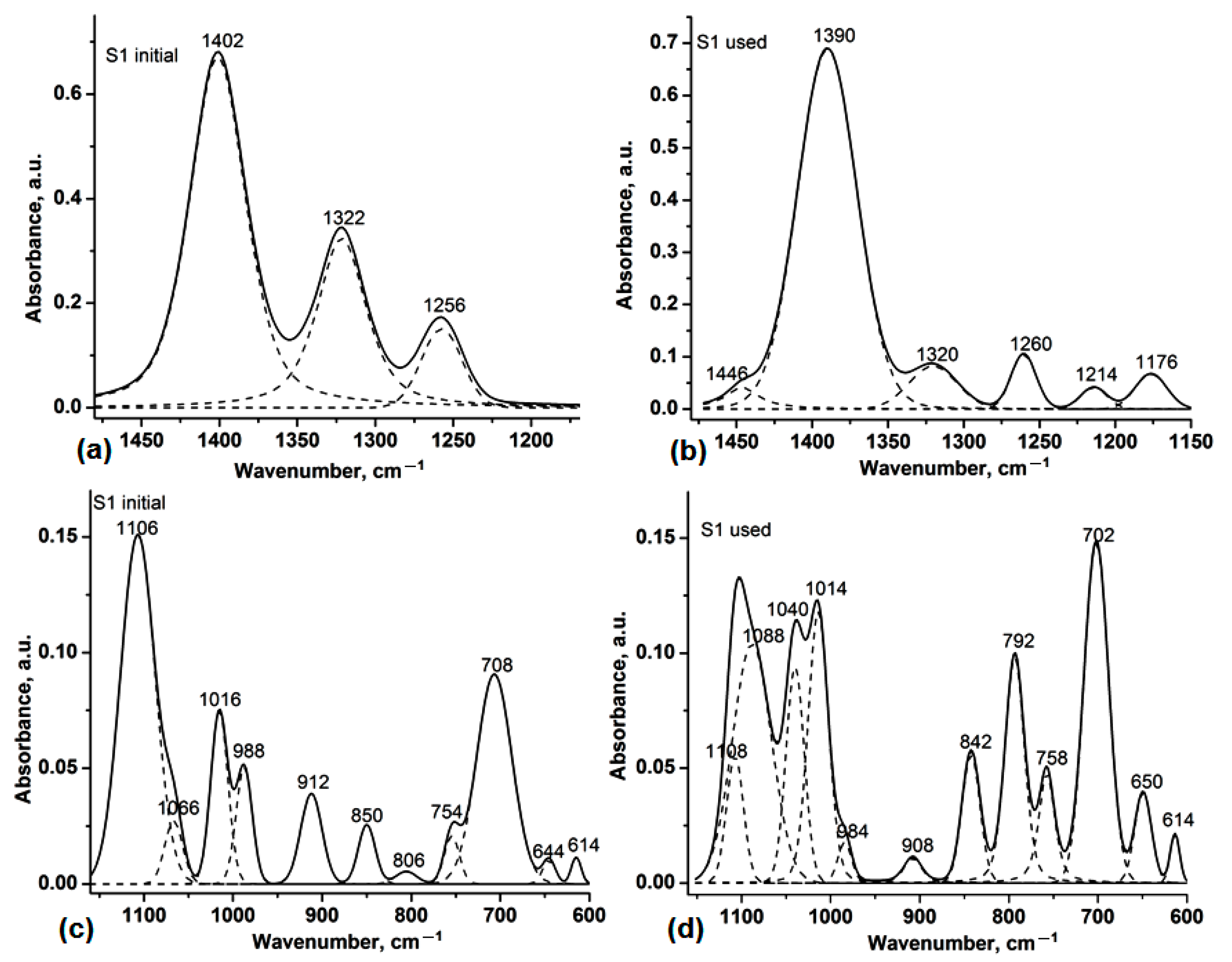


| Spectral Range | 3700–2980 cm−1 | ||||
|---|---|---|---|---|---|
| Sample | νmax (cm−1) | Area | Sample | νmax (cm−1) | Area |
| S1 initial | 3620 | 7.85 | S1 used | 3634 | 5.64 |
| 3560 | 7.59 | 3566 | 33.86 | ||
| 3494 | 20.77 | 3476 | 56.30 | ||
| 3410 | 35.67 | 3398 | 54.87 | ||
| 3328 | 29.68 | 3330 | 48.48 | ||
| 3258 | 18.07 | 3262 | 35.86 | ||
| 3196 | 18.07 | 3192 | 58.69 | ||
| 3126 | 15.46 | 3084 | 7.81 | ||
| Spectral Range | 1760–1480 cm−1 | ||||
| S1 initial | 1688 | 3.63 | S1 used | 1684 | 14.85 |
| 1636 | 71.61 | 1640 | 59.05 | ||
| 1576 | 66.93 | 1584 | 40.01 | ||
| - | - | 1542 | 28.85 | ||
| - | - | 1498 | 2.94 | ||
| Spectral Range | 1480–1160 cm−1 | ||||
|---|---|---|---|---|---|
| Sample | νmax (cm−1) | Area | Sample | νmax (cm−1) | Area |
| S1 initial | - | - | S1 used | 1446 | 1.75 |
| 1402 | 37.14 | 1390 | 34.64 | ||
| 1322 | 16.65 | 1320 | 3.28 | ||
| 1256 | 4.95 | 1260 | 2.16 | ||
| - | - | 1214 | 0.91 | ||
| - | - | 1176 | 1.76 | ||
| Spectral Range | 1160–600 cm−1 | ||||
| S1 initial | 1106 | 7.32 | S1 used | 1108 | 1.32 |
| 1066 | 0.69 | 1088 | 5.71 | ||
| - | - | 1040 | 2.43 | ||
| 1016 | 1.82 | 1014 | 3.58 | ||
| 988 | 1.15 | 984 | 0.34 | ||
| 912 | 1.16 | 908 | 0.32 | ||
| 850 | 0.61 | 842 | 1.42 | ||
| 806 | 0.16 | 792 | 3.00 | ||
| 754 | 0.44 | 758 | 1.34 | ||
| 708 | 4.52 | 702 | 5.08 | ||
| 644 | 0.18 | 650 | 0.93 | ||
| 614 | 0.16 | 614 | 0.27 | ||
| Element/Sample | S1 Initial | S1 Used |
|---|---|---|
| C | 47.04 ± 0.85 | 31.73 ± 1.13 |
| N | 5.31 ± 0.45 | 1.28 ± 0.09 |
| O | 22.95 ± 0.83 | 16.27 ± 0.47 |
| Na | 16.99 ± 0.66 | 23.47 ± 0.42 |
| Mg | - | 0.19 ± 0.03 |
| Al | - | 0.17 ± 0.02 |
| S | - | 0.82 ± 0.04 |
| Cl | - | 20.32 ± 0.67 |
| K | - | 0.04 ± 0.01 |
| Ca | - | 0.03 ± 0.01 |
| Mn | - | 0.12 ± 0.02 |
| Fe | - | 0.22 ± 0.04 |
| Pt | 7.71 ± 0.80 | 5.32 ± 0.65 |
| Samples | Sorption Capacity (% d.b., 80% RH) | BET Data | |
|---|---|---|---|
| Area (m2·g −1) | Monolayer (g·g −1) | ||
| S1 initial | 59.73 | 562.20 ± 54.28 | 0.160 |
| S1 used | 102.77 | 508.39 ± 28.86 | 0.145 |
| Sample | Decomposition Stages | Tonset (°C) | Tpeak (°C) | Tendset (°C) | W (%) | Residue | DTA Characteristics |
|---|---|---|---|---|---|---|---|
