3.1. Structural Characterization
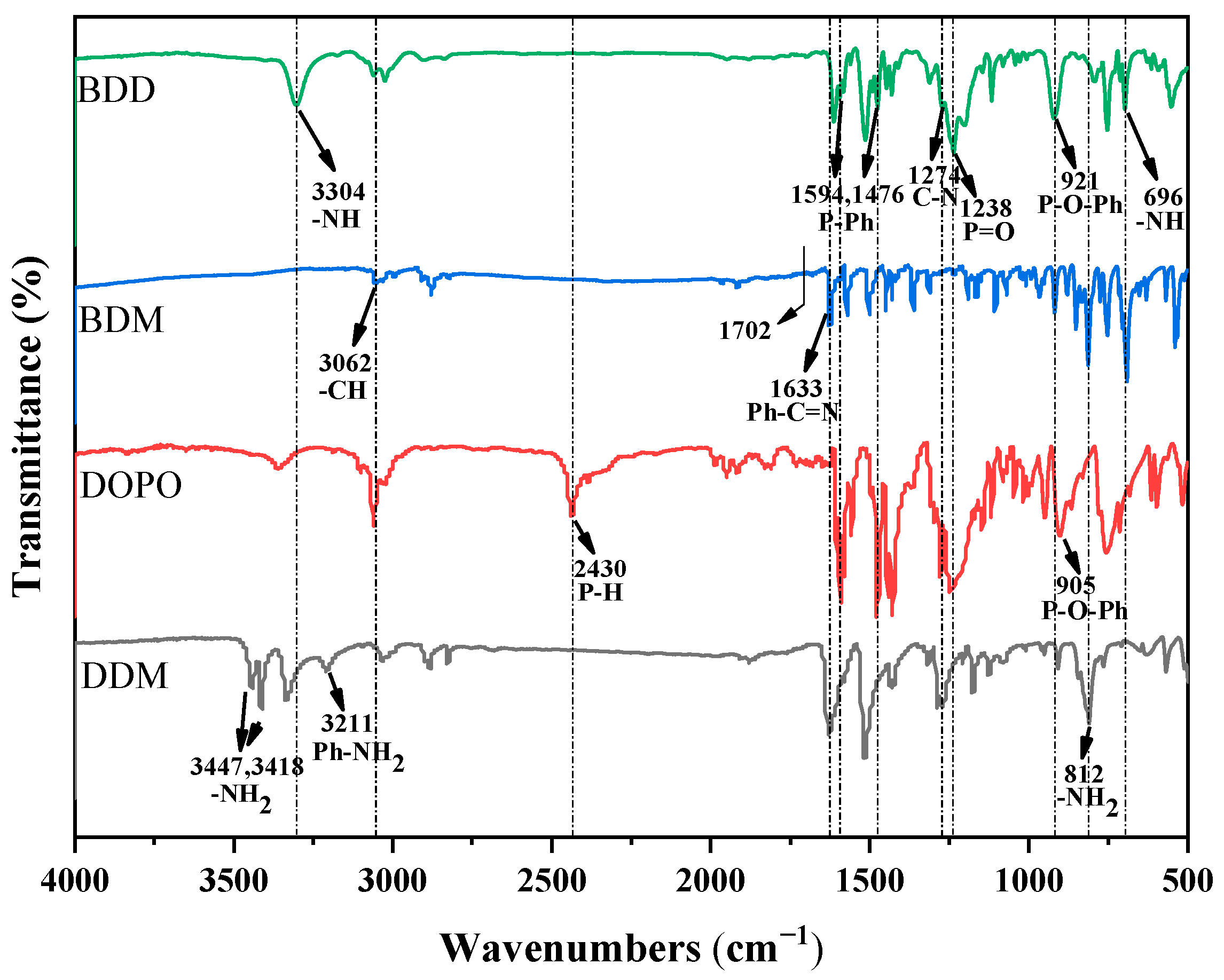
The FT-IR spectra of BDD, BDM, DOPO and DDM are shown in
Figure 2. Firstly, DDM, benzaldehyde and the intermediate product BDM were analyzed. Benzaldehyde is one of the most commonly used aromatic aldehydes in industry. Its characteristic peaks are mainly located at 3065 cm
−1, 2820 and 2738 cm
−1 and 1702 cm
−1, corresponding to the stretching vibration peaks of the -C-H bond on the benzene ring, the -C-H bond on the aldehyde group and -C=O, respectively. The group of DDM involved in the reaction was -NH
2. It can be seen that the peaks of -NH
2 at 3447, 3418 and 3211 cm
−1 in BDM all disappeared, which indicates that -NH
2 was successfully involved in the reaction. The peak at 3062 cm
−1 is attributed to the hydrocarbon stretching vibration on the benzene ring of benzaldehyde. The stretching vibration peak of -C=O of benzaldehyde generally appears at approximately 1700 cm
−1, but this peak is not observed in the spectrum of BDM, which indicates that -C=O on -CHO of benzaldehyde is also successfully involved in the reaction. In addition, the characteristic peak at 1633 cm
−1 in BDM belongs to Ph-C=N, which also exists in DDM, so it is difficult to judge. However, combined with the disappearance of the characteristic peaks of the -C=O bond of benzaldehyde and the -NH
2 bond of DDM, it can be concluded that BDM was successfully synthesized.
Subsequently, the FT-IR spectra of DDM, DOPO and BDD were analyzed. Compared with the spectrum of BDD, the bending vibration peak of -NH
2 at 812 cm
−1, the stretching vibration peak of -NH
2 at 3447 cm
−1 and 3418 cm
−1 and the binding peak of the benzene ring and amino group at 3211 cm
−1 in DDM were not observed in the BDD spectrum, indicating a successful reaction between DDM and benzaldehyde and the synthesization of the intermediate Schiff base [
3]. Compared with the FT-IR spectrum of DOPO, the peaks of BDD at 1594 cm
−1 and 1476 cm
−1 corresponded to the stretching vibration of the P-Ph bond, and the characteristic absorption peaks of the P=O bond and P-O-Ph bond appeared at 1238 cm
−1 and 921 cm
−1, respectively [
33]. The absorption peak of P-O-Ph in BDD is obtained by shifting the peak at 905 cm
−1 in the DOPO spectrum to a high wavenumber [
33]. The absorption peak of the P-H of the DOPO group near 2371 cm
−1 did not appear in the BDD spectrum, and the peak of the C-N appeared at 1274 cm
−1. In addition, the bending vibration peak of -NH at 696 cm
−1 and the tensile vibration peak of-NH at 3304 cm
−1 appear in the spectrum of BDD. This indicates that the P-H reacted with the Schiff base, confirming the synthesis of BDD.
