Exploring the Characterization, Physicochemical Properties, and Antioxidant Activities of Chitosan-Encapsulated Green Tea Extract Microsphere Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of CS-GTEMR and Chitosan Microsphere Resin (CS-MR)
2.3. Determination of Physical Properties
2.3.1. Determination of Water Absorption Capacity
2.3.2. Determination of Pile-Up Density
2.3.3. Determination of Skeleton Density
2.3.4. Determination of Pore Degree
2.3.5. Determination of Free Aldehyde Group Content
2.3.6. Determination of Weak Basic Exchange Capacity
2.4. Structure and Thermal Stability Characterization
2.5. Determination of the Polyphenolic Compounds Content
2.6. Determination of Antioxidant Activities
2.6.1. Determination of DPPH Free Radical Scavenging Activity
2.6.2. Determination of Superoxide Anion Free Radical Scavenging Activity
2.6.3. Determination of Hydroxyl Radical Scavenging Activity
2.6.4. H2O2 Scavenging Activity
2.6.5. Determination of Iron Reducing Power
2.6.6. Determination of Molybdenum Reducing Power Activity
2.7. Statistical Analysis
3. Results and Discussion
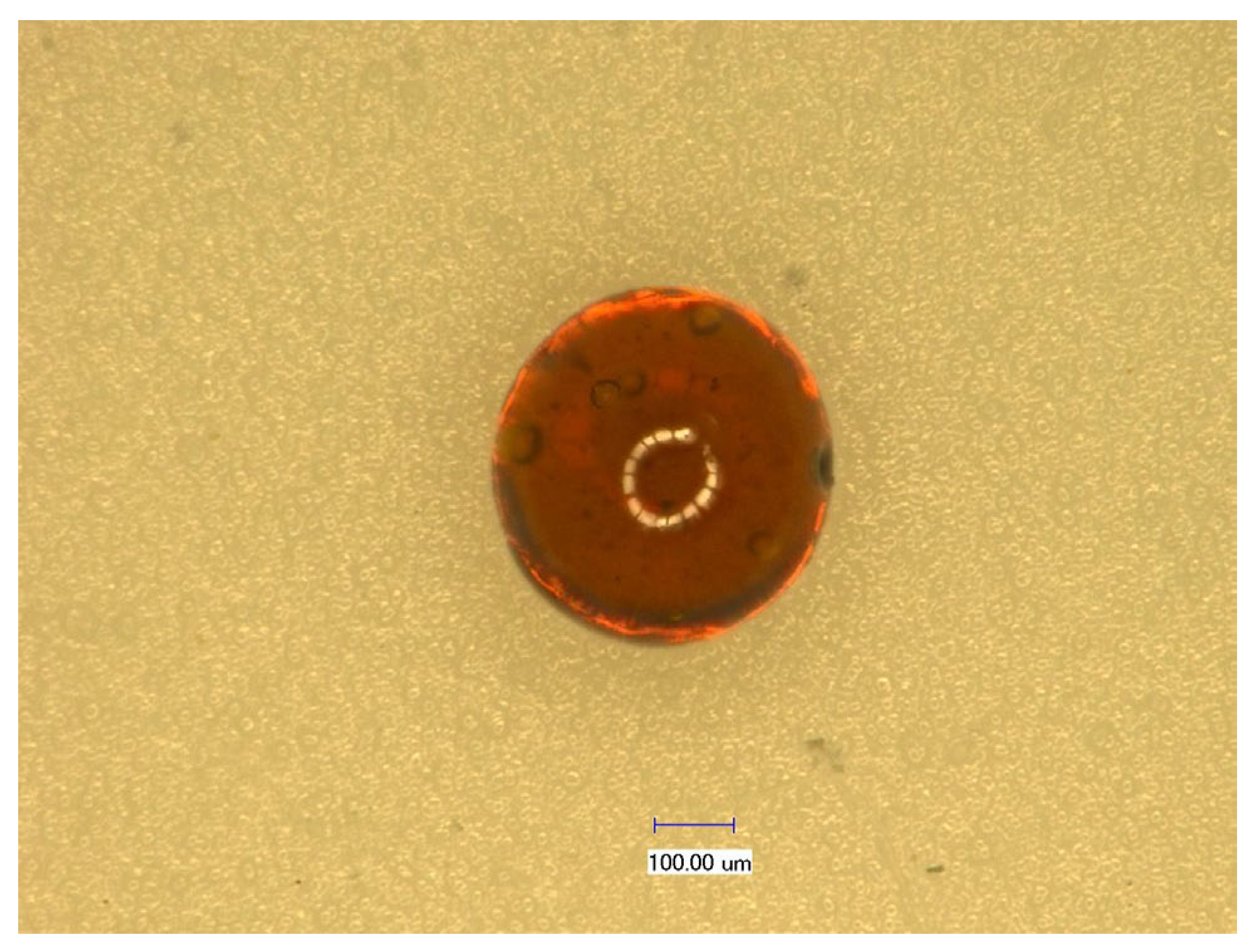
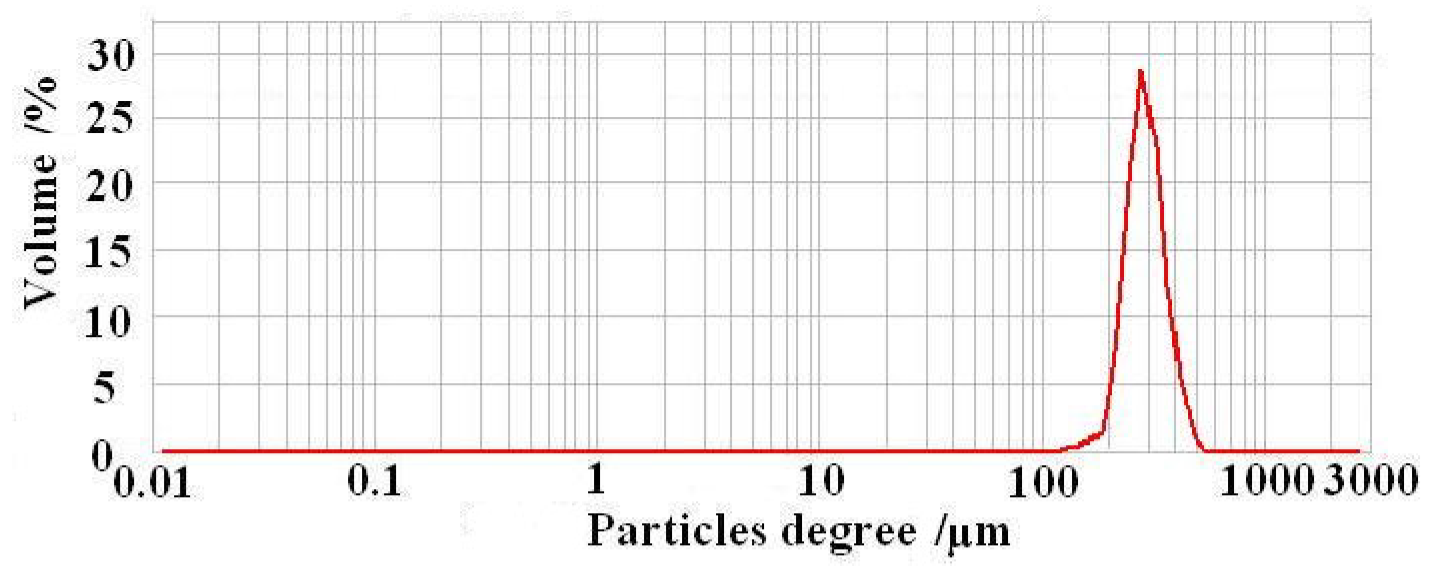
3.1. Characterization of CS-GTEMR
3.1.1. Basic Physical Properties of CS-GTEMR
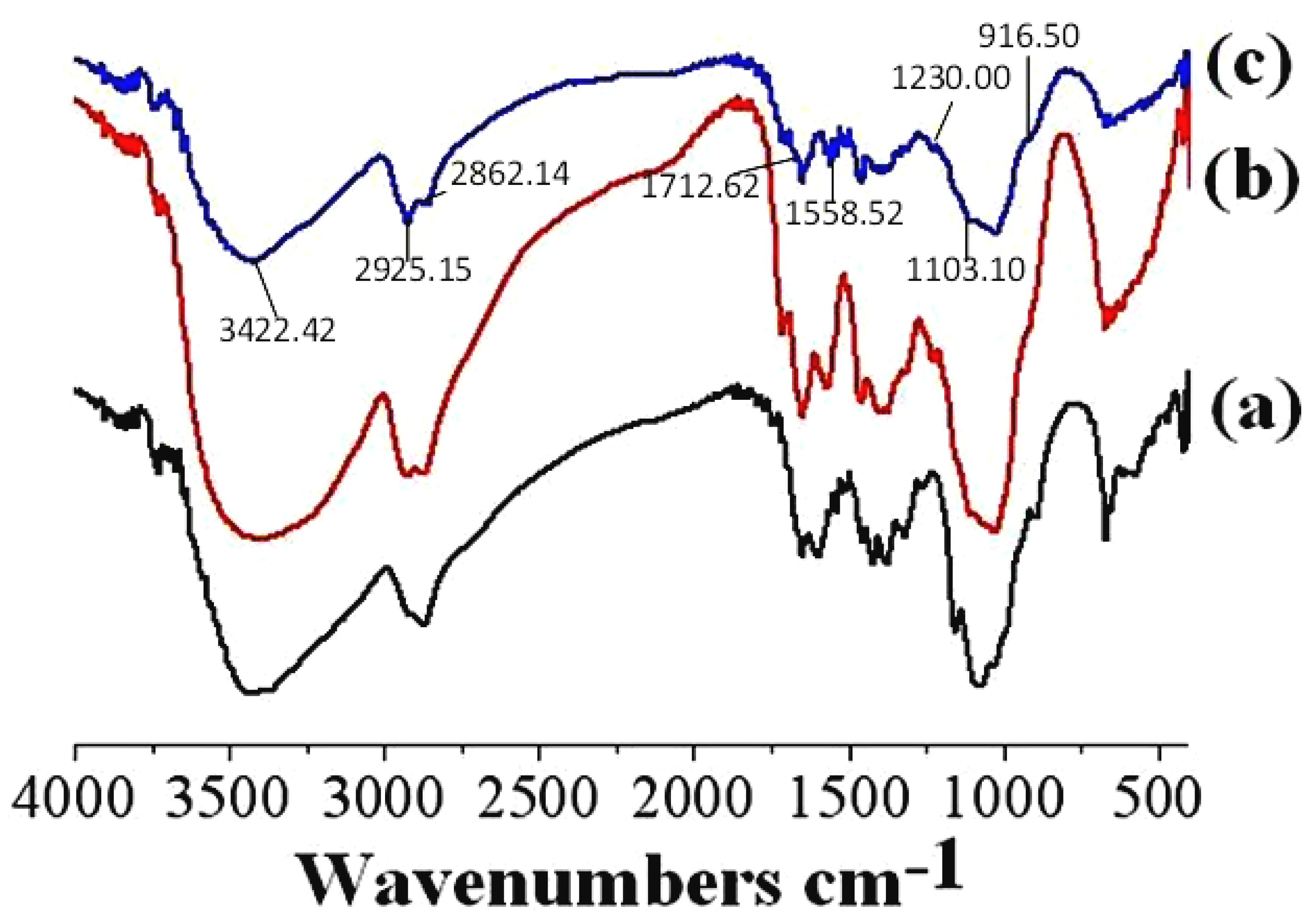
3.1.2. FTIR Spectroscopy Analysis
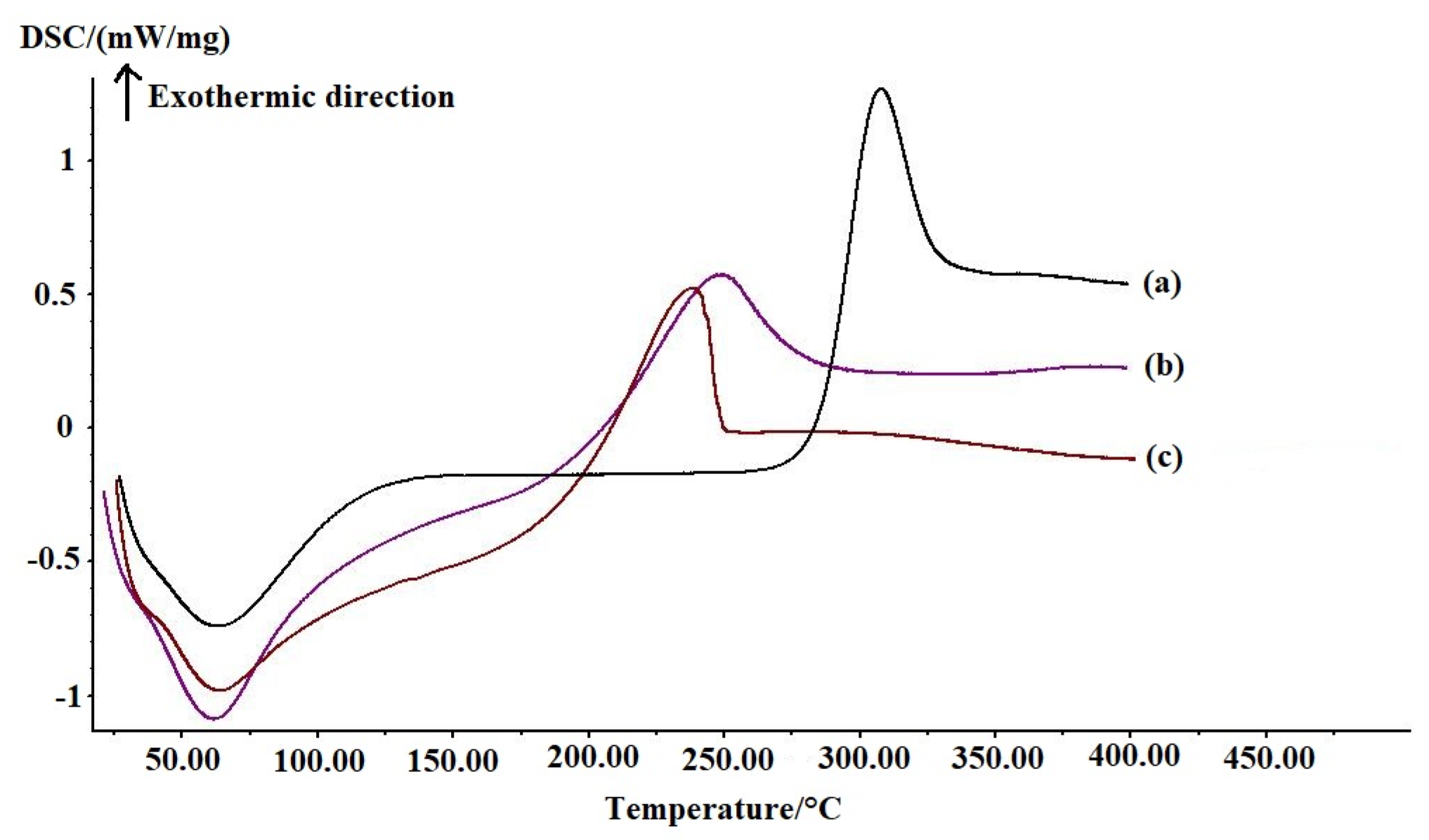
3.1.3. Differential Scanning Calorimetry (DSC) Analysis
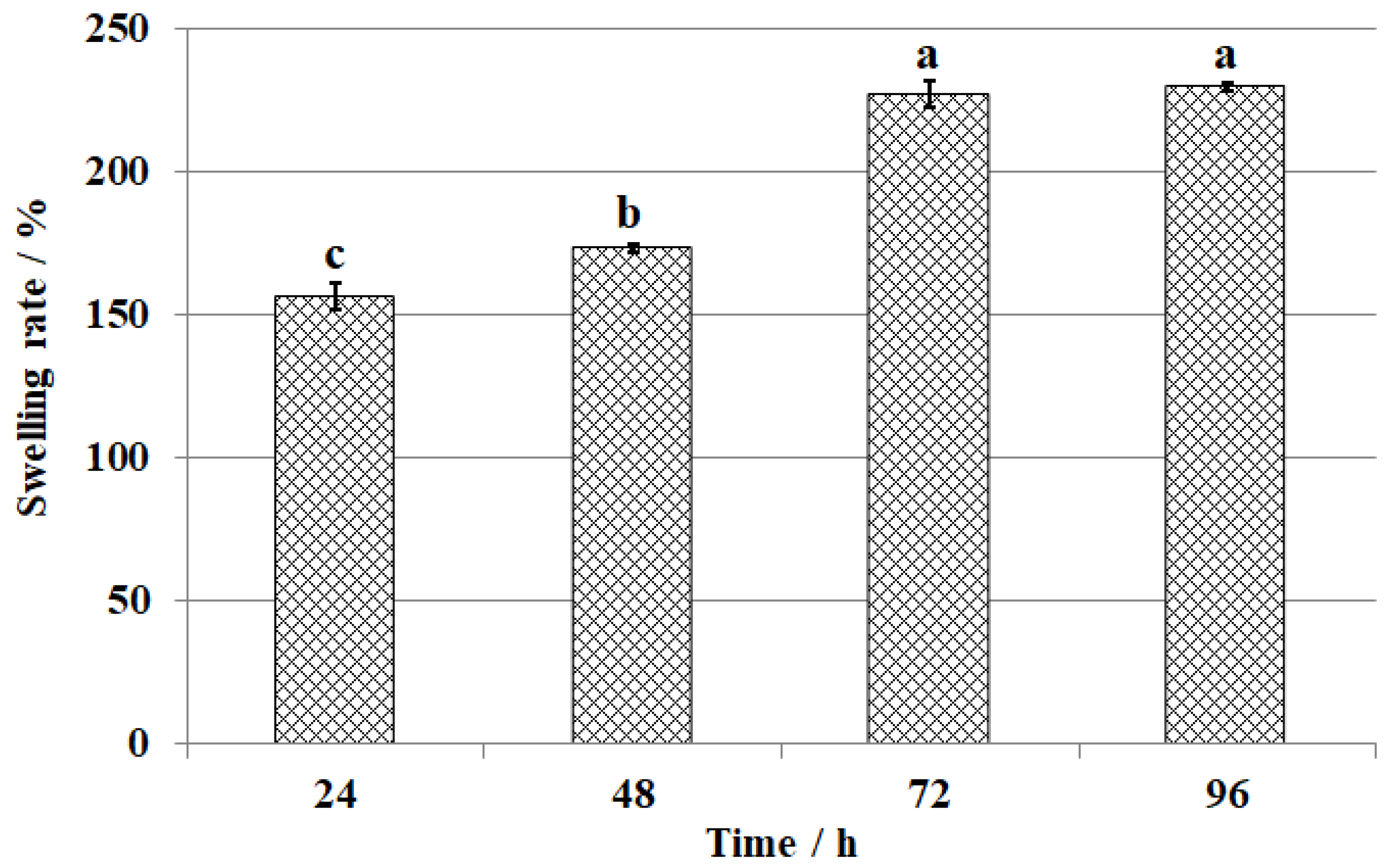
3.2. Results and Analysis of Swelling Properties of CS-GTEMR
3.2.1. Results and Analysis of CS-GTEMR Swelling Properties in Water
3.2.2. Results and Analysis of CS-GTEMR Swelling Properties in Different pH Solutions
3.3. Evaluation of the Polyphenolic Compounds in CS-GTEMR
3.3.1. Content of Polyphenols in CS-GTEMR
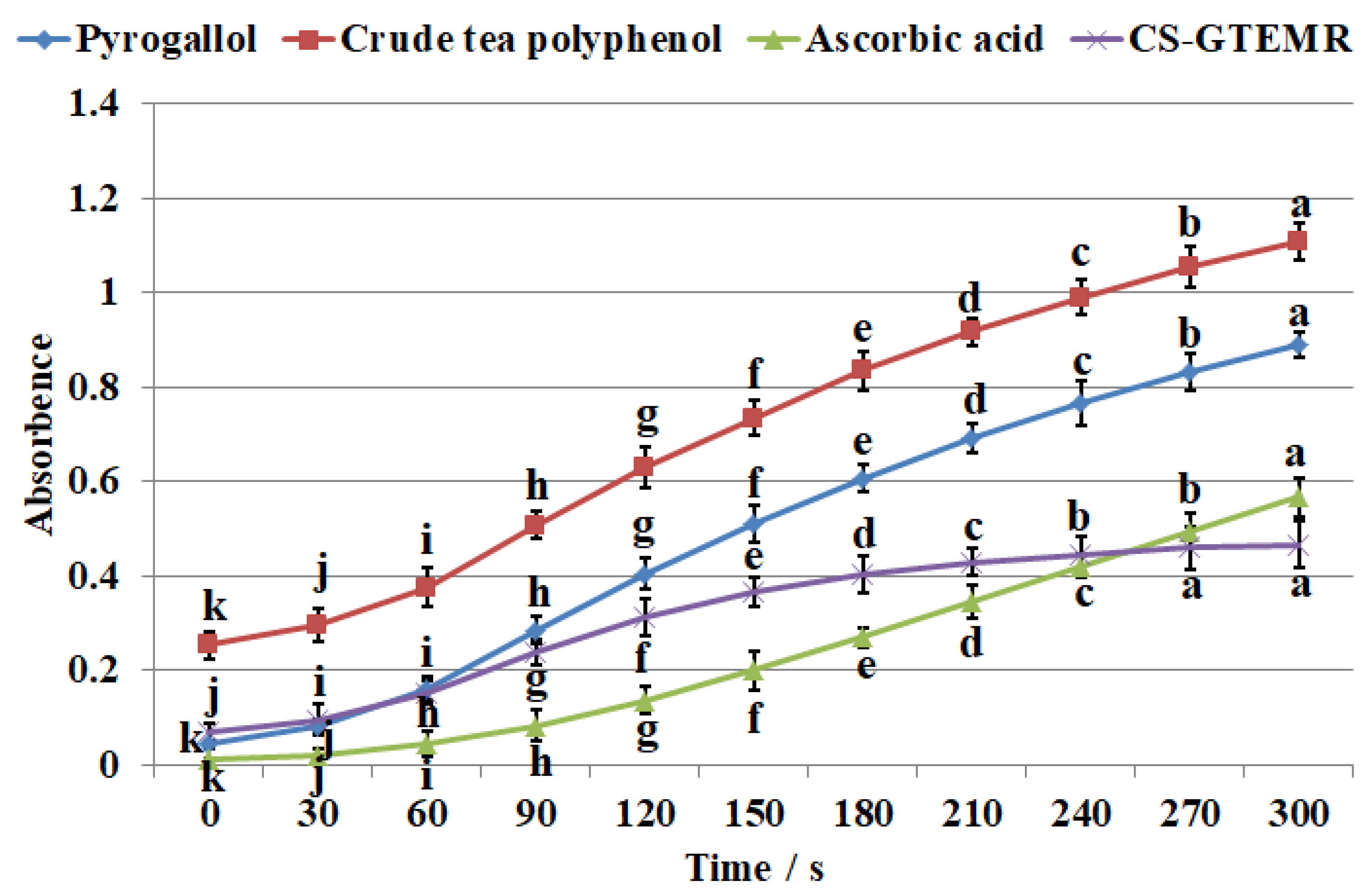
3.3.2. Results and Analysis of the Polyphenolic Compounds Release Rate in Simulated Gastric and Intestinal Fluid
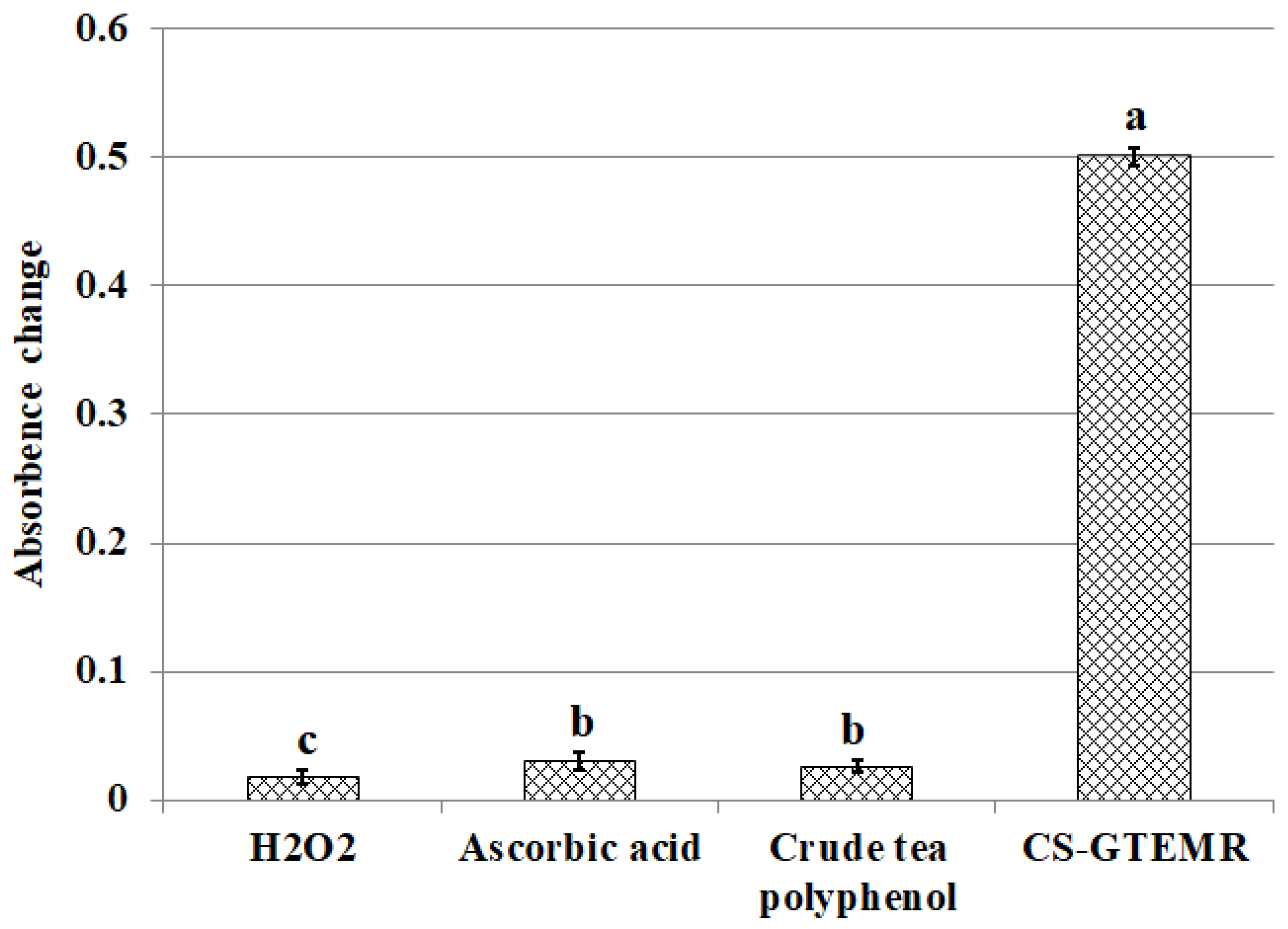
