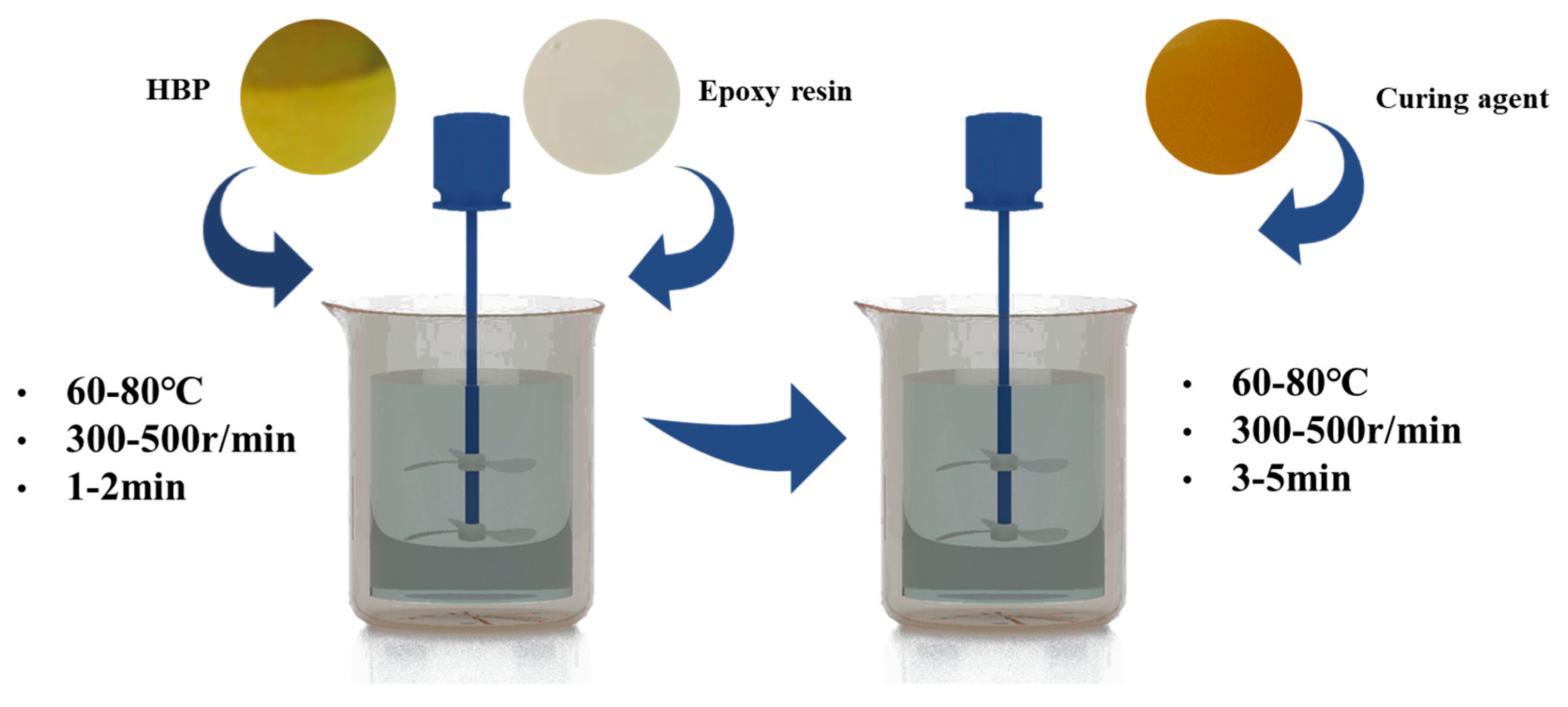
3.1.1. Non-Isothermal Curing Kinetics
The curing behavior of epoxy resin is markedly influenced by variations in curing agent and hyperbranched polymer (HBP) content, which subsequently impacts its performance characteristics [
14,
26].
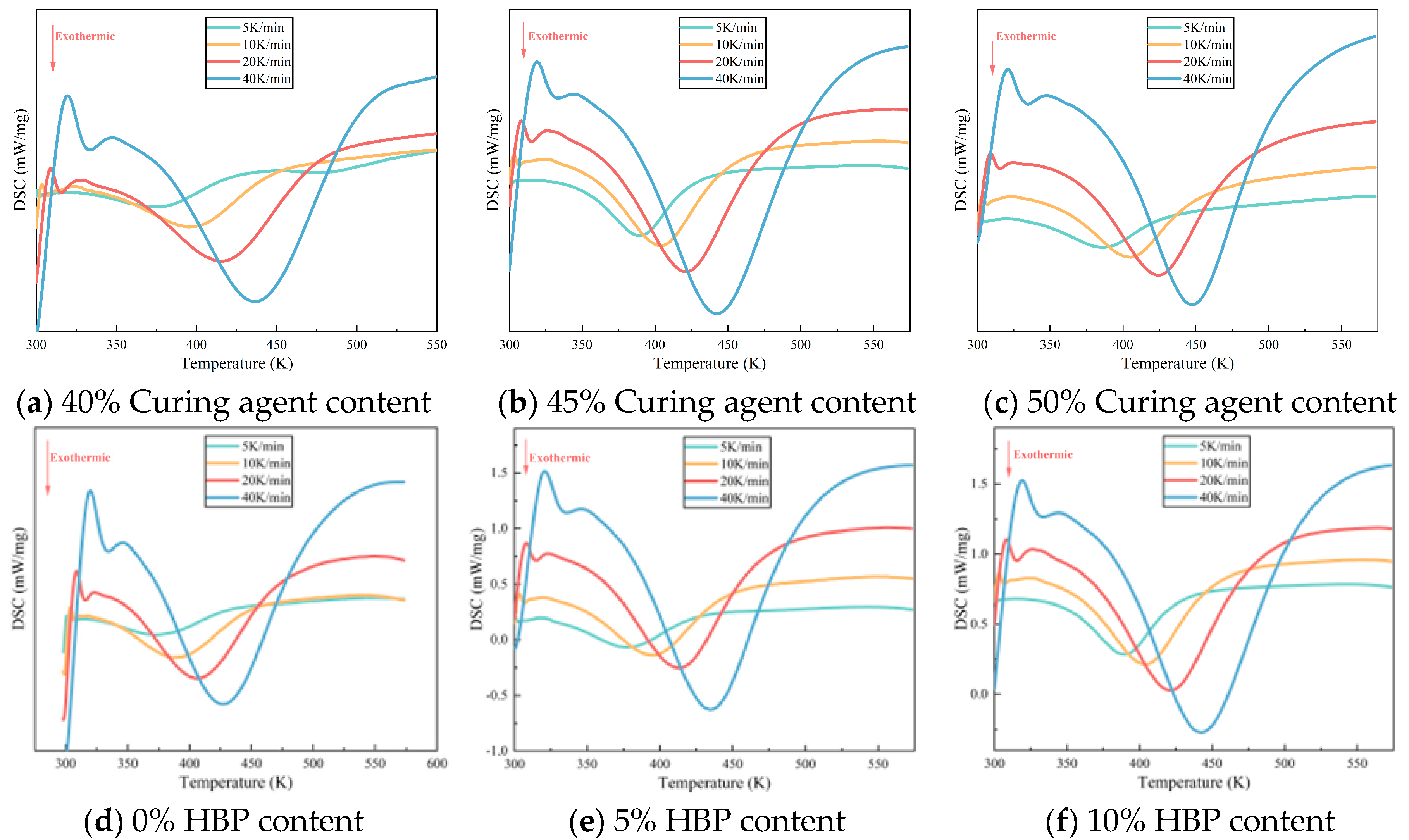
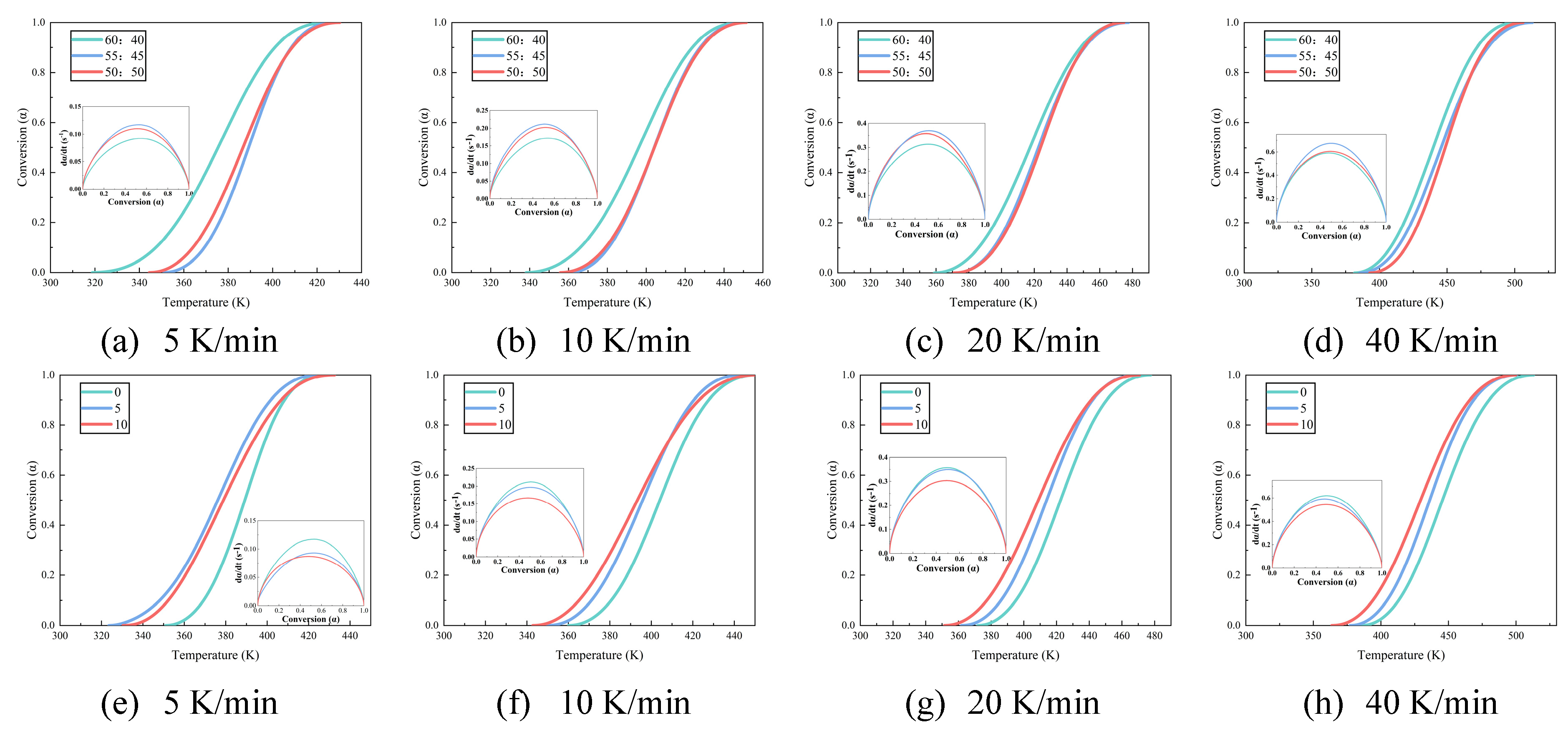
Figure 2 illustrates the differential scanning calorimetry (DSC) curves for epoxy resins with differing proportions of curing agent and HBP. As the curing process for epoxy resin is exothermic, an increase in heating rate shifts the onset, peak, and end temperatures of the exothermic peaks to higher values, resulting in a sharper exothermic profile and a reduction in curing time. This shift is primarily driven by two factors: (1) the increased heating rate augments thermal input per unit time, intensifying thermal lag and thereby shifting the reaction peak to higher temperatures, and (2) the elevated heating rate accelerates the curing reaction by increasing the reaction temperature, which shortens the curing time.
To independently assess the effects of curing agent content and hyperbranched polymer (HBP) incorporation on the properties of epoxy resins,
Figure 2a–c present data from samples formulated without HBP (0 wt%), thereby isolating the influence of varying curing agent concentrations. Conversely,
Figure 2d,e correspond to samples in which the curing agent content is fixed at 45 wt%, enabling evaluation of the effects associated solely with changes in HBP content. No further elaboration is required, as the following text remains consistent. The curing agent and HBP content in each sample is shown in
Table 2.