The
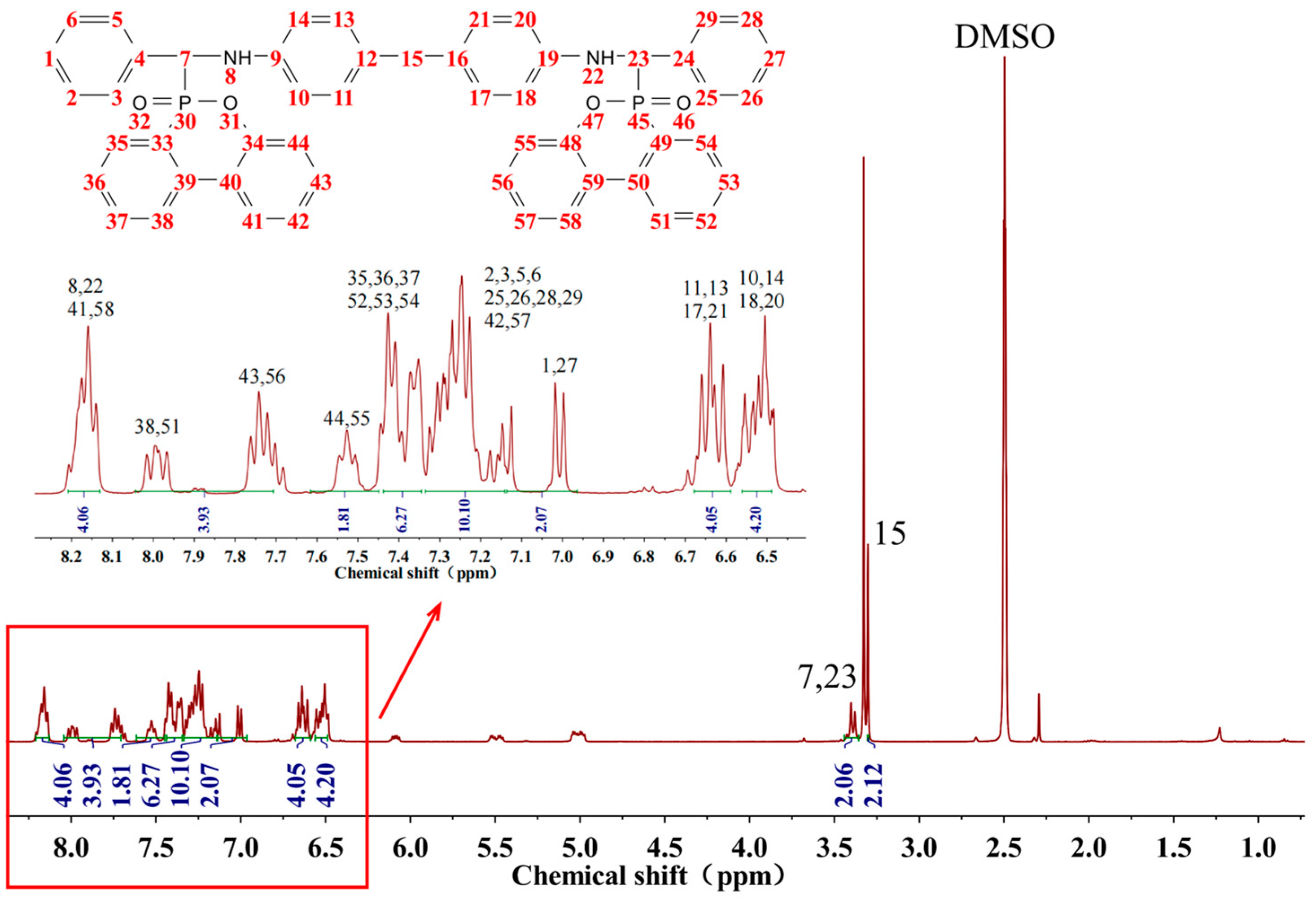
1H NMR spectrum of BDD (
Figure 3) displays two protons located on the primary carbon at 3.30 ppm, two protons located on the tertiary carbon at 3.40 ppm, aromatic hydrogen located between 6.45 and 8.25 ppm and two imino groups at 8.18 ppm. The benzene ring and the phosphaphenanthrene group’s hydrogen are challenging to distinguish and can only be roughly identified. However, after integrating all the peak areas, the ratio of the three hydrogens (C-H:N-H:Ar-H) can be calculated to be 2:1:17, consistent with each element’s proportion in BDD. In addition, the impurity peak at approximately 2.2 ppm may be the characteristic peak of -CH
3 in the residual toluene in benzaldehyde. This confirms that BDD has been successfully synthesized.
Figure 4 displays the
31P NMR spectra of BDD. The phosphorus peaks in DOPO molecules are near 31.18 ppm and 28.39 ppm, respectively [
34]. The splitting of the phosphorus peak of BDD is due to the considerable steric hindrance of the DOPO group, and the BDD molecule forms different stereoisomers under the steric hindrance of the two DOPO groups [
35,
36].
The XPS spectrum of BDD is shown in
Figure 5. According to the peak area, the ratio of C, O, N and P in the sample is 69.80:4.80:3.74:3.27. The corresponding theoretical ratio of C, O, N and P in the theoretical structure of BDD is 51:4:2:2. Although BDD may contain some impurities, such as incompletely reacted raw materials, which may cause the results to be different from the theoretical values, the two sets of data can be compared according to the cosine similarity. The calculation method is as follows:
Among them, x and y are vectors in the form of (x1, x2, x3 · · · xn), (y1, y2, y3 · · · yn), xi and yi are the proportions of each element calculated according to the BDD theoretical structure and XPS data, respectively. The value range of cosine similarity is [−1,1]. According to Formula 2.1, the similarity between the theoretical structure of BDD and the proportion of elements in XPS data is 0.9998. The closer the result is to 1, the higher the similarity is and the smaller the deviation is. Therefore, the XPS data show that the proportion of elements in BDD is highly consistent with its theoretical structure. This is an effective supplement to the results of FT-IR, 1H NMR and 31P NMR.
