Sustainable Microwave-Assisted Extraction of Hemp Seed Oil as Functional Additive into Polybutylene Succinate (PBS) Films for Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Hemp Seed Pre-Treatment
2.2. Soxhlet and Microwave-Assisted Extractions
2.3. PBS-Hemp Oil Blend and Film Preparation
2.4. Hemp Seed Extract and Film Characterization
2.4.1. Spectroscopic Analyses (FTIR-ATR)
2.4.2. DPPH Radical Scavenging Activity
2.4.3. Wide Angle X-Ray Diffraction (WAXD)
2.4.4. Scanning Electron Microscopy (SEM)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. Thermogravimetric Analyses (TGA)
2.4.7. Mechanical Properties
2.4.8. Migration Tests in Food Simulants
3. Results and Discussion
3.1. Extract Characterization
3.1.1. DPPH Assay
3.1.2. FTIR-ATR Spectroscopy of HSO and of PBS-Based Films
3.2. PBS-Oil Blend Film Characterization
3.2.1. Structural Analysis (XRD)
3.2.2. Scanning Electron Microscopy (SEM)
3.2.3. FTIR-ATR Spectroscopy of PBS-Based Films
3.2.4. Differential Scanning Calorimetry (DSC)
| 1st Heating | Cooling | 2nd Heating | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| χc xrd (%) | Tcc (°C) | Tm1 (°C) | ΔHm1 (J/g) | χc1 (%) | Tc (°C) | ΔHc (J/g) | Tg (°C) | Tm2 (°C) | ΔHm2 (J/g) | χc2 (%) | |
| PBS film | 51 | 99.4 | 119.0 | 54.9 | 49.7 | 77.5 | 65.6 | −17.0 | 117.6 | 54.4 | 49.3 |
| PBS 0.5% | 45 | 99.1 | 117.9 | 54.1 | 49.3 | 77.5 | 73.2 | −17.0 | 117.2 | 54.0 | 48.5 |
| PBS 1% | 44 | 98.9 | 113.6 | 50.2 | 45.9 | 78.4 | 61.3 | −17.0 | 114.5 | 51.2 | 46.9 |
3.2.5. Thermogravimetric Analyses (TGA)
3.2.6. Mechanical Properties
3.2.7. Migration Tests in Food Simulants—Mass Loss
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; Volume 17. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef] [PubMed]
- Canellas, V.P.E.; Nerín, C. Compounds responsible for off-odors in several samples composed by polypropylene, polyethylene, paper and cardboard used as food packaging materials. Food Chem. 2020, 309, 125792. [Google Scholar]
- Somaye, A. Polypropylene in the industry of food packaging. In Polypropylene; BoD—Books on Demand: Norderstedt, Germany, 2012; pp. 3–22. [Google Scholar]
- Ayhan, Z.; Cimmino, S.; Esturk, O.; Duraccio, D.; Pezzuto, M.; Silvestre, C. Development of films of novel polypropylene based nanomaterials for food packaging application. Packag. Technol. Sci. 2015, 28, 589–602. [Google Scholar] [CrossRef]
- Mazhandu, Z.S.; Muzenda, E.; Mamvura, T.A.; Belaid, M.; Nhubu, T. Integrated and Consolidated Review of Plastic Waste Management and Bio-Based Biodegradable Plastics: Challenges and Opportunities. Sustainability 2020, 12, 8360. [Google Scholar] [CrossRef]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Jawaid, M.; Lee, C.H. Effect of Modified Tapioca Starch on Mechanical, Thermal, and Morphological Properties of PBS Blends for Food Packaging. Polymers 2018, 10, 1187. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(Butylene Succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef]
- Mori, R. Replacing All Petroleum-Based Chemical Products with Natural Biomass-Based Chemical Products: A Tutorial Review. RSC Sustain. 2023, 1, 179–212. [Google Scholar] [CrossRef]
- Hassan, A.A.; Abbas, A.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Wang, S. Development, influencing parameters and interactions of bioplasticizers: An environmentally friendlier alternative to petro industry-based sources. Sci. Total Environ. 2019, 682, 394–404. [Google Scholar] [CrossRef]
