Optimizing Anticorrosion Coating Performance: Synthesis of Polyurethane/Epoxy Hybrids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
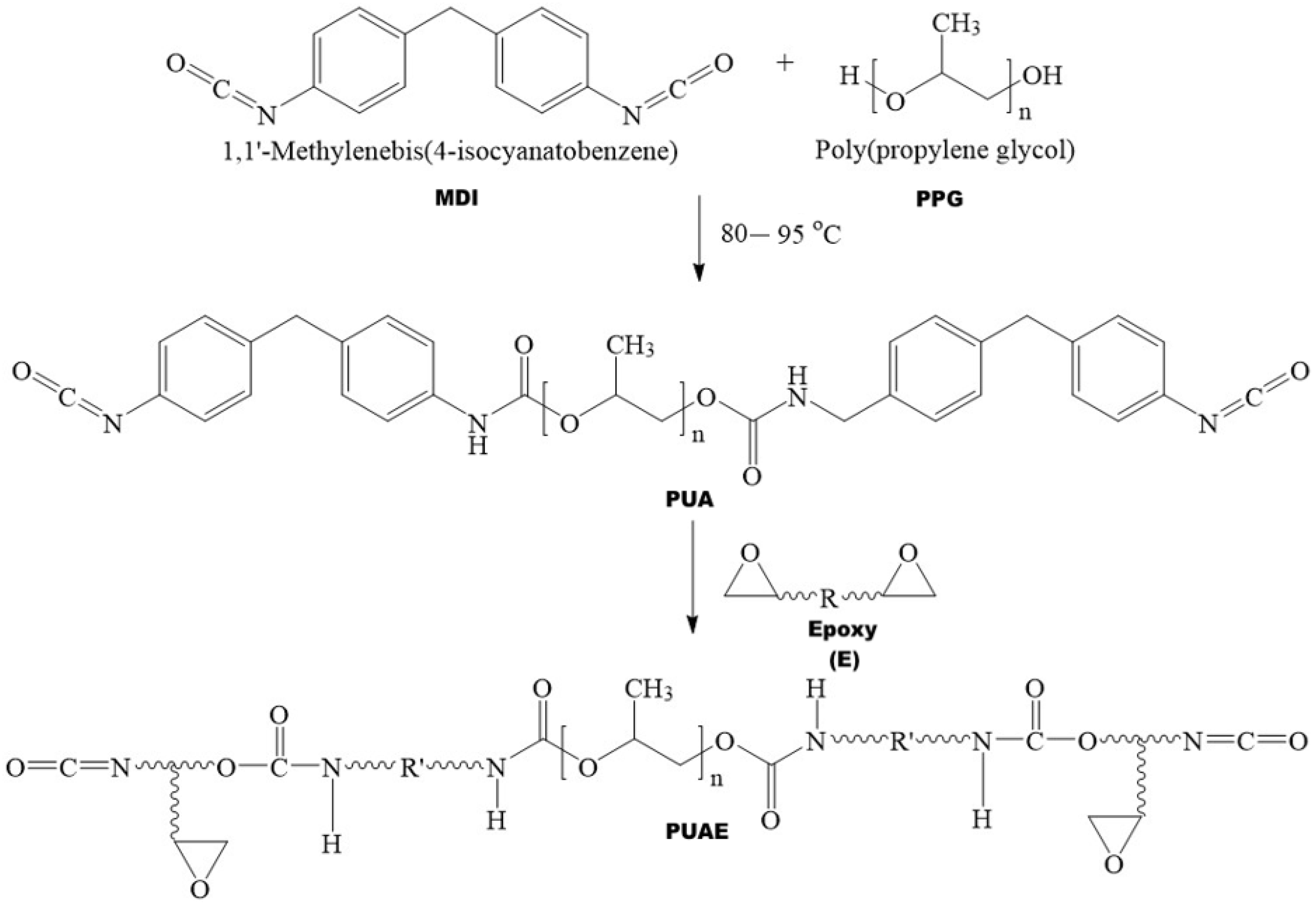
2.2. Synthesis of the Prepolymer Polyurethane (PUA)
2.3. Preparation of Polyurethane Coatings (PUAC and PUAEC)
2.4. Film Coating Preparation
2.5. Characterization of the Prepolymer Polyurethane (PUA and PUAE)
2.6. Mechanical Tests for PUA, PUAE, PUAC, and PUAEC
2.7. Corrosion Resistance Tests for PUAC and PUAEC
3. Results and Discussion
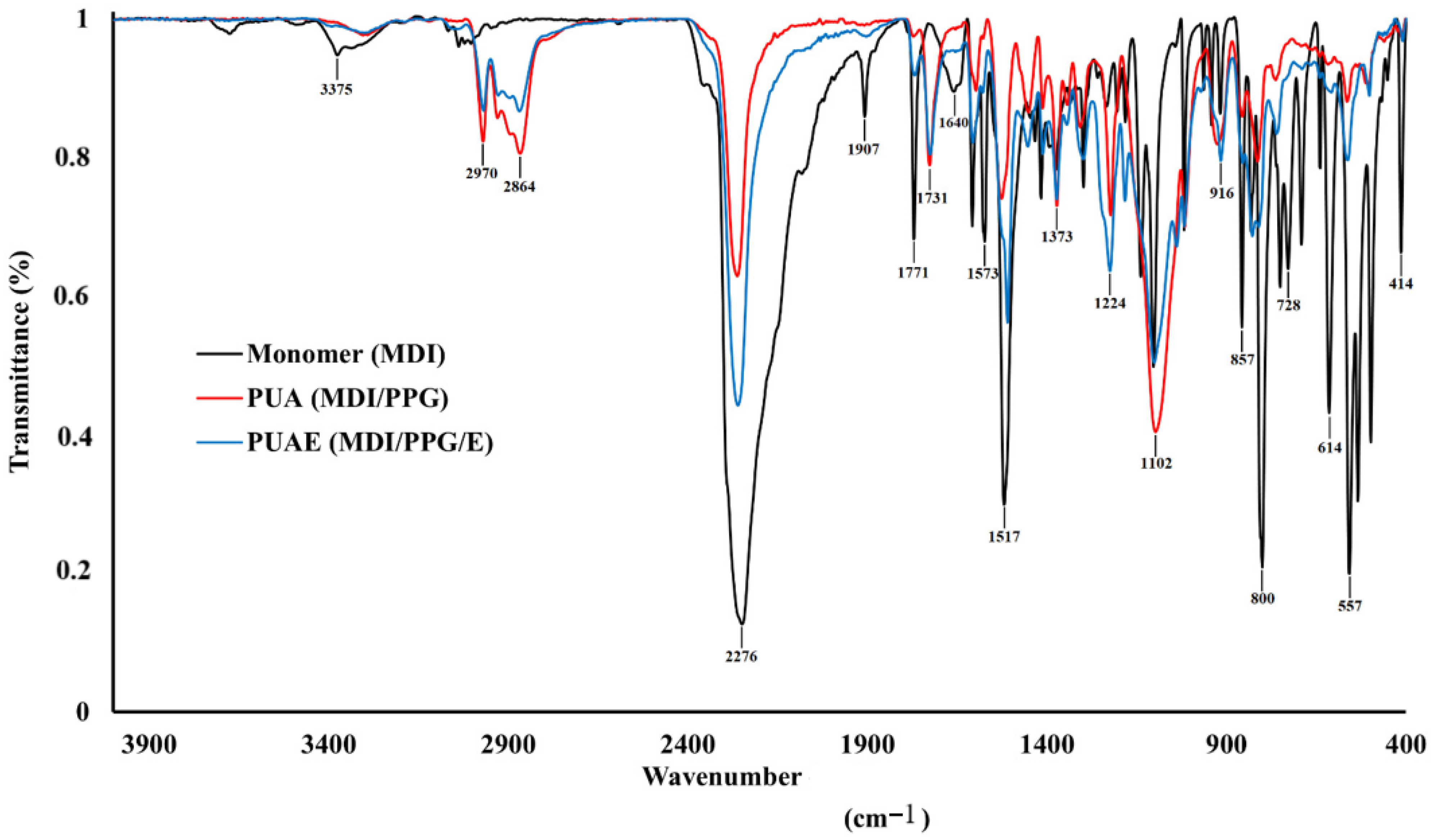
3.1. FTIR Analysis
3.2. TGA Analysis
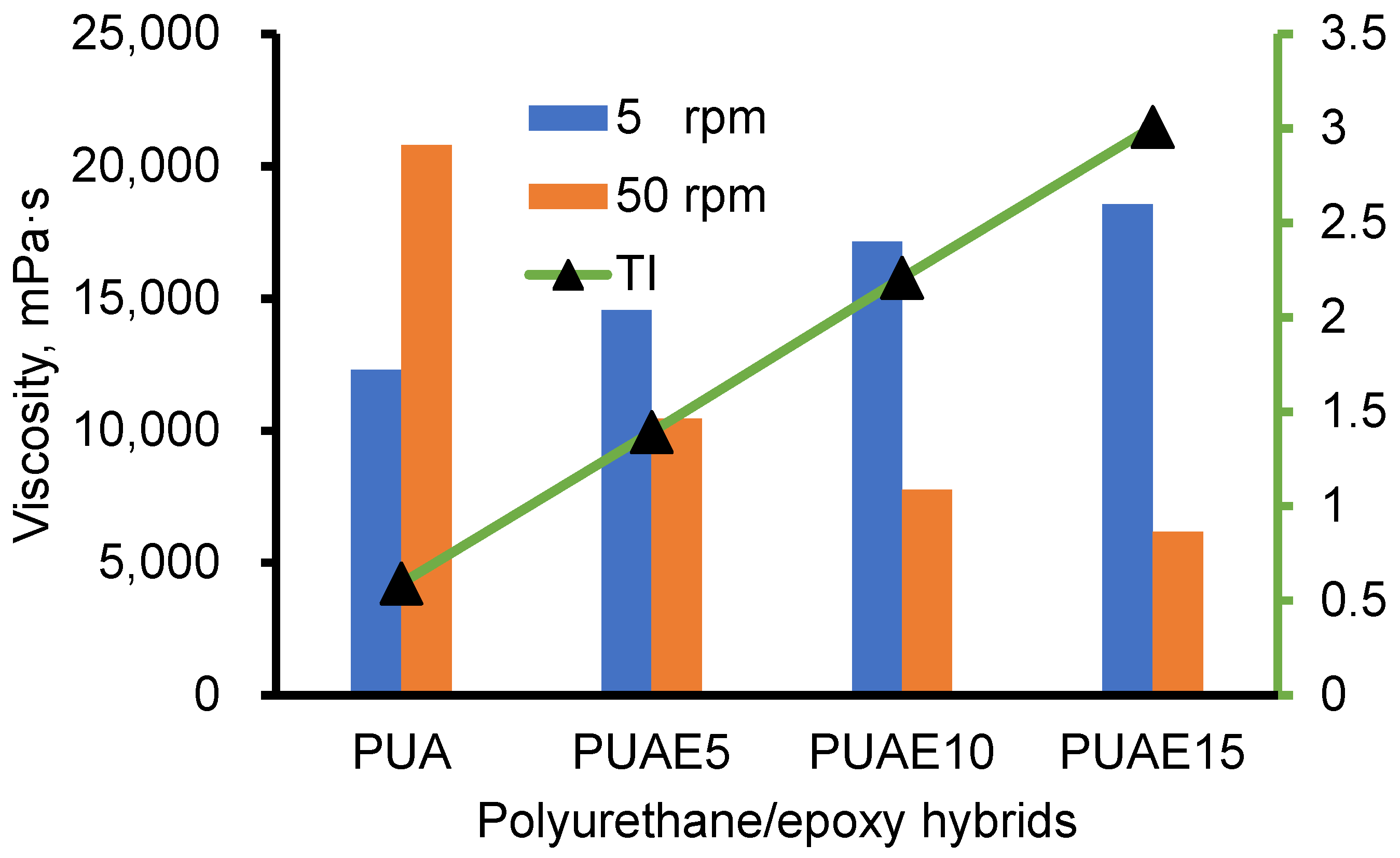
3.3. Viscosity of PUA and PUAE
3.4. Mechanical Properties
3.5. Coating Properties
Drying Time and Mechanical Properties
3.6. Chemical and Corrosion Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Janik, H.; Sienkiewicz, M.; Kucinska-Lipka, J. Polyurethanes. In Handbook of Thermoset Plastics; William Andrew Publishing: Norwich, NY, USA, 2014; pp. 253–295. [Google Scholar]
- Maurya, A.K.; de Souza, F.M.; Gupta, R.K. Polyurethane and Its Composites: Synthesis to Application. ACS Symp. Series 2023, 1452, 1–20. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Ashrafizadeh, H.; Mertiny, P.; McDonald, A. Evaluation of the effect of temperature on mechanical properties and wear resistance of polyurethane elastomers. Wear 2016, 368–369, 26–38. [Google Scholar] [CrossRef]
