Electrochemical Sensing of Metribuzin Utilizing the Synergistic Effects of Cationic and Anionic Bio-Polymers with Hetero-Doped Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
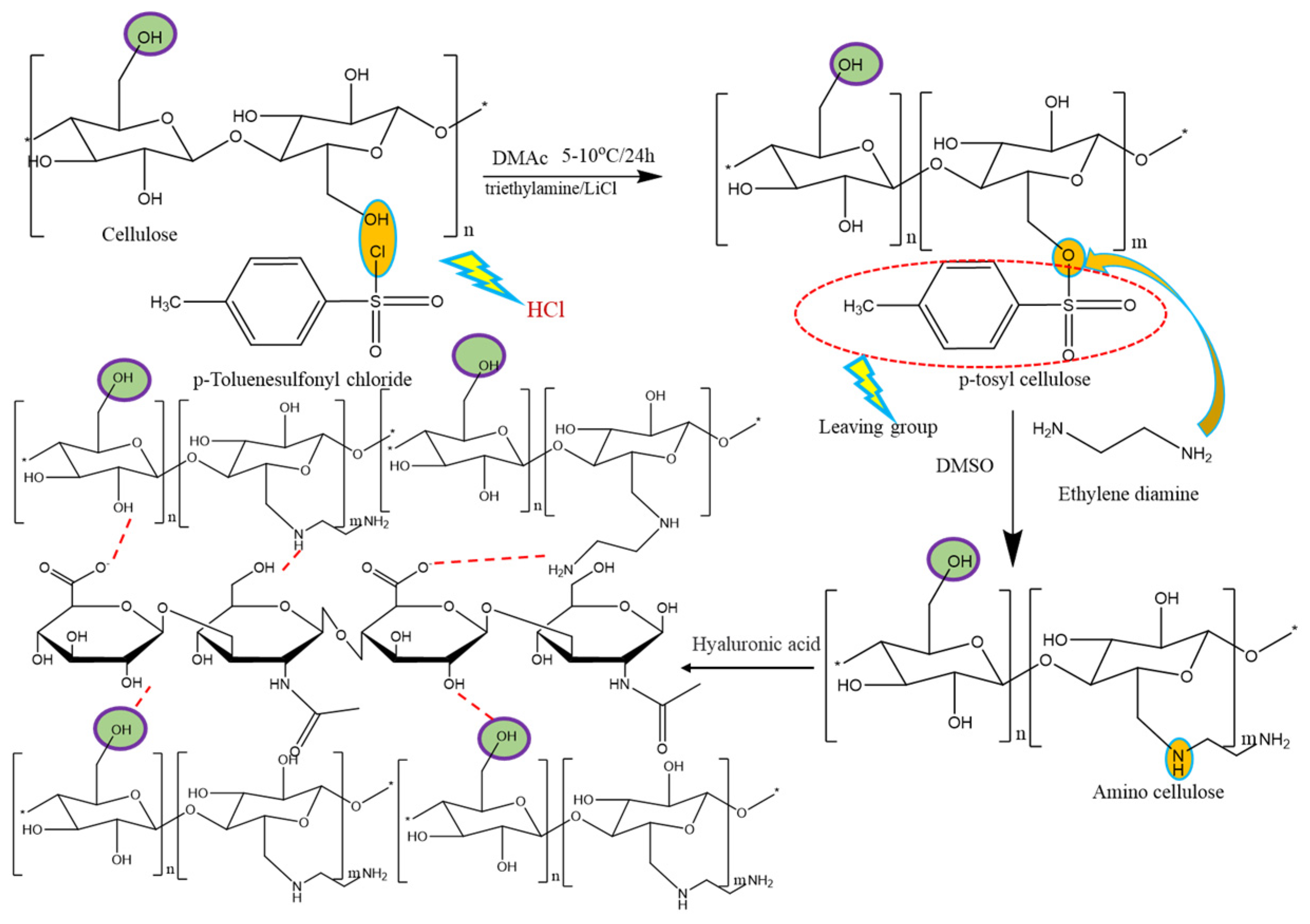
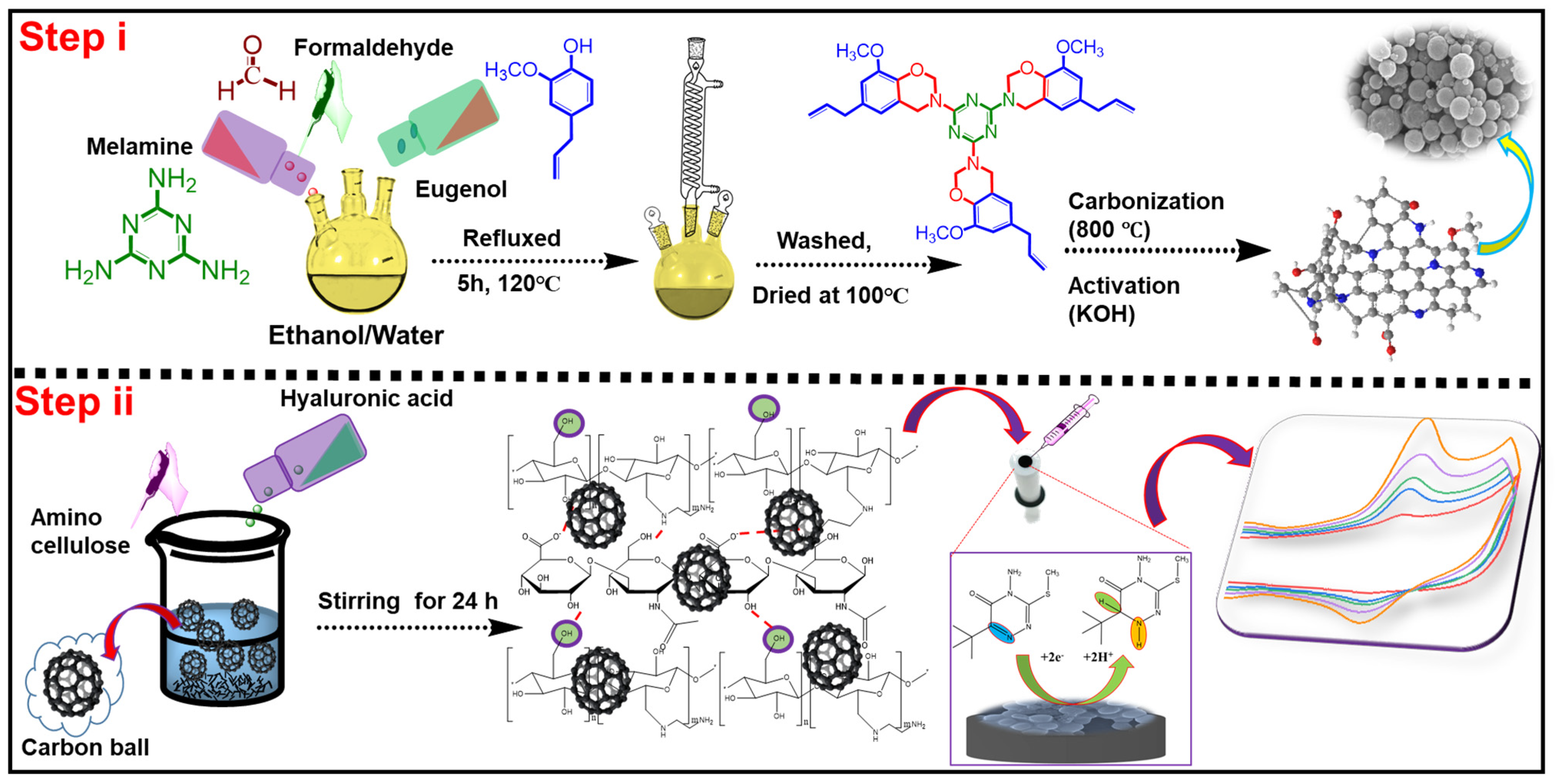
2.2. Synthesis of PBC-ACH Complex
2.3. Fabrication of GCE/PBC-ACH
3. Results and Discussion
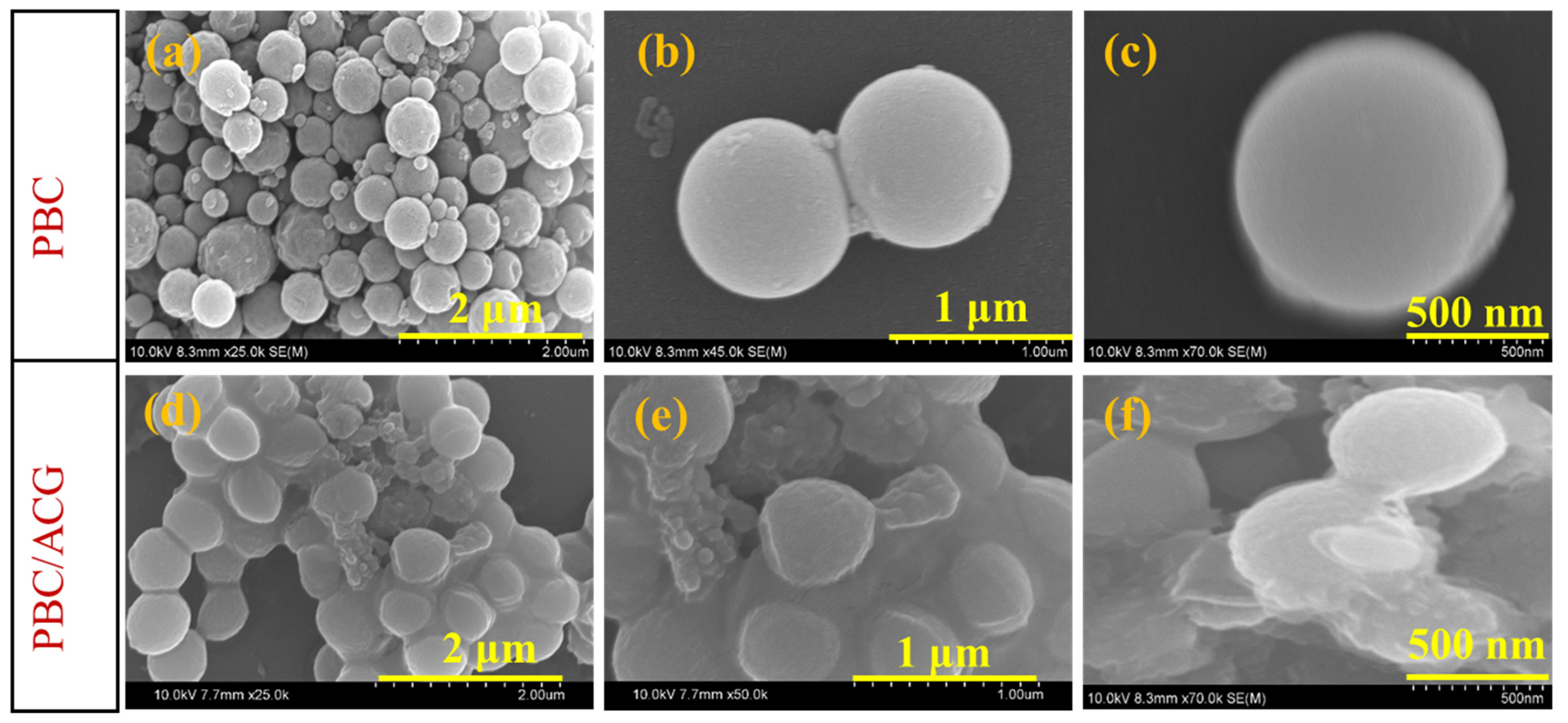
3.1. SEM Analysis
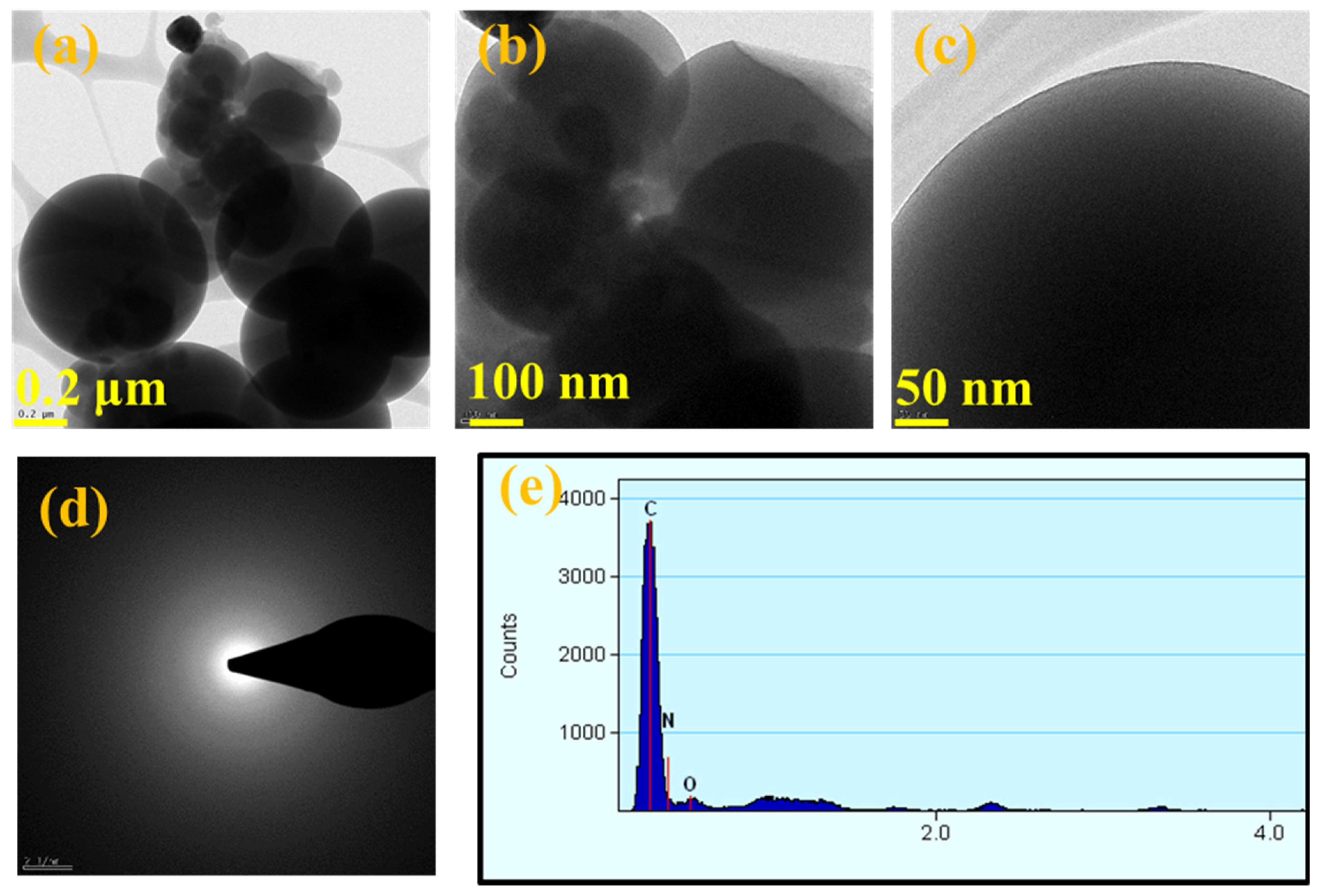
3.2. TEM Analysis
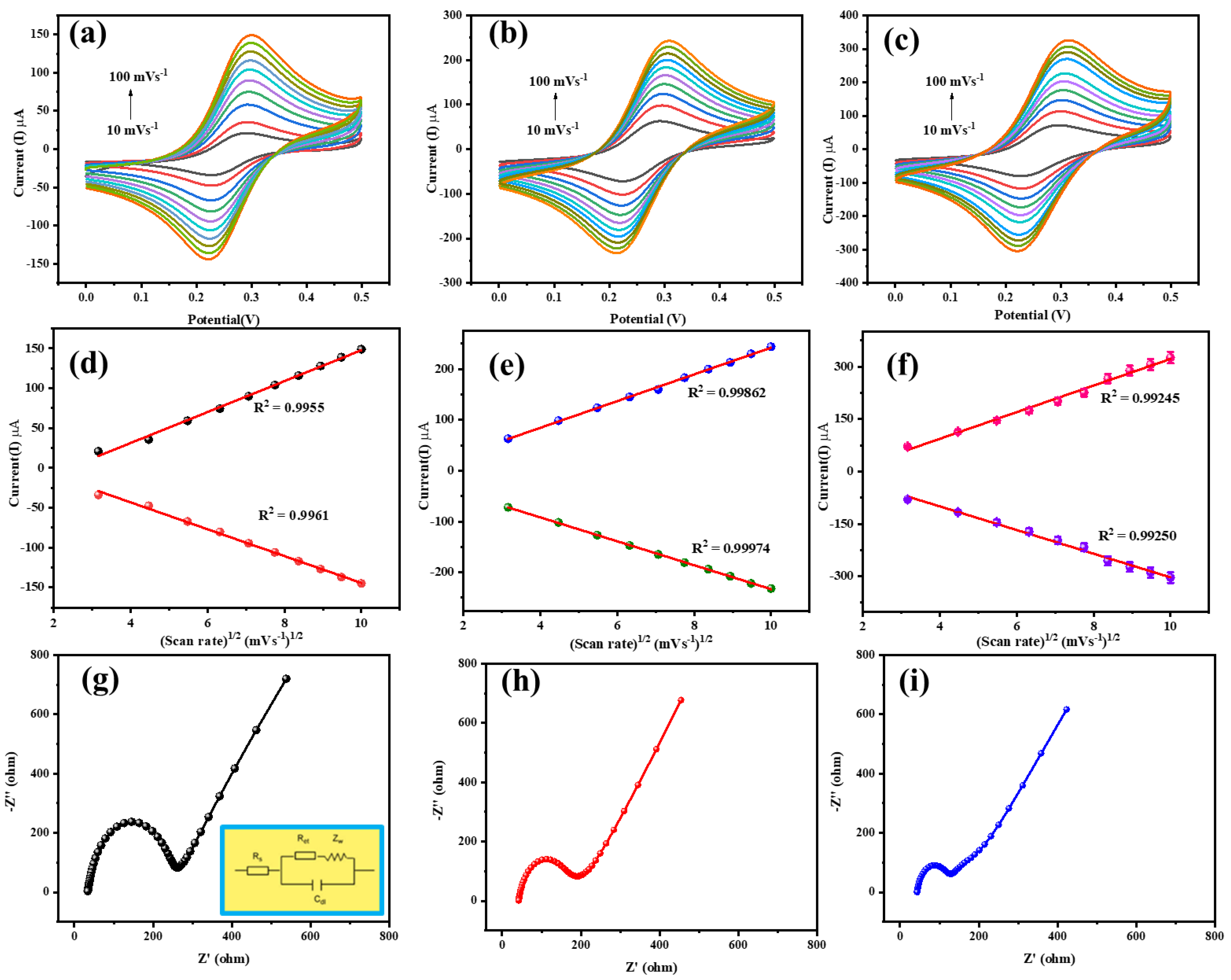
3.3. Electrochemical Results
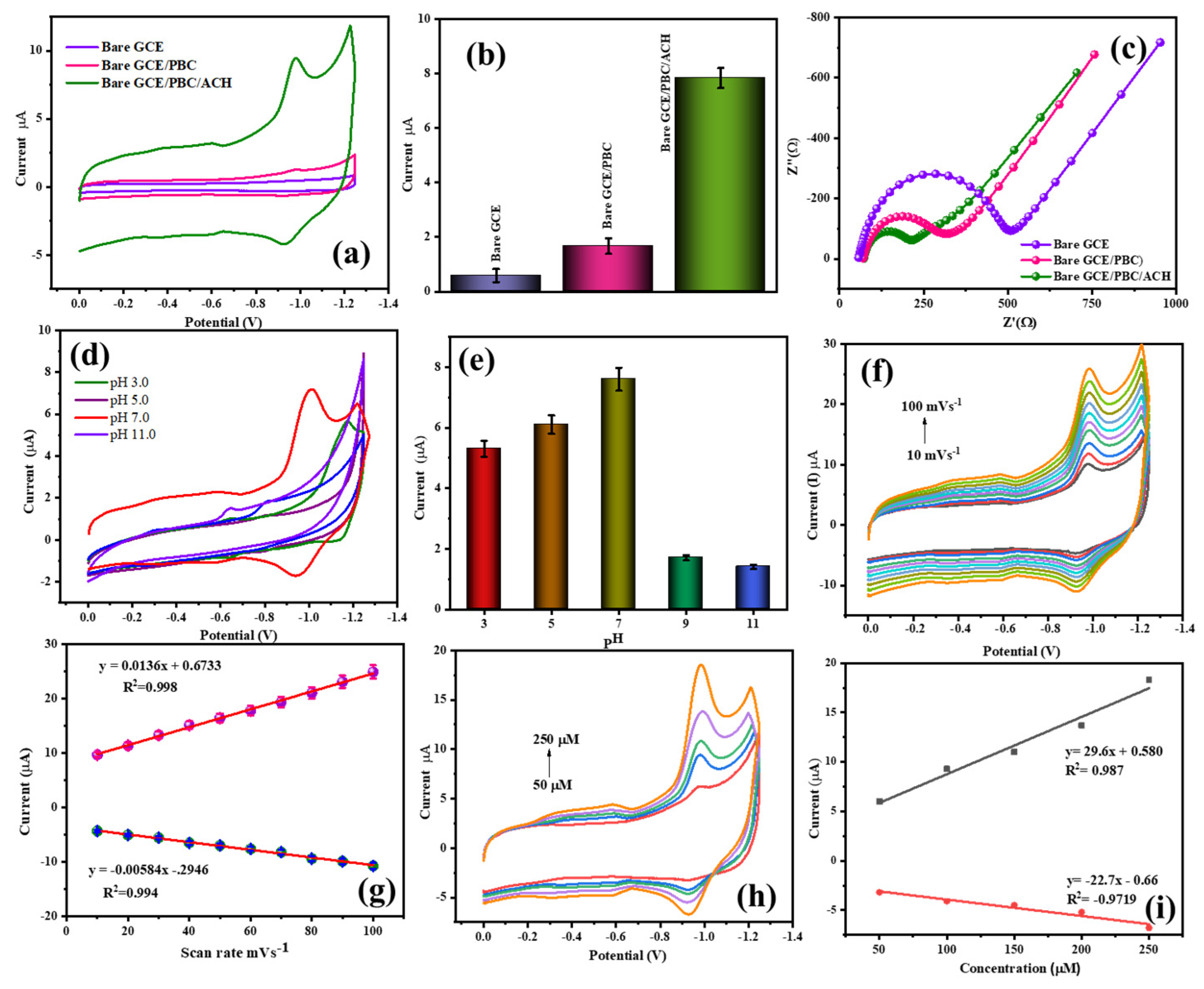
3.3.1. Electrochemical Performance of the Prepared Electrodes
3.3.2. Electrochemical Behavior of the Prepared Electrodes in Regard to MTZ Detection
3.3.3. Effect of pH
3.3.4. Effect of the Scan Rate
3.4. Material Concentration
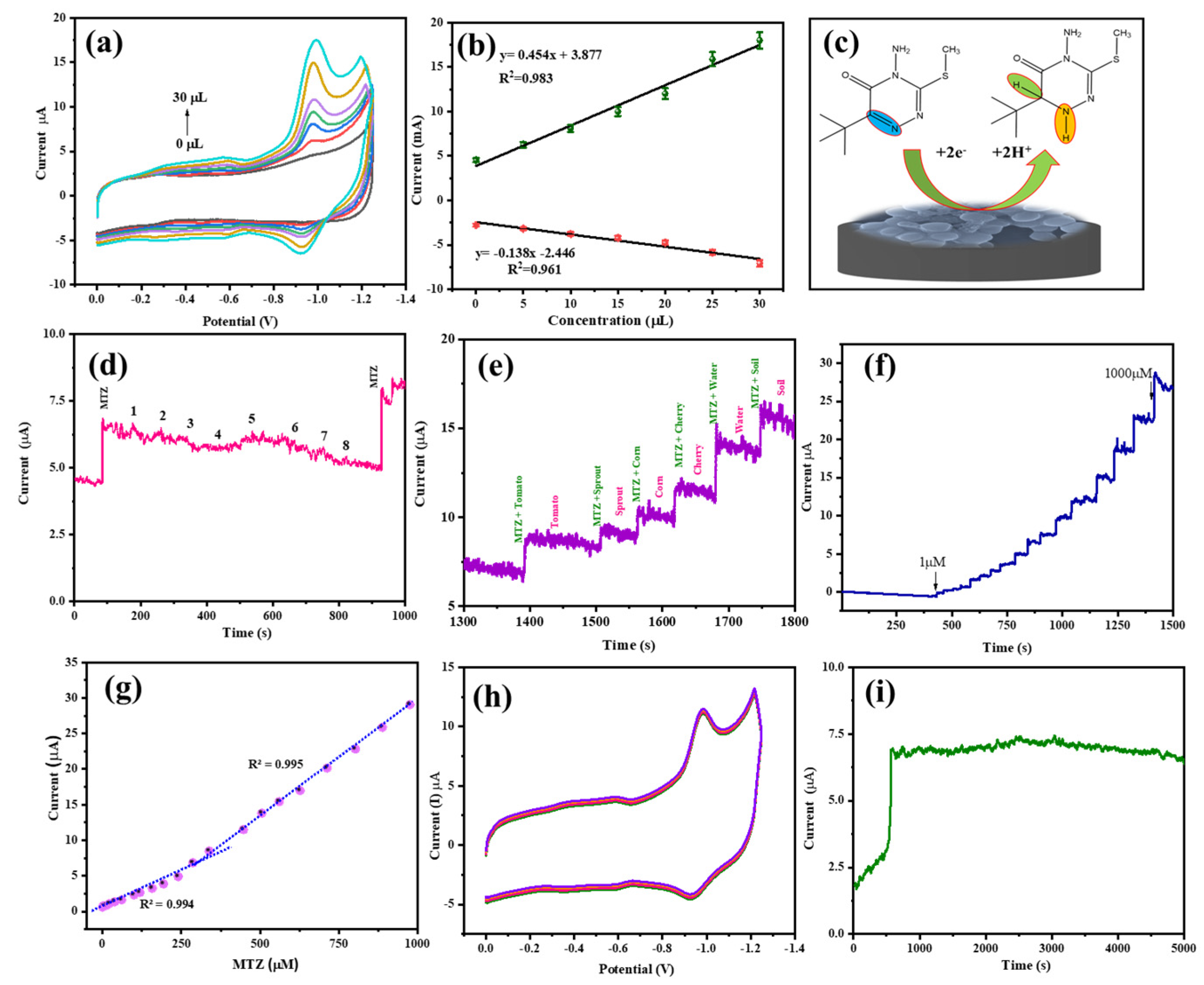
3.5. Analyte Concentration
3.6. Anti-Interference Property
3.7. Repeatability, Reproducibility, and Stability
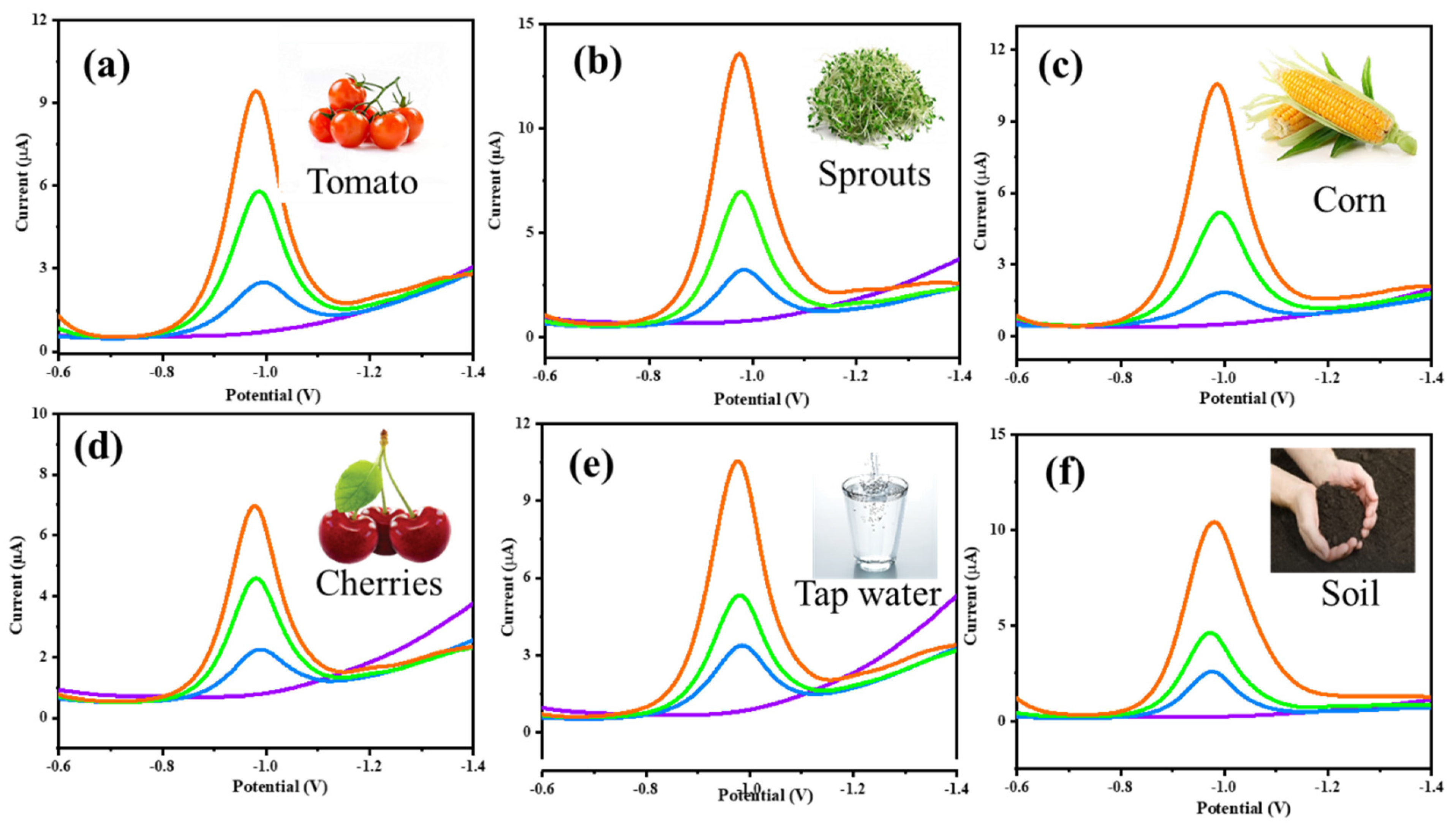
3.8. Real-Time Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mutharani, B.; Ranganathan, P.; Chen, S.M.; Deepak Vishnu, S.K. Stimuli-Enabled Reversible Switched Aclonifen Electrochemical Sensor Based on Smart PNIPAM/PANI-Cu Hybrid Conducting Microgel. Sens. Actuators B Chem. 2020, 304, 127232. [Google Scholar] [CrossRef]
- Stojanović, Z.; Durović, A.; Kravić, S.; Grahovac, N.; Suturović, Z.; Bursić, V.; Vuković, G.; Brezo, T. A Simple and Rapid Electrochemical Sensing Method for Metribuzin Determination in Tap and River Water Samples. Anal. Methods 2016, 8, 2698–2705. [Google Scholar] [CrossRef]