- Skendi, A.; Zinoviadou, K.G.; Papageorgiou, M.; Rocha, J.M. Advances on the Valorisation and Functionalization of By-Products and Wastes from Cereal-Based Processing Industry. Foods 2020, 9, 1243. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Casillo, A.; D’angelo, C.; Imbimbo, P.; Monti, D.M.; Parrilli, E.; Lanzetta, R.; D’ayala, G.G.; Mallardo, S.; Corsaro, M.M.; Duraccio, D. Aqueous Extracts from Hemp Seeds as a New Weapon against Staphylococcus epidermidis Biofilms. Int. J. Mol. Sci. 2023, 24, 16026. [Google Scholar] [CrossRef]
- Devi, V.; Khanam, S. Comparative Study of Different Extraction Processes for Hemp (Cannabis sativa) Seed Oil Considering Physical, Chemical and Industrial-Scale Economic Aspects. J. Clean. Prod. 2019, 207, 645–657. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lipid Sci. Technol. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Latif, S.; Anwar, F. Physicochemical Studies of Hemp (Cannabis sativa) Seed Oil Using Enzyme-Assisted Cold-Pressing. Eur. J. Lipid Sci. Technol. 2009, 111, 1042–1048. [Google Scholar] [CrossRef]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J.; Bilić, M.; Jokić, S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crops Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Kostić, M.D.; Joković, N.M.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of hempseed oil extraction by n-hexane. Ind. Crops Prod. 2013, 48, 133–143. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Microwave-assisted extraction of hempseed oil: Studying and comparing of fatty acid composition, antioxidant activity, physiochemical and thermal properties with Soxhlet extraction. J. Food Sci. Technol. 2019, 56, 4198–4210. [Google Scholar] [CrossRef]
- Uzel, R.A. Microwave-assisted green extraction technology for sustainable food processing. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; IntechOpen: London, UK, 2018; pp. 159–178. [Google Scholar]
- Sharma, M.; Hussain, S.; Shalima, T.; Aav, R.; Bhat, R. Valorization of seabuckthorn pomace to obtain bioactive carotenoids: An innovative approach of using green extraction techniques (ultrasonic and microwave-assisted extractions) synergized with green solvents (edible oils). Ind. Crops Prod. 2022, 175, 114257. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Gai, Q.-Y.; Jiao, J.; Mu, P.-S.; Wang, W.; Luo, M.; Li, C.-Y.; Zu, Y.-G.; Wei, F.-Y.; Fu, Y.-J. Microwave-assisted aqueous enzymatic extraction of oil from Isatis indigotica seeds and its evaluation of physicochemical properties, fatty acid compositions and antioxidant activities. Ind. Crops Prod. 2013, 45, 303–311. [Google Scholar] [CrossRef]
- Turco, R.; Mallardo, S.; Zannini, D.; Moeini, A.; Di Serio, M.; Tesser, R.; Cerruti, P.; Santagata, G. Dual role of epoxidized soybean oil (ESO) as plasticizer and chain extender for biodegradable polybutylene succinate (PBS) formulations. Giant 2024, 20, 100328. [Google Scholar] [CrossRef]
- Faga, M.G.; Duraccio, D.; Di Maro, M.; Pedraza, R.; Bartoli, M.; d’Ayala, G.G.; Torsello, D.; Ghigo, G.; Malucelli, G. Ethylene-Vinyl Acetate (EVA) Containing Waste Hemp-Derived Biochar Fibers: Mechanical, Electrical, Thermal and Tribological Behavior. Polymers 2022, 14, 4171. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.-M.; Chen, X.-T.; Liu, P.; Wu, S.-Y.; Jiang, Z.; Xiong, B.; Xu, J. Preparation of Poly(butylene succinate) Crystals with Exceptionally High Melting Point and Crystallinity from Its Inclusion Complex. Macromolecules 2017, 50, 5425–5433. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) no 10/2011 Off. J. Eur. Union. L, 12 (2011). Available online: https://eur-lex.europa.eu/eli/reg/2011/10/oj/eng (accessed on 29 April 2025).
