Tailoring CuO/Polyaniline Nanocomposites for Optoelectronic Applications: Synthesis, Characterization, and Performance Analysis
Abstract
1. Introduction
2. Experimental Techniques
2.1. Chemicals
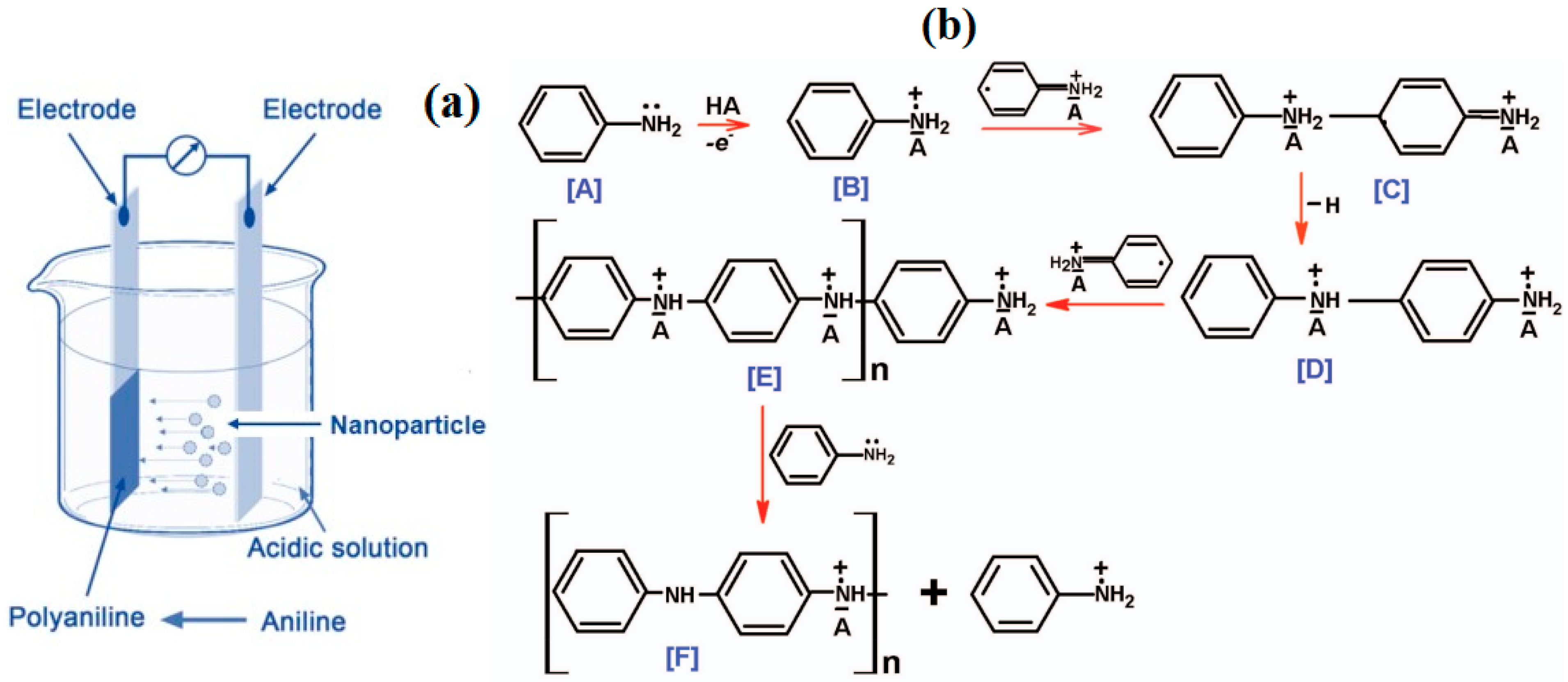
2.2. Films Electrodeposition
2.3. Electrochemical Oxidative Polymerization
2.4. Characterization Techniques
3. Results and Discussion
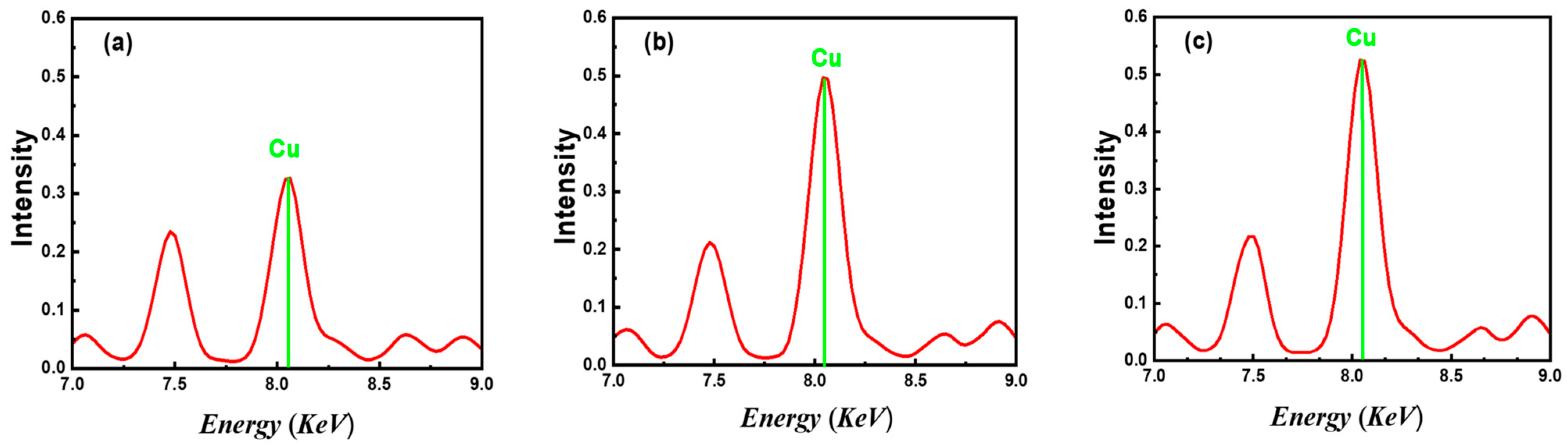
3.1. XRF Characterization
3.2. Optical Profilometer
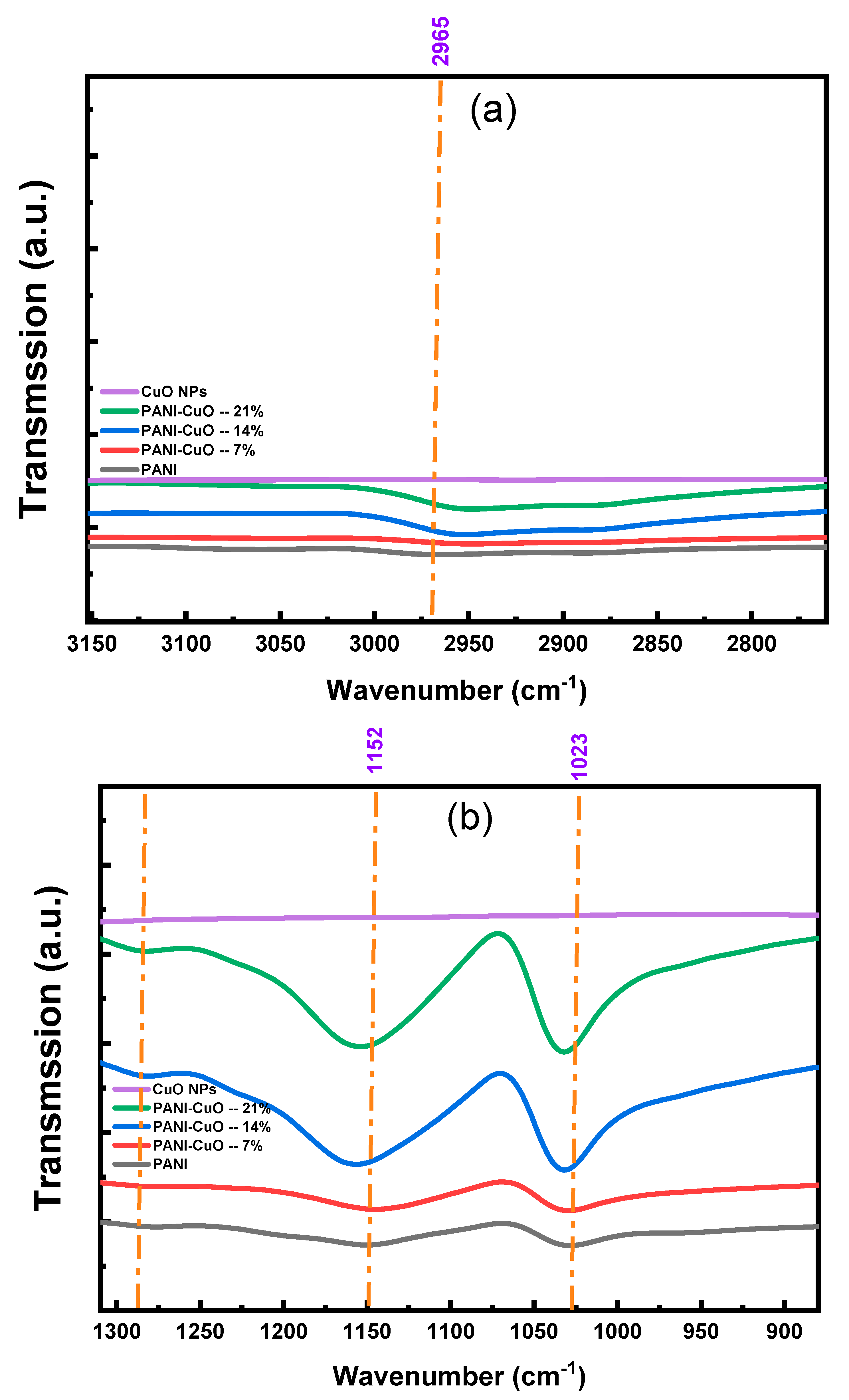
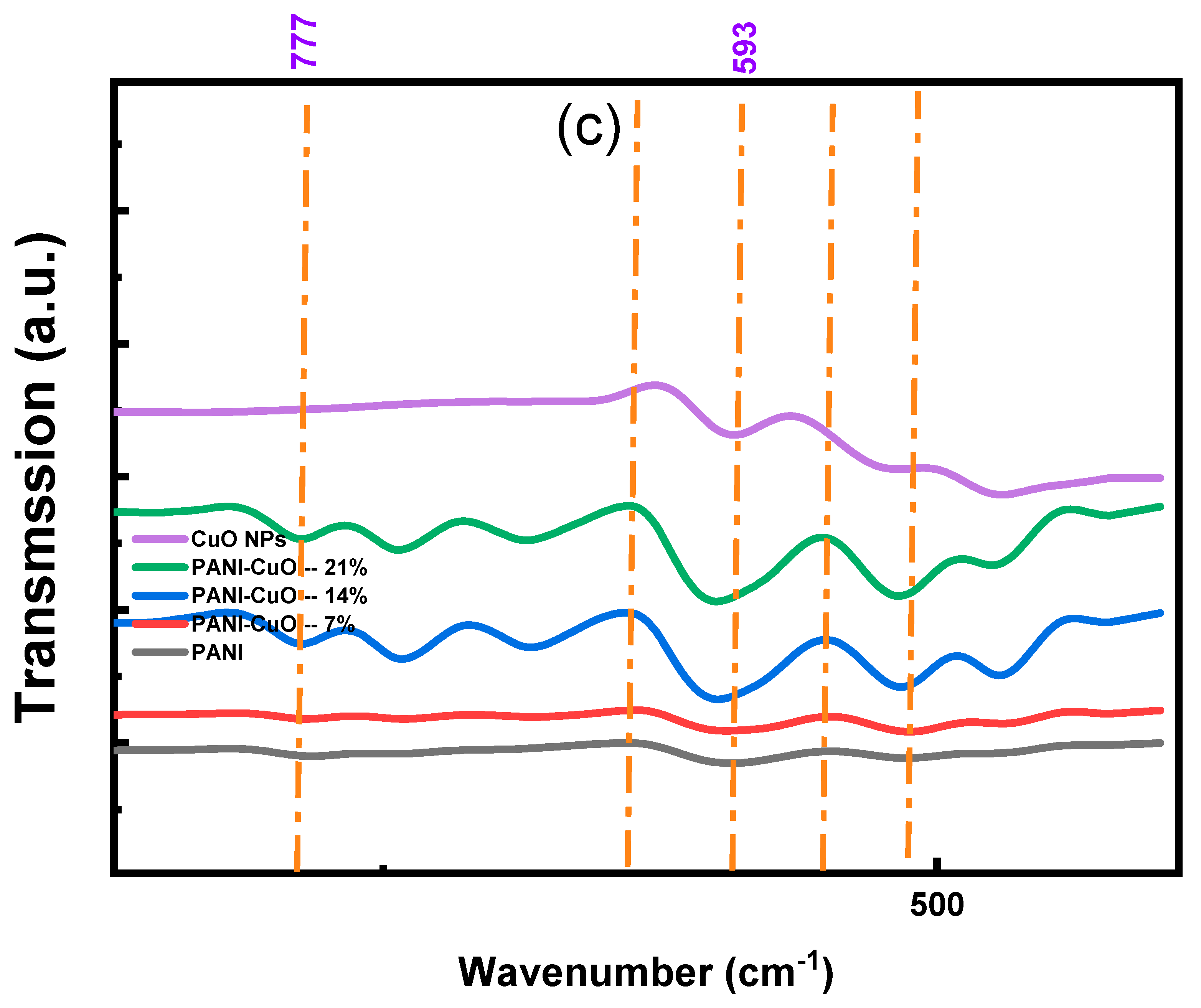
3.3. FTIR Spectra
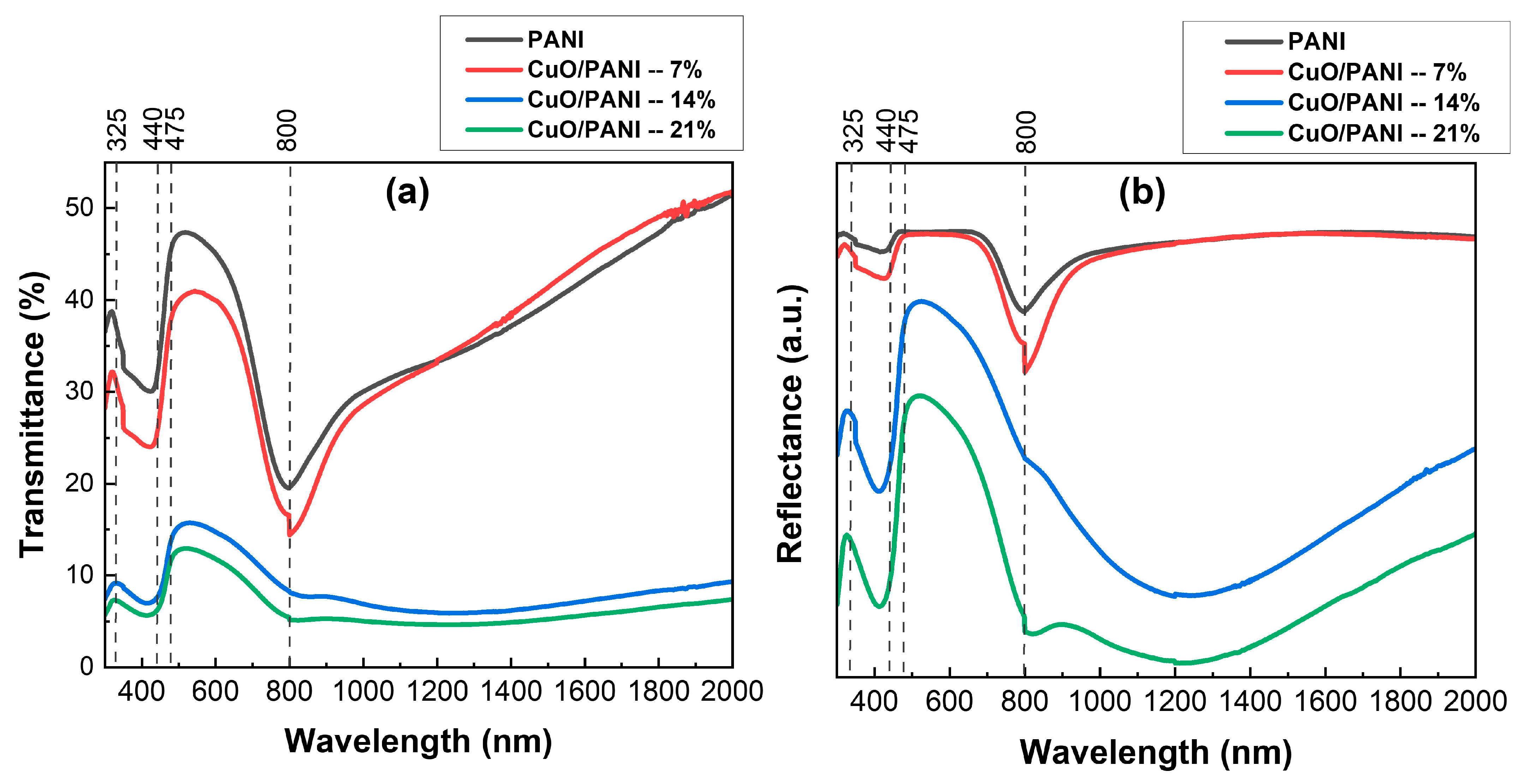
3.4. UV-Vis-NIR Analysis
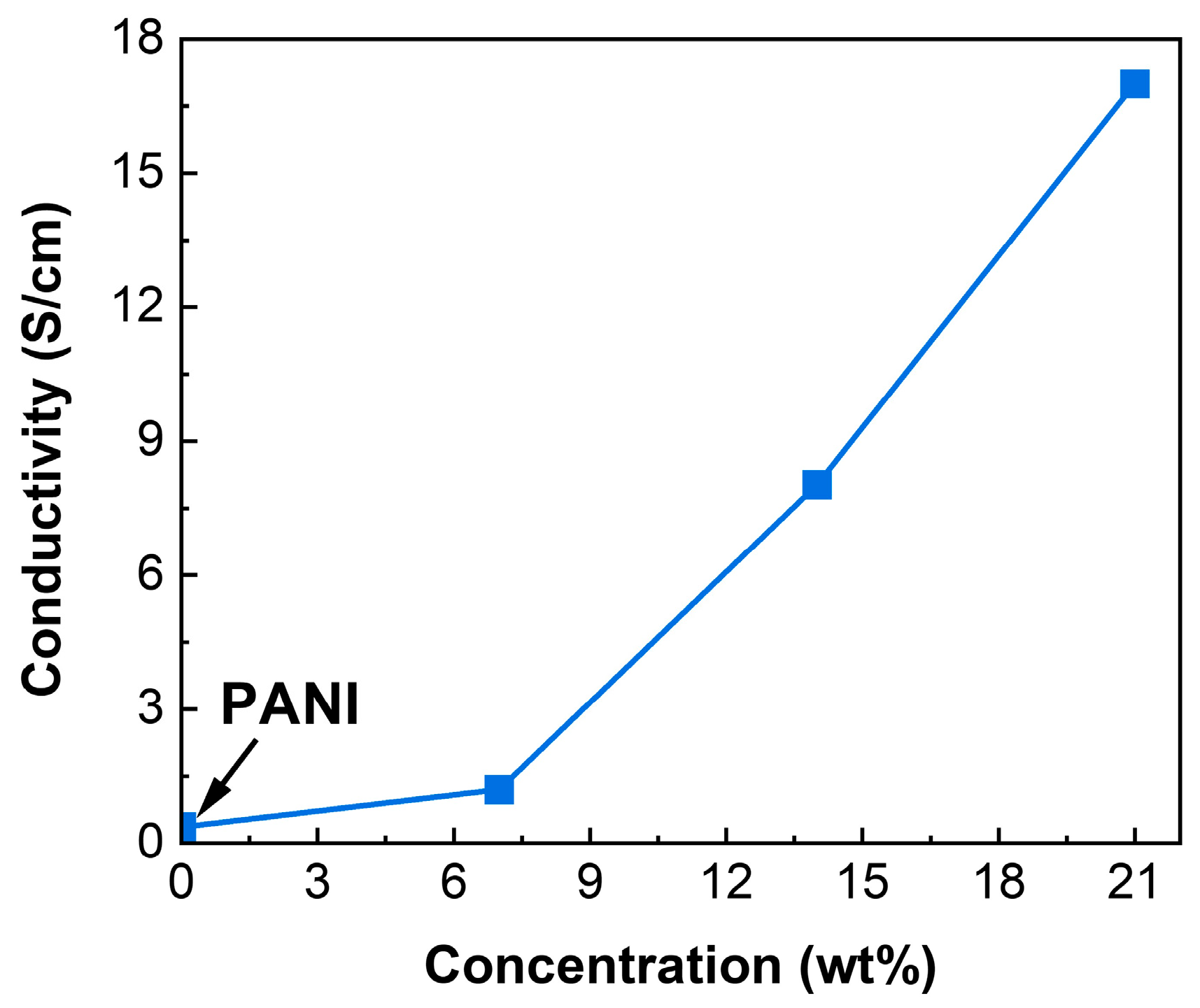
3.5. Electrical Conductivity
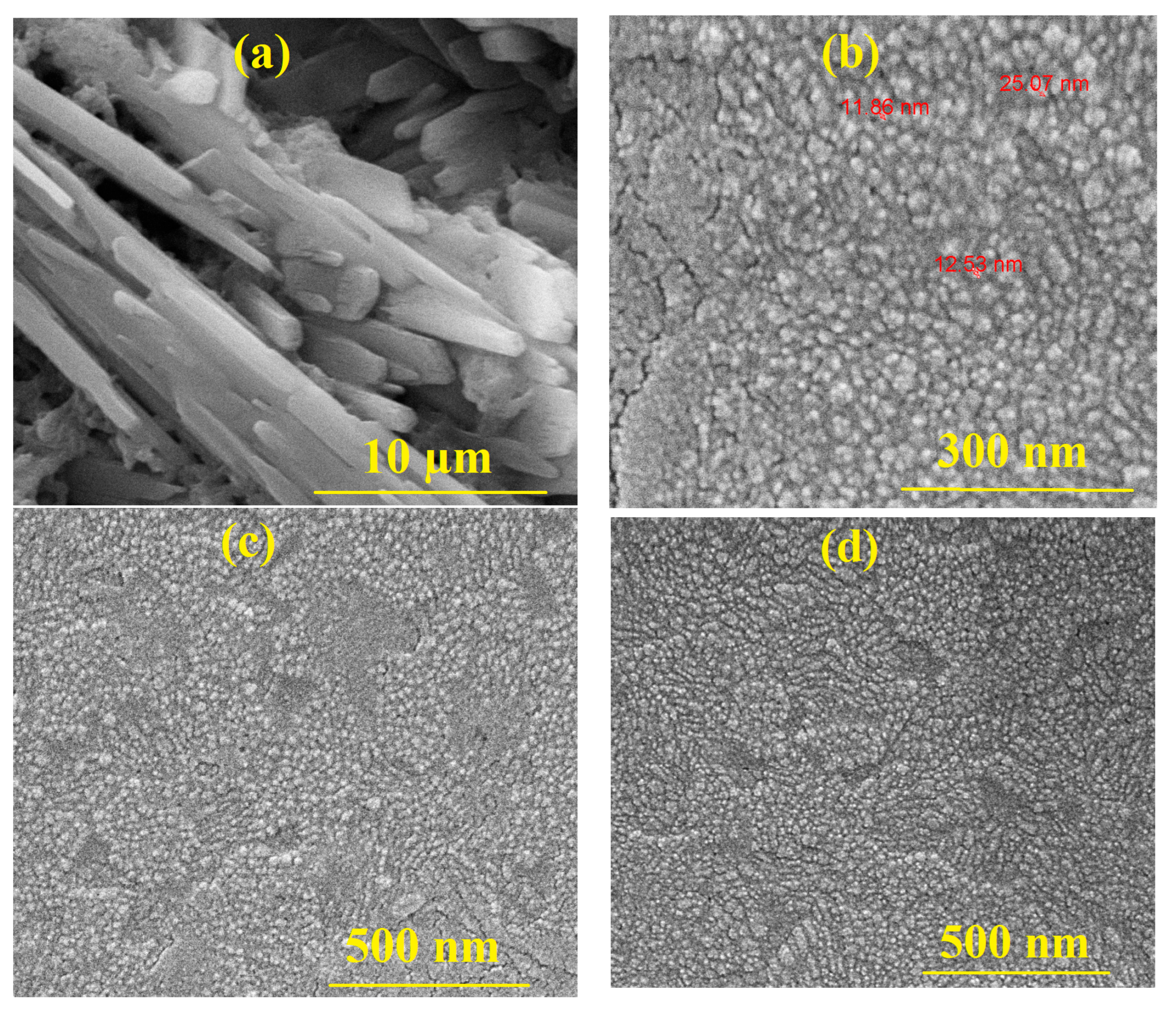
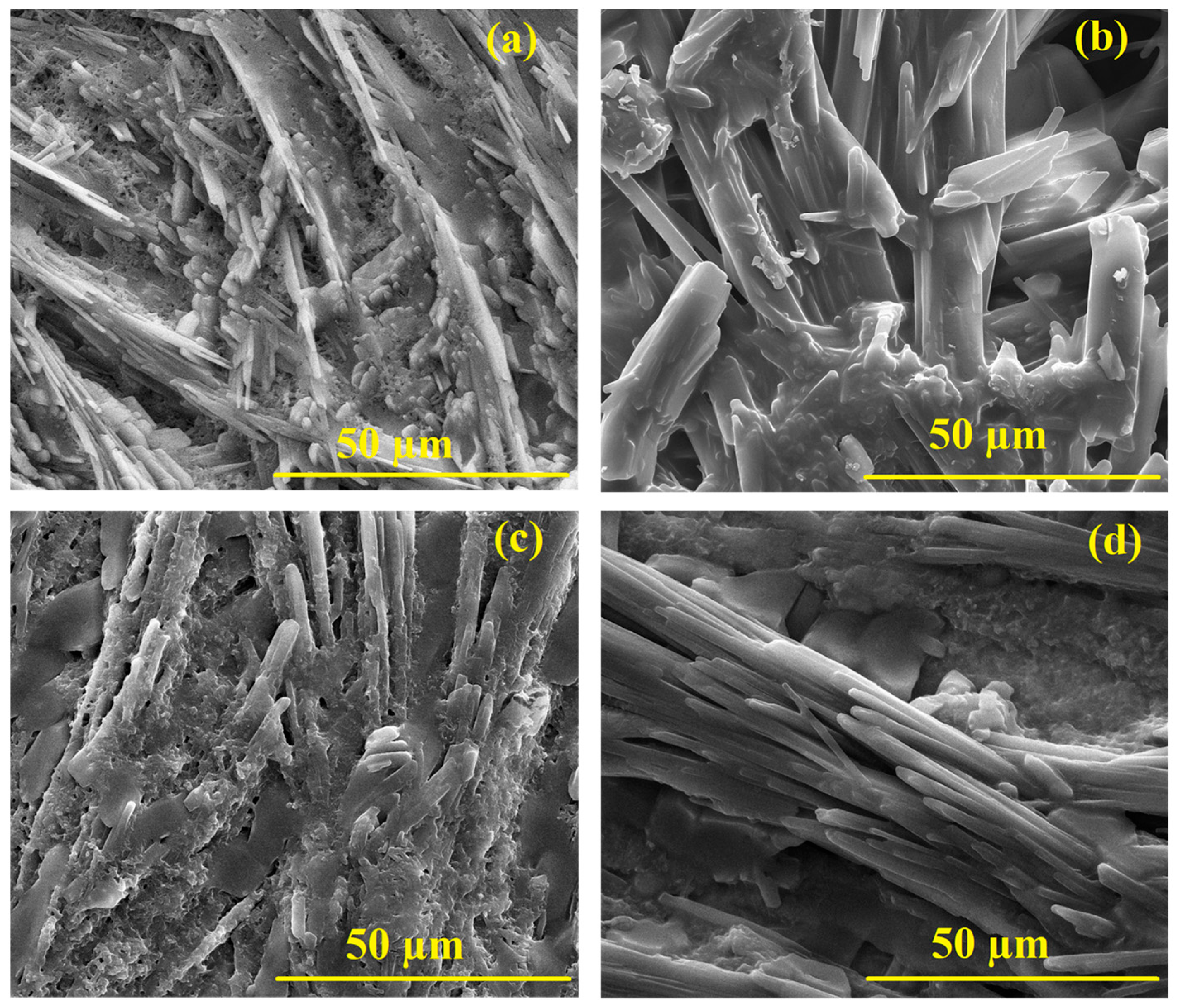
3.6. Surface Morphology
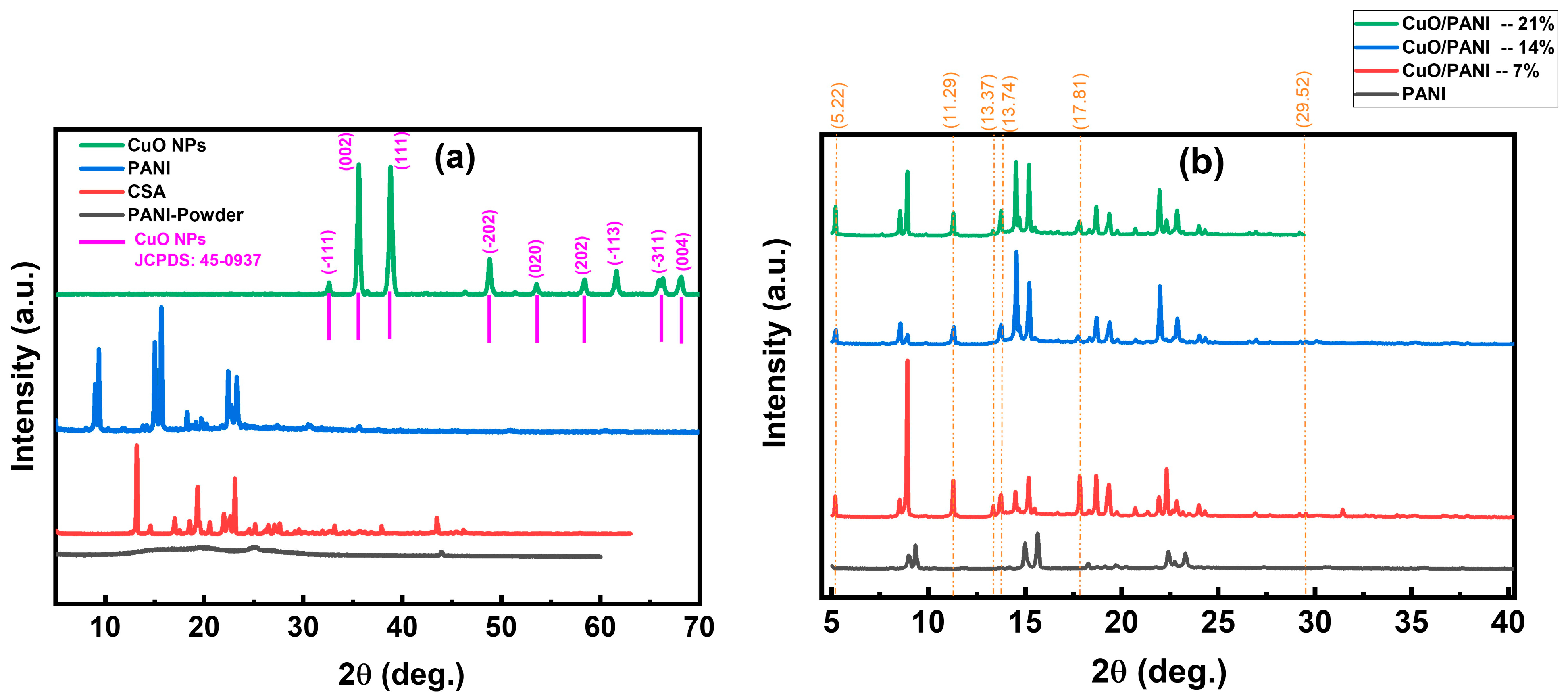
3.7. X-Ray Diffraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Supporting Information and Data
Appendix A.1. Electrochemical Oxidative Polymerization
Appendix A.2. FTIR Spectra
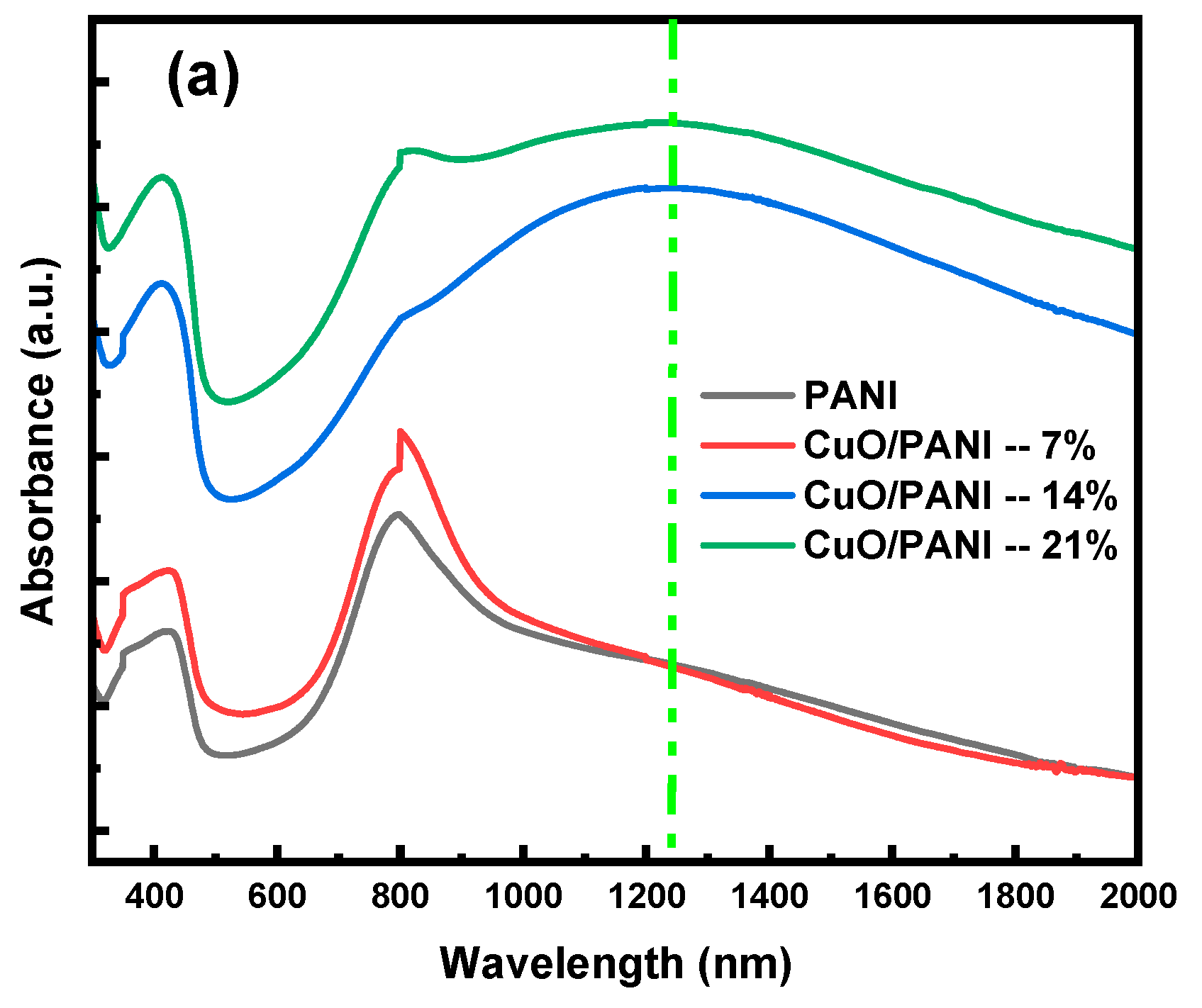
Appendix A.3. Optical Bandgap Energy
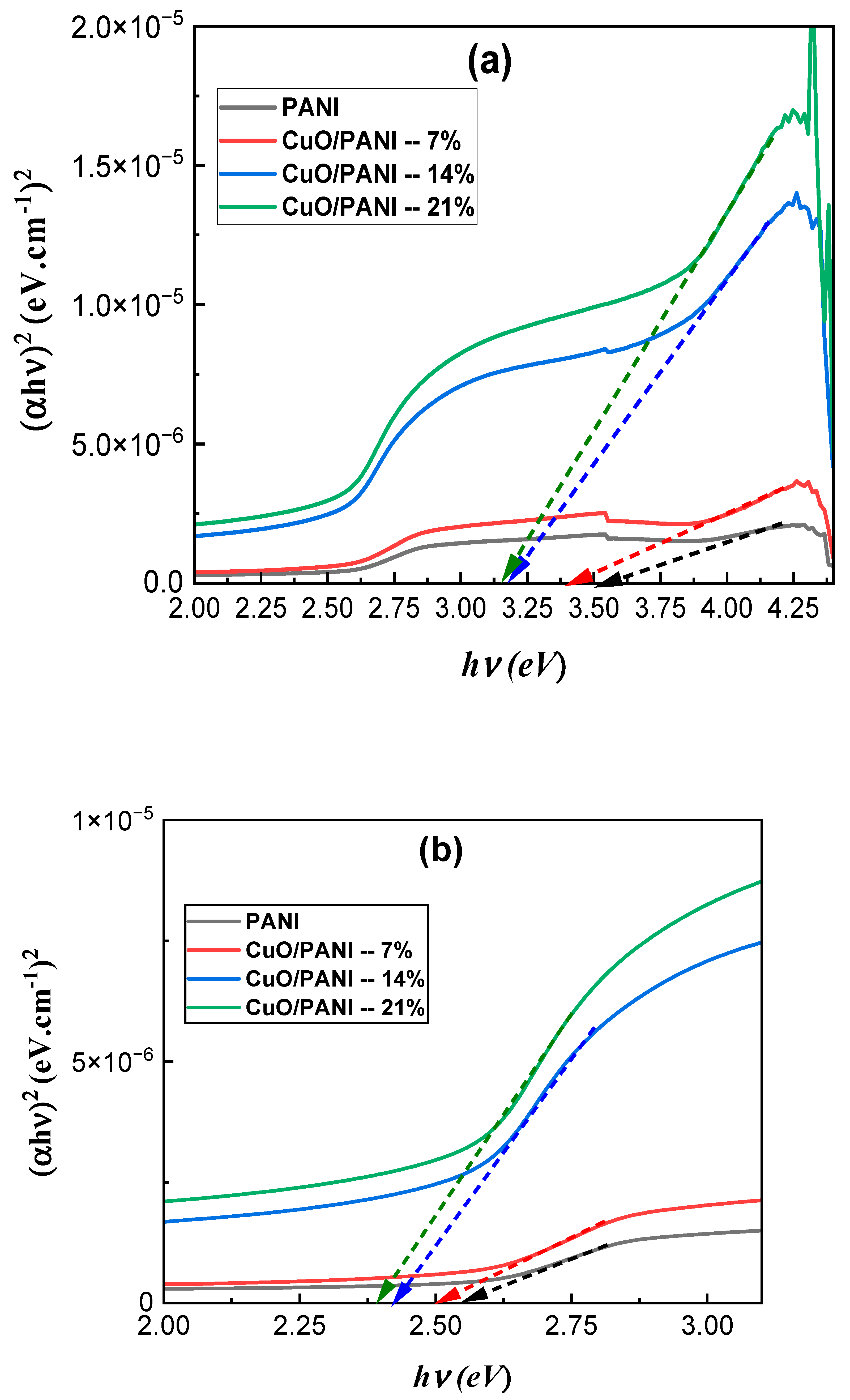
Appendix A.4. Optical Bandgaps of the PANI and CuO/PANI Nanocomposites

Appendix A.5. Surface Morphology
References
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Dakshayini, B.S.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Thomas, S.W.; Khan, R.R.; Puttananjegowda, K.; Serrano-Garcia, W. Conductive polymers and metal oxide polymeric composites for nanostructures and nanodevices. In Advances in Nanostructured Materials and Nanopatterning Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–271. [Google Scholar]
- Jadhav, A.; Qureshi, S.S.; Jadhav, H.; Nizamuddin, S.; Jatoi, A.S.; Mazari, S.A.; Mubarak, N.M. Conducting polymers and their composites. In Contemporary Nanomaterials in Material Engineering Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 147–178. [Google Scholar]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Gherasim, C.; Asandulesa, M.; Fifere, N.; Doroftei, F.; Tîmpu, D.; Airinei, A. Structural, optical and dielectric properties of some nanocomposites derived from copper oxide nanoparticles embedded in poly (vinylpyrrolidone) matrix. Nanomaterials 2024, 14, 759. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Polymer Nanocomposites: Synthesis and characterization. Environ. Nanotechnol. 2020, 4, 265–315. [Google Scholar]