| S1 initial | ET. 1 | 40 | 71 | 87 | 47.51 | 34.99 | Endothermic |
| ET. 2 | 281 | 300 | 344 | 6.25 | Exothermic | ||
| ET. 3 | 344 | 357 | 374 | 4.94 | Exothermic | ||
| ET. 4 | 444 | 461 | 473 | 6.31 | Exothermic | ||
| S1 used | ET. 1 | 50 | 78 | 93 | 47.46 | 26.07 | Endothermic |
| ET. 2 | 423 | 435 | 471 | 14.94 | Exothermic | ||
| ET. 3 | 607 | 611 | 641 | 11.53 | Exothermic |
| Sample | Decomposition Stages | Tonset (°C) | Tpeak (°C) | Tendset (°C) | W (%) | Residue | DTA Characteristics |
|---|---|---|---|---|---|---|---|
| S1 initial | ET. 1 | 46 | 87 | 107 | 56.96 | 22.63 | Endothermic |
| ET. 2 | 248 | 259 | 272 | 1.74 | Endothermic | ||
| ET. 3 | 360 | 434 | 456 | 18.67 | Exothermic | ||
| S1 used | ET. 1 | 54 | 88 | 106 | 53.55 | 26.92 | Endothermic |
| ET. 2 | 386 | 435 | 453 | 15.53 | Exothermic | ||
| ET. 3 | 453 | 511 | 533 | 4.00 | Exothermic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, L.; Postolache, L.-V.; Danilova, V.; Coman, D.; Bele, A.; Rusu, D.; Zaltariov, M.-F.; Lisa, G. Long-Term Physical and Chemical Stability and Energy Recovery Potential Assessment of a New Chelating Resin Used in Brine Treatment for Chlor-Alkali Plants. Polymers 2025, 17, 1575. https://doi.org/10.3390/polym17111575
Lazar L, Postolache L-V, Danilova V, Coman D, Bele A, Rusu D, Zaltariov M-F, Lisa G. Long-Term Physical and Chemical Stability and Energy Recovery Potential Assessment of a New Chelating Resin Used in Brine Treatment for Chlor-Alkali Plants. Polymers. 2025; 17(11):1575. https://doi.org/10.3390/polym17111575
Chicago/Turabian StyleLazar, Liliana, Loredana-Vasilica Postolache, Valeria Danilova, Dumitru Coman, Adrian Bele, Daniela Rusu, Mirela-Fernanda Zaltariov, and Gabriela Lisa. 2025. "Long-Term Physical and Chemical Stability and Energy Recovery Potential Assessment of a New Chelating Resin Used in Brine Treatment for Chlor-Alkali Plants" Polymers 17, no. 11: 1575. https://doi.org/10.3390/polym17111575
APA StyleLazar, L., Postolache, L.-V., Danilova, V., Coman, D., Bele, A., Rusu, D., Zaltariov, M.-F., & Lisa, G. (2025). Long-Term Physical and Chemical Stability and Energy Recovery Potential Assessment of a New Chelating Resin Used in Brine Treatment for Chlor-Alkali Plants. Polymers, 17(11), 1575. https://doi.org/10.3390/polym17111575