- Kalaskar, M.; Yele, S.U.; Ayyanar, M.; Gurav, N.; Beldar, V.; Surana, S.J. Methods of Extraction In Pharmacognosy and Phytochemistry: Principles, Techniques, and Clinical Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2025; pp. 121–142. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The composition of hemp seed oil and its potential as an important source of nutrition. J. Nutraceuticals Funct. Med. Foods 2020, 2, 35–53. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Marín-Aguilar, F.; García-Gimenez, M.D.; Fernández-Arche, M.A. Hemp (Cannabis sativa L.) seed oil: Analytical and phytochemical characterization of the unsaponifiable fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef]
- Kshnyakina, S.I.; Puchkovskaya, G.A. Appeaeance of methylene chain rocking vibrations in the IR spectra of crystalline dicarboxylic acids. J. Appl. Spectrosc. 1980, 32, 56–59. [Google Scholar] [CrossRef]
- Balart, J.F.; Fombuena, V.; Fenollar, O.; Boronat, T.; Sánchez-Nacher, L. Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO). Compos. Part B Eng. 2016, 86, 168–177. [Google Scholar] [CrossRef]
- Gan, Z.; Abe, H.; Kurokawa, H.; Doi, Y. Solid-state microstructures, thermal properties, and crystallization of biodegradable poly(butylene succinate)(PBS) and its copolyesters. Biomacromolecules 2001, 2, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.U.; Reiter, G. (Eds.) Polymer Crystallization—Observations, Concepts and Interpretations; Lecture Notes in Physics; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Barrino, F.; De La Rosa-Ramírez, H.; Schiraldi, C.; López-Martínez, J.; Samper, M.D. Preparation and Characterization of New Bioplastics Based on Polybutylene Succinate (PBS). Polymers 2023, 15, 1212. [Google Scholar] [CrossRef]
- Mallardo, S.; de Vito, V.; Malinconico, M.; Volpe, M.G.; Santagata, G.; di Lorenzo, M.L. Poly(butylene succinate)-based composites containing β-cyclodextrin/d-limonene inclusion complex. Eur. Polym. J. 2016, 79, 82–96. [Google Scholar] [CrossRef]
- de Matos Costa, A.R.; Crocitti, A.; Hecker de Carvalho, L.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of Biodegradable Films Based on Poly(butylene Succinate) (PBS) and Poly(butylene Adipate-co-Terephthalate) (PBAT) Blends. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, K.; Klonos, P.A.; Kyritsis, A.; Mašek, O.; Wurzer, C.; Tsachouridis, K.; Anastasiou, A.D.; Bikiaris, D.N. Synthesis and Study of Fully Biodegradable Composites Based on Poly(butylene succinate) and Biochar. Polymers 2023, 15, 1049. [Google Scholar] [CrossRef]