- Ranganathan, P.; Mutharani, B.; Chen, S.M.; Sireesha, P. Biocompatible Chitosan-Pectin Polyelectrolyte Complex for Simultaneous Electrochemical Determination of Metronidazole and Metribuzin. Carbohydr. Polym. 2019, 214, 317–327. [Google Scholar] [CrossRef]
- Akdağ, S.; Sadeghi Rad, T.; Keyikoğlu, R.; Orooji, Y.; Yoon, Y.; Khataee, A. Peroxydisulfate-Assisted Sonocatalytic Degradation of Metribuzin by La-Doped ZnFe Layered Double Hydroxide. Ultrason. Sonochem. 2022, 91, 106236. [Google Scholar] [CrossRef] [PubMed]
- Stenrød, M.; Perceval, J.; Benoit, P.; Almvik, M.; Bolli, R.I.; Eklo, O.M.; Sveistrup, T.E.; Kværner, J. Cold Climatic Conditions: Effects on Bioavailability and Leaching of the Mobile Pesticide Metribuzin in a Silt Loam Soil in Norway. Cold Reg. Sci. Technol. 2008, 53, 4–15. [Google Scholar] [CrossRef]
- Zagal, J.H.; Gulppi, M.A.; Depretz, C.; Lelièvre, D. Synthesis and Electrocatalytic Properties of Octaalkoxycobalt Phthalocyanine for the Oxidation of 2-Mercaptoethanol. J. Porphyr. Phthalocyanines 1999, 3, 355–363. [Google Scholar] [CrossRef]
- Serelis, K.; Mantzos, N.; Meintani, D.; Konstantinou, I. The Effect of Biochar, Hydrochar Particles and Dissolved Organic Matter on the Photodegradation of Metribuzin Herbicide in Aquatic Media. J. Environ. Chem. Eng. 2021, 9, 105027. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Konstantinou, I. Photocatalytic Treatment of Metribuzin Herbicide over TiO2 Aqueous Suspensions: Removal Efficiency, Identification of Transformation Products, Reaction Pathways and Ecotoxicity Evaluation. J. Photochem. Photobiol. A Chem. 2014, 294, 110–120. [Google Scholar] [CrossRef]
- Pandiyan, R.; Vinothkumar, V.; Chen, S.M.; Sangili, A.; Kim, T.H. Integrated LaFeO3/RGO Nanocomposite for the Sensitive Electrochemical Detection of Antibiotic Drug Metronidazole in Urine and Milk Samples. Appl. Surf. Sci. 2023, 635, 157672. [Google Scholar] [CrossRef]
