Elaboration of Thermally Performing Polyurethane Foams, Based on Biopolyols, with Thermal Insulating Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Synthesis of the Biopolyols: Liquefaction Process, Purification and Neutralization
Optimization of the Liquefaction Process—Ratio of Solvents
2.3. Characterization of the Biopolyols
2.4. Preparation of the Polyurethane Foams
Development of the PUF Formulations: Optimization Process
2.5. Characterization of the Polyurethane Foams
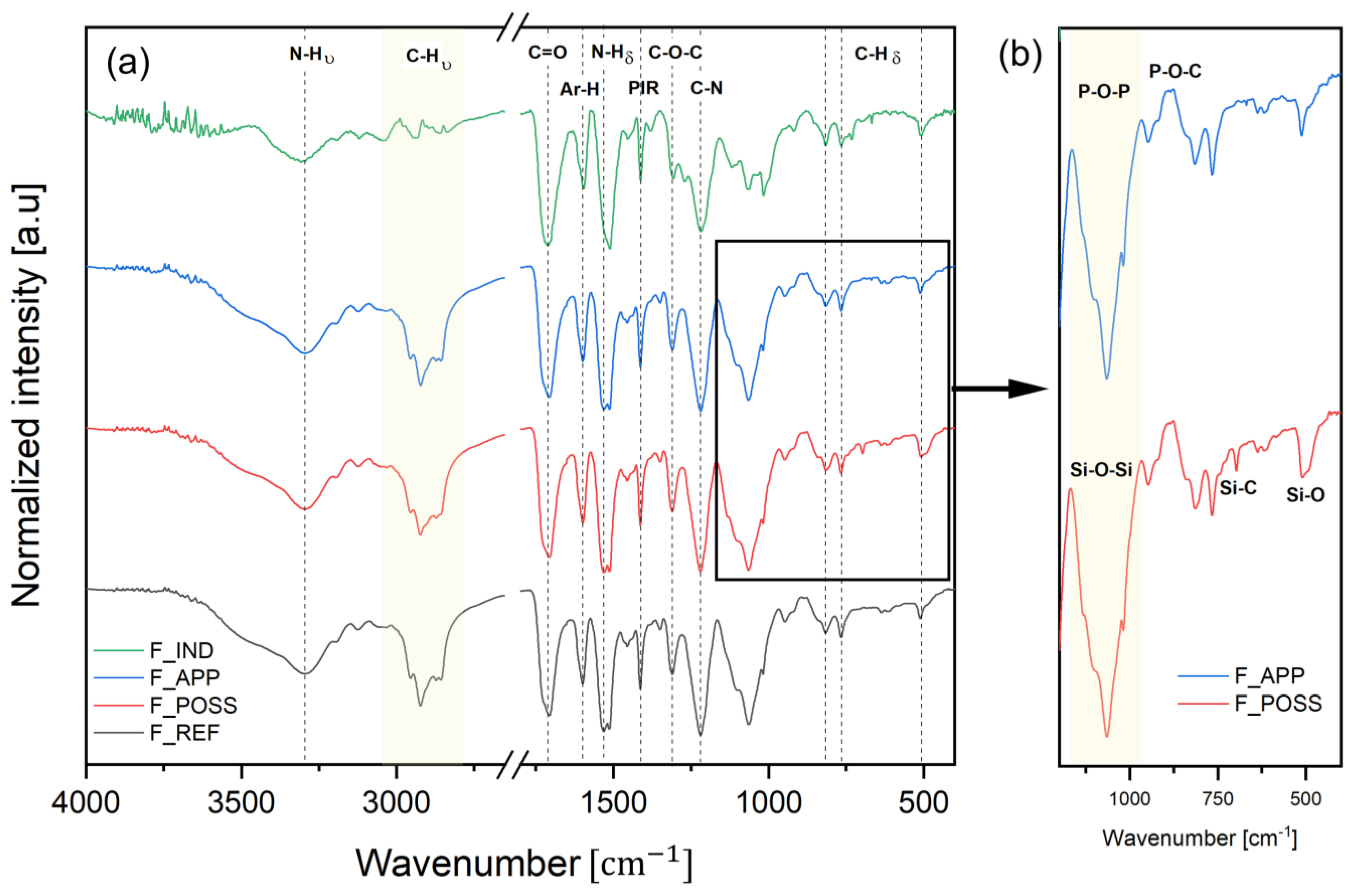
2.5.1. Structural Analysis by Fourier Transformed Infrared Spectroscopy (FT-IR)
2.5.2. Thermogravimetric Analysis (TGA)
2.5.3. Study of Polyurethane Foams Performance
3. Results and Discussion
3.1. Characterization of Raw Material and Liquefaction Process
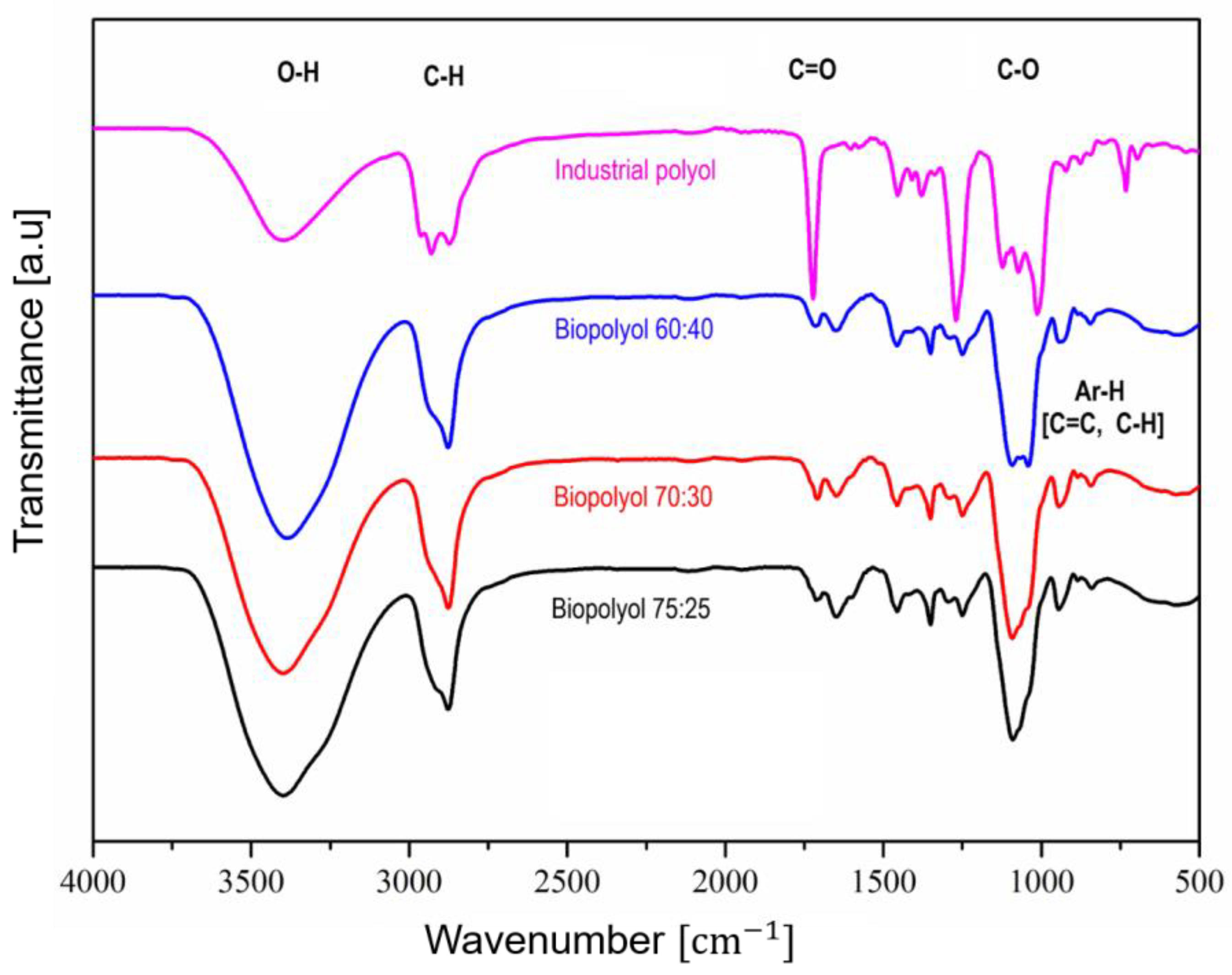
3.2. Chemical and Structural Characterization of the Polyol
3.2.1. Structure and Performance of the Polyol during the Optimization of the Liquefaction Process
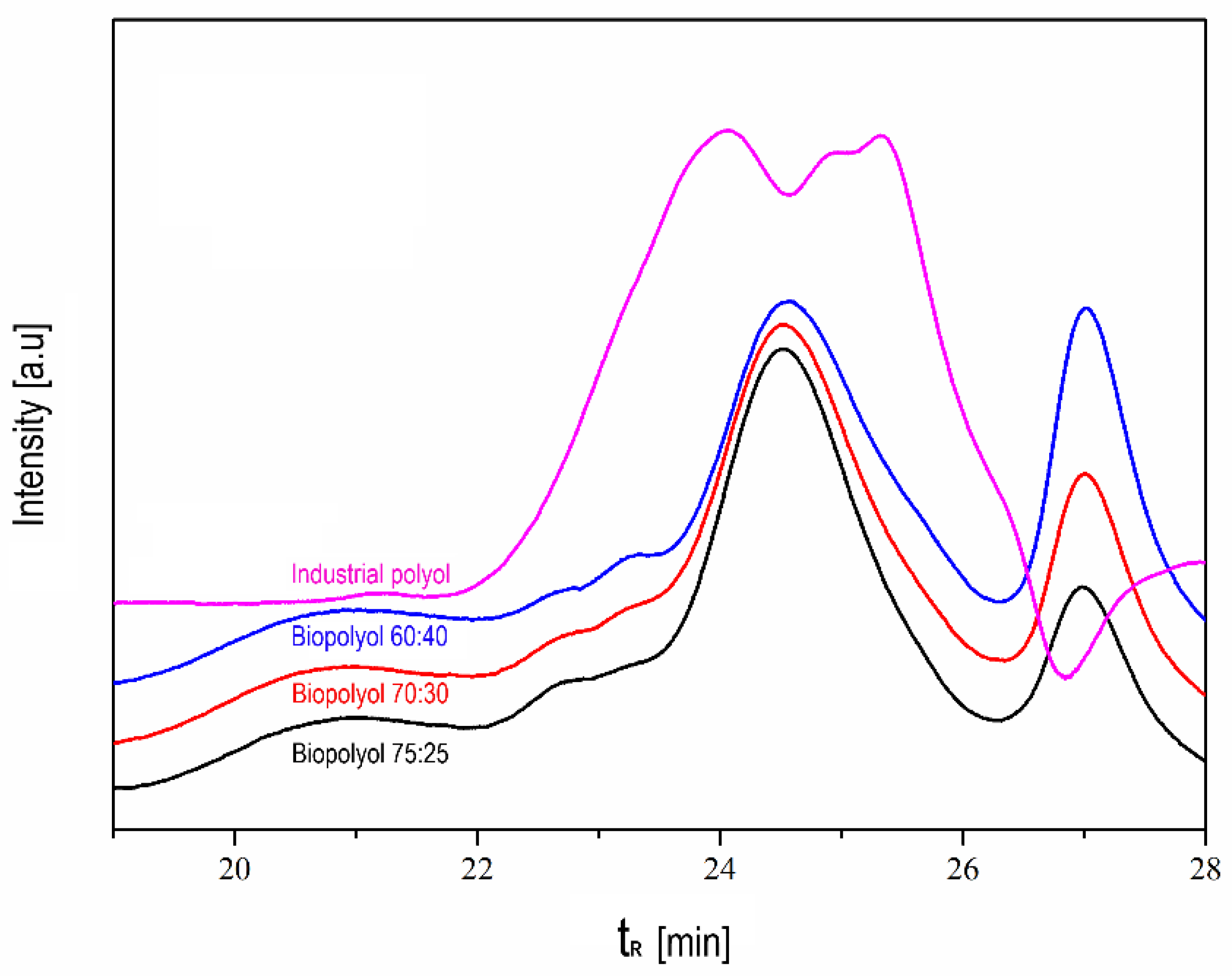
