Chromium-Doped Biomass-Based Hydrochar-Catalyzed Synthesis of 5-Hydroxymethylfurfural from Glucose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Biomass-Derived Hydrochar Catalysts
2.3. Characterization
2.4. Catalytic Conversion of Glucose to HMF
2.5. Analysis of Catalytic Products
3. Results and Discussion
3.1. Characterization of Hydrochar Catalysts
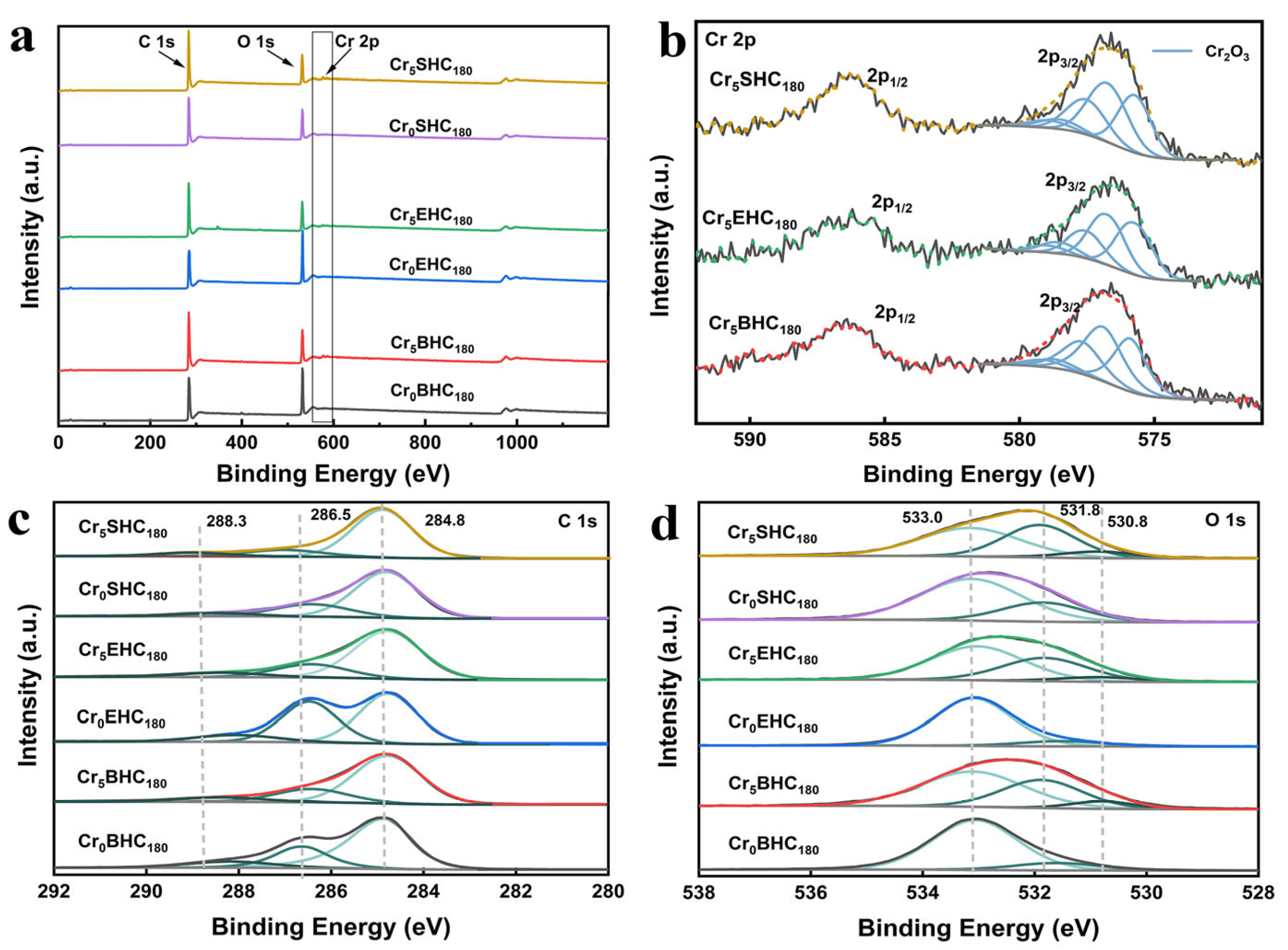
3.1.1. XPS Analysis
- Figure 1 presents the XPS spectra of all the hydrochar catalysts derived from starch, eucalyptus, and bagasse. As shown in Figure 1a, all Cr-doped catalysts of Cr5SHC180 (starch-based), Cr5EHC180 (eucalyptus-based), and Cr5BHC180 (bagasse-based) contain Cr, C, and O elements. The Cr 2p spectra (Figure 1b) display two peaks at 586.9 and 577.4 eV, which can be assigned to Cr2p1/2 and Cr2p3/2 of Cr(III) [19], respectively. Deconvolution of the Cr 2p3/2 peaks reveals five sub-peaks at 576.5, 577.5, 578.3, 579.3, and 579.7 eV, which are indicative of the presence of Cr2O3 [20], confirming successful loading of chromium oxide on all of the hydrochar catalysts.
- As shown in Figure 1c, the C 1s spectra show the peaks that can be attributed to C-C/CHx/C=C (284.6 ± 0.2 eV), C-OH/C-O-C (285.7 ± 0.2 eV), and C=O (287.3 ± 0.2 eV) [21]. In the O 1s spectra provided in Figure 1d, there are the peaks that can be assigned to C-OH/C-O-C (533.0 ± 0.2 eV), C=O (531.8 ± 0.2 eV), and Cr-O (530.8 ± 0.2 eV) [22]. In comparison to the respective undoped counterparts, all of the Cr-doped catalysts showed a significantly reduced content of C–OH groups on their surfaces, particularly Cr5SHC180 and Cr5BHC180. Such reduction is attributed to the enhanced acidity due to the introduction of CrCl3 during hydrothermal synthesis. Bagasse, with a higher hemicellulose content (23–27%) and lower lignin content (19–32%) than eucalyptus (hemicellulose: 18–23%; lignin: 29–33%) [23], was more reactive. When CrCl3 is present, the metal ions disrupt the fibrous structure of biomass by osmosis, catalyzing the dehydration and decarboxylation of α-glycosidic bonds in starch and hemicellulose [22]. As illustrated in Table 1, the content of C–OH on the surface was markedly decreased from 72.65 to 50.41 for Cr5SHC180, from 86.92 to 57.14 for Cr5BHC180, and from 89.03 to 57.21 for Cr5EHC180. Additionally, the C–C/CHx/C=C peak intensities are increased significantly, indicating that chromium salts can enhance the aromatization process during hydrothermal carbonization [24].