Figure 2a–c demonstrate that, under a constant heating rate (such as 40 K/min), an increase in curing agent content results in a higher peak temperature on the DSC curve, indicating a requirement for extended curing time and additional heat input to achieve an equivalent degree of curing. This suggests that the activation energy barrier for the curing reaction rises with increased curing agent content. An excess of curing agent, particularly amines, can lead to the formation of alternative reaction pathways or side reactions, such as secondary amine addition and epoxy group self-polymerization. These unintended reactions may involve less reactive species or result in the formation of stable intermediates, thereby increasing the overall activation energy required for the curing process. A similar pattern is observable in
Figure 2d–f, underscoring that as HBP content increases, the system’s resistance to curing also intensifies.
The study of epoxy curing kinetics based on the DSC method is mainly based on two basic assumptions: (1) the rate of the curing reaction is proportional to the rate of heat flow and (2) the exothermic enthalpy of the curing reaction is proportional to the degree of curing [
27,
28].
where
f(
α) is the reaction model and
k(
T) is the rate constant expressed by the Arrhenius equation:
where A is the pre-exponential factor,
Ea is the activation energy (kJ/mol), and R is the gas constant, i.e., 8.314 (J/mol/K). The
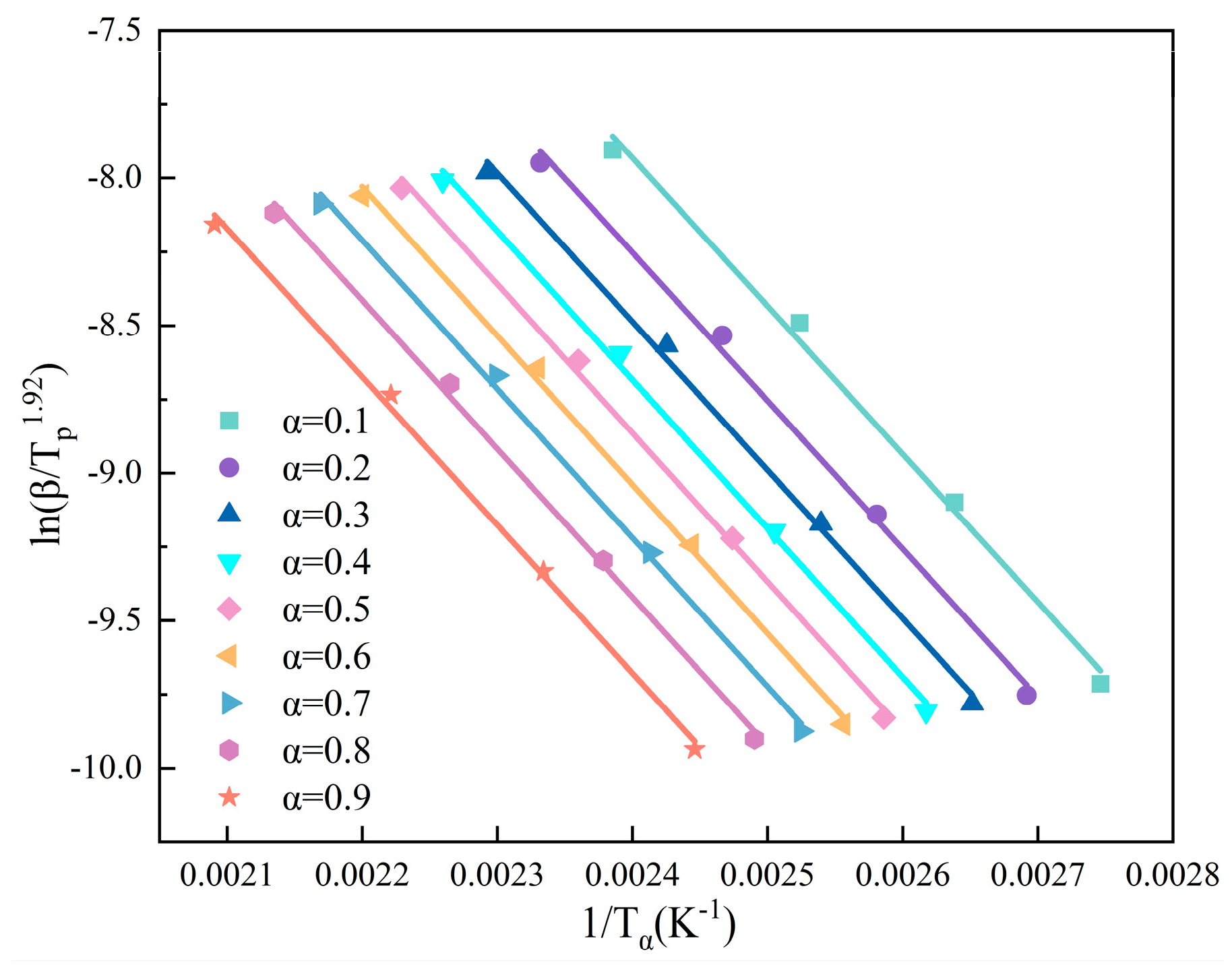
Ea was calculated by the Starink method [
9,
27,
28], as shown in
Figure 3.
where
β is the heating rate and
Tα,i is the temperature at a specific value which achieved α conversion rate.
