Flufenamic Acid-Loaded Electrospun Nanofibers Based on Chitosan/Poly(vinyl alcohol) Polymeric Composites for Drug Delivery in Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparations of Chitosan/Poly(vinyl alcohol) and Flufenamic Acid-Loaded Chitosan/Poly(vinyl alcohol) Solutions
2.3. Electrospinning Procedure
2.4. Characterizations
2.4.1. Scanning Electron Microscopy
2.4.2. Fourier-Transform Infrared Spectroscopy
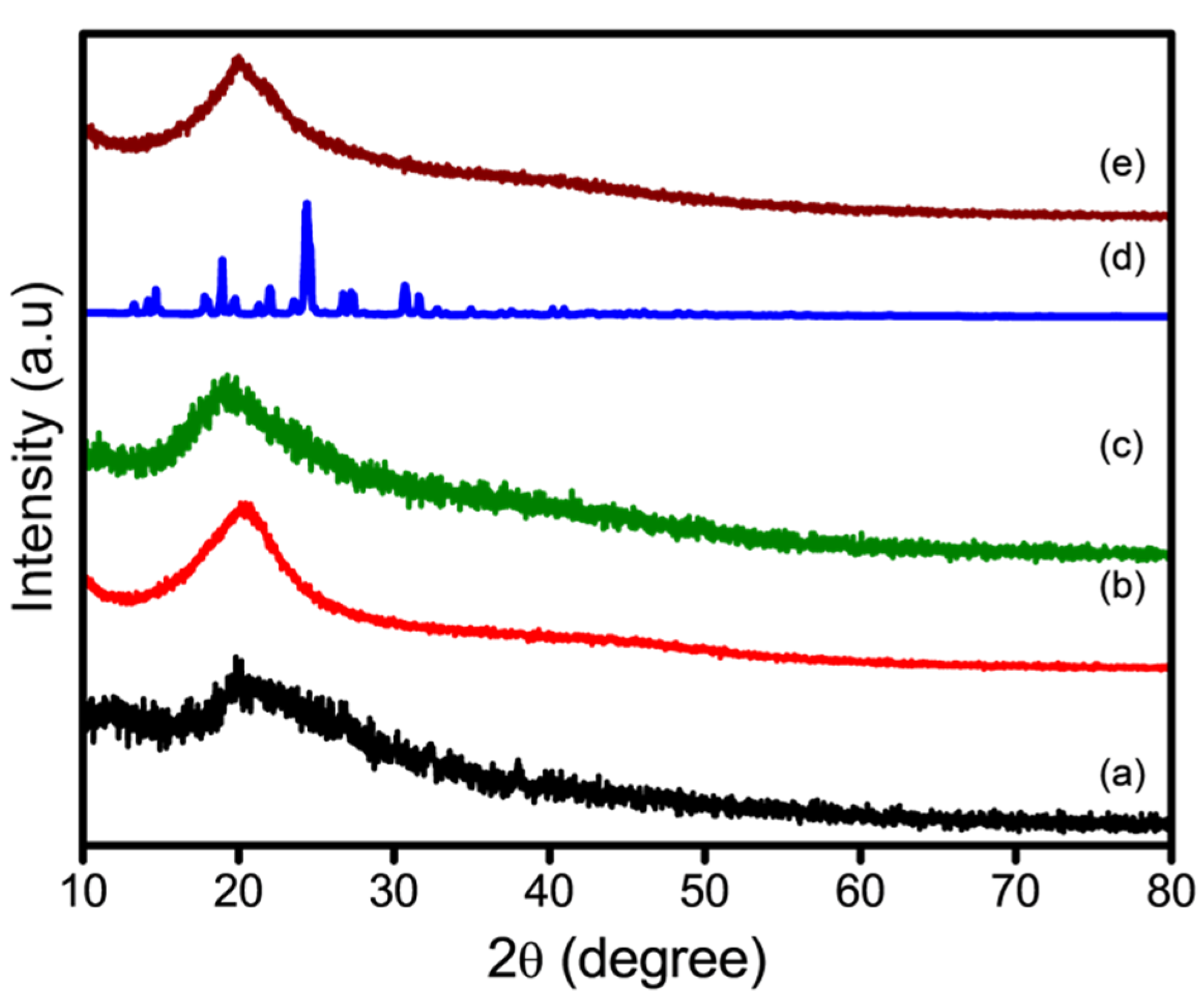
2.4.3. X-Ray Diffraction
2.5. Swelling Ratio
2.6. Degradation Profile
2.7. Drug-Encapsulation Efficiency and Drug Release
2.8. Bacterial Culture
2.9. Cytotoxicity Assay
2.10. Cell Imaging
2.11. Antioxidant Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Nanofiber Morphologies
3.2. Surface Properties of Nanofibers
3.3. Thermal Properties of Nanofibers
3.4. Swelling Evaluation
3.5. In Vitro Degradation
3.6. Loading Efficiency and In Vitro Drug Release
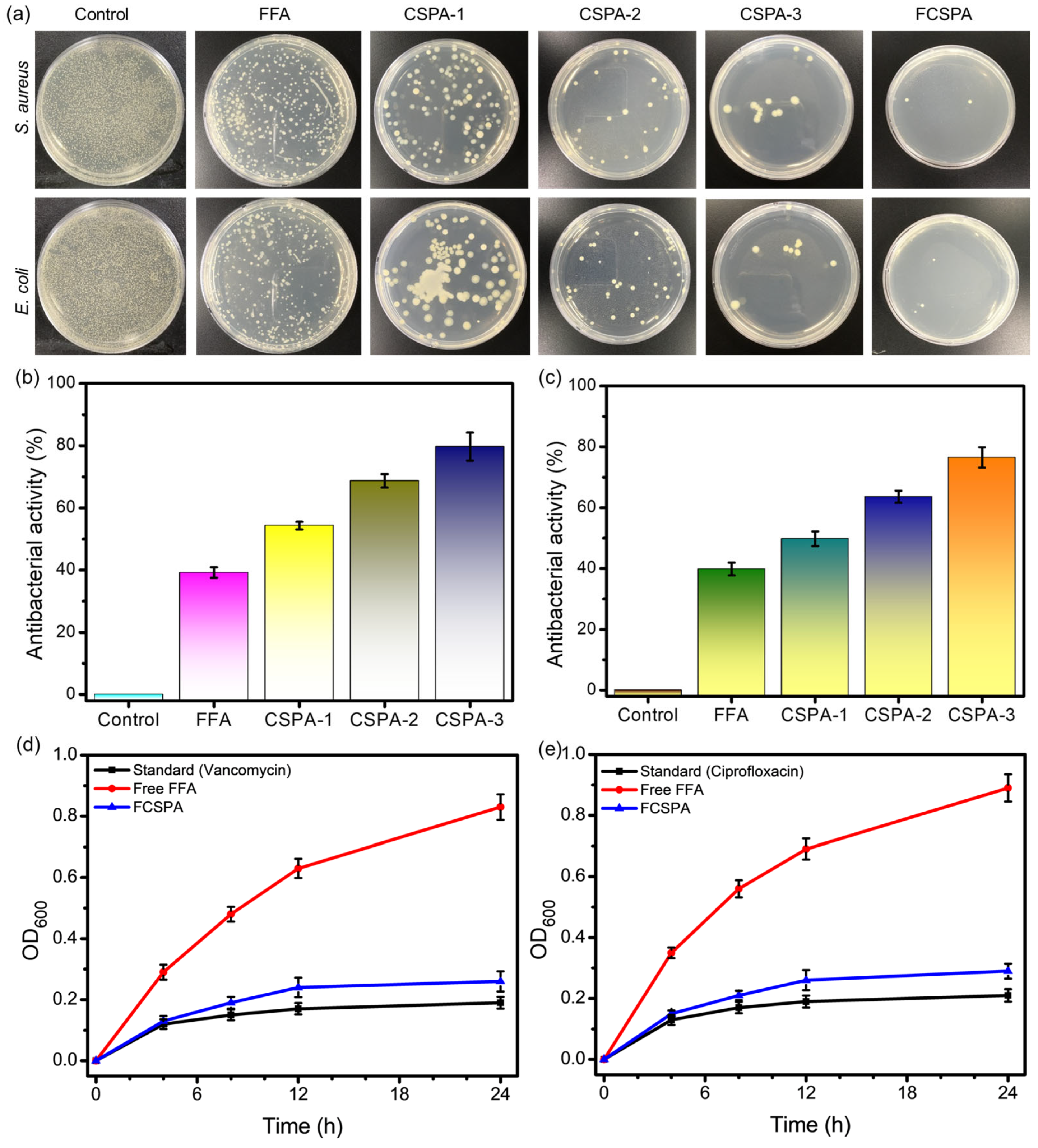
3.7. Antibacterial Activity
3.8. Anticancer Analysis
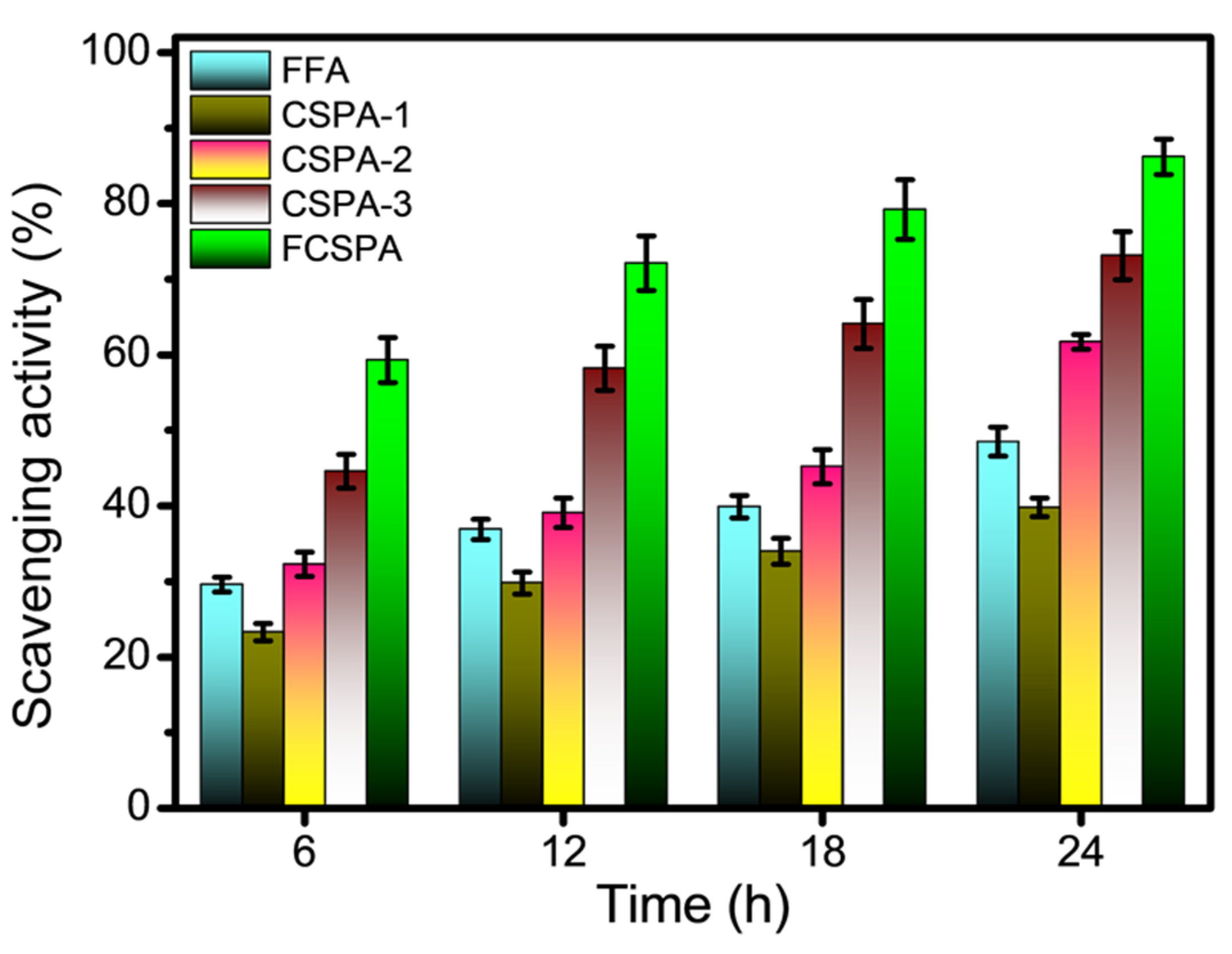
3.9. Antioxidant Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FFA | Flufenamic acid |
| CHS | Chitosan |
| PVA | Poly(vinyl alcohol) |
| CSPA | CHS/PVA |
| FCSPA | FFA-loaded CHS/PVA |
| SEM | Scanning electron microscopy |
| FTIR | Fourier-transform infrared |
| XRD | X-ray diffraction |
| DSC | Differential scanning calorimetry |
| FESEM | Field-emission scanning electron microscopy |
| E. coli | Escherichia coli |
| S. aureus | Staphylococcus aureus |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| MW | Molecular weight |
| DI | Deionized |
| NF | Nanofiber |
| PBS | Phosphate-buffered saline |
| W0 | Initial dry weight |
| W1 | Swollen weight |
| EE% | Drug-encapsulation efficiency |
| MHB | Mueller–Hinton broth |
| CFU | Colony-forming units |
| OD600 | Optical density at 600 nm |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| PI | Propidium iodide |
References