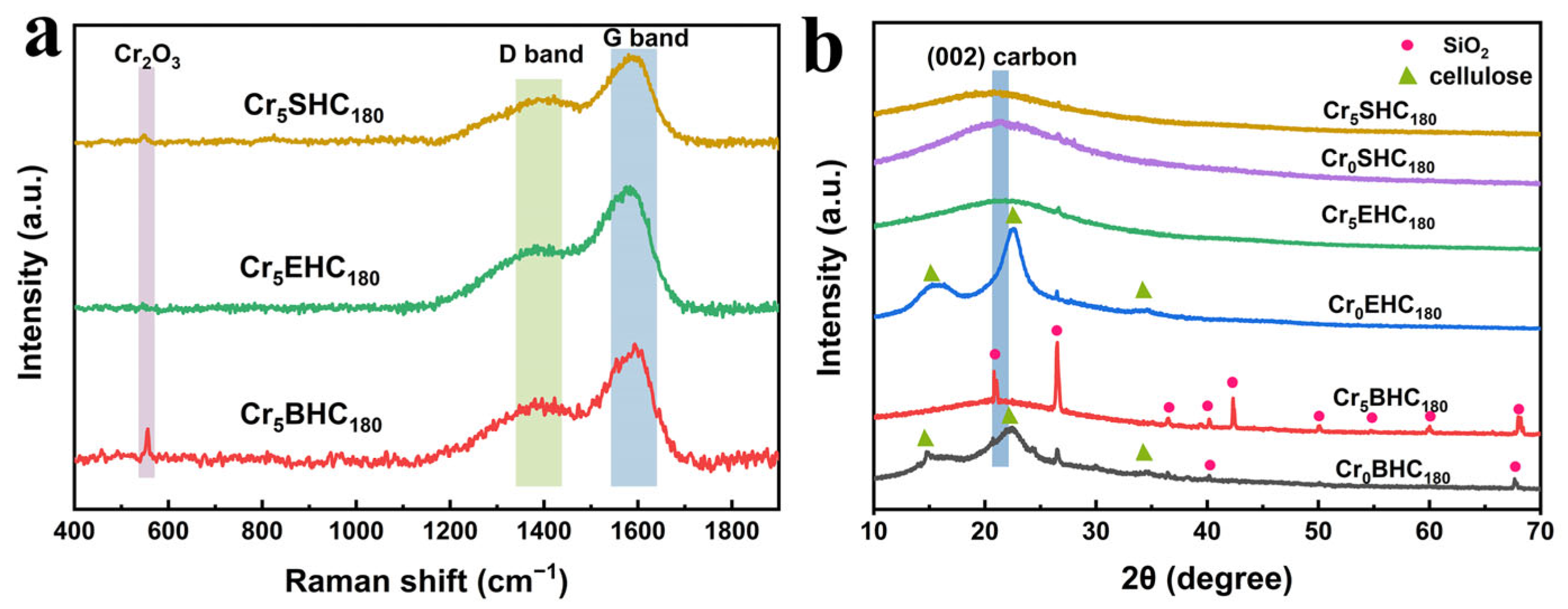
3.1.2. XRD and Raman Spectral Analyses
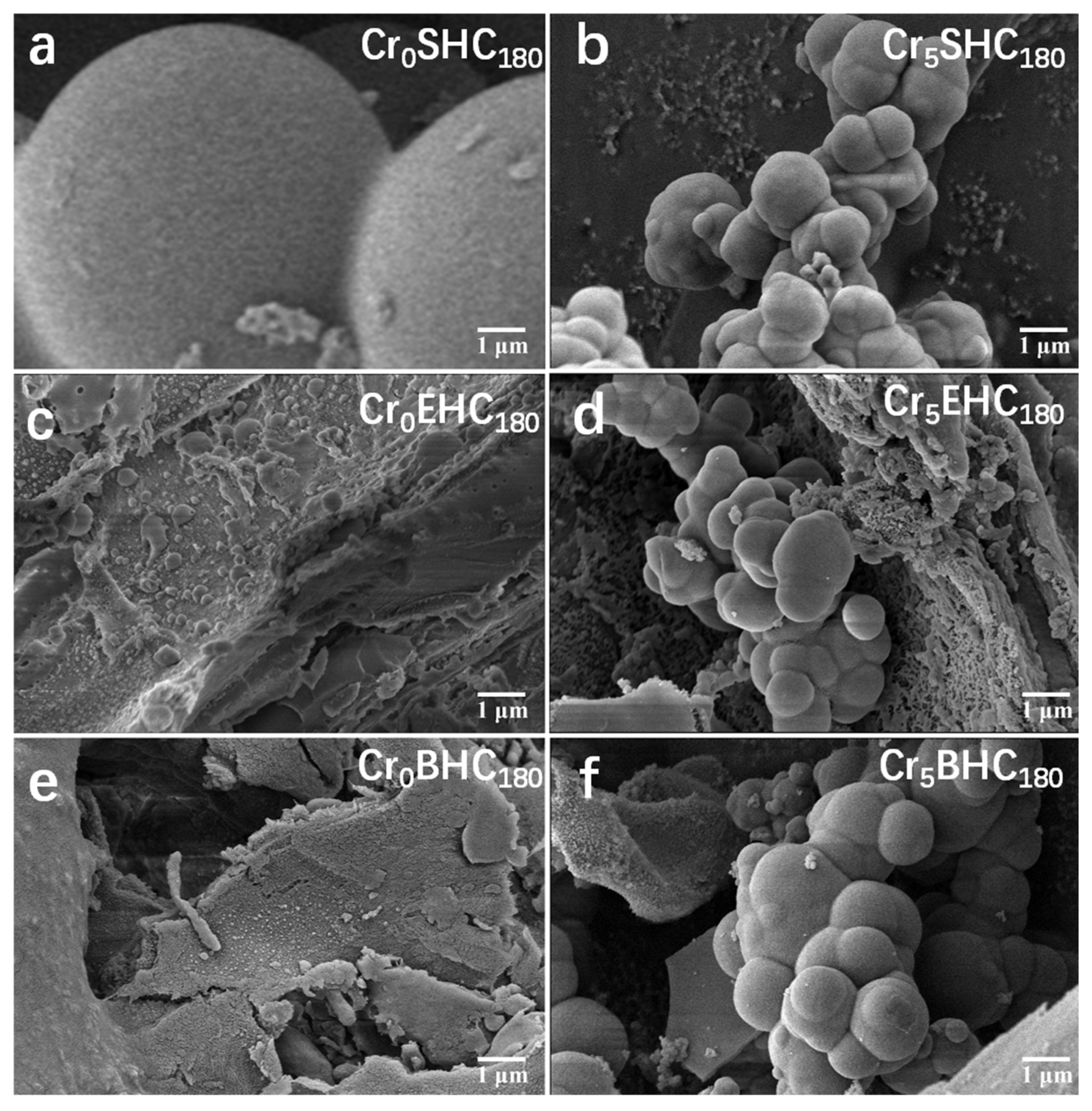
3.1.3. SEM Morphological Analysis
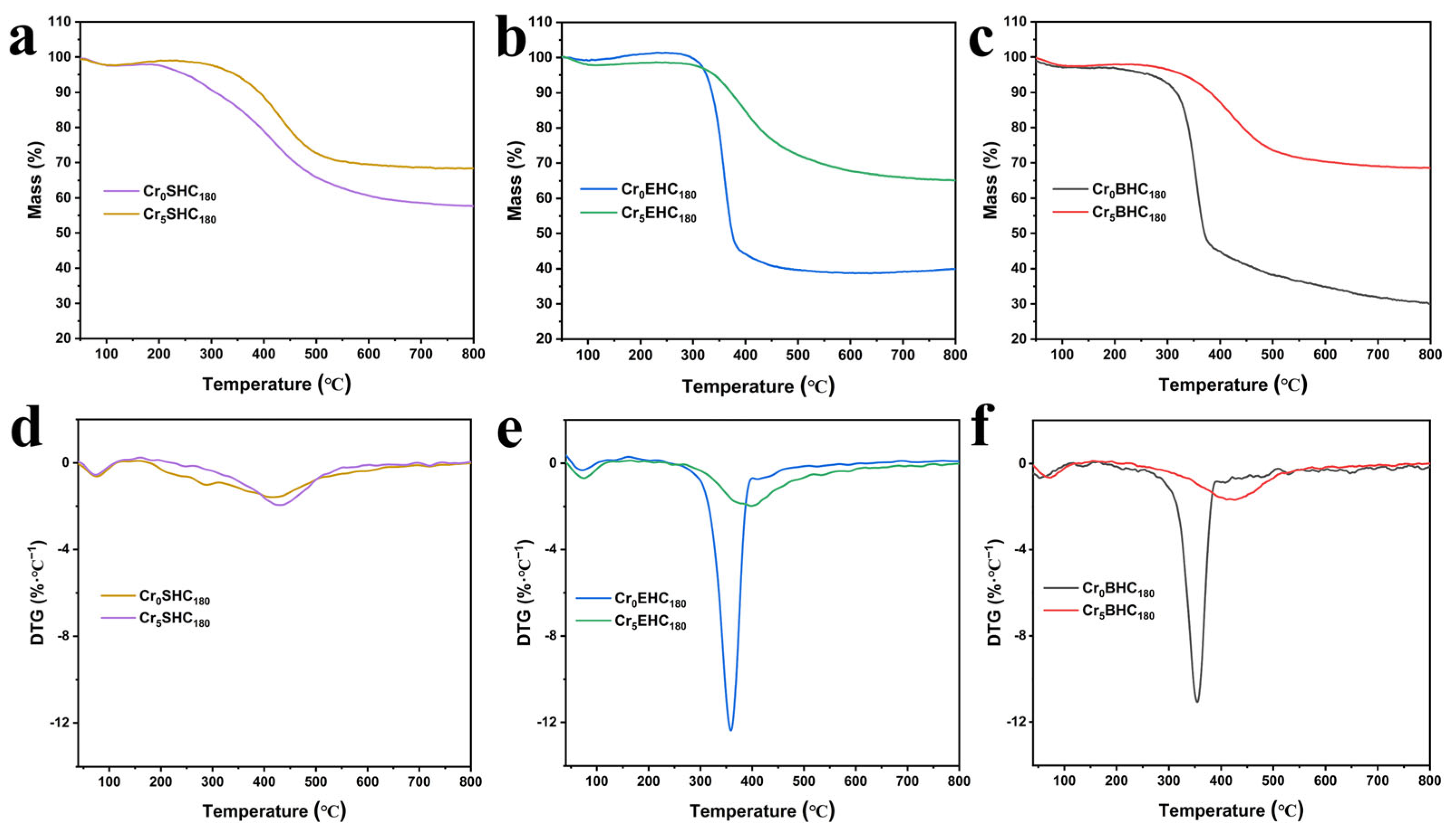
3.1.4. Thermal Stability Analysis
- The hydrochar catalysts were analyzed for their thermal stability by thermogravimetric (TG) and differential thermal analysis (DTA), and the results are presented in Figure 4, from which it is clear that both the bagasse- and eucalyptus-derived hydrochars follow similar thermal degradation patterns consisting of three main stages: dehydration, devolatilization, and combustion [36]. The thermal stability of the hydrochars is significantly enhanced with the incorporation of Cr. For the eucalyptus-based hydrochar, the temperature corresponding to the maximum degradation rate is increased from 359 °C to 400 °C, while mass loss is decreased from 60% to 36%. Similarly, in the bagasse-based hydrochar, the peak degradation temperature is shifted from 355 °C to 426 °C, and the mass loss drops from 70% to 32%. In the case of starch-based hydrochar, the maximum degradation temperature is slightly increased from 422 °C to 436 °C and the mass loss is reduced from 32% to 18% with Cr doping. All of these results demonstrate that the incorporation of Cr increases the resistance of the catalyst to thermal degradation, thereby offering improved thermal stability.
3.2. Catalytic Performance of Hydrochar Catalysts
3.2.1. Effects of Acid Density and Strength on Catalytic Performance
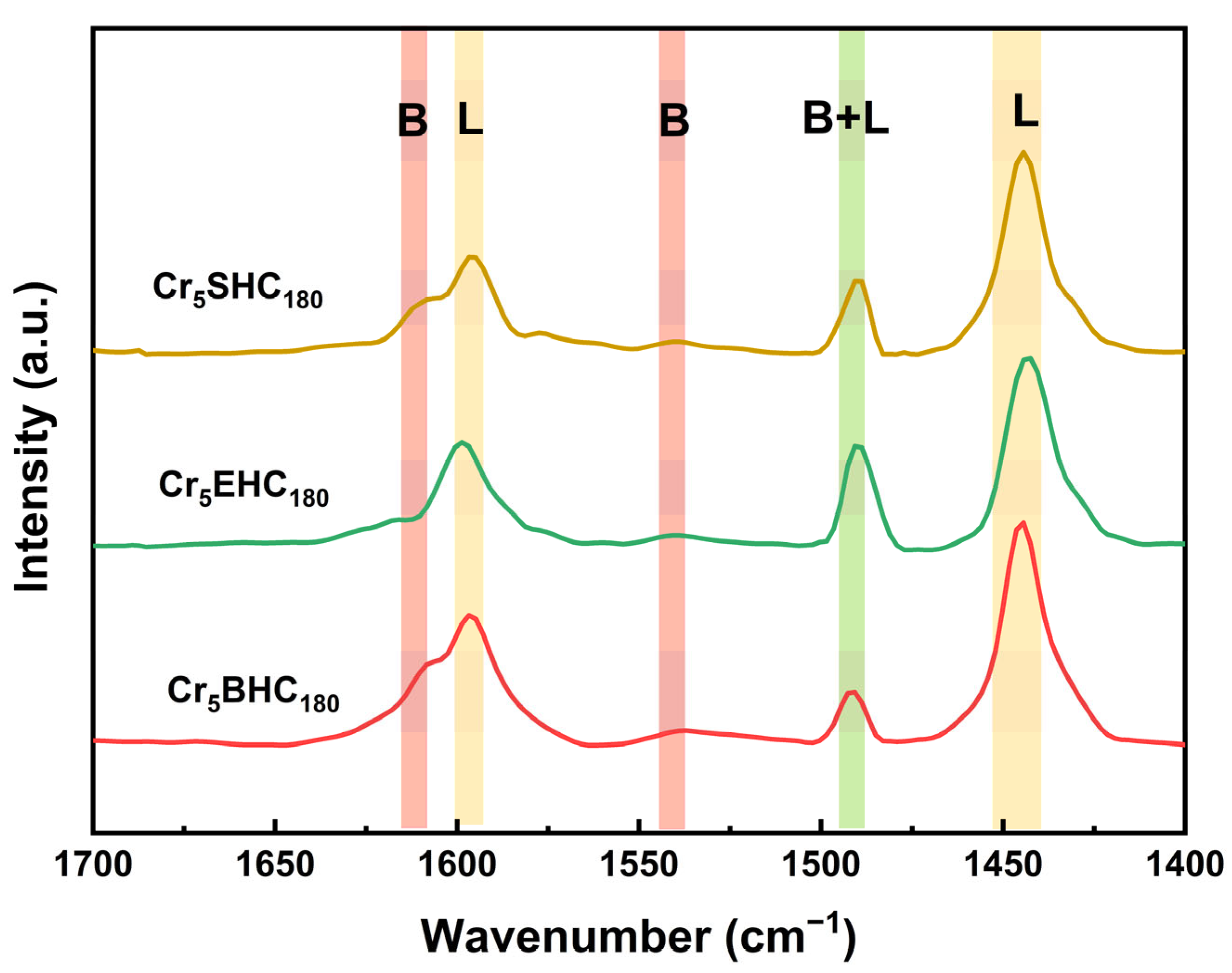