- Zadehnazari, A. Metal oxide/polymer nanocomposites: A review on recent advances in fabrication and applications. Polym. Plast. Technol. Mater. 2023, 62, 655–700. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, G.; Xu, J.L.; Zhang, M.; Kuo, C.C.; Wang, S.D. Conducting polymer-inorganic nanocomposite-based gas sensors: A review. Sci. Technol. Adv. Mater. 2020, 21, 768–786. [Google Scholar] [CrossRef]
- Yakubu, I.S.A.; Muhammad, U.; A’isha, A.M. Humidity sensing study of polyaniline/copper oxide nanocomposites. Int. J. Adv. Acad. Res. Sci. Technol. Eng. 2018, 4, 49–61. [Google Scholar]
- Jundale, D.M.; Navale, S.T.; Khuspe, G.D.; Dalavi, D.S.; Patil, P.S.; Patil, V.B. Polyaniline–CuO hybrid nanocomposites: Synthesis, structural, morphological, optical and electrical transport studies. J. Mater. Sci. Mater. Electron. 2013, 24, 3526–3535. [Google Scholar] [CrossRef]
- de Souza, V.S.; da Frota, H.O.; Sanches, E.A. Polyaniline-CuO hybrid nanocomposite with enhanced electrical conductivity. J. Mol. Struct. 2018, 1153, 20–27. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, A.; Kaur, H. Review on nanomaterials/conducting polymer-based nanocomposites for the development of biosensors and electrochemical sensors. Polym. Plast. Technol. Mater. 2021, 60, 504–521. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. Polyaniline and polypyrrole: Where are we headed? Synth. Met. 1997, 84, 27–34. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef]
- Somani, P.R.; Marimuthu, R.; Mandale, A.B. Synthesis, characterization and charge transport mechanism in conducting polyaniline/V2O5 composites. Polymer 2001, 42, 2991–3001. [Google Scholar] [CrossRef]
- Dey, A.; De, S.; De, A.; De, S.K. Characterization and dielectric properties of polyaniline–TiO2 nanocomposites. Nanotechnology 2004, 15, 1277. [Google Scholar] [CrossRef]
- Ashokkumar, S.P.; Vijeth, H.; Yesappa, L.; Niranjana, M.; Vandana, M.; Devendrappa, H. Electrochemically synthesized polyaniline/copper oxide nano composites: To study optical band gap and electrochemical performance for energy storage devices. Inorg. Chem. Commun. 2020, 115, 107865. [Google Scholar] [CrossRef]