3.2. Analysis of Curing Behavior
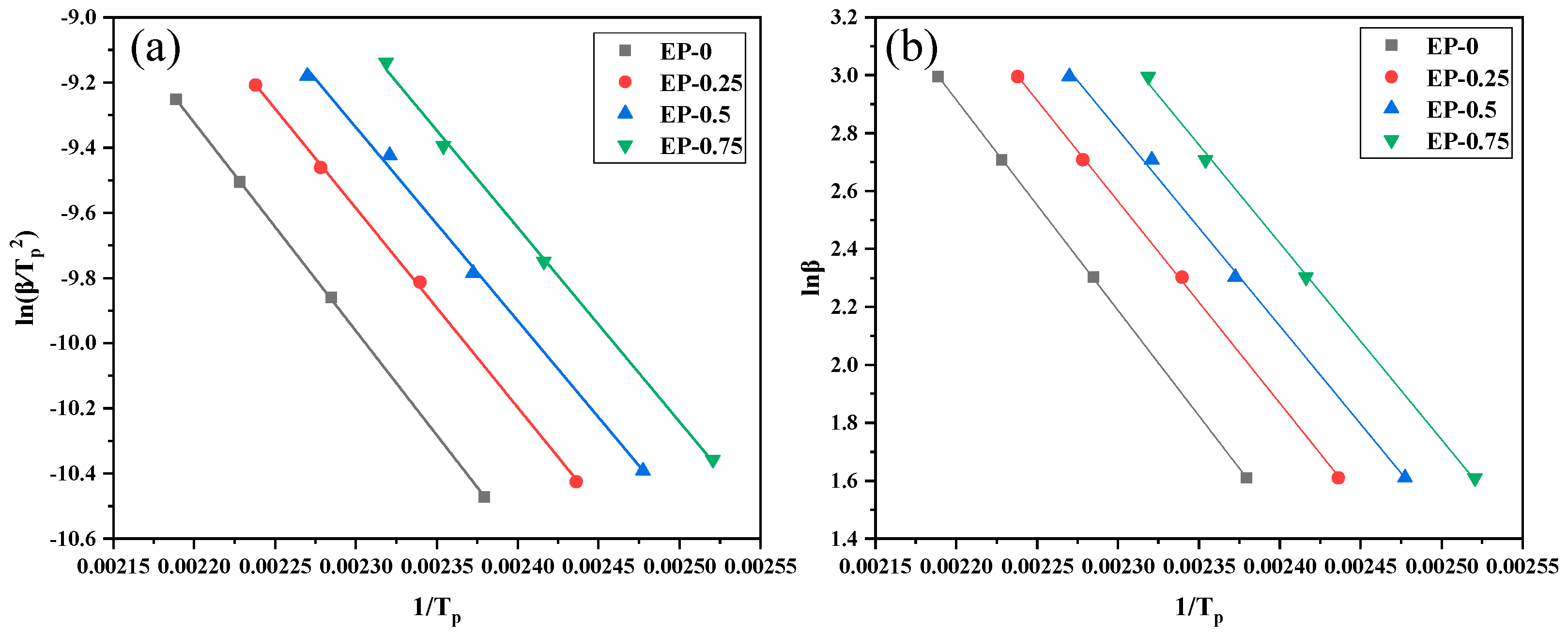
The nonisothermal curing kinetics of the EP/BDD/DDM system were studied by DSC. The activation energy of the curing reaction can be calculated using the formula of Kissinger and Ozawa [
37,
38]. Kissinger’s formula is as follows:
where
is the apparent activation energy,
is the ideal gas constant,
is the heating rate,
is the pre-exponential factor and
is the exothermic peak temperature. The peak exothermic temperatures of epoxy-cured products with different phosphorus contents measured in the test are summarized in
Table 2. The linear relationship between
and
calculated according to Kissinger’s method is shown in
Figure 6a. The Ozawa method is shown in Equation (3).
The linear relationship between
and
obtained from the above equation is shown in
Figure 6b. As shown in
Table 3, both methods can be used to calculate the apparent activation energy of the EP system by the slope of the corresponding line. It can be seen that the addition of BDD effectively reduces the activation energy of the EP system and improves the curing activity, which is because the imino group on BDD contains reactive active hydrogen. The hydrogen in the imino group can also form hydrogen bonds with the oxygen atoms in the epoxy group, which is beneficial to the ring-opening reaction of the epoxy group.
3.3. Thermal Properties
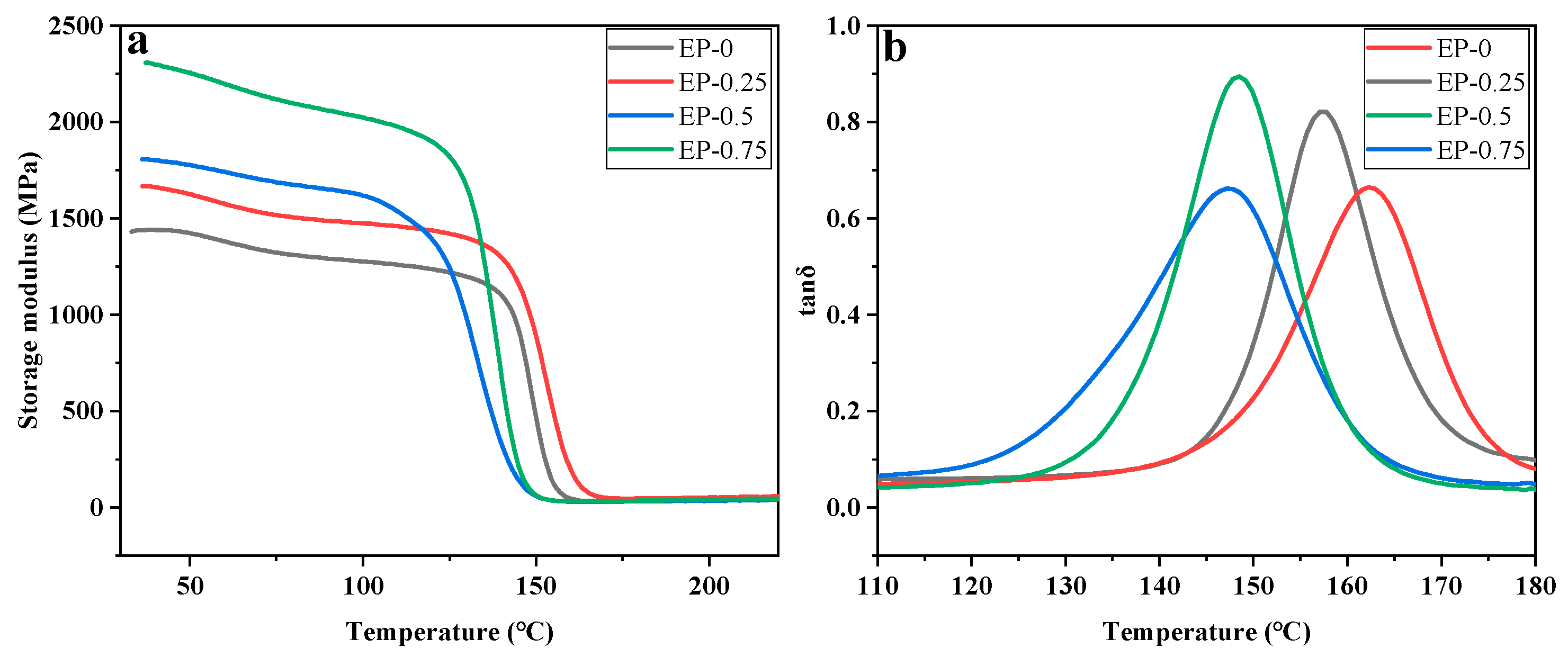
Figure 7 shows the storage modulus (E′) and loss tangent (tanδ) of EPs. Correspondingly, the storage modulus (at 35 °C), T
g and crosslinking density (ν
e) of each resin are presented in
Table 4. The DMA results demonstrated that the incorporation of BDD led to an augmentation in the E′ of EPs. For the phosphorus content of 0.75%, the storage modulus of EP-0.75 is 2296.9 MPa, 59.5% higher than EP-0. The stiffness of the EP network is increased by the substantial rigidity of the aromatic rings of BDD, as indicated by the storage modulus. The peak value of different tanδ curves in
Figure 7 represents the T
g of EP with additional phosphorus content. As the phosphorus level increases, the T
g of EP shows a downward trend, while the storage modulus is the opposite. In general, T
g depends on the stiffness of the polymer structure and its crosslinking density (ν
e) [
39]. Based on the principles of rubber elasticity theory, the ν
e can be determined using the following mathematical formula [
40]:
where
E′ is the storage modulus at T
g +45 °C,
R is the ideal gas constant, and
T is the thermodynamic temperature at T
g + 45 °C. It is evident from
Table 4 that a rise in BDD content leads to a decrease in ν
e of EP. Although the rigid group in BDD will reduce the activity of the EP segment and lead to an increase in T
g, the crosslinking density of the EP may be reduced under the influence of steric hindrance. Additionally, BDD, which has only two reactive hydrogens, reduces the bonding sites of EP, resulting in a further reduction in its crosslinking density. The T
g of EP falls when BDD is added, owing to the combined influence of these factors.