- The impact of the incorporation of Cr on the acid density and strength of the catalysts is summarized in Table 3. For the undoped catalysts of Cr0SHC180, Cr0EHC180, and Cr0BHC180, the initial potentials are 221, 159, and 225 mV, respectively, with corresponding acid densities of 0.14, 0.10, and 0.16 mmol/g. Upon Cr doping, the respective values were increased significantly to 247, 164, and 265 mV, and to 0.20, 0.18, and 0.24 mmol/g. Among the catalysts, Cr5BHC180 exhibited the highest acid strength and density, correlating with its superior catalytic performance, with both increased HMF yield from 36.7% (undoped) to 52.2% (doped) and improved HMF selectivity from 40.2% (undoped) to 52.2% (doped). In contrast, Cr5EHC180 showed the lowest HMF yield, attributed to its comparatively lower acid strength and density [37]. This enhancement of acidic functionality directly influences the catalytic activity, as demonstrated by the trends of increasing glucose conversion, fructose yield, and HMF yield and selectivity with acid strength and density [38,39]. The types of acid sites and the amounts of acid in the prepared catalysts were determined using Py-FTIR (Figure 5). Specifically, Cr5BHC180 had the highest concentration of Brønsted acid (4.11 μmol g−1) and Lewis acid (31.86 μmol g−1), with a concentration ratio of Brønsted acid to Lewis acid of 0.13. In comparison, the Brønsted acid concentrations for Cr5SHC180, and Cr5EHC180 were 2.46 and 2.84 μmol g−1, and the Lewis acid concentrations were 28.65 and 29.62 μmol g−1, with a respective concentration ratio of Brønsted acid to Lewis acid of 0.09 and 0.10. All of these results confirm that Cr5BHC180 possesses the highest acidity and, thus, the highest catalytic efficiency for converting glucose into HMF.