- Jin, F.-L.; Xiang, L.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Gul, S.; Kausar, A.; Mehmood, M.; Muhammad, B.; Jabeen, S. Progress on Epoxy/Polyamide and Inorganic Nanofiller-Based Hybrids: Introduction, Application, and Future Potential. Polym.-Plast. Technol. Eng. 2016, 55, 1842–1862. [Google Scholar] [CrossRef]
- Mirzaee1, M.; Abadchi, M.; Fateh, A.; Zolriasatein, A. Investigation of Corrosion Properties of Modified Epoxy and Polyurethane Organic Coating on Steel Substrate. Prog. Color Color. Coat. 2022, 15, 25–36. Available online: https://pccc.icrc.ac.ir/article_81741_5a9b0ce1e44cef228834e10d4f8fbb63.pdf (accessed on 15 April 2025).
- Tang, Y.; Cao, J.; Qu, S.; Quan, L.; Zhao, X.; Zuo, Y. Degradation of a High Build Epoxy Primer/Polyurethane Composite Coatings under Cyclic Wet–dry Conditions. Int. J. Electrochem. Sci. 2018, 13, 3874–3887. [Google Scholar] [CrossRef]
- Wang, T.; Li, R.; Segura, J.J.; Graversen, E.; Weinell, C.E.; Dam-Johansen, K.; Kiil, S. Interlayer molecular migration and reaction in an epoxy-polyurethane coating system: Implications for the system hardness. Prog. Org. Coat. 2021, 151, 106083. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A.; Dagdag, O.; El Gouri, M.; Sherif, E.-S.M.; Ebenso, E.E. Epoxy resins as anticorrosive polymeric materials: A review. React. Funct. Polym. 2020, 156, 104741. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Ou, B.; Wang, Y.; Lu, Y. A review on fundamentals and strategy of epoxy-resin-based anticorrosive coating materials. Polym.-Plast. Technol. Mater. 2020, 60, 601–625. [Google Scholar] [CrossRef]