- Shabani, A.; Al, G.A.; Berri, N.; Castro-Dominguez, B.; Leese, H.S.; Martinez-Hernandez, U. Electrospinning Technology, Machine Learning, and Control Approaches: A Review. Adv. Eng. Mater. 2025, 27, 2401353. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Emerine, R.; Chou, S.-F. Fast Delivery of Melatonin from Electrospun Blend Polyvinyl Alcohol and Polyethylene Oxide (PVA/PEO) Fibers. AIMS Bioeng. 2022, 9, 178–196. [Google Scholar] [CrossRef]
- Ekrami, E.; Khodabandeh Shahraky, M.; Mahmoudifard, M.; Mirtaleb, M.S.; Shariati, P. Biomedical Applications of Electrospun Nanofibers in Industrial World: A Review. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 561–575. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Kumar, M.; Ahmad, N.; Ge, Y.; Mahmood, S. Recent Applications of Chitosan and Its Derivatives in Antibacterial, Anticancer, Wound Healing, and Tissue Engineering Fields. Polymers 2024, 16, 1351. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Taokaew, S.; Chuenkaek, T. Developments of Core/Shell Chitosan-Based Nanofibers by Electrospinning Techniques: A Review. Fibers 2024, 12, 26. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Alhalafi, M.H.; Emam, E.A.M.; Ibrahim, H.; Mosaad, R.M. A Review of Chitosan and Chitosan Nanofiber: Preparation, Characterization, and Its Potential Applications. Polymers 2023, 15, 2820. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Ding, C.; Zhao, Y.; Zhang, S.; Sun, S.; Zhang, L.; Ma, S.; Ding, Q.; Liu, W. Polyvinylpyrrolidone/Chitosan-Loaded Dihydromyricetin-Based Nanofiber Membrane Promotes Diabetic Wound Healing by Anti-Inflammatory and Regulating Autophagy-Associated Protein Expression. Int. J. Biol. Macromol. 2024, 259, 129160. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, S.; Rode, S.; Chaudhary, P.K.; Khan, N.A.; Ramamurthy, P.C.; Gupta, D.N.; Kumar, R.; Das, J.; Sharma, A.K. Fabrication and Characterization of Polyvinyl Alcohol-Chitosan Composite Nanofibers for Carboxylesterase Immobilization to Enhance the Stability of the Enzyme. Sci. Rep. 2024, 14, 19615. [Google Scholar] [CrossRef] [PubMed]