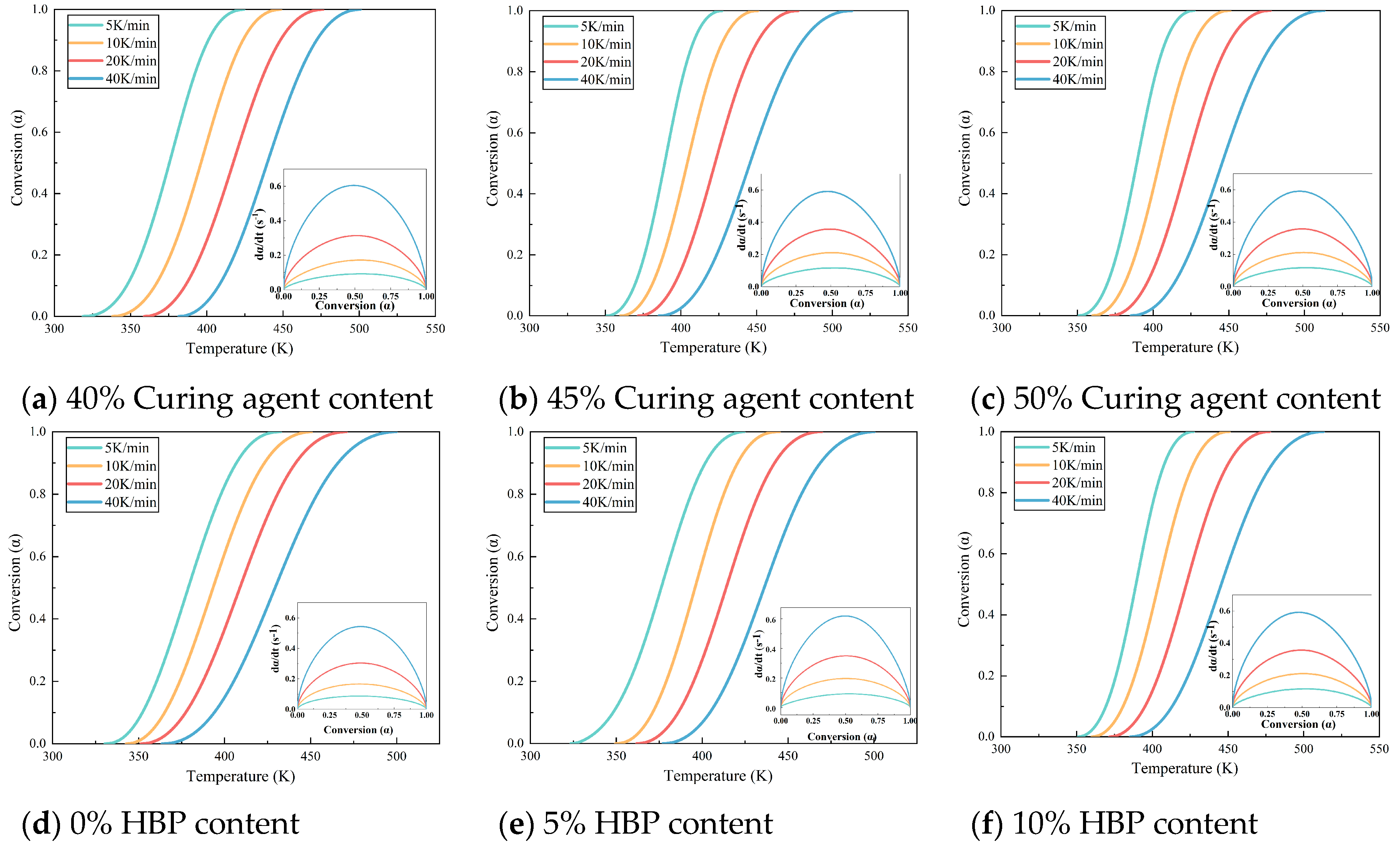
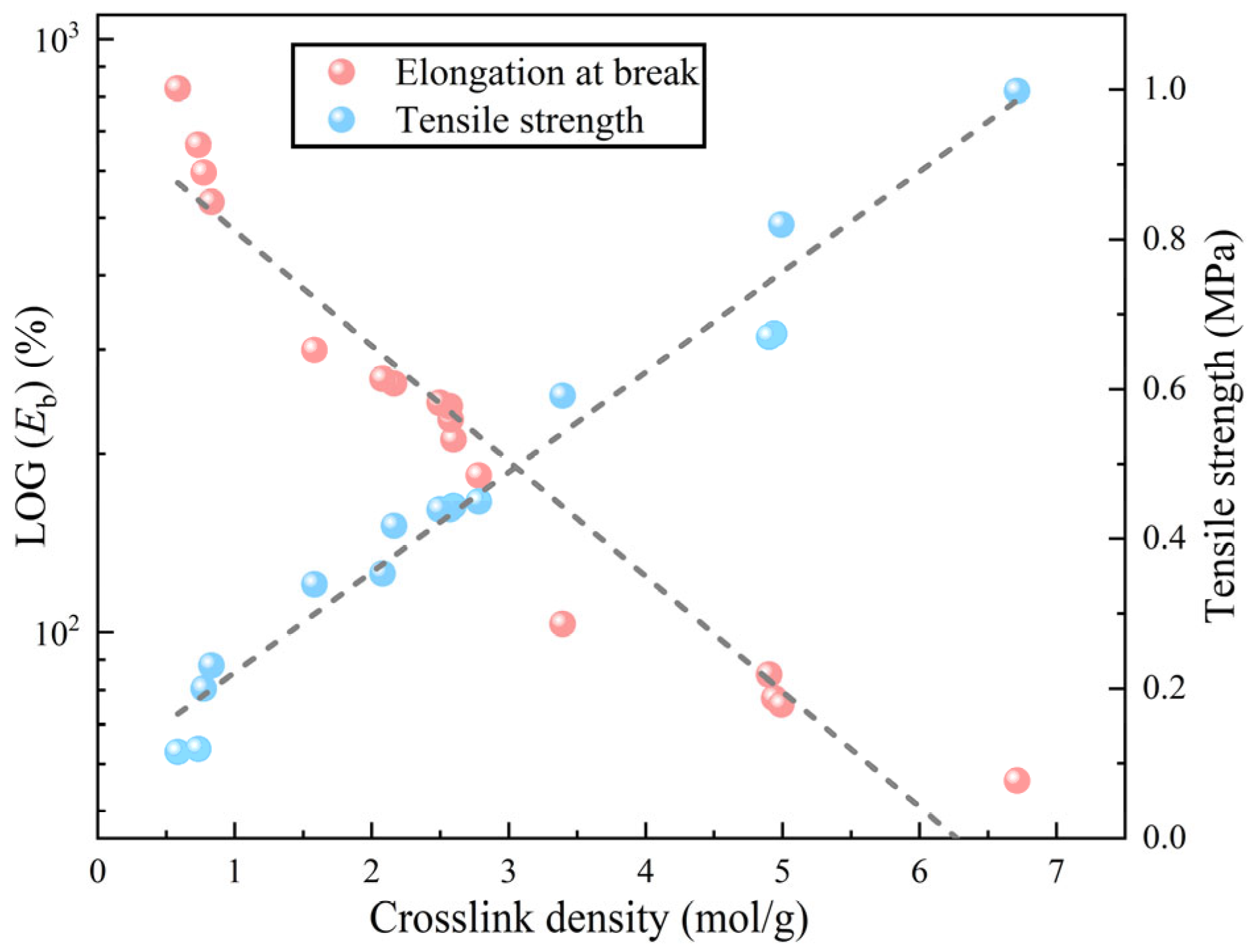
Figure 4 illustrates the temperature–conversion relationship curves for epoxy resins formulated with varying amounts of curing agent and HBP. These curves indicate that, at a constant degree of cure, the reaction rate intensifies with increasing temperature ramp rates. This trend underscores the effectiveness of elevated temperatures in accelerating the curing process, attributable to two key factors: (1) higher temperatures elevate the kinetic energy of molecular components, enabling them to more readily surpass the activation energy barrier required for the reaction, and (2) the curing process is autocatalytic, meaning that the formation of curing products actively enhances the progression of the reaction.