- Mišković-Stanković, V.B.; Zotović, J.B.; Maksimović, M.D. Corrosion Behaviour of Epoxy Coatings Investigated by EIS. Mater. Sci. Forum 1998, 289–292, 327–336. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, Q. Relationship between ion transport and the failure behavior of epoxy resin coatings. Corros. Sci. 2014, 78, 22–28. [Google Scholar] [CrossRef]
- Mushtaq, M.; Adusumalli, R.B.; Suresh, K.; Anna Abraham, A. Adhesion and tribological characteristics of modified polyurethane coating on composite substrate. Surf. Eng. 2023, 39, 836–851. [Google Scholar] [CrossRef]
- Zubielewicz, M.; Królikowska, A. The influence of ageing of epoxy coatings on adhesion of polyurethane topcoats and protective properties of coating systems. Prog. Org. Coat. 2009, 66, 129–136. [Google Scholar] [CrossRef]
- Bahramnia, H.; Mohammadian Semnani, H.; Habibolahzadeh, A.; Abdoos, H. Epoxy/polyurethane nanocomposite coatings for anti-erosion/wear applications: A review. J. Comp. Mater. 2020, 54, 3189–3203. [Google Scholar] [CrossRef]
- Agavriloaie, L.; Oprea, S.; Barbuta, M.; Luca, F. Characterisation of polymer concrete with epoxy polyurethane acryl matrix. Constr. Build. Mater. 2012, 37, 190–196. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Bae, J.C.; Choi, J.W.; Chun, B.C. The Preparation and Characterization of an Epoxy Polyurethane Hybrid Polymer Using Bisphenol A and Epichlorohydrin. Fibers Polym. 2020, 21, 447–455. [Google Scholar] [CrossRef]