- Sarac, B.; Gürbüz, R.; Soprunyuk, V.; Yüce, E.; Rezvan, A.; Schranz, W.; Eckert, J.; Ozcan, A.; Sarac, A.S. Chitosan-Containing Electrospun Poly(Ethylene Oxide)-Polybutadiene-CNT Fibers. Polym. Adv. Technol. 2024, 35, e6403. [Google Scholar] [CrossRef]
- Deng, S.; Huang, Y.; Hu, E.; Ning, L.J.; Xie, R.; Yu, K.; Lu, F.; Lan, G.; Lu, B. Chitosan/Silk Fibroin Nanofibers-Based Hierarchical Sponges Accelerate Infected Diabetic Wound Healing via a HClO Self-Producing Cascade Catalytic Reaction. Carbohydr. Polym. 2023, 321, 121340. [Google Scholar] [CrossRef]
- Zidar, A.; Zupančič, Š.; Kristl, J.; Jeras, M. Impact of Polycaprolactone, Alginate, Chitosan and Zein Nanofiber Physical Properties on Immune Cells for Safe Biomedical Applications. Int. J. Biol. Macromol. 2024, 282, 137029. [Google Scholar] [CrossRef]
- Gopakumar, A.N.; Ccanccapa-Cartagena, A.; Bell, K.; Salehi, M. Development of Crosslinked Polyvinyl Alcohol Nanofibrous Membrane for Microplastic Removal from Water. J. Appl. Polym. Sci. 2024, 141, e55428. [Google Scholar] [CrossRef]
- Liu, H.; Chen, R.; Wang, P.; Fu, J.; Tang, Z.; Xie, J.; Ning, Y.; Gao, J.; Zhong, Q.; Pan, X.; et al. Electrospun Polyvinyl Alcohol-Chitosan Dressing Stimulates Infected Diabetic Wound Healing with Combined Reactive Oxygen Species Scavenging and Antibacterial Abilities. Carbohydr. Polym. 2023, 316, 121050. [Google Scholar] [CrossRef]
- Gautam, L.; Warkar, S.G.; Ahmad, S.I.; Kant, R.; Jain, M. A Review on Carboxylic Acid Cross-Linked Polyvinyl Alcohol: Properties and Applications. Polym. Eng. Sci. 2022, 62, 225–246. [Google Scholar] [CrossRef]
- Costa-Júnior, E.S.; Barbosa-Stancioli, E.F.; Mansur, A.A.P.; Vasconcelos, W.L.; Mansur, H.S. Preparation and Characterization of Chitosan/Poly(Vinyl Alcohol) Chemically Crosslinked Blends for Biomedical Applications. Carbohydr. Polym. 2009, 76, 472–481. [Google Scholar] [CrossRef]