3.4. Results and Analysis of Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Righi, D.C.B.F.; Boeira, C.P.; Nora, F.M.D.; Schlesner, S.K.; Kaufmann, A.I.; Barin, J.S.; Machado, P.G.; Ballus, C.A.; do Nascimento, V.R.; Bizzi, C.A.; et al. Green technologies applied to extraction processes and analysis of polyphenolic compounds from Inga laurina Willd. Food Chem. 2025, 471, 142827. [Google Scholar] [CrossRef] [PubMed]
- Mashau, M.E.; Kgatla, T.E.; Makhado, M.V.; Mikasi, M.S.; Ramashia, S.E. Nutritional composition, polyphenolic compounds and biological activities of marula fruit (Sclerocarya birrea) with its potential food applications: A review. Int. J. Food Prop. 2022, 25, 1549–1575. [Google Scholar] [CrossRef]
- Castaldo, L.; Lombardi, S.; Gaspari, A.; Rubino, M.; Izzo, L.; Narváez, A.; Ritieni, A.; Grosso, M. In vitro bioaccessibility and antioxidant activity of polyphenolic compounds from spent coffee grounds-enriched cookies. Foods 2021, 10, 1837. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Zhang, Y.; Zheng, L. Determination of 19 polyphenolic compounds in tea by ultra-high performance liquid chromatography combined with quadrupole-time of flight mass spectrometry. Food Sci. Hum. Wellness 2022, 11, 719–726. [Google Scholar] [CrossRef]
- Alqathama, A.; Abdelhady, M.I.S.; Al-Omar, M.S.; Barghash, M.F.; Barghash, M.F. Antioxidant, anti-inflammatory and cytotoxic activity of Schinus terebinthifolia fruit and isolation of a new immunomodulatory polyphenolic compound. Pharmacogn. Mag. 2023, 19, 13–22. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Ng, K. Incorporating inulin and chitosan in alginate-based microspheres for targeted delivery and release of quercetin to colon. Food Res. Int. 2022, 160, 111749. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Y.; Tan, M. ROS-responsive carboxymethyl chitosan nanoparticles loaded with astaxanthin for alleviating oxidative damage in intestinal cells. Colloids Surf. B 2024, 239, 113960. [Google Scholar] [CrossRef]
- Hossain, R.; Kongchain, A.; Chatatikun, M.; Klangbud, W.K.; Yupanqui, C.T.; Majima, H.J.; Indo, H.P.; Sompol, P.; Sekeroglu, N.; Phongphithakchai, A. Green tea pressurized hot water extract in Atherosclerosis: A multi-approach study on cellular, animal, and molecular mechanisms. Antioxidants 2025, 14, 404. [Google Scholar] [CrossRef]