- Chen, S.; Tian, Y.; Chen, L.; Hu, T. Epoxy Resin/Polyurethane Hybrid Networks Synthesized by Frontal Polymerization. Chem. Mater. 2006, 18, 2159–2163. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Li, X.; Zhang, J.; Zhao, J. High-performance epoxy hybrid non-isocyanate polyurethanes prepared from diol-cyclocarbonation bisphenol A dicyclocarbonate. Polym. Eng. Sci. 2023, 63, 3025–3036. [Google Scholar] [CrossRef]
- Chen, Y.F.; Dai, Q.W.; Lin, C.W.; Feng, T. Characteristics and properties of SiO2-Al2O3/EP-PU composite. J. Cent. South Univ. 2014, 21, 4076–4083. [Google Scholar] [CrossRef]
- Song, Z.; Xie, J.; Zhou, P.; Peng, J.; Wang, X.; Deng, L. Thermal degradation of epoxy resin grafted with polyurethane. Sci. Eng. Compos. Mater. 2014, 21, 7–13. [Google Scholar] [CrossRef]
- Ghozali, M.; Triwulandari, E.; Haryono, A. Preparation and Characterization of Polyurethane-Modified Epoxy with Various Types of Polyol. Macromol. Symp. 2015, 353, 154–160. [Google Scholar] [CrossRef]
- Bahramnia, H.; Semnani, H.M.; Habibolahzadeh, A.; Abdoos, H. Epoxy/polyurethane hybrid nanocomposite coatings reinforced with MWCNTs and SiO2 nanoparticles: Processing, mechanical properties and wear behavior. Surf. Coat. Technol. 2021, 415, 127121. [Google Scholar] [CrossRef]
- Kausar, A. Interpenetrating polymer network and nanocomposite IPN of polyurethane/epoxy: A review on fundamentals and advancements. Polym.-Plast. Technol. Mater. 2019, 58, 691–706. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Wang, F.; Jiang, S.; Kang, M.; Wang, J.; Li, Q.; Wang, Z. Non-isocyanate polyurethane/epoxy hybrid materials with different and controlled architectures prepared from a CO2-sourced monomer and epoxy via an environmentally-friendly route. RSC Adv. 2017, 7, 28841–28852. [Google Scholar] [CrossRef]