3.2.2. Influence of Reaction Conditions on Catalytic Performance
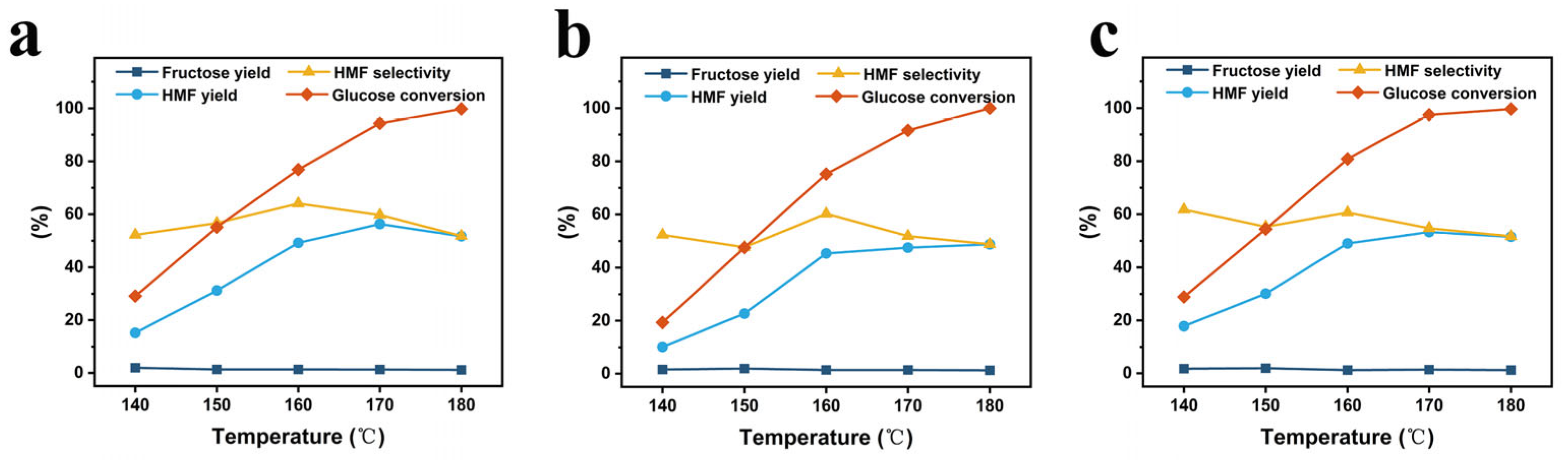
- The influence of the conditions of reaction temperature and reaction time on the catalytic performance of the catalysts was evaluated with respect to the HMF yield, HMF selectivity, fructose yield, and glucose conversion. For the reaction temperature, the analysis was performed at varying temperatures of 140 °C, 150 °C, 160 °C, 170 °C, and 180 °C with all other reaction conditions the same, and the results are provided in Figure 6. From the figure, it is evident that at 140 °C, 150 °C, 160 °C, 170 °C, and 180 °C, the HMF yields are 15.2%, 31.2%, 49.2%, 56.3%, and 51.7%, respectively, for the starch-based Cr5SHC180; they are 10.1%, 22.6%, 45.3%, 47.5%, and 48.8% for the eucalyptus-based Cr5EHC180; and 17.8%, 30.1%, 49.0%, 53.3%, and 51.5%, respectively, for the bagasse-based Cr5BHC180. With the increase in the reaction temperature, the glucose conversion rate also increased from 20.0% to 99.6%, and the fructose yield remained stable at 1.5%. In all cases, the HMF yield presented a trend of increasing at first and then decreasing with temperature due to side reactions like HMF rehydration and condensation after reaching certain high temperatures, and the selectivity of HMF also decreased [41]. As a result, the optimal reaction temperatures for maximum HMF yield were identified as 170 °C for Cr5SHC180 and Cr5EHC180, and 180 °C for Cr5BHC180.