- De la Fuente-Muñoz, M.; Román-Carmena, M.; Amor, S.; de la Cruz, M.C.; Martorell, P.; Guilera-Bermell, S.; Bou, R.G.; Inarejos-García, A.M.; García-Villalón, A.L.; Granado, M. Supplementation with standardized green/black or white tea extracts attenuates hypertension and ischemia-reperfusion-induced myocardial damage in mice infused with Angiotensin II. Antioxidants 2025, 14, 47. [Google Scholar] [CrossRef]
- Sulaimani, N.; Rosbotham, E.J.; Warnock, R.; Polzella, L.; Judowski, R.; Nicolotti, L.; Houghton, M.J.; Williamson, G.; Bonham, M.P. Time-of-day-dependent effects of a green tea extract on postprandial glycemia and insulinemia in healthy adults: A randomized, controlled, double-blind, cross-over intervention. Food Funct. 2025, 16, 4122–4133. [Google Scholar] [CrossRef]
- Joo, Y.; Lee, H.Y.J.; Jeong, H.; Suh, C.; Hong, H.J.; Kim, Y.; Yu, S.Y.; Lee, C.R.; Shim, Y.; Yoon, S. Effects of Heat-Treated Green Tea Extract on Memory Function and Default Mode Network Connectivity in Individuals with Subjective Memory Impairment: A Randomized, Double-Blinded, Placebo-Controlled Trial. J. Med. Food 2025. [Google Scholar] [CrossRef] [PubMed]
- Moalemi, S.F.S.S.; Safari, F.; Ahvati, H. Suppression of cellular proliferation in PC3 prostate cancer cells by green tea extract through induction of miR-34a expression. Food Sci. Nutr. 2025, 13, e70215. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tao, H.; Xu, C.; Song, J.; Teng, C.; Pan, C.; Wei, S. Selenium-enriched green tea extracts: Chemical constituents and effects on antioxidant and anti-inflammatory factors and four major intestinal flora in mice with intestinal disorders. J. Sci. Food Agric. 2025, 105, 4472–4482. [Google Scholar] [CrossRef] [PubMed]
- Ogundare, T.E.; Kulkarni, R.R.; Omaliko, P.C.; Iwuozo, O.C.; Enenya, I.G.; Orimaye, O.E.; Suberu, S.A.; Jeje, O.; Fasina, Y.O. Effect of green tea (Camellia sinensis) extract on growth performance, intestinal health, and immune response of Broiler chickens during subclinical necrotic enteritis. Pathogens 2025, 14, 260. [Google Scholar] [CrossRef]