- Ling, Z.; Zhang, C.; Zhou, Q. Synthesis and characterization of 1K waterborne non-isocyanate polyurethane epoxy hybrid coating. Prog. Org. Coat. 2022, 169, 106915. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Wang, Y.; Zhao, J. Epoxy-free synthesis of aromatic dicyclocarbonates and the related strong epoxy hybrid non-isocyanate polyurethanes. Mater. Today Commun. 2023, 34, 105263. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, S.-R.; Lee, D.-S. Self-healing of cross-linked PU via dual-dynamic covalent bonds of a Schiff base from cystine and vanillin. Mater. Design. 2019, 172, 107774. [Google Scholar] [CrossRef]
- ASTM D2572-19; Standard Test Methods for Isocyanate Group in Urethane Materials or Prepolymer. American Society for Testing and Materials (ASTM): Conshohocken, PA, USA, 2019.
- ASTM D4147-18; Outlines a Standard Procedure for Applying Uniform Film Coatings on Flat Panels Using a Wire-Wound. American Society for Testing and Materials (ASTM): Conshohocken, PA, USA, 2018.
- ISO 12058-1; International Standard: Determination of Viscosity Using a Falling-Ball Viscometer. ISO: Geneva, Switzerland, 2018.
- ASTM D2240-15; Standard Test Method for Rubber Property-Durometer Hardness. American Society for Testing and Materials (ASTM): Conshohocken, PA, USA, 2015.
- ASTM D522; Standard Test Methods for Mandrel Bend Test of Attached Organic Coatings. American Society for Testing and Materials (ASTM): Conshohocken, PA, USA, 2001.