- Ravikiran, Y.T.; Lagare, M.T.; Sairam, M.; Mallikarjuna, N.N.; Sreedhar, B.; Manohar, S.; Aminabhavi, T.M. Synthesis, characterization and low frequency AC conduction of polyaniline/niobium pentoxide composites. Synth. Met. 2006, 156, 1139–1147. [Google Scholar] [CrossRef]
- Kuzmanović, B.; Vujković, M.J.; Tomić, N.; Bajuk-Bogdanović, D.; Lazović, V.; Šljukić, B.; Mentus, S. The influence of oxygen vacancy concentration in nanodispersed non-stoichiometric CeO2-δ oxides on the physico-chemical properties of conducting polyaniline/CeO2 composites. Electrochim. Acta 2019, 306, 506–515. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Wee, Y.K.; Mahmood, W.A.K. Effect of CuO on the thermal stability of polyaniline nanofibers. Int. J. Chem. React. Eng. 2014, 12, 215–221. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Wee, Y.K.; Mahmood, W.A.K. Effects of CuO on the morphology and conducting properties of PANI nanofibers. Synth. Met. 2012, 162, 1065–1072. [Google Scholar] [CrossRef]
- Mishra, D.; Anand, S.; Panda, R.K.; Das, R.P. Hydrothermal preparation and characterization of boehmites. Mater. Lett. 2000, 42, 38–45. [Google Scholar] [CrossRef]
- Guo, Z.; Pereira, T.; Choi, O.; Wang, Y.; Hahn, H.T. Surface functionalized alumina nanoparticle filled polymeric nanocomposites with enhanced mechanical properties. J. Mater. Chem. 2006, 16, 2800–2808. [Google Scholar] [CrossRef]
- Proença, M.; Borges, J.; Rodrigues, M.S.; Domingues, R.P.; Dias, J.P.; Trigueiro, J.; Bundaleski, N.; Teodoro, O.M.N.D.; Vaz, F. Development of Au/CuO nanoplasmonic thin films for sensing applications. Surf. Coat. Technol. 2018, 343, 178–185. [Google Scholar] [CrossRef]
- Vijayan, A.; Ramadoss, S.; Sisubalan, N.; Gnanaraj, M.; Chandrasekaran, K.; Kokkarachedu, V. Cerium Oxide Nanoparticles for Biomedical Applications. In Nanoparticles in Modern Antimicrobial and Antiviral Applications; Springer International Publishing: Cham, Germany, 2024; pp. 175–200. [Google Scholar]
- Mir, S.H.; Ochiai, B. Development of Hierarchical Polymer@Pd Nanowire-Network: Synthesis and Application as Highly Active Recyclable Catalyst and Printable Conductive Ink. Chem. Open 2016, 5, 213. [Google Scholar] [CrossRef]