- Zhang, X.; Yong, Z. Reinforcement effect of poly (butylene succinate)(PBS)-grafted cellulose nanocrystal on toughened PBS/polylactic acid blends. Carbohydr. Polym. 2016, 140, 374–382. [Google Scholar] [CrossRef]
- Mosén, K.; Bäckström, K.; Thalberg, K.; Schaefer, T.; Axelsson, A.; Kristensen, H.G. The apparent plasticizing effect of polyethylene glycol (PEG) on the crystallinity of spray dried lactose/PEG composites. Eur. J. Pharm. Biopharm. 2006, 64, 206–211. [Google Scholar] [CrossRef]
- Fadda, H.M.; Khanna, M.; Santos, J.C.; Osman, D.; Gaisford, S.; Basit, A.W. The use of dynamic mechanical analysis (DMA) to evaluate plasticization of acrylic polymer films under simulated gastrointestinal conditions. Eur. J. Pharm. Biopharm. 2010, 76, 493–497. [Google Scholar] [CrossRef]
- Immergut, E.H.; Herman, F.M. Principles of plasticization. Adv. Chem. 1965, 48, 1–26. [Google Scholar]
- Hammer, C.F. Chapter 17—Polymeric Plasticizers. In Polymer Blends; Paul, D.R., Newman, S., Eds.; Academic Press: Cambridge, MA, USA, 1978; pp. 219–241. ISBN 9780125468022. [Google Scholar] [CrossRef]