- Salleh, N.A.M.; Afifi, A.M.; Zuki, F.M.; SalehHudin, H.S. Enhancing Mechanical Properties of Chitosan/PVA Electrospun Nanofibers: A Comprehensive Review. Beilstein J. Nanotechnol. 2025, 16, 286–307. [Google Scholar] [CrossRef]
- Olvera Bernal, R.A.; Olekhnovich, R.O.; Uspenskaya, M.V. Chitosan/PVA Nanofibers as Potential Material for the Development of Soft Actuators. Polymers 2023, 15, 2037. [Google Scholar] [CrossRef]
- Menazea, A.A.; Ahmed, M.K. Wound Healing Activity of Chitosan/Polyvinyl Alcohol Embedded by Gold Nanoparticles Prepared by Nanosecond Laser Ablation. J. Mol. Struct. 2020, 1217, 128401. [Google Scholar] [CrossRef]
- Jia, Y.T.; Gong, J.; Gu, X.H.; Kim, H.Y.; Dong, J.; Shen, X.Y. Fabrication and Characterization of Poly (Vinyl Alcohol)/Chitosan Blend Nanofibers Produced by Electrospinning Method. Carbohydr. Polym. 2007, 67, 403–409. [Google Scholar] [CrossRef]
- Hang, A.T.; Tae, B.; Park, J.S. Non-Woven Mats of Poly(Vinyl Alcohol)/Chitosan Blends Containing Silver Nanoparticles: Fabrication and Characterization. Carbohydr. Polym. 2010, 82, 472–479. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Martínez, E.K.T.; Bravo, J.M.C.; Medina, A.S.; González, G.L.P.; Gómez, L.J.V. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Gaydhane, M.K.; Sharma, C.S.; Majumdar, S. Electrospun Nanofibres in Drug Delivery: Advances in Controlled Release Strategies. RSC Adv. 2023, 13, 7312–7328. [Google Scholar] [CrossRef]
- Chi, Y.; Li, K.; Yan, Q.; Koizumi, S.; Shi, L.; Takahashi, S.; Zhu, Y.; Matsue, H.; Takeda, M.; Kitamura, M.; et al. Nonsteroidal Anti-Inflammatory Drug Flufenamic Acid Is a Potent Activator of AMP-Activated Protein Kinase. J. Pharmacol. Exp. Ther. 2011, 339, 257–266. [Google Scholar] [CrossRef]
- Lazar, A.D.; Dinescu, S.; Albu-Kaya, M.G.; Gharbia, S.; Hermenean, A.; Costache, M. Release of the Non-Steroidal Anti-Inflammatory Drug Flufenamic Acid by Multiparticulate Delivery Systems Promotes Adipogenic Differentiation of Adipose-Derived Stem Cells. Materials 2020, 13, 1550. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Y.; Wang, L.; Kong, J.; Pan, W.; Zhang, X.; Chen, L.; Yao, Z.; Zhou, T.; Cao, J. Flufenamic Acid, a Promising Agent for the Sensitization of Colistin-Resistant Gram-Negative Bacteria to Colistin. Microbiol. Spectr. 2023, 11, e0405222. [Google Scholar] [CrossRef]