- Benítez-Martínez, S.; López-Lorente, Á.I.; Valcárcel, M. Multilayer Graphene-Gold Nanoparticle Hybrid Substrate for the SERS Determination of Metronidazole. Microchem. J. 2015, 121, 6–13. [Google Scholar] [CrossRef]
- Jin, W.; Li, W.; Xu, Q.; Dong, Q. Quantitative Assay of Metronidazole by Capillary Zone Electrophoresis with Amperometric Detection at a Gold Microelectrode. Electrophoresis 2000, 21, 1409–1414. [Google Scholar] [CrossRef]
- Orooji, Y.; Haddad Irani-nezhad, M.; Hassandoost, R.; Khataee, A.; Rahim Pouran, S.; Joo, S.W. Cerium Doped Magnetite Nanoparticles for Highly Sensitive Detection of Metronidazole via Chemiluminescence Assay. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118272. [Google Scholar] [CrossRef]
- Daeseleire, E.; De Ruyck, H.; Van Renterghem, R. Rapid Confirmatory Assay for the Simultaneous Detection of Ronidazole, Metronidazole and Dimetridazole in Eggs Using Liquid Chromatography-Tandem Mass Spectrometry. Analyst 2000, 125, 1533–1535. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Karuppiah, C.; Chen, S.M.; Mani, V.; Vasantha, V.S.; Ramaraj, S. Highly Selective Determination of Cysteine Using a Composite Prepared from Multiwalled Carbon Nanotubes and Gold Nanoparticles Stabilized with Calcium Crosslinked Pectin. Microchim. Acta 2015, 182, 727–735. [Google Scholar] [CrossRef]
- Pasini Cabello, S.D.; Ochoa, N.A.; Takara, E.A.; Mollá, S.; Compañ, V. Influence of Pectin as a Green Polymer Electrolyte on the Transport Properties of Chitosan-Pectin Membranes. Carbohydr. Polym. 2017, 157, 1759–1768. [Google Scholar] [CrossRef]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Rachamalla, S.S.; Sistla, R. Xanthan Gum Stabilized Gold Nanoparticles: Characterization, Biocompatibility, Stability and Cytotoxicity. Carbohydr. Polym. 