- Bavi, E.P.; Shakerinasab, E.; Hamidinezhad, H.; Nazifi, E. A green and facile approach for fabrication of biocompatible anti-Parkinson chitosan-gelatin-green tea extract composite particles with neuroprotective and Neurotherapeutic effects: In vitro evaluation. Int. J. Biol. Macromol. 2022, 224, 1183–1195. [Google Scholar] [CrossRef]
- Piran, F.; Khoshkhoo, Z.; Hosseini, S.E.; Azizi, M.H. Controlling the antioxidant activity of green tea extract through encapsulation in chitosan-citrate nanogel. J. Food Qual. 2020, 2020, 7935420. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Chunshom, N.; Kotcharat, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. The encapsulation of green tea extract in cyclodextrin and loading into chitosan-based composites: Controlled-release behavior and antioxidant properties. J. Polym. Environ. 2021, 29, 2628–2638. [Google Scholar] [CrossRef]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Gajda, P.; Jaromin, A.; Czopek, A.; Zagórska, A.; Tyliszczak, B. Synthesis and physicochemical evaluation of bees’ chitosan-based hydrogels modified with yellow tea extract. Materials 2021, 14, 3379. [Google Scholar] [CrossRef]
- Yu, L.; Bi, J.; Song, Y.; Wang, M. Isotherm, thermodynamics, and kinetics of methyl orange adsorption onto magnetic resin of chitosan microspheres. Int. J. Mol. Sci. 2022, 23, 13839. [Google Scholar] [CrossRef]
- Yu, L.; Song, Y.; Bi, J.; Gao, Y.; Jiang, C.; Yang, Z.; Qi, H.; Yu, H.; Yang, W.; Gong, Q.; et al. Exploring the potent hydrolytic activity of chitosan-cerium complex microspheres resin for organophosphorus pesticide degradation. Heliyon 2024, 10, e33642. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The chemistry behind the Folin-Ciocalteu method for the estimation of (poly)phenol content in food: Total phenolic intake in a mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Bi, J.; Song, Y.; Jiang, C.; Qi, H.; Chi, X.; Yang, W.; Shi, C.; Gong, Q.; Wang, M. Products and properties of components from heat-denatured peanut meal following solid-state fermentation by Aspergillus oryzae and Saccharomyces cerevisiae. Fermentation 2023, 9, 425. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Wang, X.; Song, Y.; Fan, X.H.; García Martín, J.F. Optimization of pyrogallol autoxidation conditions and its application in evaluation of superoxide anion radical scavenging capacity for four antioxidants. J. AOAC Int. 2016, 99, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Falcone, E.; Vigna, V.; Schueffl, H.; Stellato, F.; Vileno, B.; Bouraguba, M.; Mazzone, G.; Proux, O.; Morante, S.; Heffeter, P.; et al. When metal complexes evolve, and a minor species is the most active: The case of bis(phenanthroline)copper in the catalysis of glutathione oxidation and hydroxyl radical generation. Angew. Chem. Int. Ed. Engl. 2025, 64, e202414652. [Google Scholar] [CrossRef]
- Porcher, A.; Guérin, V.; Montrichard, F.; Lebrec, A.; Lothier, J.; Vian, A. Ascorbate glutathione-dependent H2O2 scavenging is an important process in axillary bud outgrowth in rosebush. Ann. Bot. 2020, 126, 1049–1062. [Google Scholar] [CrossRef]
- Uma, T.K.N.N.; Szewczyk, P.K.; Karbowniczek, J.E.; Polak, M.; Knapczyk-Korczak, J.; Stachewicz, U. Improving stability and mechanical strength of electrospun chitosan-polycaprolactone scaffolds using genipin cross-linking for biomedical applications. Macromol. Rapid Commun. 2024, 2400869. [Google Scholar] [CrossRef]
- Salah, M.; Huang, J.; Zhu, C.; Sobhy, M.; Farag, M.A.; Fang, Y.; Sobhy, R.; Walayat, N.; Khalifa, I.; Maqsood, S.; et al. Chitosan dual gel-like functionalized with flavonoid extract and cinnamaldehyde oil using dual cross-linking agents: Characterization, antioxidant, and antimicrobial effects. Curr. Res. Food Sci. 2024, 9, 100826. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, Z.; Chen, Z.; Li, P.; Du, B.; Li, L. Modification of Inca peanut albumin-polyphenol conjugates by chitosan through laccase catalysis: Structural, interfacial, and functional properties. Int. J. Biol. Macromol. 2025, 289, 138898. [Google Scholar] [CrossRef]
- Miyauchi, M. Water adsorption on hydrophilic fibers and porous and deliquescent materials: Cellulose, polysaccharide, silica, inorganic salt, sugar alcohol, and amino acid. ACS Omega 2023, 8, 44212–44220. [Google Scholar] [CrossRef]