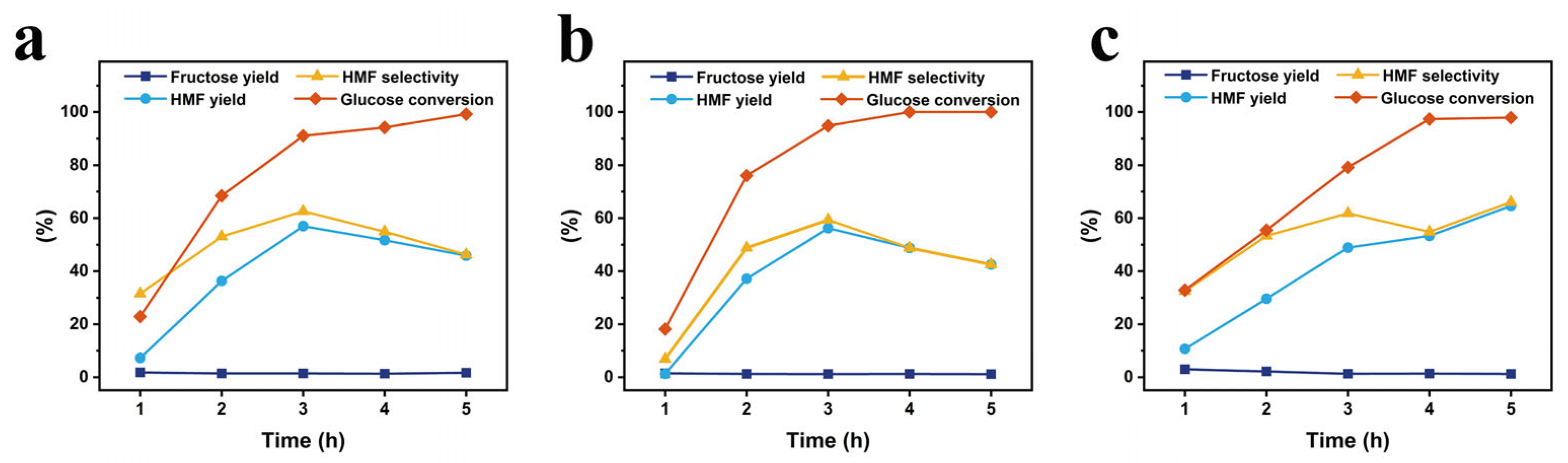
- For the reaction time, the experiments were performed at the respective optimal temperature for each the catalysts and the reaction times of 1, 2, 3, 4, and 5 h, with all other reaction conditions the same, and the results are depicted in Figure 7. As indicated in the figure, the HMF yields at 1, 2, 3, 4, and 5 h are 7.2%, 36.3%, 57.0%, 51.7%, and 45.8%, respectively, for the starch-based Cr5SHC180; 1.24%, 37.14%, 56.2%, 48.8%, and 42.5%, respectively, for eucalyptus-based Cr5EHC180; and 10.7%, 29.6%, 48.9%, 53.3%, and 64.5%, respectively, for the bagasse-based Cr5BHC180. The optimal reaction times and performance metrics for each catalyst were as follows: (1) Cr5SHC180: at 170 °C for 3 h, the HMF yield was 57.0%, HMF selectivity was 62.6%, glucose conversion was 91.0%, and fructose yield was 1.5%; (2) Cr5EHC180: at 180 °C for 3 h, the HMF yield was 56.2%, HMF selectivity was 59.3%, glucose conversion was 94.8%, and fructose yield was 1.2%; (3) Cr5BHC180: at 170 °C for 5 h, the HMF yield reached 64.5%, with an HMF selectivity of 66.0%, glucose conversion of 97.8%, and fructose yield of 1.3% (the HPLC chromatograms of the final HMF detection of the catalysts are shown in Figures S2 and S3).
3.2.3. Catalyst Recyclability
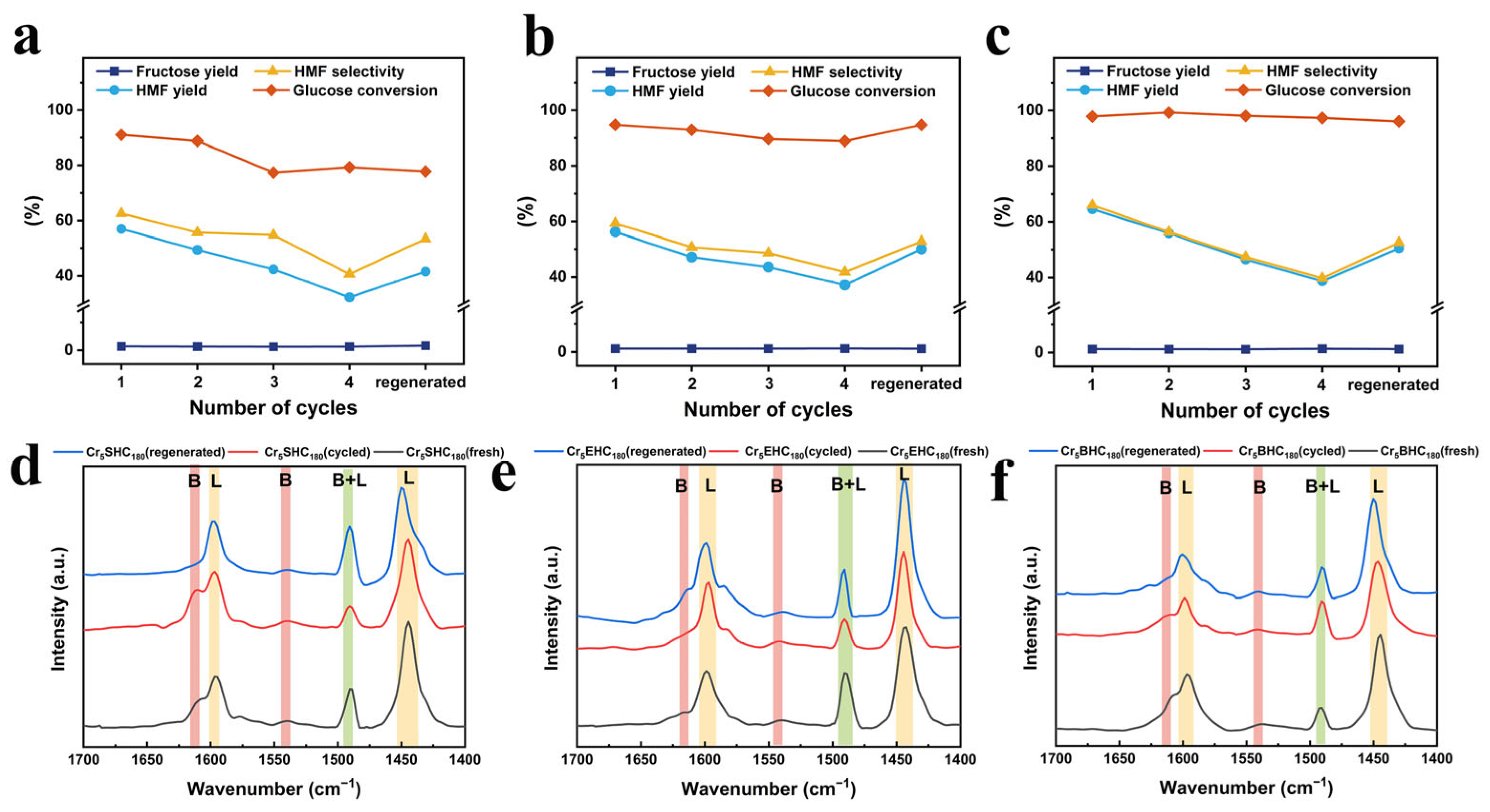
- The reusability of the Cr5SHC180, Cr5EHC180, and Cr5BHC180 catalysts was evaluated over four consecutive reaction cycles under the optimal conditions, and the results are presented in Figure 8 and Table 4. After every reaction cycle, the catalysts were recovered through centrifugation, washing, and drying and then were reused in the next cycle. From the results, it is evident that all of the HMF yields showed a trend of slightly decreasing over the cycles, with values of 32.2%, 37.1%, and 38.7% for Cr5SHC180, Cr5EHC180 and Cr5BHC180, respectively, in the last cycle. To investigate the cause of this decline, the spent catalysts were regenerated by aerobic calcination at 300 °C for 1 h. After the regeneration, the HMF yield was recovered to 41.6%, 50.1%, and 50.5%, respectively, suggesting that deactivation is primarily due to the deposition of carbonaceous by-products, such as humins, on active sites.