2014, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kadir, M.F.Z.; Hamsan, M.H. Green Electrolytes Based on Dextran-Chitosan Blend and the Effect of NH4SCN as Proton Provider on the Electrical Response Studies. Ionics 2018, 24, 2379–2398. [Google Scholar] [CrossRef]
- Zhong, X.; Yuan, R.; Chai, Y.Q. Synthesis of Chitosan-Prussian Blue-Graphene Composite Nanosheets for Electrochemical Detection of Glucose Based on Pseudobienzyme Channeling. Sens. Actuators B Chem. 2012, 162, 334–340. [Google Scholar] [CrossRef]
- Rassas, I.; Braiek, M.; Bonhomme, A.; Bessueille, F.; Rafin, G.; Majdoub, H.; Jaffrezic-Renault, N. Voltammetric Glucose Biosensor Based on Glucose Oxidase Encapsulation in a Chitosan-Kappa-Carrageenan Polyelectrolyte Complex. Mater. Sci. Eng. C 2019, 95, 152–159. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Ionic Conductivity and Dielectric Properties of Potato Starch-Magnesium Acetate Biopolymer Electrolytes: The Effect of Glycerol and 1-Butyl-3-Methylimidazolium Chloride. Ionics 2016, 22, 1113–1123. [Google Scholar] [CrossRef]
- Espíndola-González, A.; Martínez-Hernández, A.L.; Fernández-Escobar, F.; Castaño, V.M.; Brostow, W.; Datashvili, T.; Velasco-Santos, C. Natural-Synthetic Hybrid Polymers Developed via Electrospinning: The Effect of PET in Chitosan/Starch System. Int. J. Mol. Sci. 2011, 12, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Tiwari, A.; Gaur, A. Role of Excipients and Polymeric Advancements in Preparation of Floating Drug Delivery Systems. Int. J. Pharm. Investig. 