- Gupta, K.; Jana, P.C.; Meikap, A.K. Electrical transport and optical properties of the composite of polyaniline nanorod with gold. Solid State Sci. 2012, 14, 324–329. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar]
- Wu, G.; Zhang, H. Synthesis and characterization of camphor sulfonic acid fully doped polyaniline. Res. Appl. Mater. 2013, 1, 5–8. [Google Scholar] [CrossRef]
- Kaner, R.B. Electrochemical Science Technology of Polymers; Elsevier: New York, NY, USA, 1990; Volume 97. [Google Scholar]
- Rao, P.S.; Sathyanarayana, D.N. Advanced Functional Molecules and Polymers; Nalwa, H.S., Ed.; Gordon Breach: Tokyo, Japan, 2001. [Google Scholar]
- Kryszewaski, M. Heterogeneous conducting polymeric systems: Dispersions, blends, crystalline conducting networks—An introductory presentation. Synth. Met. 1991, 45, 289–296. [Google Scholar] [CrossRef]
- Li, G.R.; Feng, Z.P.; Zhong, J.H.; Wang, Z.L.; Tong, Y.X. Electrochemical synthesis of polyaniline nanobelts with predominant electrochemical performances. Macromolecules 2010, 43, 2178–2183. [Google Scholar] [CrossRef]
- Chebakova, K.A.; Dzidziguri, E.L.; Sidorova, E.N.; Vasiliev, A.A.; Ozherelkov, D.Y.; Pelevin, I.A.; Nalivaiko, A.Y. X-ray fluorescence spectroscopy features of micro-and nanoscale copper and nickel particle compositions. Nanomaterials 2021, 11, 2388. [Google Scholar] [CrossRef]
- Al-Gharram, M.; AlZoubi, T. Electrochemical synthesis of a novel hybrid nanocomposite based on Co3O4 nanoparticles embedded in PANI-camphor sulfonic Acid matrix for optoelectronic applications. Ceram. Int. 2024, 50, 5473–5482. [Google Scholar] [CrossRef]
- Palaniappan, S. Chemical and electrochemical polymerization of aniline using tartaric acid. Eur. Polym. J. 2001, 37, 975–981. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Polyaniline by new miniemulsion polymerization and the effect of reducing agent on conductivity. Synth. Met. 2006, 156, 1148–1154. [Google Scholar] [CrossRef]
- Namazi, H.; Kabiri, R.; Entezami, A. Determination of extremely low percolation threshold electroactivity of the blend polyvinyl chloride/polyaniline doped with camphorsulfonic acid by cyclic voltammetry method. Eur. Polym. J. 2002, 38, 771–777. [Google Scholar] [CrossRef]