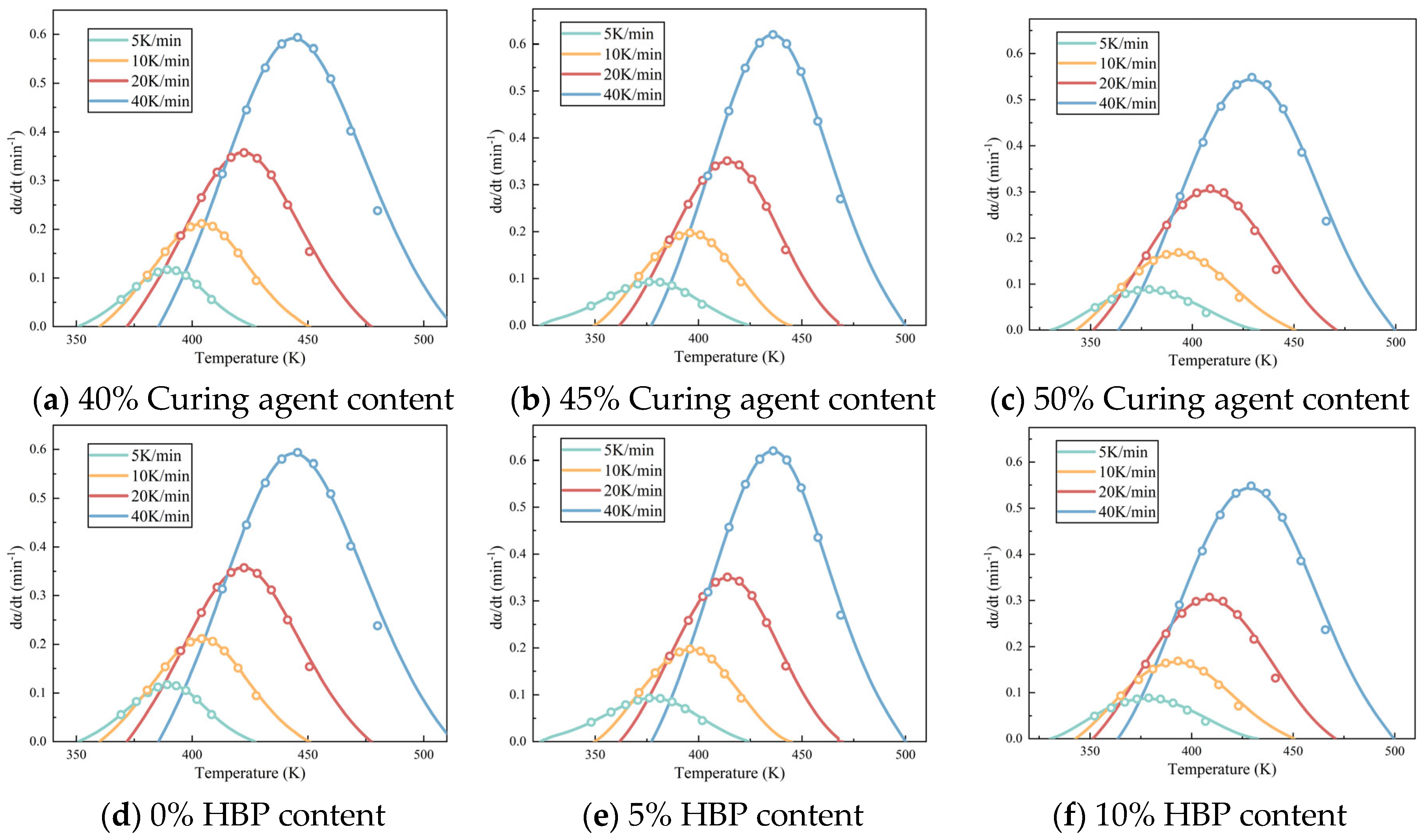
Figure 5 illustrates the relationship between temperature and conversion rate for epoxy resin at different heating rates.
Figure 5a–d examine the influence of systematic variation in the epoxy resin to curing agent ratio on the curing kinetics while
Figure 5e–h assess how graded additions of HBP modifiers alter these curing parameters. The data indicate that, when the same conversion rate is achieved, the curing rate of the epoxy resin initially increases and then decreases as the proportion of curing agent increases, which can be attributed to the intricate interplay between reactant concentration and reaction order. Specifically, the reaction order
n between the epoxy resin and curing agent is a non-integer, which results in a complex dependency of the curing rate on reactant concentration [
27]. Furthermore, the curing rate of epoxy resin decreases with increasing HBP content, likely due to the initiation of secondary reactions triggered by the HBP. These secondary reactions create competing pathways that diminish the efficiency of the primary curing reaction, thereby slowing the overall curing process.
It was shown that the autocatalytic model (Šesták–Berggren model) (Equation (4)) is a suitable choice for characterizing the intricate curing process of epoxy materials [
27]:
where
m is the number of reaction stages with the epoxy resin,
n is the number of reaction stages with the curing agent, and
p =
m/
n =
. Equation (4) can be, therefore, transformed into Equation (5):
The curing kinetics parameters, including the pre-exponential factor
A and reaction stage
n, can be determined by Equation (5). The reaction rate equations and kinetic parameters for different samples are listed in
Table 3 and
Table 4.