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.W.W.; Lincy, V.; Selvi, S.V.; Prasannan, A.; Sambasivam, S.; Nimita Jebaranjitham, J. Carrageenan Derived Polyelectrolyte Complexes Material: An Effective Bifunctional for Electrochemical Sensing of Sulfamethazine and Antibacterial Activity. Int. J. Biol. Macromol. 2024, 264, 130445. [Google Scholar] [CrossRef] [PubMed]
- Novotný, V.; Barek, J. Voltammetric Determination of Aclonifen at a Silver Amalgam Electrode in Drinking and River Water. Ecol. Chem. Eng. S 2017, 24, 277–284. [Google Scholar] [CrossRef][Green Version]
- Rodrigo, M.A.; Oturan, M.A.; Oturan, N. Electrochemically Assisted Remediation of Pesticides in Soils and Water: A Review. Chem. Rev. 2014, 114, 8720–8745. [Google Scholar] [CrossRef] [PubMed]
- Guziejewski, D.; Smarzewska, S.; Skowron, M.; Ciesielski, W.; Nosal-Wiercińska, A.; Skrzypek, S. Rapid and Sensitive Voltammetric Determination of Aclonifen in Water Samples. Acta Chim. Slov. 2016, 63, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inam, R.; Çakmak, Z. A Simple Square Wave Voltammetric Method for the Determination of Aclonifen Herbicide. Anal. Methods 2013, 5, 3314–3320. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Kim, S.C. Heteroatom-Enhanced Porous Carbon Materials Based on Polybenzoxazine for Supercapacitor Electrodes and CO2 Capture. Polymers 2023, 15, 1564. [Google Scholar] [CrossRef]
- Bigucci, F.; Luppi, B.; Cerchiara, T.; Sorrenti, M.; Bettinetti, G.; Rodriguez, L.; Zecchi, V. Chitosan/pectin polyelectrolyte complexes: Selection of suitable preparative conditions for colon-specific delivery of vancomycin. Eur. J. Pharm. Sci. 2008, 35, 435–441. [Google Scholar] [CrossRef]