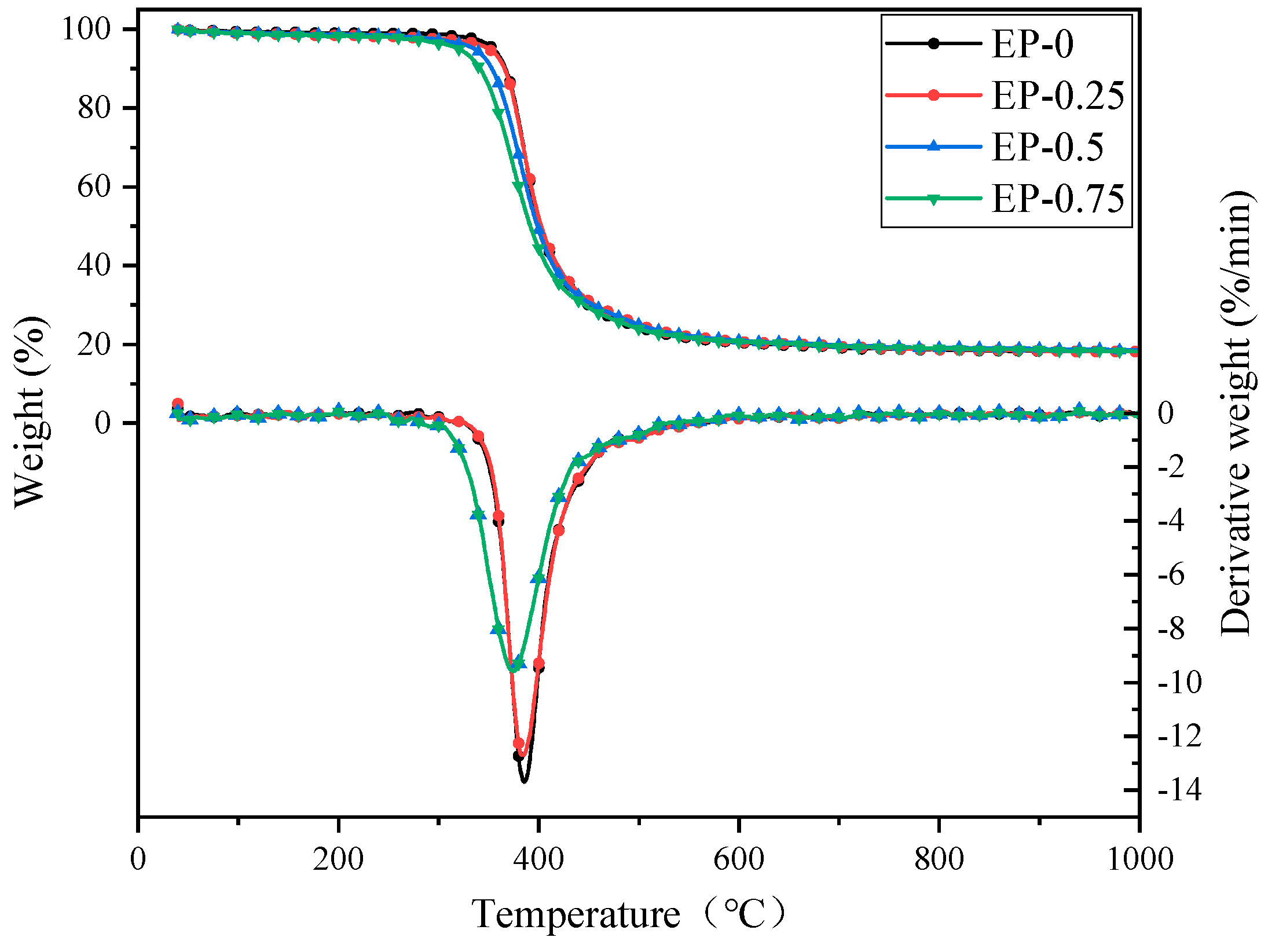
The TGA and DTG curves of EP are shown in
Figure 8, with the data of T
5% (5% weight loss temperature), T
max (maximum weight loss rate temperature), R
max (maximum weight loss rate) and R
700 (residual mass at 700 °C) collected in
Table 5. The T
5% of EP exhibits a steady decline with increasing phosphorus levels. This is because the flame retardant BDD contains an unstable O=P-O bond [
41], causing it to decompose at a lower temperature and generate substances such as phosphoric and polyphosphoric acids. The DTG curves in
Figure 8 exhibit a single sharp weight loss peak for each resin in a nitrogen atmosphere, with R
max decreasing as the phosphorus level increases. The decrease in R
max is due to the instability of BDD macromolecules, which will undergo pyrolysis at a lower temperature. The pyrolysis products of BDD are evenly distributed on the surface of EP, which promotes the formation of a protective carbon layer and prevents its further decomposition. Therefore, as the proportion of BDD in the EP system increases, the R
700 of the cured EP gradually increases, exhibiting gradually enhanced thermal stability of the EP.