- Further analyses of deactivation mechanisms included assessments of Cr leaching, the BET surface area, and the pore diameter. After 7 days in water, the content of leached Cr was measured at 1.12, 0.56, and 0.89 mg/L for Cr5SHC180, Cr5EHC180, and Cr5BHC180, respectively (Figure S4). The specific surface areas of the fresh catalysts were 7.84, 20.59, and 19.43 m2/g, which were decreased to 5.20, 12.16, and 12.71 m2 g−1 after four reaction cycles, but then increased significantly to 96.94, 105.43, and 42.97 m2 g−1 (Table 4, Figure S5) after the generation, indicating a strengthened pore structure.
- The acidic properties were evaluated using Py-FTIR, with the results illustrated in Figure 8d,e, and provided in Table 4. As indicated in the figure, the Lewis acid contents are 28.65, 29.62, and 31.86 μmol g−1, and Brønsted acid contents are 2.46, 2.84, and 4.11 μmol g−1 for Cr5SHC180, Cr5EHC180, and Cr5BHC180, respectively; after four reaction cycles, the contents were decreased to 19.94, 26.52, and 27.71 μmol g−1 for Lewis acid, and to 2.23, 2.92, and 2.87 μmol g−1 for Brønsted acid, respectively. Moreover, the total acid content also declined from 31.11–35.97 μmol g−1 to 22.19–30.58 μmol g−1.
- Following the calcination, the acid content of Cr5SHC180, Cr5EHC180, and Cr5BHC180 was restored to 26.76, 33.87, and 32.15 μmol g−1 for the Lewis acid, and to 2.30, 2.00, and 3.46 μmol g−1 for the Brønsted acid, giving total acid amounts of 29.06, 35.87, and 35.61 μmol g−1, respectively. In conclusion, catalyst deactivation is primarily attributed to the degradation of the pore structure, loss of acidic sites, and humin deposition, all of which are partially reversible and can be restored through regeneration by calcination.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Shao, L.; Wu, Z.; Zhan, P.; Zhang, L. Design and Synthesis of Porous Organic Polymers: Promising Catalysts for Lignocellulose Conversion to 5-Hydroxymethylfurfural and Derivates. Polymers 2023, 15, 2630. [Google Scholar] [CrossRef] [PubMed]
- Messori, A.; Fasolini, A.; Mazzoni, R. Advances in Catalytic Routes for the Homogeneous Green Conversion of the Bio-Based Platform 5-Hydroxymethylfurfural. ChemSusChem 2022, 15, e202200228. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Zhen, M.; Li, W.; Liu, L.; Liu, J.; Zhang, S.; Nie, Y.; Bai, C.; Bai, X.; Ju, M. Efficient catalytic conversion of glucose into 5-hydroxymethylfurfural by aluminum oxide in ionic liquid. Appl. Catal. B Environ. 2019, 253, 1–10. [Google Scholar] [CrossRef]
- Ke, K.; Ji, H.; Shen, X.; Kong, F.; Li, B. Pressure Reduction Enhancing the Production of 5-Hydroxymethylfurfural from Glucose in Aqueous Phase Catalysis System. Polymers 2021, 13, 2096. [Google Scholar] [CrossRef]
- You, L.; Liu, T.; Liu, D.; Zhao, X. Efficient conversion of glucose to 5-hydroxymethylfurfural by synergetic catalysis of enzymes and chemical catalysts towards potential large-scale continuous operation. Chem. Eng. J. 2023, 459, 141552. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M.M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, H.; Xiao, H.; Li, W.; Jin, Y.; Wei, W.; Wu, S. Advance in constructing acid catalyst-solvent combinations for efficient transformation of glucose into 5-Hydroxymethylfurfural. Mol. Catal. 2020, 498, 111254. [Google Scholar] [CrossRef]
- Rajabbeigi, N.; Torres, A.I.; Lew, C.M.; Elyassi, B.; Ren, L.; Wang, Z.; Je Cho, H.; Fan, W.; Daoutidis, P.; Tsapatsis, M. On the kinetics of the isomerization of glucose to fructose using Sn-Beta. Chem. Eng. Sci. 2014, 116, 235–242. [Google Scholar] [CrossRef]
- Adedeji, J.; Smarte Anekwe, I.M.; Okiemute Akpasi, S.O.; Lewis Kiambi, S. Biochar Development as a Catalyst and Its Application. In Biochar—Productive Technologies, Properties and Applications; Bartoli, M., Giorcelli, M., Tagliaferro, A., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, G.Y.; Gao, M.M.; Cao, S.S.; Li, L.; Liu, S.W.; Xie, C.X.; Huang, L.; Yu, S.T.; Ragauskas, A.J. Clean production of 5-hydroxymethylfurfural from cellulose using a hydrothermal/biomass-based carbon catalyst. J. Clean. Prod. 2019, 213, 1096–1102. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, F.; Lang, Q.; Liu, T.; Peng, N.; Liu, Z. Facile one-pot synthesis of iron nanoparticles immobilized into the porous hydrochar for catalytic decomposition of phenol. Appl. Catal. B Environ. 2017, 204, 566–576. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Shuang, E.; Jin, C.; Gong, C.; Sheng, K.; Zhang, X. Facile one-pot synthesis of functional hydrochar catalyst for biomass valorization. Fuel 2022, 315, 123172. [Google Scholar] [CrossRef]
- Kang, X.; You, Z.; Huang, Y.; Peng, J.; Zhang, J.; Ragauskas, A.J.; Zhang, Z.; Song, X. Multi-catalytic active site biochar-based catalysts for glucose isomerized to fructose: Experiments and density functional theory study. Adv. Compos. Hybrid Mater. 2024, 7, 54. [Google Scholar] [CrossRef]