3.2.2. Assessment of the Optimized Biopolyol with the Industrial Polyol
3.3. Characterization of the Foams
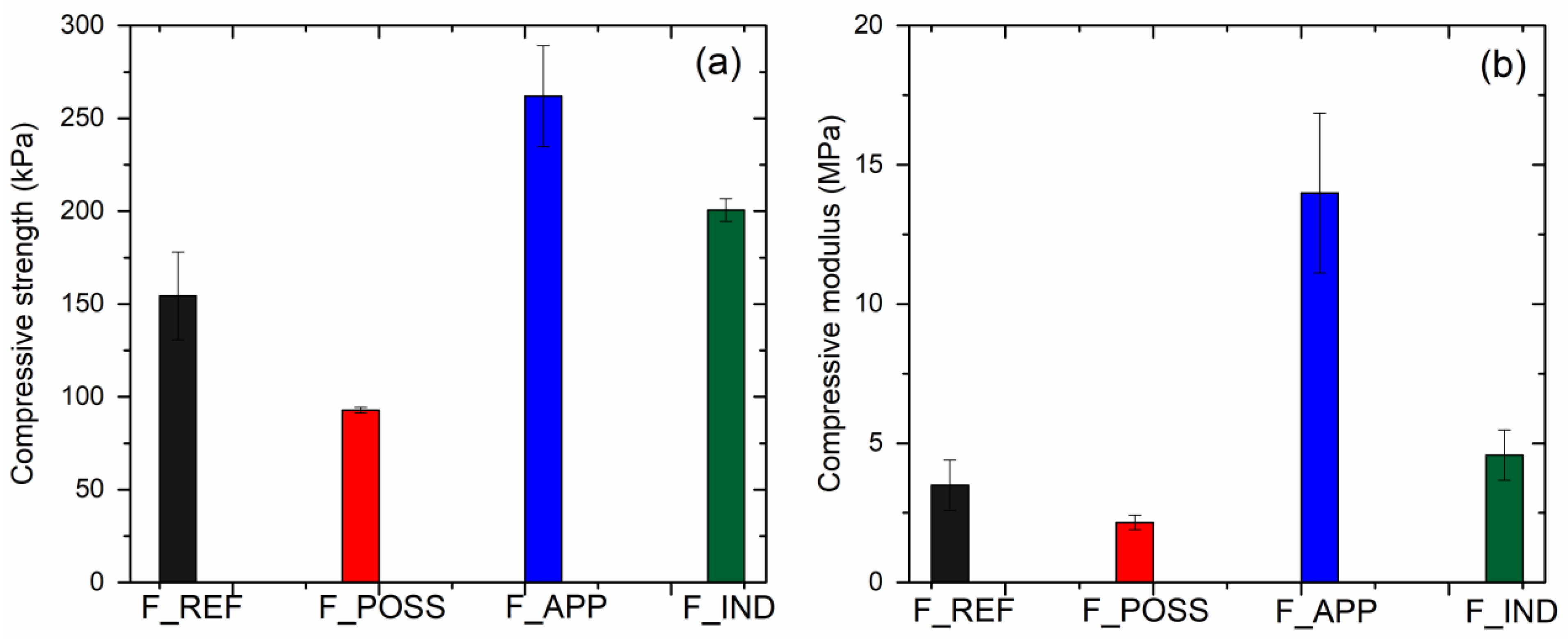
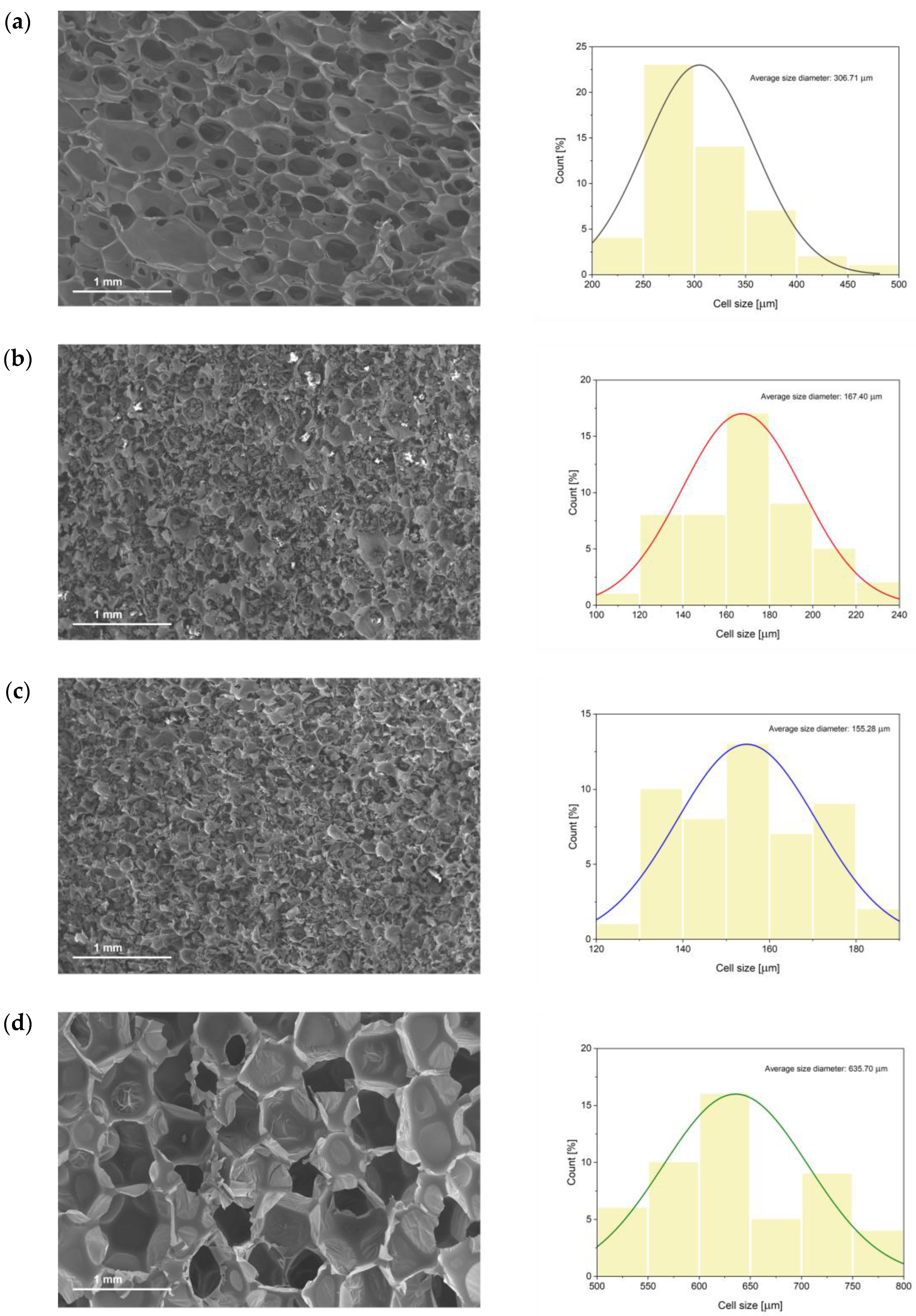
3.4. Physical, Mechanical and Morphological Properties of the Foams
3.5. Flame Retardancy and Fireproofing Properties
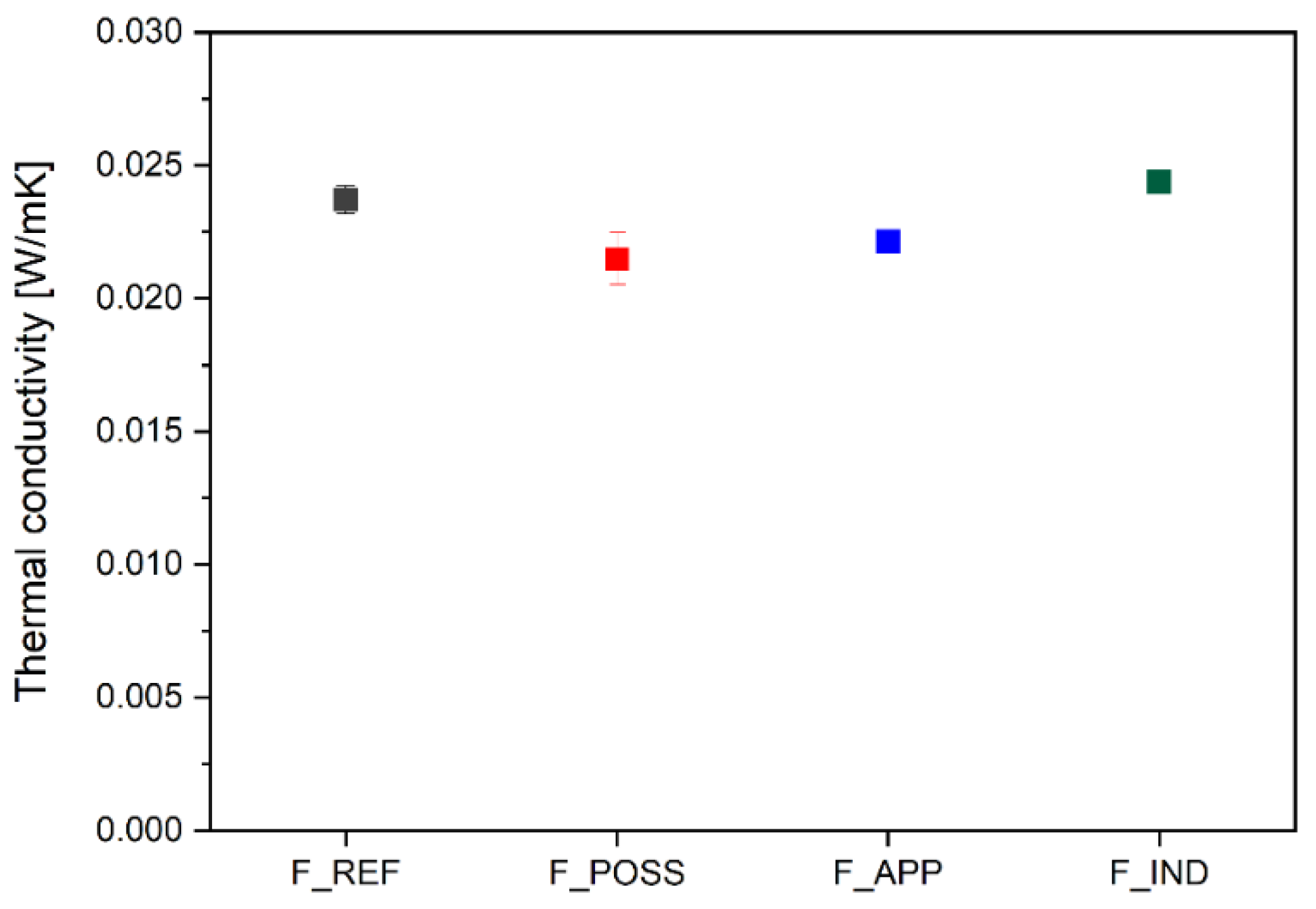
3.6. Thermal Insulating Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Austin, A.; Hicks, D. A Review of the Global PU Industry 2016 and Outlook for 2017. Plast. Eur. Eur. Plast. Ind. Mark. Data 2017, 14, 1–15. [Google Scholar]
- Global Polyurethane Market Volume 2015–2021. Available online: https://www.statista.com/statistics/720449/global-polyurethane-market-size-forecast/ (accessed on 15 March 2022).
- Grandviewresearch Polyurethane Market Size, Share & Trends Report, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/polyurethane-pu-market (accessed on 14 March 2023).