- Maciel, V.B.V.; Yoshida, C.M.P.; Franco, T.T. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr. Polym. 2015, 132, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, T.; Zhao, H.; Zhu, G.; Li, F.; Guo, M.; Ran, Q.; Komarneni, S. An Electrochemical Sensor Modified with Novel Nanohybrid of Super-P Carbon Black@zeolitic-Imidazolate-Framework-8 for Sensitive Detection of Carbendazim. Ceram. Int. 2023, 49, 23775–23787. [Google Scholar] [CrossRef]
- Shu, X.; Zhao, H.; Hu, Y.; Liu, J.; Tan, M.; Liu, S.; Zhang, M.; Ran, Q.; Li, H.; Liu, X. Magnesium and Silicon Co-Doped LiNi0.5Mn1.5O4 Cathode Material with Outstanding Cycling Stability for Lithium-Ion Batteries. Vacuum 2018, 156, 1–8. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Shen, D.; Yin, Q.; Ran, Q. Significantly Enhanced Electrochemical Properties of LiMn2O4-Based Composite Microspheres Embedded with Nano-Carbon Black Particles. J. Mater. Res. Technol. 2020, 9, 7027–7033. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, M.; Li, F.; Zhou, Y.; Zhu, G.; Liu, Y.; Ran, Q.; Nie, F.; Dubovyk, V. Fabrication of Gallic Acid Electrochemical Sensor Based on Interconnected Super-P Carbon Black@mesoporous Silica Nanocomposite Modified Glassy Carbon Electrode. J. Mater. Res. Technol. 2023, 24, 2100–2112. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, G.; Li, F.; Liu, Y.; Guo, M.; Zhou, L.; Liu, R.; Komarneni, S. 3D Interconnected Honeycomb-like Ginkgo Nut-Derived Porous Carbon Decorated with β-Cyclodextrin for Ultrasensitive Detection of Methyl Parathion. Sens. Actuators B Chem. 2023, 380, 133309. [Google Scholar] [CrossRef]