- Al-Gharram, M.; AlZoubi, T. Linear and nonlinear optical properties of hybrid Polyaniline/CoFe2O4 nanocomposites: Electrochemical synthesis, characterization, and analysis. Ceram. Int. 2024, 50, 32841–32852. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchová, M.; Konyushenko, E.N.; Holler, P. The genesis of polyaniline nanotubes. Polymer 2006, 47, 8253–8262. [Google Scholar] [CrossRef]
- Abdiryim, T.; Zhang, X.G.; Jamal, R. Comparative studies of solid-state synthesized polyaniline doped with inorganic acids. Mater. Chem. Phys. 2005, 90, 367–372. [Google Scholar] [CrossRef]
- Abdiryim, T.; Jamal, R.; Nurulla, I. Doping effect of organic sulphonic acids on the solid-state synthesized polyaniline. J. Appl. Polym. Sci. 2007, 105, 576–584. [Google Scholar] [CrossRef]
- Nagaraja, M.; Pattar, J.; Shashank, N.; Manjanna, J.; Kamada, Y.; Rajanna, K.; Mahesh, H.M. Electrical, structural and magnetic properties of polyaniline/pTSA-TiO2 nanocomposites. Synth. Met. 2009, 159, 718–722. [Google Scholar] [CrossRef]
- Wang, J.S.; Wang, J.X.; Yang, Z.; Wang, Z.; Zhang, F.B.; Wang, S.C. A novel strategy for the synthesis of polyaniline nanostructures with controlled morphology. React. Funct. Polym. 2008, 68, 1435–1440. [Google Scholar] [CrossRef]
- Epstein, A.J.; Joo, J.; Kohlman, R.S.; Du, G.; MacDiarmid, A.G.; Oh, E.J.; Min, Y.; Tsukamoto, J.; Kaneko, H.; Pouget, J.P. Inhomogeneous disorder and the modified Drude metallic state of conducting polymers. Synth. Met. 1994, 65, 149–157. [Google Scholar] [CrossRef]
- Šuligoj, A.; Arčon, I.; Mazaj, M.; Dražić, G.; Arčon, D.; Cool, P.; Tušar, N.N. Surface modified titanium dioxide using transition metals: Nickel as a winning transition metal for solar light photocatalysis. J. Mater. Chem. A 2018, 6, 9882–9892. [Google Scholar] [CrossRef]
- Habeeb, M.A.; Oreibi, I.; Hamza, R.S.A.; Mamoun, F. Fabrication and unraveling the morphological, structural, optical and dielectric features of PMMA-SiO2/CuO promising ternary nanostructures for Nanoelectronic and photonic applications. Silicon 2024, 16, 5947–5960. [Google Scholar] [CrossRef]
- Hashim, A.; Abbas, M.H.; Al-Aaraji, N.A.H.; Hadi, A. Facile fabrication and developing the structural, optical and electrical properties of SiC/Y2O3 nanostructures doped PMMA for optics and potential nanodevices. Silicon 2023, 15, 1283–1290. [Google Scholar] [CrossRef]