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane Types, Synthesis and Applications—A Review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Wendels, S.; Avérous, L. Biobased Polyurethanes for Biomedical Applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef] [PubMed]
- Abu-Jdayil, B.; Mourad, A.H.; Hittini, W.; Hassan, M.; Hameedi, S. Traditional, State-of-the-Art and Renewable Thermal Building Insulation Materials: An Overview. Constr. Build. Mater. 2019, 214, 709–735. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-Based Polyurethane Foams toward Applications beyond Thermal Insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Manzardo, A.; Marson, A.; Roso, M.; Boaretti, C.; Modesti, M.; Scipioni, A.; Lorenzetti, A. Life Cycle Assessment Framework to Support the Design of Biobased Rigid Polyurethane Foams. ACS Omega 2019, 4, 14114–14123. [Google Scholar] [CrossRef] [PubMed]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to Bioplastics: How Close Are We to Sustainable Polyhydroxyalkanoates Production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Błażek, K.; Datta, J. Renewable Natural Resources as Green Alternative Substrates to Obtain Bio-Based Non-Isocyanate Polyurethanes-Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 173–211. [Google Scholar] [CrossRef]
- Monie, F.; Grignard, B.; Thomassin, J.M.; Mereau, R.; Tassaing, T.; Jerome, C.; Detrembleur, C. Chemo- and Regioselective Additions of Nucleophiles to Cyclic Carbonates for the Preparation of Self-Blowing Non-Isocyanate Polyurethane Foams. Angew. Chemie Int. Ed. 2020, 59, 17033–17041. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, J.; Loos, K. Bio-Based Polyurethane Films Using White Dextrins. J. Appl. Polym. Sci. 2019, 136, 47454. [Google Scholar] [CrossRef]
- Bossa, F.D.L.; Verdolotti, L.; Russo, V.; Campaner, P.; Minigher, A.; Lama, G.C.; Boggioni, L.; Tesser, R. Upgrading Sustainable Polyurethane Foam Based On. Materials 2020, 13, 3170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wu, Y.; Chen, W.; Han, D.; Lin, X.; Xu, G.; Zhang, Q. Open-Cell Rigid Polyurethane Foams from Peanut Shell-Derived Polyols Prepared under Different Post-Processing Conditions. Polymers 2019, 11, 1392. [Google Scholar] [CrossRef]