- Suea-Ngam, A.; Rattanarat, P.; Chailapakul, O.; Srisa-Art, M. Electrochemical Droplet-Based Microfluidics Using Chip-Based Carbon Paste Electrodes for High-Throughput Analysis in Pharmaceutical Applications. Anal. Chim. Acta 2015, 883, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Mutharani, B.; Rajakumaran, R.; Chen, S.M.; Ranganathan, P.; Tsai, H.C. Hierarchical Polyacrylonitrile-Derived Nitrogen Self-Doped 3D Carbon Superstructures Enabling Electrochemical Detection of Calcium Channel Blocker Nimodipine in Real Human Blood Serum. ACS Sustain. Chem. Eng. 2021, 9, 6586–6598. [Google Scholar] [CrossRef]
- Mutharani, B.; Ranganathan, P.; Yang, J.M.; Chang, Y.H.; Chiu, F.C.; Tsai, H.C. Rational Assembly of Polymer-Metal Coordination Hierarchical Superstructures for Azathioprine-Responsive Electrodes in Biological Samples. ACS Appl. Nano Mater. 2022, 5, 16207–16219. [Google Scholar] [CrossRef]
- Yao, Y.; Wen, Y.; Zhang, L.; Wang, Z.; Zhang, H.; Xu, J. Electrochemical Recognition and Trace-Level Detection of Bactericide Carbendazim Using Carboxylic Group Functionalized Poly(3,4-Ethylenedioxythiophene) Mimic Electrode. Anal. Chim. Acta 2014, 831, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, H.; Li, X.; Liu, B.; Liu, R.; Komarneni, S. Nanocomposite of Halloysite Nanotubes/Multi-Walled Carbon Nanotubes for Methyl Parathion Electrochemical Sensor Application. Appl. Clay Sci. 2021, 200, 105907. [Google Scholar] [CrossRef]
| Electrode | Rs (Ω) | Rct (Ω) | Zw (Ω) |
|---|---|---|---|
| Bare GCE | 76 | 233 | 724 |
| GCE/PBC | 100 | 162 | 680 |
| GCE/PBC-ACH | 105 | 89 | 616 |
| Sensor Material | Electrode Type | Detection Technique | Linear Range | Limit of Detection (LoD) | Sensitivity | Reference |
|---|---|---|---|---|---|---|
| Chitosan–Pectin Bio-Polyelectrolyte (CS-PC BPE) | GCE | Voltammetry | nM range | N/A | High selectivity and stability | [3] |
| LaFeO3/RGO Nanocomposite | Modified Electrode | Voltammetry | N/A | N/A | Sensitive detection in real samples | [9] |