- Turco, R.; Zannini, D.; Mallardo, S.; Poggetto, G.D.; Tesser, R.; Santagata, G.; Malinconico, M.; Di Serio, M. Biocomposites based on Poly(lactic acid), Cynara Cardunculus seed oil and fibrous presscake: A novel eco-friendly approach to hasten PLA biodegradation in common soil. Polym. Degrad. Stab. 2021, 188, 109576. [Google Scholar] [CrossRef]
- Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Oz, F.; Khan, M.H.; Roy, S.; Esatbeyoglu, T.; Pratap-Singh, A. Thermal properties of biopolymer films: Insights for sustainable food packaging applications. Food Eng. Rev. 2024, 16, 497–512. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Microwave Food Packaging Material and Package Designs. In Microwave Processing of Foods: Challenges, Advances and Prospects: Microwaves and Food; Springer International Publishing: Cham, Switzerland, 2024; pp. 591–604. [Google Scholar]
- Griffin, R.C. Retortable plastic packaging. In Modern Processing, Packaging and Distribution Systems for Food; Paine, F.A., Ed.; Springer: Boston, MA, USA, 1987. [Google Scholar] [CrossRef]
- Majeed, T.; Dar, A.H.; Pandey, V.K.; Dash, K.K.; Srivastava, S.; Shams, R.; Jeevarathinam, G.; Singh, P.; Echegaray, N.; Pandiselvam, R. Role of additives in starch-based edible films and coating: A review with current knowledge. Prog. Org. Coat. 2023, 181, 107597. [Google Scholar] [CrossRef]