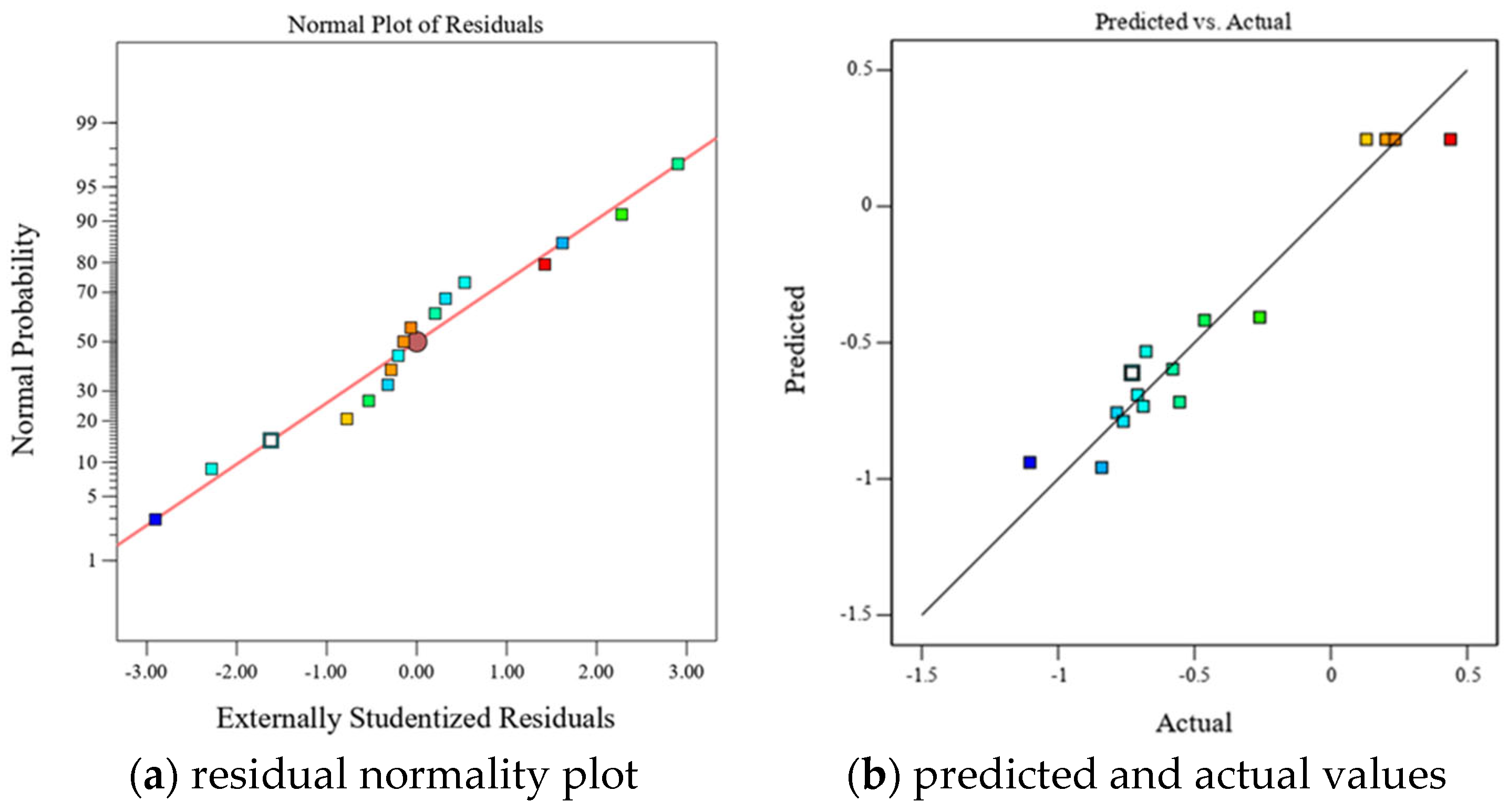
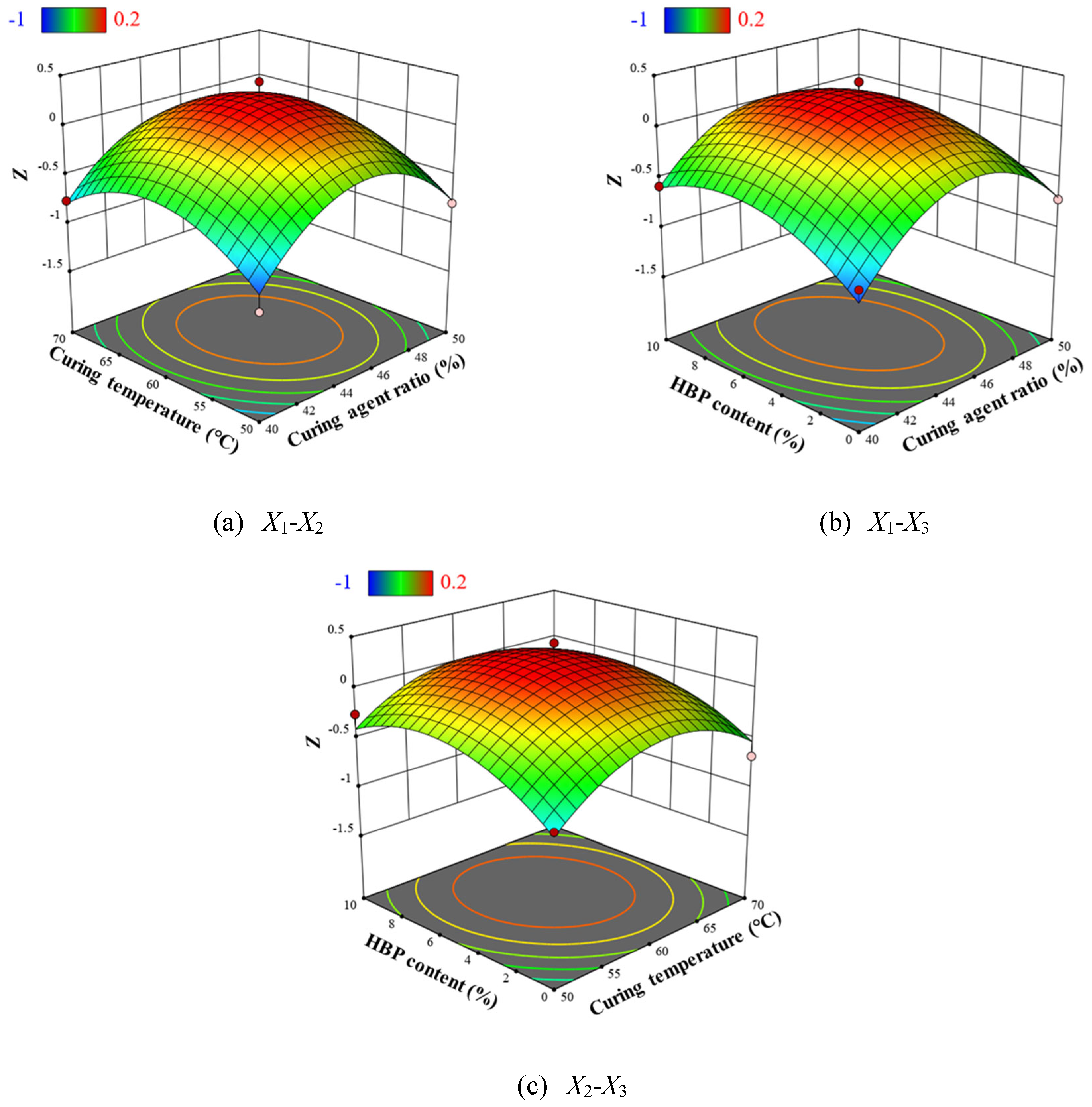
A comparative analysis of the calculated values and experimental curves (
Figure 6) demonstrates that the Šesták–Berggren model provides an accurate representation of the non-isothermal curing process of the epoxy system, underscoring its effectiveness in capturing the complex kinetics of epoxy resin curing. The results of
Table 3 indicate that as the curing agent content increases, the activation energy of the epoxy resin system also increases. This phenomenon can be attributed to two primary factors: first, the reduction in the concentration of epoxy resin, which diminishes the collision efficiency between reactants; and second, the accumulation of unreacted epoxy resin or curing agent, which can initiate side reactions that require higher activation energies to proceed.