- Al-Amsyar, S.M.; Adam, F.; Ng, E.-P. Aluminium oxide-silica/carbon composites from rice husk as a bi-functional heterogeneous catalyst for the one-pot sequential reaction in the conversion of glucose. Surf. Interfaces 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Shao, Y.C.; Ding, Y.; Dai, J.H.; Long, Y.Y.; Hu, Z.T. Synthesis of 5-hydroxymethylfurfural from dehydration of biomass-derived glucose and fructose using supported metal catalysts. Green Synth. Catal. 2021, 2, 187–197. [Google Scholar] [CrossRef]
- Rakngam, I.; Khemthong, P.; Osakoo, N.; Rungnim, C.; Youngjan, S.; Thongratkaew, S.; Pengsawang, A.; Rungtaweevoranit, B.; Faungnawakij, K.; Kidkhunthod, P.; et al. Unraveling Structural and Acidic Properties of Al-SBA-15-supported Metal Phosphates: Assessment for Glucose Dehydration. ChemPlusChem 2023, 88, e202300326. [Google Scholar] [CrossRef]
- Perez, G.A.P.; Pandey, S.; Dumont, M.J. Sulfosuccinic acid-based metal-center catalysts for the synthesis of HMF from carbohydrates. Catal. Today 2023, 418, 114127. [Google Scholar] [CrossRef]
- Chen, Y.; An, D.; Sun, S.; Gao, J.; Qian, L. Reduction and Removal of Chromium VI in Water by Powdered Activated Carbon. Materials 2018, 11, 269. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Chai, X.; He, H.; Fan, H.; Kang, X.; Song, X. A hydrothermal-carbonization process for simultaneously production of sugars, graphene quantum dots, and porous carbon from sugarcane bagasse. Bioresour. Technol. 2019, 282, 142–147. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, H.; Li, Y.; Yi, L.; Zhang, X.; Hu, H.; Yao, H. Correlations between hydrochar properties and chemical constitution of orange peel waste during hydrothermal carbonization. Bioresour. Technol. 2018, 265, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.M.d.; Sevastyanova, O.; Pennaa, L.S.; Silvaa, B.P.d.; Lindströmb, M.E.; Colodette, J.L. Assessment of chemical transformations in eucalyptus, sugarcane bagasse and straw during hydrothermal, dilute acid, and alkaline pretreatments. Ind. Crops Prod. 2015, 73, 118–126. [Google Scholar] [CrossRef]
- Zheng, K.; Hu, S.; Li, A.; Ren, Q.; Xu, K.; Xu, J.; Jiang, L.; Wang, Y.; Su, S.; Xiang, J. Catalytic effect of metal salts on deoxygenation and aromatization reaction during pressurized pyrolysis of corncob waste at mild temperatures. Energy 2024, 291, 130338. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Xu, R.; Ning, X.; Zhang, J.; Guo, X.; Ye, L.; Li, J.; Jiang, C.; Wang, P.; et al. Hydrothermal carbonization of forest waste into solid fuel: Mechanism and combustion behavior. Energy 2022, 246, 123343. [Google Scholar] [CrossRef]
- Xu, S.Q.; Pan, D.H.; Hu, F.; Wu, Y.F.; Wang, H.Z.; Chen, Y.; Yuan, H.; Gao, L.J.; Xiao, G.M. Highly efficient Cr/beta zeolite catalyst for conversion of carbohydrates into 5-hydroxymethylfurfural: Characterization and performance. Fuel Process. Technol. 2019, 190, 38–46. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, S.; Liu, J.; E, S.; Jin, C.; Xu, Z.; Zhang, X. Hydrothermal carbonization of cellulose and xylan into hydrochars and application on glucose isomerization. J. Clean. Prod. 2019, 237, 117831. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Song, X.; Yang, F.; Cheng, F. Co-hydrothermal carbonization of sewage sludge and coal slime with sulfuric acid for N, S doped hydrochar. J. Clean. Prod. 2022, 354, 131615. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Qureshi, S.S.; Baloch, H.A.; Siddiqui, M.T.; Takkalkar, P.; Mubarak, N.M.; Dumbre, D.K.; Griffin, G.J.; Madapusi, S.; Tanksale, A. Microwave Hydrothermal Carbonization of Rice Straw: Optimization of Process Parameters and Upgrading of Chemical, Fuel, Structural and Thermal Properties. Materials 2019, 12, 403. [Google Scholar] [CrossRef]
- Melo, C.A.; Junior, F.H.S.; Bisinoti, M.C.; Moreira, A.B.; Ferreira, O.P. Transforming Sugarcane Bagasse and Vinasse Wastes into Hydrochar in the Presence of Phosphoric Acid: An Evaluation of Nutrient Contents and Structural Properties. Waste Biomass Valorization 2017, 8, 1139–1151. [Google Scholar] [CrossRef]
- Saqib, N.U.; Baroutian, S.; Sarmah, A.K. Physicochemical, structural and combustion characterization of food waste hydrochar obtained by hydrothermal carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef]
- Yu, X.; Peng, L.; Gao, X.; He, L.; Chen, K. One-step fabrication of carbonaceous solid acid derived from lignosulfonate for the synthesis of biobased furan derivatives. RSC Adv. 2018, 8, 15762–15772. [Google Scholar] [CrossRef] [PubMed]
- Seroka, N.S.; Taziwa, R.T.; Khotseng, L. Extraction and Synthesis of Silicon Nanoparticles (SiNPs) from Sugarcane Bagasse Ash: A Mini-Review. Appl. Sci. 2022, 12, 2310. [Google Scholar] [CrossRef]
- Wei, W.; Wu, M.; Xu, H.; Zhang, X.; Ren, W. Modulation of the properties of starch gels by a one-step extrusion modification method based on Ca2+-citric acid synergistic crosslinking. Int. J. Biol. Macromol. 2024, 257, 128607. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Romero, I.; Moya, M.; Cara, C.; Ruiz, E.; Castro, E. Pretreatment of olive tree biomass with FeCl3 prior enzymatic hydrolysis. Bioresour. Technol. 2013, 128, 180–187. [Google Scholar] [CrossRef]