- Bor, Y.; Alin, J.; Hakkarainen, M. Electrospray ionization-mass spectrometry analysis reveals migration of cyclic lactide oligomers from polylactide packaging in contact with ethanolic food simulant. Packag. Technol. Sci. 2012, 25, 427–433. [Google Scholar] [CrossRef]
- Baner, A.L.; Franz, R.; Piringer, O. Alternative fatty food simulants for polymer migration testing. In Food Packaging and Preservation; Mathlouthi, M., Ed.; Springer: Boston, MA, USA, 1994. [Google Scholar] [CrossRef]
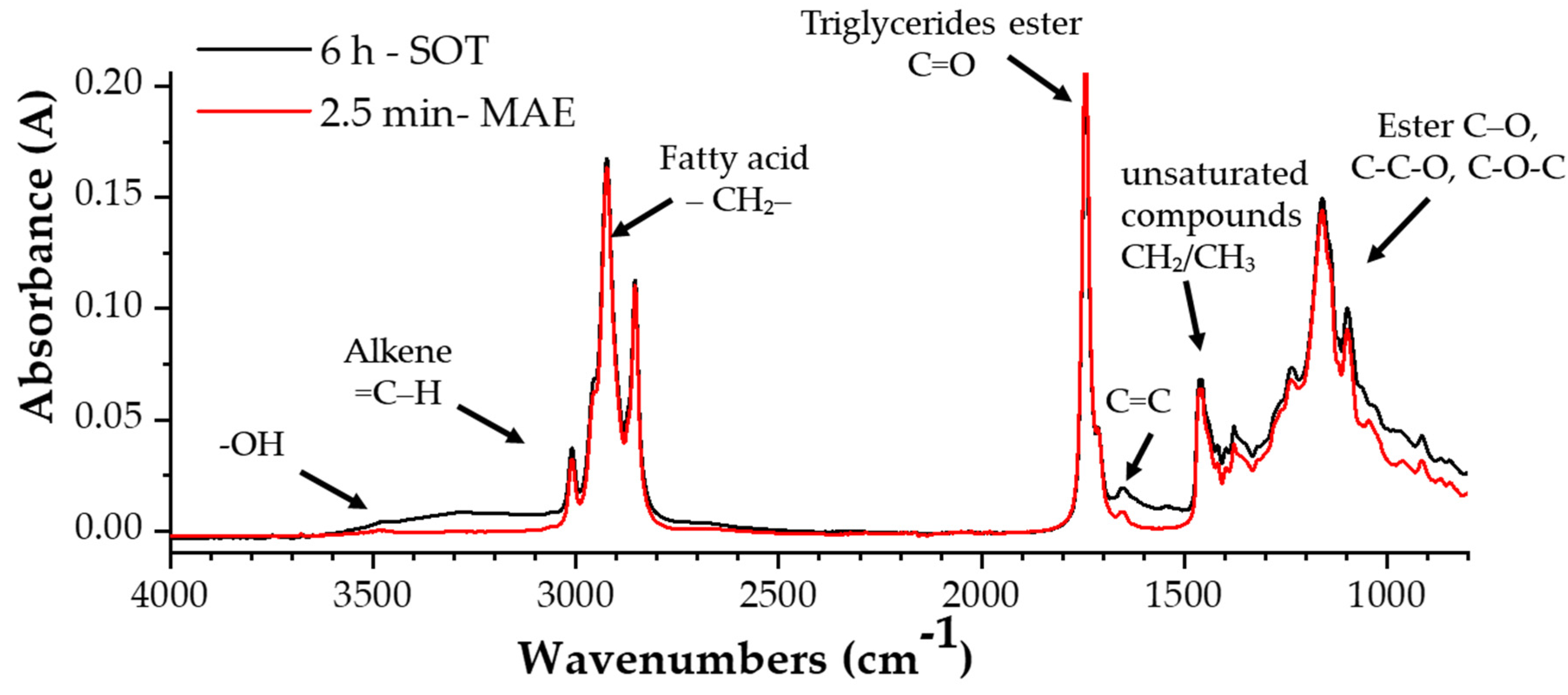

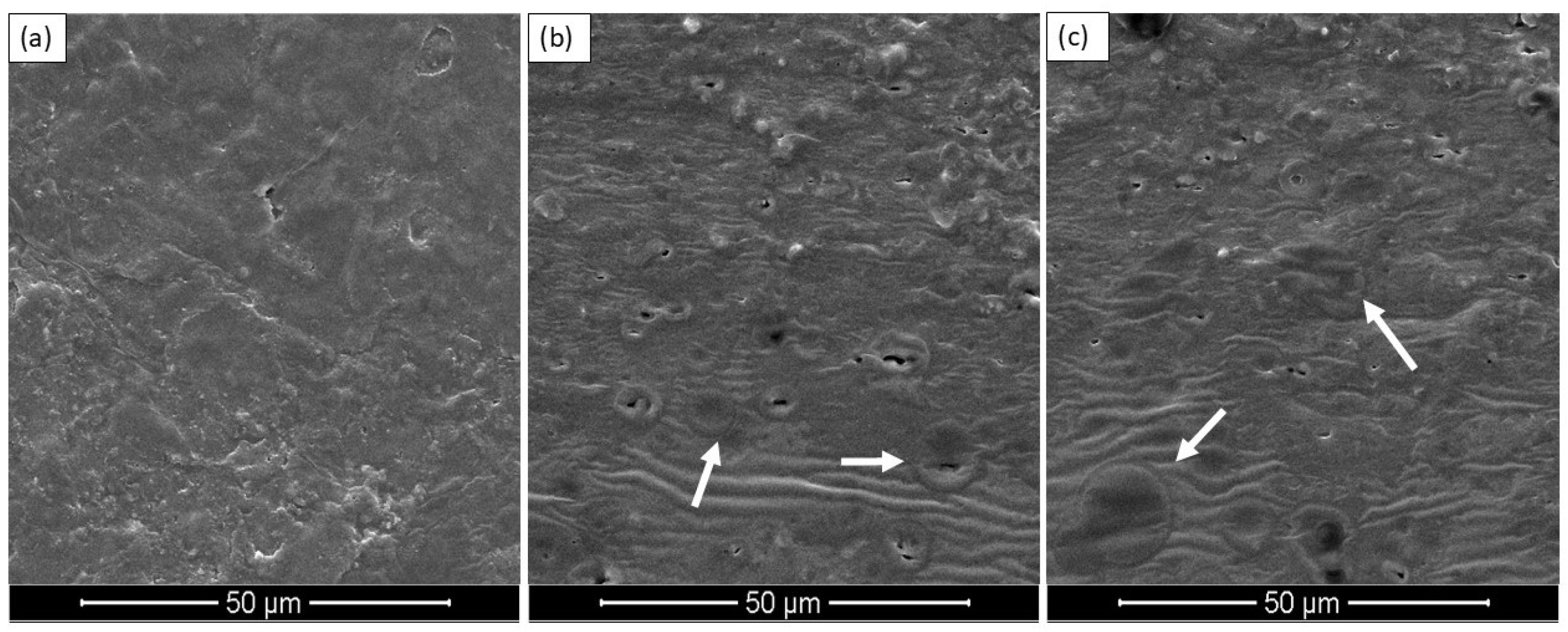
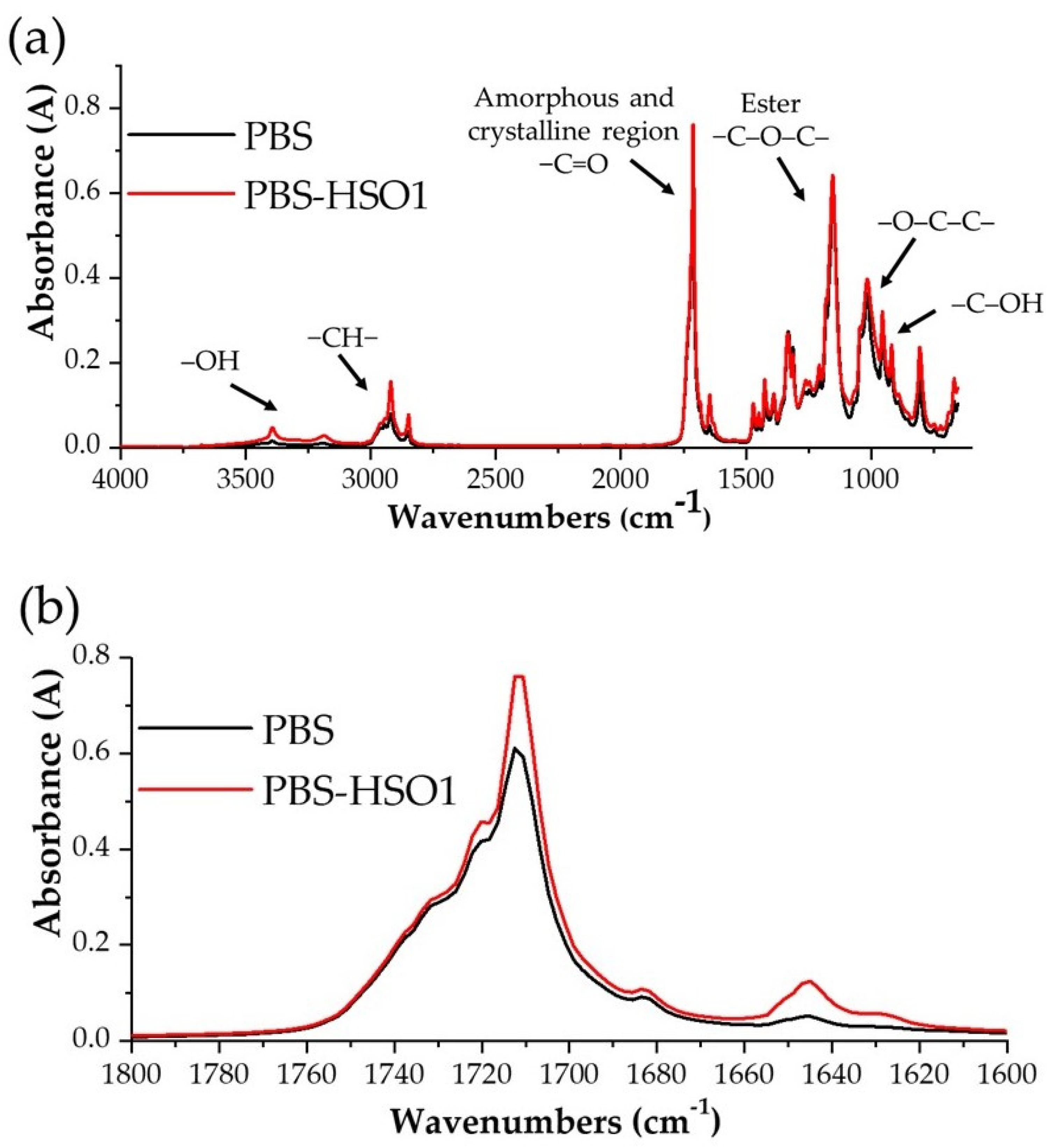


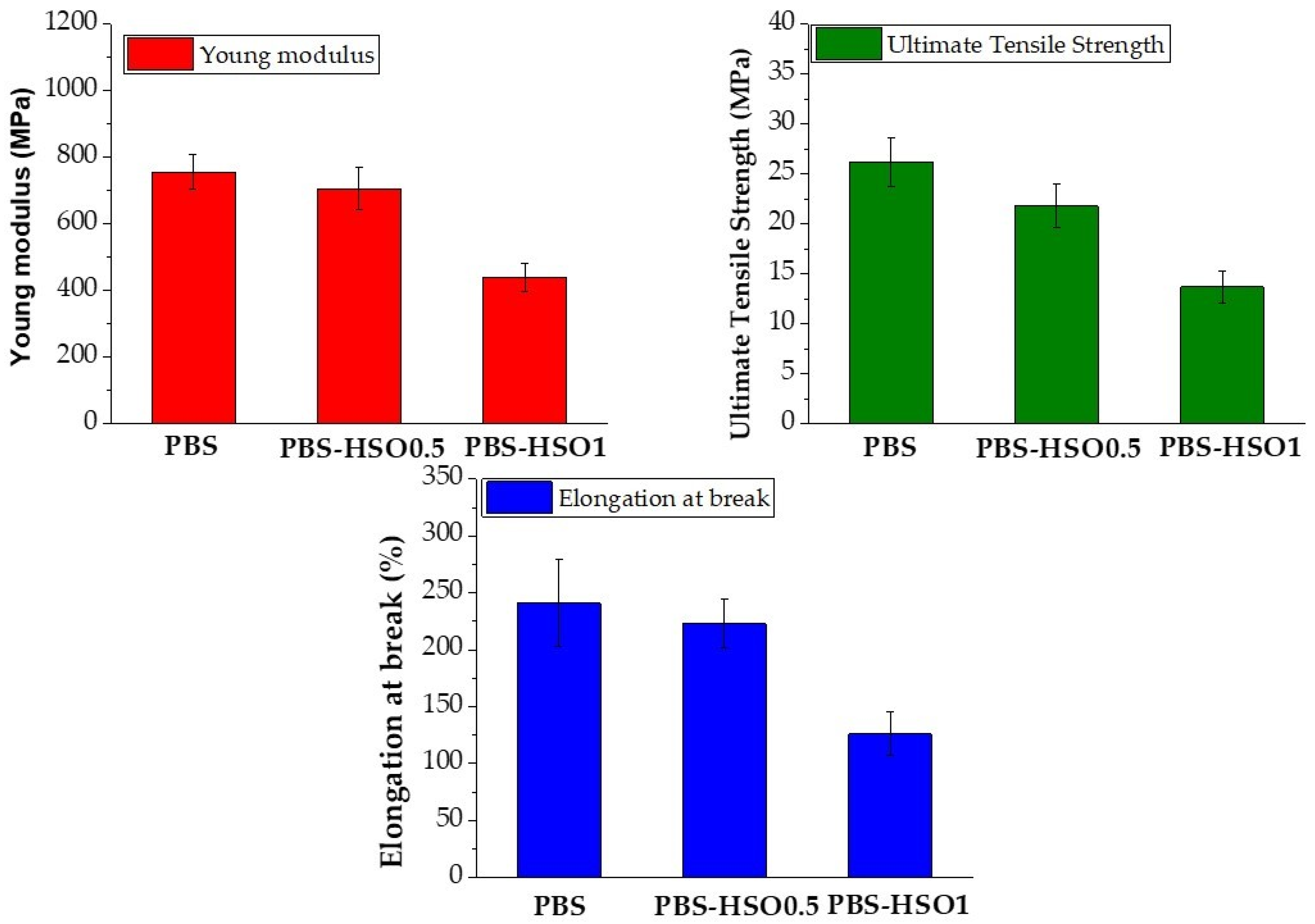

| Extraction Method | Time of Extraction (min) | Yield (%) | DPPH (%) | |
|---|---|---|---|---|
| Initial * | After thermal treatment ** | |||
| MAE | 2.5 | 27 ± 2 | 67 ± 3 | 68 ± 2 |
| MAE | 5 | 26 ± 1 | 68 ± 2 | 67 ± 1 |
| MAE | 15 | 26 ± 1 | 71 ± 2 | 71 ± 3 |
| MAE | 30 | 30 ± 2 | 68 ± 1 | 69 ± 1 |
| MAE | 60 | 30 ± 3 | 68 ± 2 | 69 ± 2 |
| SOT | 360 | 29 ± 3 | 68 ± 1 | 68 ± 1 |
| Sample | Tonset (°C) | Tpeak (°C) |
|---|---|---|
| PBS | 324.7 | 397.2 |
| PBS-HSO0.5 | 301.3 | 393.3 |