- ASTM D2794; Standard Test Methods for Resistance of Organic Coatings to the Effects of Rapid Deformation (Impact). American Society for Testing and Materials (ASTM): Conshohocken, PA, USA, 2019.
- ASTM D3359; Standard Test Methods for Rating Adhesion by Tap Test. American Society for Testing and Materials (ASTM): Conshohocken, PA, USA, 2001.
- BS EN 1542; Standard Test Method, Measurement Of Bond Strength By Pull-Off. British Standards Institution (BSI): London, UK, 1999.
- ASTM D5402-19; Standard Practice for Assessing the Solvent Resistance of Organic Coatings Using Solvent Rubs. American Society for Testing and Materials (ASTM): Conshohocken, PA, USA, 2019.
- ASTM D1647-89; Standard Test Method for Resistance of Dried Films of Varnishes to Water and Alkali. American Society for Testing and Materials (ASTM): Conshohocken, PA, USA, 2017.
- Xiaojuan, L.; Peiyan, S.; Lei, W. Preparation and Properties of Epoxy-modified Waterborne Polyurethane/polyacrylate Composite Emulsion with the Action of Polmerizable Emulsifier. J. Appl. Sci. Eng. 2017, 20, 87–94. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Huang, Z.; Cai, C.; Tusiime, R.; Li, Z.; Wang, H.; Cheng, C.; Liu, Y.; Sun, Z.; et al. Synergistic toughen epoxy resin by incorporation of polyetherimide and amino groups grafted MWCNTs. Comp. Commun. 2020, 21, 100377. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Zhang, P.; Xue, K.; Yao, T.; Liu, L.; Huang, Y. POSS-polyurethane prepolymer strengthened and toughened CF/epoxy resin composites for room and simulated Arctic ambient temperature. Polymer 2024, 294, 126692. [Google Scholar] [CrossRef]
- Velayati, M.; Sabouri, Z.; Masoudi, A.; Mostafapour, A.; Khatami, M.; Darroudi, M. Thermal Stability Investigation of Synthesized Epoxy-Polyurethane/Silica Nanocomposites. Silicon 2022, 14, 7541–7554. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Malitowski, N.M.; Zamboulis, A.; Friebel, S.; Bikiaris, D.; Robert, T. Influence of bio-based 2,5-furandicarboxylic acid on the properties of water-borne polyurethane dispersions. React. Func. Polym. 2023, 190, 105622. [Google Scholar] [CrossRef]
- Kluge, M.; Bikiaris, D.N.; Robert, T. Enhancing the properties of poly(propylene succinate) by the incorporation of crystallizable symmetrical amido diols. Eur. Polym. J. 2019, 120, 109195. [Google Scholar] [CrossRef]
- Volkova, E.R.; Savchuk, A.V.; Slodobenyuk, A.I.; Strel’nikov, V.N. Rheological Properties of Epoxy Urethane Oligomers and Curing Kinetics of Polymer Composites on Their Basis. Inorg. Mater. Appl. Res. 2020, 11, 147–153. [Google Scholar] [CrossRef]
- Deka, A.; Dey, N. Rheological studies of two component high build epoxy and polyurethane based high performance coatings. J. Coat. Technol. Res. 2013, 10, 305–315. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.; Jang, S.; Park, J.; Choi, J.; Lee, S.; Jung, J.; Park, J. Exploring the Effect of the Polyol Structure and the Incorporation of Lignin on the Properties of Bio-Based Polyurethane. Polymers 2025, 17, 604. [Google Scholar] [CrossRef]