3.4. Flame-Retardant Property
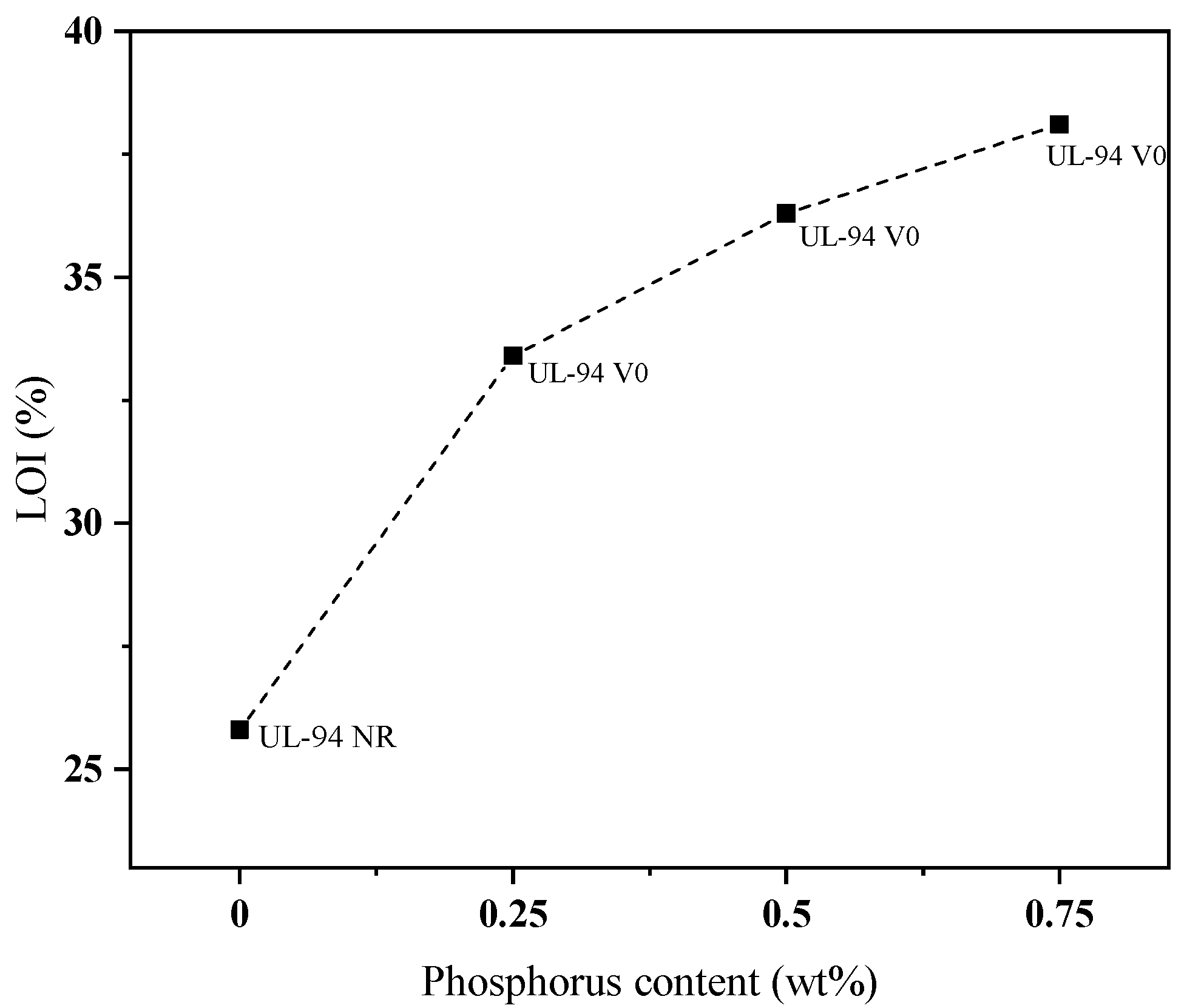
Figure 9 shows the vertical burning rating and LOI value of EP with different BDD additions, which are elaborated in
Table 6. The flame-retardant qualities of BDD-modified EP are very commendable. An EP-0.25 material can earn a V-0 rating in the UL-94 classification when its phosphorus concentration is limited to 0.25 wt%. The LOI value of EP-0 without BDD was 25.8%. Nevertheless, it exhibits a significant increase when BDD is introduced, ultimately peaking at 38.1% when the percentage of phosphorus reaches 0.75 wt% [
42].
Phosphorus is the essential ingredient that imparts flame retardancy to EP in BDD. During combustion, PO· released by BDD decomposition can effectively capture the surrounding active free radicals, thereby reducing the energy of the flame. Furthermore, the presence of phosphate and polyphosphoric acid in the condensed phase facilitates the creation of a compact carbon layer, effectively shielding the polymer from both heat and air [
43,
44]. Moreover, the nitrogen-containing groups produce nonflammable gases when burned, which have a flame-retardant action in the gas phase [
45]. Therefore, incorporating BDD into the EP results in a remarkable enhancement of flame retardancy. Xu et al. [
3] synthesized D-bp with a molecular structure similar to BDD to modify EP. When the phosphorus content of the modified resin is 0.25 wt %, the LOI value of EP is 30.5%, which can pass UL-94 V-1 certification. By comparing the LOI value and UL 94 test results at the same phosphorus content, it can be determined that the flame-retardant efficiency of BDD is higher than that of D-bp. The only difference between the two flame retardants is that D-bp has two extra hydroxyl groups. In the initial stage of the thermal decomposition of EP, the nonchain-breaking reactions of dehydrogenation and dehydration occur, forming an unstable double bond related to the secondary alcohol group in the EP [
46]. However, the hydroxyl groups in D-bp are not entirely reacted, and they promote the generation of methane, carbon dioxide, formaldehyde and hydrogen during combustion, which are flammable gases except for carbon dioxide. Thus, the EP cured with BDD, which does not contain a hydroxyl group, is more effective in terms of flam-retardant properties than D-bp. It can be inferred that the elimination of certain unstable groups from the current flame retardants is a feasible strategy to enhance efficiency.