- Çelikçi, N.; Zıba, C.A.; Tümer, M. Chitosan-Based Schiff Base Compounds: Synthesis, Chemical Characterization and Antibacterial Properties. J. Fluoresc. 2025, 23, 839–1106. [Google Scholar] [CrossRef]
- Chang, X.; Mubarak, N.M.; Karri, R.R.; Tan, Y.; Khalid, M.; Dehghani, M.H.; Tyagi, I.; Khan, N.A. Insights into chitosan-based cellulose nanowhiskers reinforced nanocomposite material via deep eutectic solvent in green chemistry. Environ. Res. 2023, 219, 115089. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, H.S. Microwaved schiff base dialdehyde cellulose-chitosan hydrogels for sustained drug release with DFT calculations. BMC Chem. 2025, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Akhtar, N.; Arshad, M.; Azhar, U.; Ullah, S.; Waris, T.S.; Jabbar, F.; Hasan, A.; Iqbal, F.; Chaudhry, A.A.; et al. A novel methodology for stabilization of silver nanoparticles on cotton, nylon and cotton/nylon fabrics using chitosan and triethyl orthoformate for enhanced and elongated antibacterial performance. Int. J. Biol. Macromol. 2024, 267, 129256. [Google Scholar] [CrossRef] [PubMed]
- Chinarak, K.; Wongnen, C.; Chaijan, M.; Tamman, A.; Donlao, N.; Cheong, L.Z. Unveiling the transformative influence of sonochemistry on formation of whey protein isolate and green tea extract (WPI-GTE) conjugates. Ultrason. Sonochem. 2024, 110, 107037. [Google Scholar] [CrossRef]
- Ata, T.; Al-Ani, I.; Karameh, N.; Atta, M.R.; Dayyih, W.A. Alectinib-loaded chitosan-alginate nanoparticles: A novel synthesis method with in vitro and in vivo evaluations. Pharmaceutics 2025, 17, 492. [Google Scholar] [CrossRef]
- Kadam, D.; Lele, S.S. Cross-linking effect of polyphenolic extracts of Lepidium sativum seedcake on physicochemical properties of chitosan films. Int. J. Biol. Macromol. 2018, 114, 1240–1247. [Google Scholar] [CrossRef]
- Zhu, L.; Ouyang, F.; Fu, X.; Wang, Y.; Li, T.; Wen, M.; Zha, G.; Yang, X. Tannic acid modified keratin/sodium alginate/carboxymethyl chitosan biocomposite hydrogels with good mechanical properties and swelling behavior. Sci. Rep. 2024, 14, 12864. [Google Scholar] [CrossRef]