- Chavez-Dozal, A.A.; Jahng, M.; Rane, H.S.; Asare, K.; Kulkarny, V.V.; Bernardo, S.M.; Lee, S.A. In Vitro Analysis of Flufenamic Acid Activity against Candida Albicans Biofilms. Int. J. Antimicrob. Agents 2014, 43, 86–91. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Liu, H.; Zhu, Y.; Xia, D.; Wang, S.; Gu, R.; Zhang, P.; Liu, Y.; Zhou, Y. Flufenamic Acid Inhibits Adipogenic Differentiation of Mesenchymal Stem Cells by Antagonizing the PI3K/AKT Signaling Pathway. Stem Cells Int. 2020, 2020, 1540905. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ouyang, W.C.; Zhou, X.H.; Jin, T.; Wu, Z.W. Antibacterial Activity and Drug Loading of Moxifloxacin-Loaded Poly(Vinyl Alcohol)/Chitosan Electrospun Nanofibers. Front. Mater. 2021, 8, 36. [Google Scholar] [CrossRef]
- Cheng, Y.; Farasati Far, B.; Jahanbakhshi, M.; Bahrami, S.; Tamimi, P.; Sedaghat, M.; Ghazizadeha, E. Exploring the Potential of a Polyvinyl Alcohol/Chitosan-Based Nanofibrous Matrix for Erythromycin Delivery: Fabrication, in Vitro and in Vivo Evaluation. RSC Adv. 2023, 13, 18450–18460. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, P.; Datta, P. Tranexamic Acid-Loaded Chitosan Electrospun Nanofibers as Drug Delivery System for Hemorrhage Control Applications. J. Drug Deliv. Sci. Technol. 2019, 52, 559–567. [Google Scholar] [CrossRef]
- Pang, Y.; Buanz, A.; Gaisford, S.; Magdysyuk, O.V.; Williams, G.R. Monitoring Polymorphic Phase Transitions in Flufenamic Acid Amorphous Solid Dispersions Using Hyphenated X-Ray Diffraction-Differential Scanning Calorimetry. Mol. Pharm. 2022, 19, 1477–1487. [Google Scholar] [CrossRef]