| PBS-HSO1 | 301.5 | 391.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Poggetto, G.; Di Maro, M.; Gargiulo, L.; Duraccio, D.; Santagata, G.; Gomez d’Ayala, G. Sustainable Microwave-Assisted Extraction of Hemp Seed Oil as Functional Additive into Polybutylene Succinate (PBS) Films for Food Packaging. Polymers 2025, 17, 1376. https://doi.org/10.3390/polym17101376
Dal Poggetto G, Di Maro M, Gargiulo L, Duraccio D, Santagata G, Gomez d’Ayala G. Sustainable Microwave-Assisted Extraction of Hemp Seed Oil as Functional Additive into Polybutylene Succinate (PBS) Films for Food Packaging. Polymers. 2025; 17(10):1376. https://doi.org/10.3390/polym17101376
Chicago/Turabian StyleDal Poggetto, Giovanni, Mattia Di Maro, Luca Gargiulo, Donatella Duraccio, Gabriella Santagata, and Giovanna Gomez d’Ayala. 2025. "Sustainable Microwave-Assisted Extraction of Hemp Seed Oil as Functional Additive into Polybutylene Succinate (PBS) Films for Food Packaging" Polymers 17, no. 10: 1376. https://doi.org/10.3390/polym17101376
APA StyleDal Poggetto, G., Di Maro, M., Gargiulo, L., Duraccio, D., Santagata, G., & Gomez d’Ayala, G. (2025). Sustainable Microwave-Assisted Extraction of Hemp Seed Oil as Functional Additive into Polybutylene Succinate (PBS) Films for Food Packaging. Polymers, 17(10), 1376. https://doi.org/10.3390/polym17101376