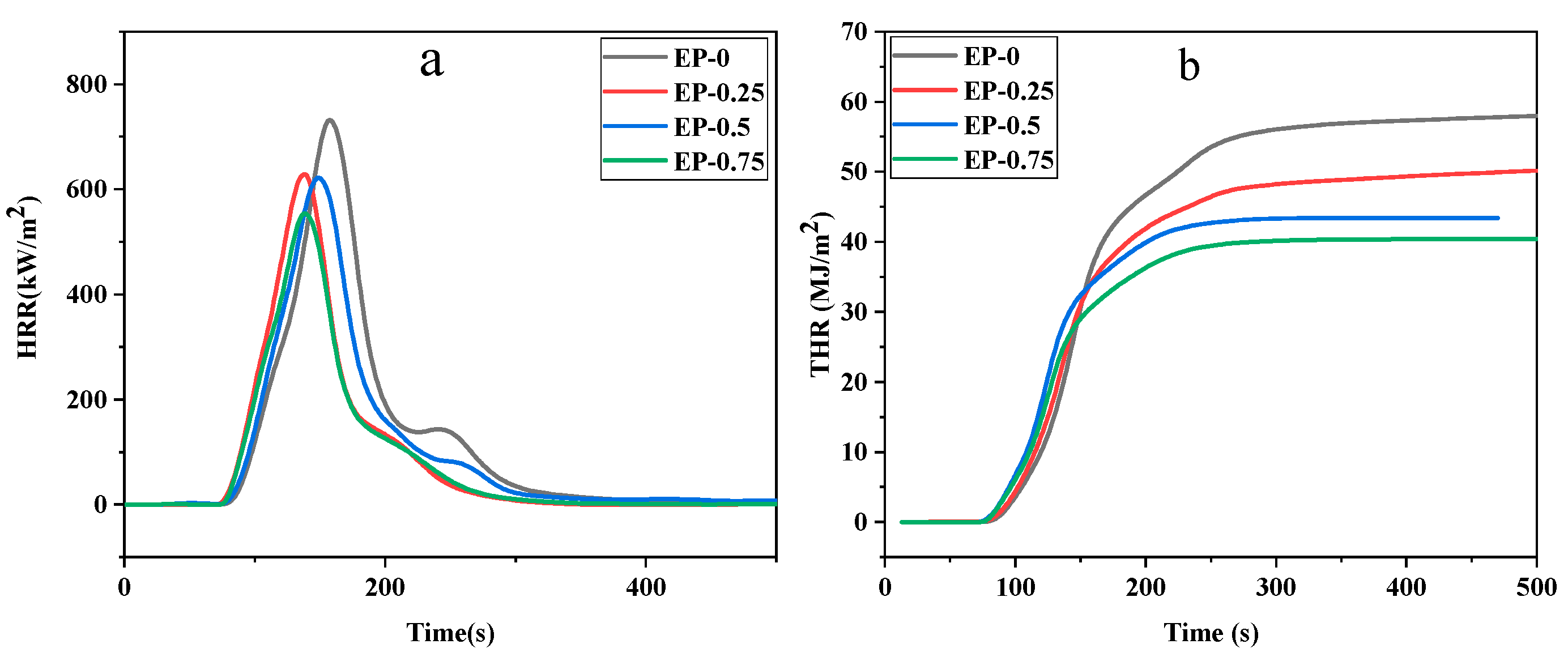
The cone calorimeter test is employed to further assess the combustibility of EP. The requested data include heat release rate (HRR), peak heat release rate (P-HRR), total heat release (THR), effective heat of combustion (EHC) and time to ignition (TTI).
Figure 10 displays the relationship between the HRR and the THR over time for various phosphorus concentrations in the EP. The P-HRR and EHC data in
Table 7 showed that with the addition of BDD, the P-HRR and THR of EP decreased significantly with the increase in phosphorus content, and EHC also decreased gradually. BDD offers a prosperous enhancement to the flame retardancy of EP.
Table 7 demonstrates that the TTI of EP-0 was 55 s, and that of EP-0.75 declined to 44 s with a 20% reduction. This is because the phosphorus-containing groups begin to pyrolyze at lower temperatures and subsequently catalyze the creation of a durable carbon layer on the surface of the polymer [
39]. As for the residue, EP-0 was almost completely burned, with a mass loss of 94.37% or so. The addition of BDD resulted in a notable rise in the char amount of the resins, which confirms the mechanism by which BDD facilitates the creation of a carbon layer so as to isolate heat and limit the emission of flammable gases [
47].
It is crucial to highlight that the residue of EP-0.25 shows a substantial increase when compared to EP-0. The quantity of residual carbon remains nearly constant as the phosphorus content steadily rises. The phosphorus content does not appear to dramatically affect the char yield. This is because more EP has been pyrolyzed, forming a dense carbon layer. The experiments reveal that a small amount of BDD improves the flame retardancy of EP effectively. Nevertheless, increasing the percentage of BDD does not result in an enormous leap in the flame-retardant properties of EP. Therefore, the inclusion of a minimal quantity of BDD can effectively enhance the flame-retardant properties of EP without compromising its mechanical capability.
3.5. Morphologies and Components of Char Residue
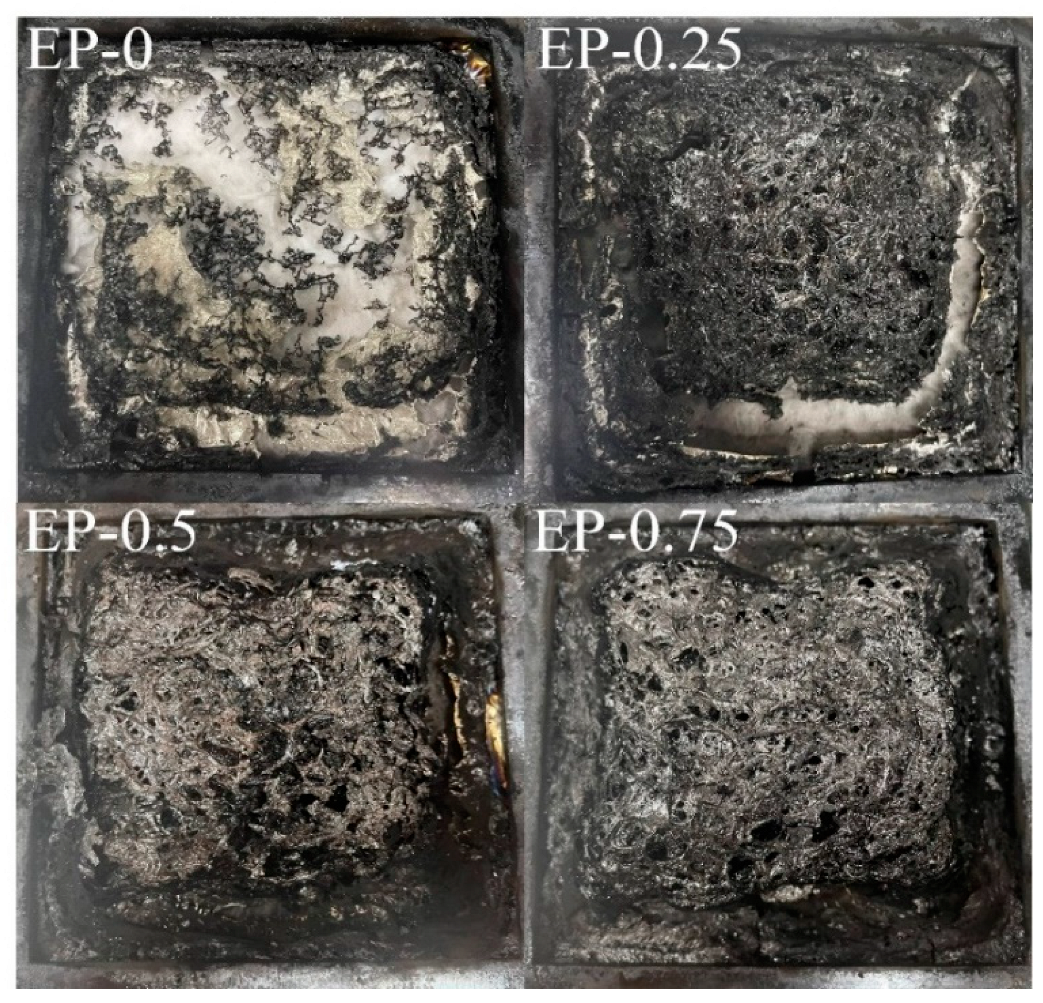
The photos of the residual of the EP sample following the cone calorimeter test are displayed in
Figure 11. Only scattered residual carbon can be observed in EP-0. In contrast, the EP incorporated with BDD retained its cubic shape after combustion, demonstrating the role of BDD in enhancing and accelerating char residue formation.