- Mohandoss, S.; Velu, K.S.; Wahab, R.; Al-Khedhairy, A.A.; Tamizhselvi, R.; Napoleon, A.A.; Palanisamy, S.; You, S.; Lee, Y.R. Enhanced Solubility and Biological Activities of Flufenamic Acid through β-Cyclodextrin Derivatives Inclusion Complexes: A Comprehensive Study. J. Mol. Liq. 2024, 402, 124765. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, L.; Ma, J.; Wang, X.; Wang, Y.; Ran, F.; Wang, Y.; Ma, H.; Yu, S. Electrospinning of Fucoidan/Chitosan/Poly(Vinyl Alcohol) Scaffolds for Vascular Tissue Engineering. Fibers Polym. 2017, 18, 922–932. [Google Scholar] [CrossRef]
- Rahnama, S.; Movaffagh, J.; Shahroodi, A.; Jirofti, N.; Fazly Bazzaz, B.S.; Beyraghdari, M.; Hashemi, M.; Kalalinia, F. Development and Characterization of the Electrospun Melittin-Loaded Chitosan Nanofibers for Treatment of Acne Vulgaris in Animal Model. J. Ind. Text. 2022, 52, 152808372211124. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Razak, S.I.A.; Bohari, S.P.M.; Shahir, S.; Salihu, R.; Kadir, M.R.A.; Nayan, N.H.M. Vitamin D3-Loaded Electrospun Cellulose Acetate/Polycaprolactone Nanofibers: Characterization, in-Vitro Drug Release and Cytotoxicity Studies. Int. J. Biol. Macromol. 2021, 181, 82–98. [Google Scholar] [CrossRef]
- Yu, H.; Chen, D.; Lu, W.; Zhang, C.; Wang, H.; Peng, Z.; Jiang, H.; Xiao, C. Characterization of Polyvinyl Alcohol/Chitosan Nanofibers Loaded with Royal Jelly by Blending Electrospinning for Potential Wound Dressings. Int. J. Biol. Macromol. 2025, 307, 141977. [Google Scholar] [CrossRef]
- Iqbal, H.; Khan, B.A.; Khan, Z.U.; Razzaq, A.; Khan, N.U.; Menaa, B.; Menaa, F. Fabrication, Physical Characterizations and in Vitro Antibacterial Activity of Cefadroxil-Loaded Chitosan/Poly(Vinyl Alcohol) Nanofibers against Staphylococcus Aureus Clinical Isolates. Int. J. Biol. Macromol. 2020, 144, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Amiri Darban, S.; Fazly Bazzaz, B.S.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-Loaded Chitosan-PEO Nanofibers for Local Antibiotic Delivery and Wound Healing. Int. J. Biol. Macromol. 2020, 162, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hu, Q.; Liu, S.; Wang, Y.; Zhang, H.; Chen, J.; Yao, G. Electrospinning/3D Printing Drug-Loaded Antibacterial Polycaprolactone Nanofiber/Sodium Alginate-Gelatin Hydrogel Bilayer Scaffold for Skin Wound Repair. Int. J. Biol. Macromol. 2024, 275, 129705. [Google Scholar] [CrossRef]