- Bratasyuk, N.A.; Zuev, V.V. The effect molecular weight of polyol components on shape memory effect of epoxy-polyurethane composites. Polym. Eng. Sci. 2021, 61, 2674–2690. [Google Scholar] [CrossRef]
- Białkowsk, A.; Bakar, M.; Przybyłek, M. Effect of Nonisocyanate Polyurethane and Nanoclay on the Mechanical Properties of an Epoxy Resin. Mech. Comp. Mater. 2018, 54, 665–674. [Google Scholar] [CrossRef]
- Ismail, E.A.; Motawie, A.M.; Sadek, E.M. Synthesis and characterization of polyurethane coatings based on soybean oil–polyester polyols. Egypt. J. Pet. 2011, 20, 1–8. [Google Scholar] [CrossRef]
- Doley, S.; Sarmah, A.; Sarkar, C.; Dolui, S.K. In situ development of bio-based polyurethane-blend-epoxy hybrid materials and their nanocomposites with modified graphene oxide via non-isocyanate route. Polym. Int. 2018, 67, 1062–1069. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, P.; Zhao, Z.; Sun, A.; Wei, L.; Zhu, J.; Li, Y. Co-enhancement of toughness and strength of room-temperature curing epoxy adhesive derived from hydroxyl-terminated polybutadiene based polyurethane resin. Eur. Polym. J. 2024, 219, 113373. [Google Scholar] [CrossRef]
- Kausar, A. Polyurethane/Epoxy Interpenetrating Polymer Network. In Aspects of Polyurethanes; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Kausar, A. Polyurethane Composite Foams in High-Performance Applications: A Review. Polym.-Plast. Technol. Eng. 2017, 57, 346–369. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Ma, J.; Huang, L.; Huang, L.; Chen, X.; Zeng, H.; Ma, S. Preparation and Properties of Corrosion-Resistant Coatings from Waterborne Polyurethane Modified Epoxy Emulsion. Front. Mater. 2019, 6, 185. [Google Scholar] [CrossRef]



| Samples | PUA | PUAE5 | PUAE10 | PUAE15 | ||||
|---|---|---|---|---|---|---|---|---|
| Wt (g) | Wt (%) | Wt (g) | Wt (%) | Wt (g) | Wt (%) | Wt (g) | Wt (%) | |
| Polyols, OH | ||||||||
| PP-1200 | 30.64 | 20.32 | 30.64 | 22,21 | 30.64 | 24.10 | 30.64 | 25.98 |
| PP-2000 | 66.39 | 44.03 | 46.73 | 33.87 | 30.05 | 23.63 | 15.85 | 13.44 |
| PP-2700 | 13.78 | 9.15 | 13.78 | 9.99 | 13.78 | 10.85 | 13.78 | 11.69 |
| E | 0.0 | 0.0 | 6882 | 5.0 | 12.72 | 10.00 | 17.69 | 15.00 |
| Mole of OH (gm/mole) | 0.06384 | 0.06384 | 0.06384 | 0.06384 | ||||
| Isocyanate, NCO | ||||||||
| MDI | 39.93 | 26.50 | 39.93 | 28.94 | 39.93 | 31.41 | 39.93 | 33.87 |
| Total | 150.74 | 100 | 137.98 | 100 | 127.15 | 100 | 117.91 | 100 |
| Mole of NCO (gm/mole) | 0.1596 | 0.1596 | 0.1596 | 0.1596 | ||||
| NCO/OH | 2.5 | 2.5 | 2.5 | 2.5 | ||||
| Raw Materials | Weight Percent |
|---|---|
| Prepolymer PUA or PUAE | 35 |
| Rheological agent | 0.5 |
| Titanium dioxide | 30 |
| Anti-settling additive | 1.5 |
| Dispersion agent | 0.03 |
| Pigment | 10.22 |
| Xylene | 15 |
| Butanol | 6 |
| DBTDL | 1.75 |
| Samples | Stages | Decomposition Temperature, °C | Weight Percentage, % | PDTmax (°C) | |
|---|---|---|---|---|---|