The reaction order parameters,
n and
m, which correspond to the number of reaction stages for the epoxy resin and curing agent, respectively, provide insight into the complexity of the curing process. An increase in these parameters signifies a more complex reaction mechanism. As can be seen in
Table 3 and
Table 4, the increase in both
n and
m with rising curing agent content suggests that a more intricate curing reaction mechanism is involved as the proportion of curing agent increases. Furthermore, the observed trend of the curing rate initially increasing and then decreasing with higher curing agent content, as shown in
Figure 5, can be explained by the inverse relationship between the epoxy resin and curing agent proportions, with a higher curing agent content leading to a corresponding decrease in epoxy resin content.
Similarly, as shown in
Table 4, the activation energy of the epoxy resin system increases with higher hyperbranched polymer (HBP) content. This increase is likely due to the side reactions between HBP and epoxy resin, which generally require higher activation energies. As HBP primarily interacts with the epoxy resin components, the number of reaction stages increases, further complicating the curing reaction mechanism as the curing agent content increases.
The curing kinetics equation allows for the calculation of the time required for epoxy resin to reach a specific conversion rate at a given temperature. Prior research [
29] has established that a conversion rate of 95% or greater signifies a near-complete reaction of the epoxy resin with the curing agent.
Table 5 and
Table 6 present the calculated times for achieving conversion rates between 95% and 99% under curing conditions of 140 °C, 150 °C, and 160 °C, which can be calculated using Equation (4). As shown, the time to achieve a given conversion rate decreases with increasing temperature, indicating that raising the curing temperature is an effective method to shorten curing times. Moreover, under constant temperature conditions, the relationship between conversion rate and curing time is non-linear. Specifically, the time to reach a 99% conversion rate is more than double that required to achieve 98%, illustrating the substantial increase in curing time as the reaction nears completion. As a result, the time required to attain 98% conversion was selected as the primary reference for determining subsequent curing conditions in this study.