- Karabulut, H.; Xu, D.; Ma, Y.; Tut, T.A.; Ulag, S.; Pinar, O.; Kazan, D.; Guncu, M.M.; Sahin, A.; Wei, H.; et al. A New Strategy for the Treatment of Middle Ear Infection Using Ciprofloxacin/Amoxicillin-Loaded Ethyl Cellulose/Polyhydroxybutyrate Nanofibers. Int. J. Biol. Macromol. 2024, 269, 131794. [Google Scholar] [CrossRef]
- Khasteband, M.; Sharifi, Y.; Akbari, A. Chrysin Loaded Polycaprolactone-Chitosan Electrospun Nanofibers as Potential Antimicrobial Wound Dressing. Int. J. Biol. Macromol. 2024, 263, 130250. [Google Scholar] [CrossRef]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Sagharjoghi Farahani, M.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of Curcumin Loaded Chitosan Nanoparticle within Poly (ε-Caprolactone) and Gelatin Fiber Mat for Wound Healing and Layered Dermal Reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef]
- Karuppannan, S.K.; Dowlath, M.J.H.; Ramalingam, R.; Musthafa, S.A.; Ganesh, M.R.; Chithra, V.; Ravindran, B.; Arunachalam, K.D. Quercetin Functionalized Hybrid Electrospun Nanofibers for Wound Dressing Application. Mater. Sci. Eng. B 2022, 285, 115933. [Google Scholar] [CrossRef]
- Mohiti-Asli, M.; Saha, S.; Murphy, S.V.; Gracz, H.; Pourdeyhimi, B.; Atala, A.; Loboa, E.G. Ibuprofen Loaded PLA Nanofibrous Scaffolds Increase Proliferation of Human Skin Cells in Vitro and Promote Healing of Full Thickness Incision Wounds in Vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 327–339. [Google Scholar] [CrossRef]
- Yu, F.; Li, M.; Yuan, Z.; Rao, F.; Fang, X.; Jiang, B.; Wen, Y.; Zhang, P. Mechanism Research on a Bioactive Resveratrol– PLA–Gelatin Porous Nano-Scaffold in Promoting the Repair of Cartilage Defect. Int. J. Nanomed. 2018, 13, 7845–7858. [Google Scholar] [CrossRef]