- Al-Gharram, M.; AlZoubi, T.; Noqta, O.A.; Makhadmeh, G.N. Comprehensive study of linear and nonlinear optical properties of polyaniline-Al2O3 nanocomposites for optoelectronic applications. Surf. Interfaces 2024, 52, 104939. [Google Scholar] [CrossRef]
- Ambalagi, S.M.; Devendrappa, M.; Nagaraja, S.; Sannakki, B. Dielectric properties of PANI/CuO nanocomposites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 310, p. 012081. [Google Scholar]
- AlZoubi, T.; Mourched, B.; Al Gharram, M.; Makhadmeh, G.; Abu Noqta, O. Improving photovoltaic performance of hybrid organic-inorganic MAGeI3 perovskite solar cells via numerical optimization of carrier transport materials (HTLs/ETLs). Nanomaterials 2023, 13, 2221. [Google Scholar] [CrossRef]
- Al Zoubi, T.; Al-Gharram, M.; Moustafa, M. Insights into the impact of defect states and temperature on the performance of kesterite-based thin-film solar cells. Optik 2022, 264, 169442. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Plenum: New York, NY, USA, 1974; Volume 159. [Google Scholar]
- Al-Gharram, M.; Uhlmann, P.; Al-Hussein, M. Highly dispersed crystalline magnetic and conductive polyaniline/iron oxide nanocomposite films. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133212. [Google Scholar] [CrossRef]
- Alzoubi, F.; Al-Gharram, M.; AlZoubi, T.; Noqta, O.A.; Makhadmeh, G.; Al-Khateeb, H.; Al-Qadi, M. Advanced electrochemical synthesis and characterization of Al2O3 nanoparticles embedded in polyaniline matrix for optoelectronic applications. Ceram. Int. 2024, 50, 37968–37977. [Google Scholar] [CrossRef]
- Bandgar, D.K.; Khuspe, G.D.; Pawar, R.C.; Lee, C.S.; Patil, V.B. Facile and novel route for preparation of nanostructured polyaniline (PANI) thin films. Appl. Nanosci. 2014, 4, 27–36. [Google Scholar]
- Chandrasekhar, P. Conducting Polymers, Fundamentals and Applications: A Practical Approach; Springer Science Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Li, X.; Zhang, Y.; Wang, H.; Zhao, J. Electronic Structure Modulation in Conducting Polymer Nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47238. [Google Scholar]
- Alipour, A.; Mansour Lakouraj, M.; Tashakkorian, H. Study of the effect of band gap and photoluminescence on biological properties of polyaniline/CdS QD nanocomposites based on natural polymer. Sci. Rep. 2021, 11, 1913. [Google Scholar] [CrossRef]