- Chen, S.; Tang, X.; Chen, J.; Xue, Y.; Wang, Y.; Xu, D. Regulation of slow-release performance of high-sugar biomass waste filter mud and sugarcane bagasse by co-hydrothermal carbonization and potential evaluation of hydrochar-based slow-release fertilizers. Biomass Bioenergy 2025, 193, 107557. [Google Scholar] [CrossRef]
- Shan, J.; Guo, H.; Zhou, J.; Shen, F.; Qiu, M.; Yang, J.; Smith Jr, R.L.; Qi, X. Efficient isomerization of glucose to fructose over Al-loaded functional lignin biopolymer. Appl. Catal. B Environ. Energy 2024, 353, 124095. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Y.; Dai, F.; Liu, C.; Liu, Y. Host–guest synthesis and encapsulation of phosphotungstic acid in MIL-101 via “bottle around ship”: An effective catalyst for oxidative desulfurization. Microporous Mesoporous Mater. 2013, 170, 36–44. [Google Scholar] [CrossRef]
- Wang, K.; Rezayan, A.; Si, L.; Zhang, Y.; Nie, R.; Lu, T.; Wang, J.; Xu, C. Highly Efficient 5-Hydroxymethylfurfural Production from Glucose over Bifunctional SnOx/C catalyst. ACS Sustain. Chem. Eng. 2021, 9, 11351–11360. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, J.; Wang, Y.; Ma, Y. Synthesis of phosphotungstic acid-supported versatile metal–organic framework PTA@MIL-101(Fe)–NH2–Cl. RSC Adv. 2015, 5, 97589–97597. [Google Scholar] [CrossRef]
- Tang, Z.; Liang, J.; Su, J. High efficient production of 5-hydroxymethyl-furfural from glucose over AACH @ γ-AlOOH catalyst: Insights into structure, acidic properties and performance. Appl. Catal. A Gen. 2025, 696, 120184. [Google Scholar] [CrossRef]
| Samples | C 1s | O 1s | Cr 2p | ||||
|---|---|---|---|---|---|---|---|
| C=C/ CHx/C-C (%) | C-OH/ C-O-C (%) | -C=O (%) | C-OH/ C-O-C (%) | -C=O (%) | Cr-O (%) | Cr3+ (%) | |
| Cr0SHC180 | 71.09 | 23.04 | 5.86 | 72.65 | 27.35 | -- | -- |
| Cr5SHC180 | 74.34 | 17.76 | 7.90 | 50.41 | 41.08 | 8.51 | 100 |
| Cr0EHC180 | 50.51 | 36.64 | 9.86 | 89.03 | 10.97 | -- | -- |
| Cr5EHC180 | 71.35 | 20.70 | 7.95 | 57.21 | 36.11 | 6.68 | 100 |
| Cr0BHC180 | 65.15 | 24.07 | 10.78 | 86.92 | 13.08 | -- | -- |
| Cr5BHC180 | 74.12 | 17.97 | 7.91 | 57.14 | 35.63 | 7.24 | 100 |
| Samples | BET * Surface Area(m2 g−1) | Average Pore Size (Diameter, nm) | Carbon Yield (%) | |
|---|---|---|---|---|
| Starch-based | Cr0SHC180 | 2.47 | 19.42 | 4.61 |
| Cr5SHC180 | 7.84 | 17.68 | 18.06 | |
| Eucalyptus-based | Cr0EHC180 | 2.21 | 22.73 | 60.72 |
| Cr5EHC180 | 20.59 | 25.64 | 36.36 | |
| Bagasse-based | Cr0BHC180 | 2.51 | 20.00 | 29.12 |
| Cr5BHC180 | 19.43 | 16.95 | 23.96 | |
| Samples | Acid Density /mmol g−1 | Initial Potential /mV | HMF Yield /% | HMF Selectivity /% | Glucose Conversion /% | Fructose Yield /% | |
|---|---|---|---|---|---|---|---|
| Starch-based | Cr0SHC180 | 0.14 | 221 | 28.3 | 32.4 | 87.6 | 1.4 |
| Cr5SHC180 | 0.20 | 247 | 51.7 | 51.7 | 100.0 | 1.2 | |
| Eucalyptus-based | Cr0EHC180 | 0.1 | 159 | 23.1 | 26.4 | 87.3 | 1.5 |
| Cr5EHC180 | 0.18 | 164 | 48.8 | 48.8 | 100.0 | 1.3 | |
| Bagasse-based | Cr0BHC180 | 0.16 | 225 | 36.7 | 40.2 | 91.3 | 1.4 |
| Cr5BHC180 | 0.24 | 265 | 52.5 | 52.5 | 100.0 | 1.3 | |
| Samples | BET * Surface Area (m2 g−1) | Average Pore Size (Diameter, nm) | Brønsted Acid (μmol g−1) | Lewis Acid (μmol g−1) | Total Acids (μmol g−1) | |
|---|---|---|---|---|---|---|
| Starch-based | Cr5SHC180(fresh) | 7.84 | 17.68 | 2.46 | 28.65 | 31.11 |
| Cr5SHC180(recycled) | 5.20 | 19.98 | 2.25 | 19.94 | 22.19 | |
| Cr5SHC180(regenerated) | 96.94 | 9.83 | 2.30 | 26.76 | 29.06 | |
| Eucalyptus-based | Cr5EHC180(fresh) | 20.59 | 25.64 | 2.84 | 29.62 | 32.46 |
| Cr5EHC180(recycled) | 12.16 | 28.39 | 2.92 | 26.52 | 29.44 | |
| Cr5EHC180(regenerated) | 105.43 | 16.98 | 2.00 | 33.87 | 35.87 | |
| Bagasse-based | Cr5BHC180(fresh) | 19.43 | 16.95 | 4.11 | 31.86 | 35.97 |
| Cr5BHC180(recycled) | 12.71 | 18.74 | 2.87 | 27.71 | 30.58 | |
| Cr5BHC180(regenerated) | 42.97 | 15.19 | 3.46 | 32.15 | 35.61 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Mao, W.; Xiao, P.; Ling, C.; Wu, Z.; Zhou, J. Chromium-Doped Biomass-Based Hydrochar-Catalyzed Synthesis of 5-Hydroxymethylfurfural from Glucose. Polymers 2025, 17, 1413. https://doi.org/10.3390/polym17101413
Gao H, Mao W, Xiao P, Ling C, Wu Z, Zhou J. Chromium-Doped Biomass-Based Hydrochar-Catalyzed Synthesis of 5-Hydroxymethylfurfural from Glucose. Polymers. 2025; 17(10):1413. https://doi.org/10.3390/polym17101413
Chicago/Turabian StyleGao, Huimin, Wei Mao, Pize Xiao, Chutong Ling, Zhiming Wu, and Jinghong Zhou. 2025. "Chromium-Doped Biomass-Based Hydrochar-Catalyzed Synthesis of 5-Hydroxymethylfurfural from Glucose" Polymers 17, no. 10: 1413. https://doi.org/10.3390/polym17101413
APA StyleGao, H., Mao, W., Xiao, P., Ling, C., Wu, Z., & Zhou, J. (2025). Chromium-Doped Biomass-Based Hydrochar-Catalyzed Synthesis of 5-Hydroxymethylfurfural from Glucose. Polymers, 17(10), 1413. https://doi.org/10.3390/polym17101413