| Sample ID | CHS (3% w/v) | PVA (10% w/v) | CHS:PVA Ratio (w/w) | FFA Content |
|---|---|---|---|---|
| CSPA-1 | 0.9 g in 30 mL | 7 g in 70 mL | 30:70 | - |
| CSPA-2 | 0.9 g in 30 mL | 7 g in 70 mL | 50:50 | - |
| CSPA-3 | 0.9 g in 30 mL | 7 g in 70 mL | 70:30 | - |
| FCSPA | 0.9 g in 30 mL | 7 g in 70 mL | 70:30 (CSPA-3) | 25 mg in 5 mL |
| Drug | Polymer Matrix | EE (%) | Release Duration (h) | Bioactivity | Ref. |
|---|---|---|---|---|---|
| FFA | CSPA | 91.2 | 72 | Antioxidant | This study |
| Curcumin | PCL/PEG | 70 | 48 | Anti-inflammatory | [46] |
| Quercetin | PVA/CHS | 78 | 12 | Antioxidant | [47] |
| Ibuprofen | PLA | 65–75 | 8–24 | Anti-inflammatory | [48] |
| Resveratrol | Gelatin | 80–90 | 48 | Antioxidant | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakthi Velu, K.; Aslam, M.; Srinivasan, R.; Somu, P.; Mohandoss, S. Flufenamic Acid-Loaded Electrospun Nanofibers Based on Chitosan/Poly(vinyl alcohol) Polymeric Composites for Drug Delivery in Biomedical Applications. Polymers 2025, 17, 1411. https://doi.org/10.3390/polym17101411
Sakthi Velu K, Aslam M, Srinivasan R, Somu P, Mohandoss S. Flufenamic Acid-Loaded Electrospun Nanofibers Based on Chitosan/Poly(vinyl alcohol) Polymeric Composites for Drug Delivery in Biomedical Applications. Polymers. 2025; 17(10):1411. https://doi.org/10.3390/polym17101411
Chicago/Turabian StyleSakthi Velu, Kuppu, Mohammad Aslam, Ramachandran Srinivasan, Prathap Somu, and Sonaimuthu Mohandoss. 2025. "Flufenamic Acid-Loaded Electrospun Nanofibers Based on Chitosan/Poly(vinyl alcohol) Polymeric Composites for Drug Delivery in Biomedical Applications" Polymers 17, no. 10: 1411. https://doi.org/10.3390/polym17101411
APA StyleSakthi Velu, K., Aslam, M., Srinivasan, R., Somu, P., & Mohandoss, S. (2025). Flufenamic Acid-Loaded Electrospun Nanofibers Based on Chitosan/Poly(vinyl alcohol) Polymeric Composites for Drug Delivery in Biomedical Applications. Polymers, 17(10), 1411. https://doi.org/10.3390/polym17101411