| T1 | T2 | Δ W | |||
| PUA | 1 | 29 | 150 | 2.7 | 320 |
| 2 | 150 | 545 | 92 | ||
| 3 | 545 | 819 | 3.6 | ||
| PUAE5 | 1 | 29 | 160 | 3.1 | 320 |
| 2 | 160 | 535 | 91.5 | ||
| 3 | 535 | 845 | 5.1 | ||
| PUAE10 | 1 | 30 | 174 | 3.7 | 310 |
| 2 | 174 | 407 | 80.2 | ||
| 3 | 407 | 897 | 15.1 | ||
| PUAE15 | 1 | 39 | 786 | 99.9 | 390 |
| PUA | PUAE5 | PUAE10 | PUAE15 | |
|---|---|---|---|---|
| Tensile strength (MPa) | 39.1 ± 1.18 | 53.5 ± 1.28 *** | 64.9 ± 1.22 **** | 86.3 ± 1.65 **** |
| Elongation (%) | 158 ± 3.61 | 130 ± 4.58 *** | 112 ± 4.00 **** | 95 ± 4.58 **** |
| Hardness (shore A) | 68.3 ± 1.15 | 81.3 ± 0.58 **** | 87.3 ± 1.53 **** | 98.0 ± 1.73 **** |
| Adhesion (MPa) | 2.5 ± 0.3 | 3.8 ± 0.4 ** | 5.9 ± 0.3 *** | 8.3 ± 0.4 **** |
| CPUA | CPUAE5 | CPUAE10 | CPUAE15 | |
|---|---|---|---|---|
| Pot Life and Dry Time | ||||
| Pot life (25 °C, min) | 105 | 80 | 68 | 51 |
| Dry time (set to touch), h | 13 | 9 | 8.5 | 7 |
| Dry time (dry hard), h | 22 | 18 | 17 | 15 |
| Mechanical Properties | ||||
| Tensile strength (N/m2) | 62 | 79 | 86 | 94 |
| Elongation (%) | 243 | 221 | 205 | 197 |
| Adhesion (MPa) | 7.2 | 8.9 | 9.9 | 11.1 |
| Crosshatch (%) | 100 | 100 | 100 | 100 |
| Impact resistance (1 m/1 kg) | F | P | P | P |
| Hardness (Shore A) | 77 | 85 | 91 | 96 |
| Conical-Mandrel (¼″) | P | P | P | P |
| Contact angle | 105 | 127 | 135 | 149 |
| Corrosion resistance | ||||
| NaCl (10%) | O | O | O | O |
| NaOH (1.0 M) | O | O | O | O |
| HCl (1.0 M) | X | O | O | O |
| H2SO4 (1.0 M) | X | Δ | O | O |
| HNO3 (1.0 M) | X | Δ | O | O |
| Water | O | O | O | O |
| Chemical resistance | ||||
| Acetone | Δ | O | O | O |
| Xylene | X | O | O | O |
| Toluene | X | O | O | O |
| Benzene | X | Δ | O | O |
| Isopropyl alcohol | X | Δ | Δ | O |
| Chloroform | X | O | O | O |
| Cyclohexane | O | O | O | O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekbayeva, L.; Negim, E.-S.; Al Azzam, K.M.; Zhanibekov, R.; Yeligbayeva, G.; Moldabayeva, G.Z.; Ewies, E.F. Optimizing Anticorrosion Coating Performance: Synthesis of Polyurethane/Epoxy Hybrids. Polymers 2025, 17, 1516. https://doi.org/10.3390/polym17111516
Bekbayeva L, Negim E-S, Al Azzam KM, Zhanibekov R, Yeligbayeva G, Moldabayeva GZ, Ewies EF. Optimizing Anticorrosion Coating Performance: Synthesis of Polyurethane/Epoxy Hybrids. Polymers. 2025; 17(11):1516. https://doi.org/10.3390/polym17111516
Chicago/Turabian StyleBekbayeva, Lyazzat, El-Sayed Negim, Khaldun M. Al Azzam, Rinat Zhanibekov, Gulzhakhan Yeligbayeva, Gulnaz Zhaksylykovna Moldabayeva, and Ewies F. Ewies. 2025. "Optimizing Anticorrosion Coating Performance: Synthesis of Polyurethane/Epoxy Hybrids" Polymers 17, no. 11: 1516. https://doi.org/10.3390/polym17111516
APA StyleBekbayeva, L., Negim, E.-S., Al Azzam, K. M., Zhanibekov, R., Yeligbayeva, G., Moldabayeva, G. Z., & Ewies, E. F. (2025). Optimizing Anticorrosion Coating Performance: Synthesis of Polyurethane/Epoxy Hybrids. Polymers, 17(11), 1516. https://doi.org/10.3390/polym17111516