- Hameed, A.R.; Majdoub, H.; Jabrail, F.H. Effects of surface morphology and type of cross-lnking of chitosan-pectin microspheres on their degree of swelling and favipiravir release behavior. Polymers 2023, 15, 3173. [Google Scholar] [CrossRef]
- Cheng, B.; Feng, H.; Li, C.; Jia, F.; Zhang, X. The mutual effect of dietary fiber and polyphenol on gut microbiota: Implications for the metabolic and microbial modulation and associated health benefits. Carbohydr. Polym. 2025, 358, 123541. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary polyphenol, gut microbiota, and health benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Gerasopoulos, A.; Mantzouridou, F.T.; Nenadis, N. Physicochemical properties of olive leaf powders and incorporation in chitosan-based edible films for improved functionality. Food Hydrocoll. 2025, 160, 110748. [Google Scholar] [CrossRef]
- Carrapiso, A.I.; Pimienta, M.; Martín, L.; Cardenia, V.; Andrés, A.I. Effect of a chitosan coating enriched with an olive leaf extract on the characteristics of pork burgers. Foods 2023, 12, 3757. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Zhang, X.; Wang, M.H. Enhancing the antioxidant, antibacterial, and wound healing effects of melaleuca alternifolia oil by microencapsulating it in chitosan-sodium alginate mcrospheres. Nutrients 2023, 15, 1319. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z. Preparation and characterization of catechin-grafted chitosan with antioxidant and antidiabetic potential. Int. J. Biol. Macromol. 2014, 70, 150–155. [Google Scholar] [CrossRef]



| Physical Properties | CS-GTEMR | CS-MR [20] |
|---|---|---|
| Water absorption capacity/M (%) | 64.296 ± 1.588 | 51.982 ± 1.944 |
| Pile-up density/ρP (g/mL) | 0.843 ± 0.087 | 0.862 ± 0.007 |
| Skeletal density/ρT (g/cm3) | 1.248 ± 0.480 | 1.212 ± 0.453 |
| Pore degree/P | 0.679 ± 0.075 | 0.554 ± 0.097 |
| Free aldehyde group/(mmol/g) | 0.267 ± 0.012 | 0.315 ± 0.009 |
| Weak basic exchange capacity/(mmol/g) | 1.409 ± 0.084 | 1.311 ± 0.084 |
| Peaks Attribution | Chitosan Powder (cm−1) | CS-MR (cm−1) | CS-GTEMR (cm−1) |
|---|---|---|---|