| Multilayer Graphene–Gold Nanoparticle | Modified Electrode | Surface-Enhanced Raman Scattering (SERS) | N/A | N/A | High sensitivity for low concentrations | [10] |
| Chitosan/K-Carrageenan Polyelectrolyte | Glassy Carbon Electrode (GCE) | Voltammetry | 5 µM–7 mM | 5 µM | N/A | [19] |
| K-Carrageenan-Derived Polyelectrolyte (k-CGN/P(Am-co-DMDAAc)-GO) | GCE | Electrochemical Sensing | nM range | N/A | Excellent anti-interference ability | [23] |
| PBC-ACH Composite (Current Study) | GCE/PBC-ACH | Cyclic Voltammetry (CV) | 0.01–30 µM | 0.217 µM | 1.40 µA µM−1 cm−2 | Present Study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periyasamy, T.; Asrafali, S.P.; Kim, S.-C.; Lee, J. Electrochemical Sensing of Metribuzin Utilizing the Synergistic Effects of Cationic and Anionic Bio-Polymers with Hetero-Doped Carbon. Polymers 2025, 17, 39. https://doi.org/10.3390/polym17010039
Periyasamy T, Asrafali SP, Kim S-C, Lee J. Electrochemical Sensing of Metribuzin Utilizing the Synergistic Effects of Cationic and Anionic Bio-Polymers with Hetero-Doped Carbon. Polymers. 2025; 17(1):39. https://doi.org/10.3390/polym17010039
Chicago/Turabian StylePeriyasamy, Thirukumaran, Shakila Parveen Asrafali, Seong-Cheol Kim, and Jaewoong Lee. 2025. "Electrochemical Sensing of Metribuzin Utilizing the Synergistic Effects of Cationic and Anionic Bio-Polymers with Hetero-Doped Carbon" Polymers 17, no. 1: 39. https://doi.org/10.3390/polym17010039
APA StylePeriyasamy, T., Asrafali, S. P., Kim, S.-C., & Lee, J. (2025). Electrochemical Sensing of Metribuzin Utilizing the Synergistic Effects of Cationic and Anionic Bio-Polymers with Hetero-Doped Carbon. Polymers, 17(1), 39. https://doi.org/10.3390/polym17010039