- Tripathi, A.; Dixit, T.; Agrawal, J.; Singh, V. Bandgap engineering in CuO nanostructures: Dual-band, broadband, and UV-C photodetectors. Appl. Phys. Lett. 2020, 116, 111102. [Google Scholar] [CrossRef]
- Varughese, G.; Rini, V.; Suraj, S.P.; Usha, K.T. Characterisation and optical studies of copper oxide nanostructures doped with lanthanum ions. Adv. Mater. Sci. 2014, 14, 49. [Google Scholar] [CrossRef]
- Smits, F.M. Measurement of Sheet Resistivities with the Four-Point Probe. Bell Syst. Tech. J. 1958, 37, 711–718. [Google Scholar] [CrossRef]
- Schroder, D.K. Semiconductor Material and Device Characterization; John Wiley Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- AlZoubi, T.; Kadhem, W.J.; Al Gharram, M.; Makhadmeh, G.; Abdelfattah, M.A.; Abuelsamen, A.; Mourched, B. Advanced Optoelectronic Modeling and Optimization of HTL-Free FASnI3/C60 Perovskite Solar Cell Architecture for Superior Performance. Nanomaterials 2024, 14, 1062. [Google Scholar] [CrossRef]
- Ahlskog, M.; Menon, R. The localization-interaction model applied to the direct-current conductivity of metallic conducting polymers. J. Phys. Condens. Matter 1998, 10, 7171. [Google Scholar] [CrossRef]
- Krinichnyi, V.I.; Chemerisov, S.D.; Lebedev, Y.S. EPR and charge-transport studies of polyaniline. Phys. Rev. B 1997, 55, 16233. [Google Scholar] [CrossRef]
- Ansari, M.O.; Oves, M.; Salah, N.; Asad, M.; Kumar, R.; Hasan, P.M.Z.; Darwesh, R. DC electrical conductivity retention and antibacterial aspects of microwave-assisted ultrathin CuO@ polyaniline composite. Chem. Pap. 2020, 74, 3887–3898. [Google Scholar] [CrossRef]
- Djurado, D.; Nicolau, Y.F.; Dalsegg, I.; Samuelsen, E.J. X-ray scattering study of CSA protonated polyaniline films and powders. Synth. Met. 1997, 84, 121–122. [Google Scholar] [CrossRef]
- Pouget, J.P.; Hsu, C.H.; Mac Diarmid, A.G.; Epstein, A.J. Structural investigation of metallic PAN-CSA and some of its derivatives. Synth. Met. 1997, 69, 119–120. [Google Scholar] [CrossRef]
- Patil, S.L.; Chougule, M.A.; Pawar, S.G.; Sen, S.; Patil, V.B. Effect of camphor sulfonic acid doping on structural, morphological, optical and electrical transport properties on polyaniline-ZnO nanocomposites. Soft Nanosci. Lett. 2012, 2, 46–53. [Google Scholar]