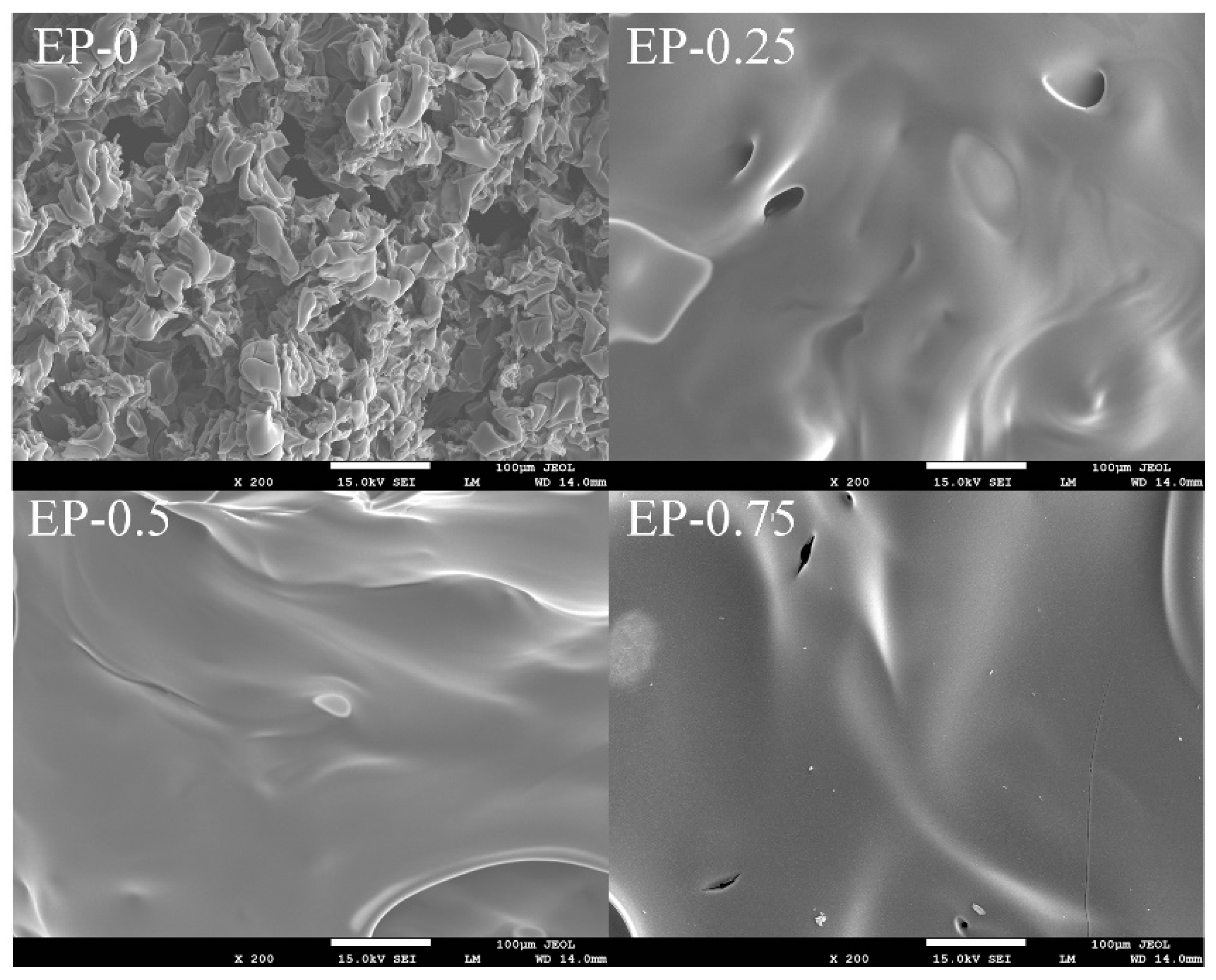
Figure 12 displays the microscopic structure of residual carbon as observed through the cone calorimeter. The results indicate that EP-0 is severely damaged, with a charred surface full of holes and cracks that help transfer oxygen and heat. Conversely, the carbon residue surface of EP containing BDD is smooth and dense, with only a tiny number of micropores, which is consistent with the observations shown in
Figure 11. The EP containing BDD forms a dense carbon layer that successfully blocks the heat transfer during combustion [
21], thereby showing excellent flame retardancy. The elemental distribution of chars was analyzed using EDS element mapping, as shown in
Figure 13. The element mapping corresponding to the external residual carbon shows that C, O and P are evenly distributed, which may be related to the carbonization catalysis of phosphorus and the quenching effect of PO free radicals, indicating that the phosphorus-containing macromolecules are pyrolyzed into phosphorus-containing derivatives such as phosphoric acid during combustion and uniformly attached to the surface of the substrate to catalyze dehydration and dehydrogenation, thus forming a dense carbon layer [
48,
49]. This discovery offers more substantiation for the effectiveness of integrating phosphorus into the EP as a flame retardant.