Furthermore, the time required to achieve the same conversion rate at a given temperature increases with higher curing agent content. This trend offers crucial information for optimizing curing conditions in epoxy resin systems.
3.1.4. ATR-FTIR
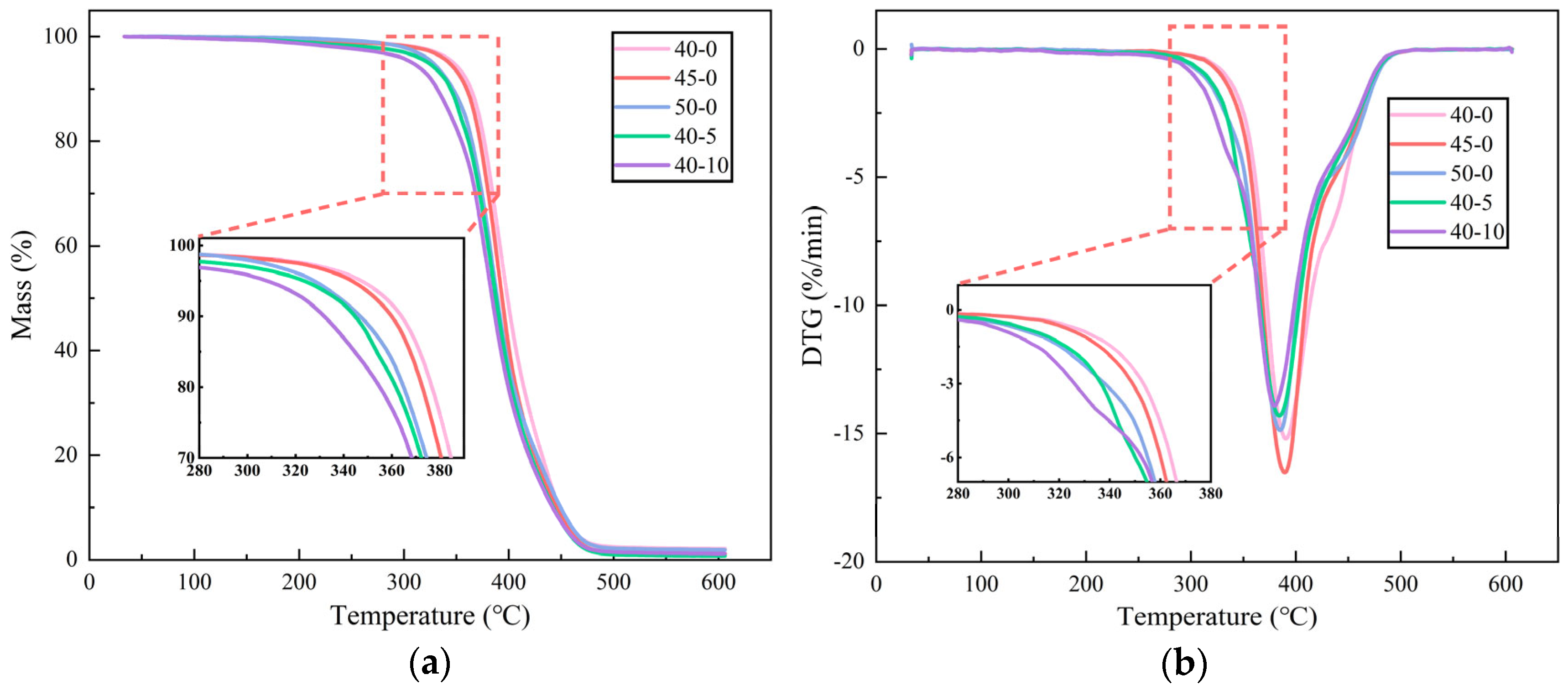
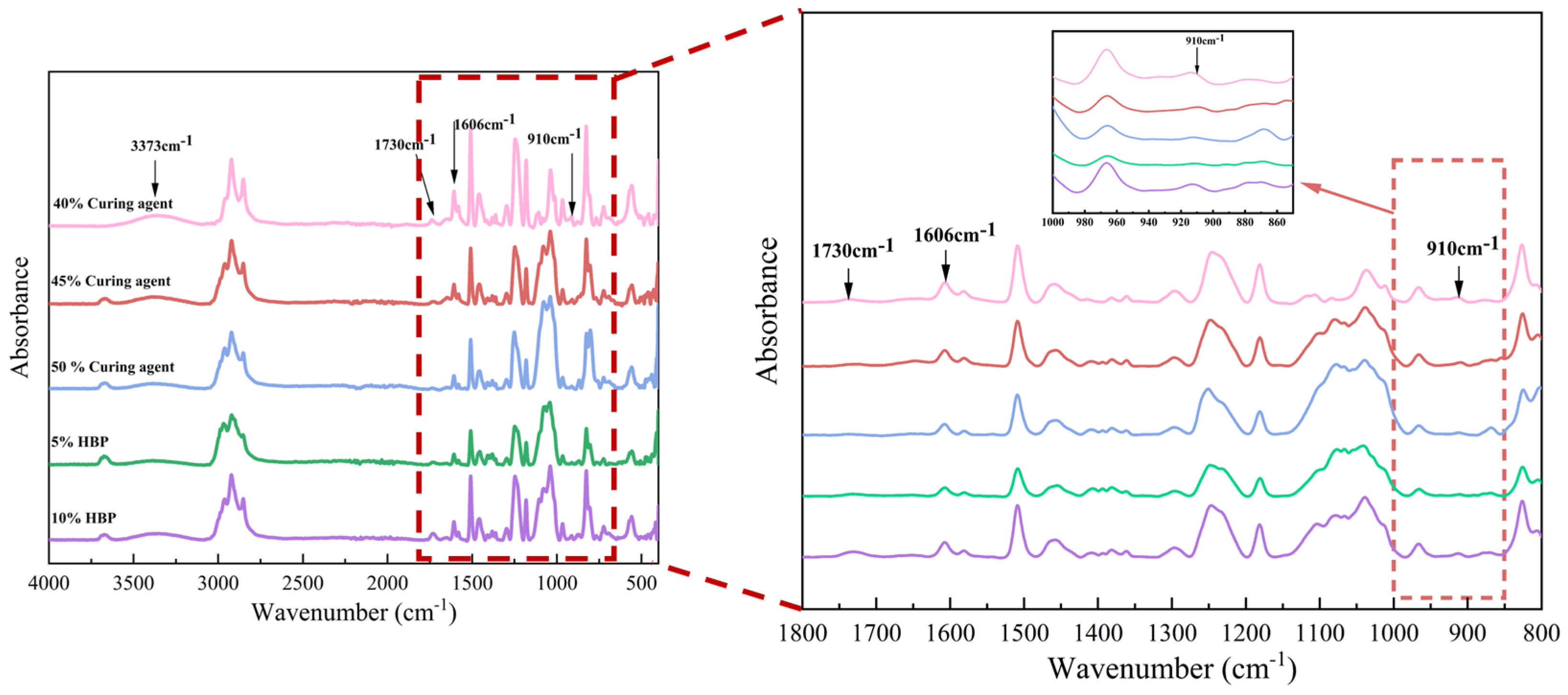
The impact of curing agent and hyperbranched polymer (HBP) content on the chemical composition of epoxy resins was systematically evaluated using Fourier Transform Infrared (FTIR) spectroscopy, as depicted in
Figure 9. The findings demonstrate a reduction in the absorption intensity of the epoxy group (910 cm
−1) with an increasing curing agent proportion. This decline is attributed to two factors: the decreased relative content of epoxy resin and the ring-opening reactions between epoxy groups and the curing agent, which consume these functional groups [
30]. In contrast, the epoxy group absorption peak initially increases and subsequently decreases with the progressive addition of HBP. This behavior is attributed to the highly branched architecture of HBPs, which facilitates the reaction of terminal epoxy groups with the curing agent. However, excessive HBP content leads to an accumulation of unreacted epoxy groups due to incomplete reactions.
The ring-opening reaction between the epoxy groups in the resin and amino groups in the curing agent results in the formation of hydroxyl groups, as indicated by the absorption peak at 3373 cm−1. A slight decrease in the intensity of this peak is observed with higher curing agent proportions, potentially due to a reduction in hydroxyl group formation when excess curing agent is present. Conversely, the incorporation of HBP enhances the reaction extent, increasing hydroxyl group generation and intensifying the corresponding absorption peak.
The absorption peak associated with the C=C bonds in aromatic rings (1606 cm−1) remains largely unchanged, signifying that aromatic rings do not participate in the chemical reactions between the epoxy resin and curing agent. However, at an HBP content of 10%, a significant enhancement in the carbonyl group absorption peak (1730 cm−1) is observed. This increase might be attributed to oxidative side reactions induced by excessive HBP.
These results elucidate the intricate interactions between curing agent and HBP content in shaping the chemical composition and reaction mechanisms of epoxy resins. Such insights are critical for optimizing resin formulations to achieve tailored mechanical and chemical properties for advanced applications.