| ν(O-H) and ν(N-H) | 3444.86 | 3420.21 | 3422.42 |
| ν(-CH3) | 2917.82 | 2925.88 | 2925.15 |
| ν(-CH2) | 2875.65 | 2875.98 | 2862.14 |
| ν(-CHO) | - | 1715.07 | 1712.62 |
| amide I (ν(C=O) and ν(C-N)) | 1649.84 | 1650.65 | 1650.48 |
| δ(-NH2) | 1597.87 | - | - |
| ν(C=N) | - | 1573.36 | 1558.52 |
| amide II (δ(N-H) and ν(C-N)) | 1540.99 | - | 1541.28 |
| amide III (ν(C-N) and δ(N-H)) | 1259.77 | 1227.50 | 1227.18 |
| ν(C-O-C) | 1154.80 | 1102.21 | 1103.10 |
| C6-OH (ν(C-O)) | 1087.55 | weaker | weaker |
| C3-OH (ν(C-O)) | 1029.13 | 1031.00 | 1024.82 |
| characteristic absorption peak of β-D-glucopyranoside | 897.47 | weaker | 916.50 |
| phenolic hydroxyl group (ν(H-O)) | - | - | 1230.00 |
| Peaks Attribution | CS-GTEMR | Crude Tea Polyphenol | Ascorbic Acid |
|---|---|---|---|
| IC50 | 0.16 g/mL | 61.49 μg/mL | 70.66 μg/mL |
| scavenging rate/% | 59.42 ± 3.99 | 64.78 ± 1.46 | 40.35 ± 4.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Feng, S.; Song, Y.; Bi, J.; Gao, Y.; Wang, L.; Jiang, C.; Wang, M. Exploring the Characterization, Physicochemical Properties, and Antioxidant Activities of Chitosan-Encapsulated Green Tea Extract Microsphere Resin. Polymers 2025, 17, 1633. https://doi.org/10.3390/polym17121633
Yu L, Feng S, Song Y, Bi J, Gao Y, Wang L, Jiang C, Wang M. Exploring the Characterization, Physicochemical Properties, and Antioxidant Activities of Chitosan-Encapsulated Green Tea Extract Microsphere Resin. Polymers. 2025; 17(12):1633. https://doi.org/10.3390/polym17121633
Chicago/Turabian StyleYu, Lina, Siyu Feng, Yu Song, Jie Bi, Yuan Gao, Luhui Wang, Chen Jiang, and Mingqing Wang. 2025. "Exploring the Characterization, Physicochemical Properties, and Antioxidant Activities of Chitosan-Encapsulated Green Tea Extract Microsphere Resin" Polymers 17, no. 12: 1633. https://doi.org/10.3390/polym17121633
APA StyleYu, L., Feng, S., Song, Y., Bi, J., Gao, Y., Wang, L., Jiang, C., & Wang, M. (2025). Exploring the Characterization, Physicochemical Properties, and Antioxidant Activities of Chitosan-Encapsulated Green Tea Extract Microsphere Resin. Polymers, 17(12), 1633. https://doi.org/10.3390/polym17121633