- Duhan, M.; Kaur, R. Nano-structured polyaniline as a potential adsorbent for methylene blue dye removal from effluent. J. Compos. Sci. 2020, 5, 7. [Google Scholar] [CrossRef]



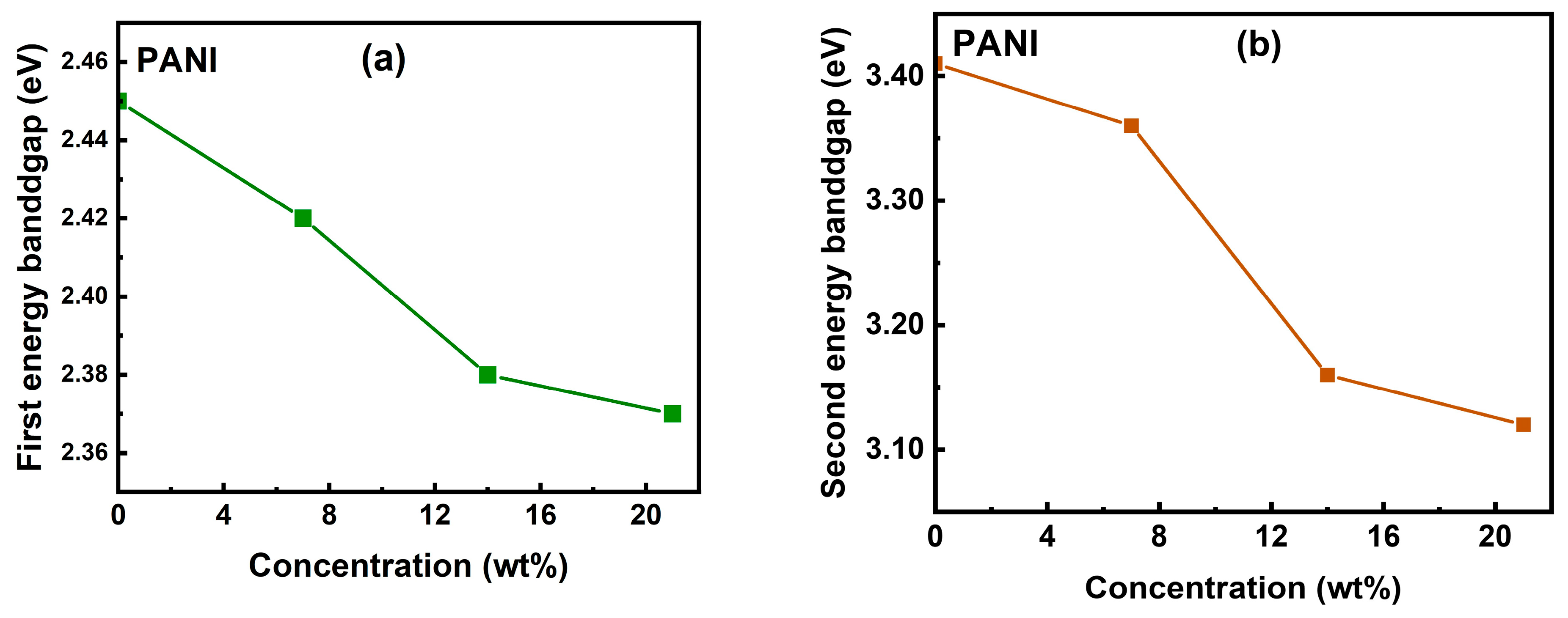
| Sample | Optical Bandgap 1 (Eg1) (eV) | Optical Bandgap 2 (Eg2) (eV) |
|---|---|---|
| PANI | 2.45 | 3.41 |
| CuO/PANI—7 wt.% | 2.42 | 3.36 |
| CuO/PANI—14 wt.% | 2.38 | 3.16 |
| CuO/PANI—21 wt.% | 2.37 | 3.12 |
| Sample | Conductivity σ [S/cm] |
|---|---|
| PANI | 0.36 |
| CuO/PANI—7 wt.% | 1.20 |
| CuO/PANI—14 wt.% | 8.02 |
| CuO/PANI—21 wt.% | 17.00 |
| Sample | Crystalline Grain Size (D) (nm) at (2θ = 15.2 °) | Strain (ε) (× 10−4) | Dislocation Density (δ) (× 1013 m−2) |
|---|---|---|---|
| PANI | 80 | 4.55 | 15.8 |
| CuO/PANI—7 wt.% | 108 | 3.37 | 8.65 |
| CuO/PANI—14 wt.% | 99 | 3.66 | 1.02 |
| CuO/PANI—21 wt.% | 121 | 2.99 | 6.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzoubi, F.; Al-Gharram, M.; AlZoubi, T.; Al-Khateeb, H.; Al-Qadi, M.; Abu Noqta, O.; Makhadmeh, G.; Mouhtady, O.; Al-Hmoud, M.; Mandumpal, J. Tailoring CuO/Polyaniline Nanocomposites for Optoelectronic Applications: Synthesis, Characterization, and Performance Analysis. Polymers 2025, 17, 1423. https://doi.org/10.3390/polym17101423
Alzoubi F, Al-Gharram M, AlZoubi T, Al-Khateeb H, Al-Qadi M, Abu Noqta O, Makhadmeh G, Mouhtady O, Al-Hmoud M, Mandumpal J. Tailoring CuO/Polyaniline Nanocomposites for Optoelectronic Applications: Synthesis, Characterization, and Performance Analysis. Polymers. 2025; 17(10):1423. https://doi.org/10.3390/polym17101423
Chicago/Turabian StyleAlzoubi, Fedda, Mahmoud Al-Gharram, Tariq AlZoubi, Hasan Al-Khateeb, Mohammed Al-Qadi, Osamah Abu Noqta, Ghaseb Makhadmeh, Omar Mouhtady, Mohannad Al-Hmoud, and Jestin Mandumpal. 2025. "Tailoring CuO/Polyaniline Nanocomposites for Optoelectronic Applications: Synthesis, Characterization, and Performance Analysis" Polymers 17, no. 10: 1423. https://doi.org/10.3390/polym17101423
APA StyleAlzoubi, F., Al-Gharram, M., AlZoubi, T., Al-Khateeb, H., Al-Qadi, M., Abu Noqta, O., Makhadmeh, G., Mouhtady, O., Al-Hmoud, M., & Mandumpal, J. (2025). Tailoring CuO/Polyaniline Nanocomposites for Optoelectronic Applications: Synthesis, Characterization, and Performance Analysis. Polymers, 17(10), 1423. https://doi.org/10.3390/polym17101423