3.6. TG-IR Analysis
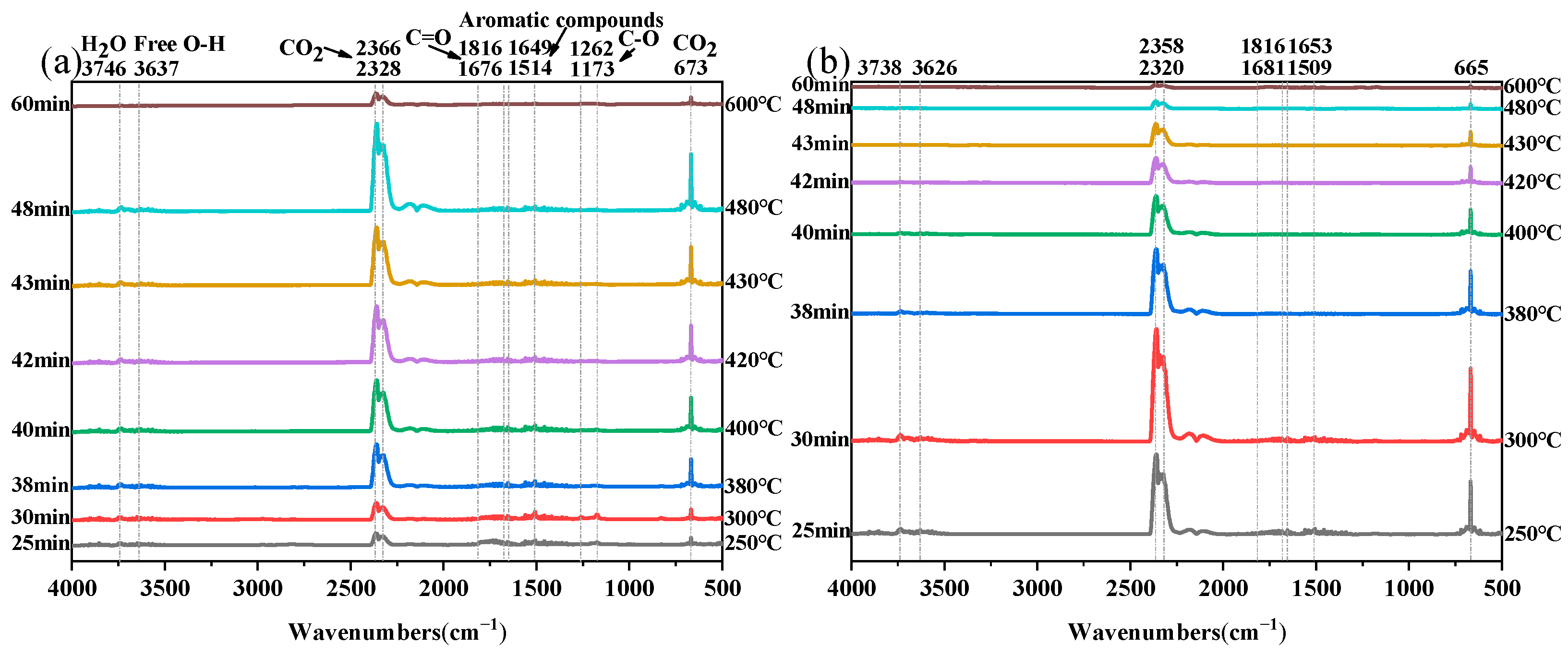
The volatile components of EPs (EP-0 and EP-0.25) were continually monitored using TGA-FTIR. As depicted in
Figure 14a and
Figure 15a, the primary gaseous products of pure EP volatiles include H
2O (3746 cm
−1), free O-H (3637 cm
−1), CO
2 (2366, 2328 and 673 cm
−1), carbonyl C=O (1816−1676 cm
−1), aromatic compounds (1649 and 1514 cm
−1) and ester or ether components (1262 and 1173 cm
−1) [
50,
51]. Aromatic amine, phenolic and carbonyl-containing substances are the primary components of these structures [
52], in which carbonyl-containing substances are highly flammable volatile fuels.
As observed in
Figure 15a,b, the main absorption peaks of EP-0.25 are essentially the same as those of EP-0, except for the different peak intensities in the corresponding regions. The area of the peak can represent the amount of the corresponding volatiles. It can be seen that a significant amount of CO
2 was generated during the initial phase of EP-0.25 combustion, with the maximum reached at 30 min or so, when CO
2 had just begun to generate for EP-0. This is strongly correlated with the TGA results above. The phosphorus-containing functional group is unstable, and it rapidly decomposes into free radicals during the initial moments of burning and catalyzes the pyrolysis of EP to form a dense protective carbon layer, which inhibits further combustion. Due to the lack of an effective protective carbon layer formed at the initial reaction stage, the pure EP will quickly burn out as the reaction intensifies. In addition, the absorption peaks of EP-0.25 at 1816–1676 cm
−1, 1649 cm
−1, 1514 cm
−1, 1262 cm
−1 and 1173 cm
−1 are much lower than those of EP-0. Carbonyl C=O (1816−1676 cm
−1), aromatic compounds (1649 and 1514 cm
−1) and ether components (1173 cm
−1) are considered to be combustible gases released during the combustion of epoxy. This phenomenon can be explained by the fact that the PO· free radicals, which are produced when phosphorus-containing flame retardants undergo pyrolysis, are able to capture O· free radicals. This reduces the attack on the carbon chain and leads to a decrease in the oxygen content of the air, which further inhibits the combustion of the EP and reduces the combustible gases from the pyrolysis of the carbon chain. The dense carbon layer formed due to the incorporation of the flame retardant also inhibits further cracking of the EPs. These factors play together and lead to a significant reduction in the emission of combustible gases, which also offers flame-retarding effects in the gas phase.
3.7. Mechanical Properties
Figure 16 illustrates a comparison of the mechanical characteristics of EPs with varying phosphorus amounts. The mean and standard deviation of the mechanical properties of each sample are shown in
Table 8. The mechanical properties of EPs show a consistent pattern. As the phosphorus content increases, the tensile and flexural strengths steadily fall, but the tensile and flexural moduli increase. For D-bp with a similar structure to BDD, when the D-bp modified EP reached UL 94 V-0 level, the tensile and flexural strengths decreased by 13.1% and 10.3% compared to EP-0, respectively. In this paper, as far as EP-0.25 is concerned, the V-0 rating in the UL-94 test is achieved with the average tensile and flexural strengths of 77.1 MPa and 131.02 MPa, respectively, only 6.4% and 10.5% lower than those of the EP-0. However, the tensile and flexural strengths of EPs decrease significantly for higher phosphorus contents of 0.5 wt% and 0.75 wt%. This is attributed to the rigid structures, such as benzene and heterophenanthrene rings, introduced into the epoxy-cured network by BDD [
53]. On one hand, the molecular structures of BDD occupy the crosslinking sites of the EP network. This reduces the crosslinking density of EP and leads to a decline in both its tensile strength and modulus. On the other hand, these macromolecular structures have considerable steric hindrance, resulting in a decrease in the flexibility of molecular chains and an increase in moduli. The weak bonds in the phosphorus-containing groups such as O=P-O and P-C on BDD also cause an elevation in stiffness and a reduction in strength, making the cured epoxy products more brittle and less tough [
41]. For the combined consideration of fire retardancy and mechanical behavior, it is suggested that the optimal amount of phosphorus content is 0.25 wt% for the flame-retardant EP.