- Gosz, K.; Tercjak, A.; Olszewski, A.; Haponiuk, J.; Piszczyk, Ł. Bio-Based Polyurethane Networks Derived from Liquefied Sawdust. Materials 2021, 14, 3138. [Google Scholar] [CrossRef] [PubMed]
- Mori, R. Inorganic–Organic Hybrid Biodegradable Polyurethane Resin Derived from Liquefied Sakura Wood. Wood Sci. Technol. 2015, 49, 507–516. [Google Scholar] [CrossRef]
- Gosz, K.; Kosmela, P.; Hejna, A.; Gajowiec, G.; Piszczyk, Ł. Biopolyols Obtained via Microwave-Assisted Liquefaction of Lignin: Structure, Rheological, Physical and Thermal Properties. Wood Sci. Technol. 2018, 52, 599–617. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.K. Overview of the Recent Advances in Lignocellulose Liquefaction for Producing Biofuels, Bio-Based Materials and Chemicals. Bioresour. Technol. 2019, 279, 373–384. [Google Scholar] [CrossRef]
- Daneshvar, S.; Behrooz, R.; Najafi, S.K.; Mir, G.; Sadeghi, M. Characterization of Polyurethane Wood Adhesive Prepared from Liquefied Sawdust by Ethylene Carbonate. Bioresources 2019, 14, 796–815. [Google Scholar] [CrossRef]
- Vale, M.; Mateus, M.M.; Galhano dos Santos, R.; Nieto de Castro, C.; de Schrijver, A.; Bordado, J.C.; Marques, A.C. Replacement of Petroleum-Derived Diols by Sustainable Biopolyols in One Component Polyurethane Foams. J. Clean. Prod. 2019, 212, 1036–1043. [Google Scholar] [CrossRef]
- Huang, X.; De Hoop, C.F.; Xie, J.; Wu, Q.; Boldor, D.; Qi, J. High Bio-Content Polyurethane (PU) Foam Made from Bio-Polyol and Cellulose Nanocrystals (CNCs) via Microwave Liquefaction. Mater. Des. 2018, 138, 11–20. [Google Scholar] [CrossRef]
- Rastegarfar, N.; Behrooz, R.; Barikani, M. Characterization of Polyurethane Foams Prepared from Liquefied Sawdust by Crude Glycerol and Polyethylene Glycol. J. Polym. Res. 2018, 25, 154. [Google Scholar] [CrossRef]
- Patel, C.M.; Dhore, N.R.; Barot, A.A.; Kothapalli, R.V.S.N. An Efficient and Environment Friendly Bio-Based Polyols through Liquefaction: Liquefaction Temperature and Catalyst Concentration Optimization and Utilized for Rigid Polyurethane Foams. Cell. Polym. 2021, 40, 212–227. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, W.S.; Lee, M.W.; Goh, M. Liquefaction of Lignin Using Chemical Decomposition and Its Application to Polyurethane Foam. ACS Omega 2021, 6, 10745–10751. [Google Scholar] [CrossRef]
- Abd Hilmi, N.H.; Lodin, V.; Gilbert Jesuet, M.S.; Salim, S.; Lee, S.H.; Hori, N.; Takemura, A.; Palle, I. Producing Eucalyptus Pellita Wood Polyol through Liquefaction for Polyurethane Film Production. Ind. Crops Prod. 2023, 205, 117431. [Google Scholar] [CrossRef]
- Jiang, W.; Hosseinpourpia, R.; Biziks, V.; Ahmed, S.A.; Militz, H.; Adamopoulos, S. Preparation of Polyurethane Adhesives from Crude and Purified Liquefied Wood Sawdust. Polymers 2021, 13, 3267. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, A.; Kosmela, P.; Piszczyk, Ł. Bio-Polyols Synthesized by Liquefaction of Cellulose: Influence of Liquefaction Solvent Molecular Weight. J. Appl. Polym. Sci. 2023, 140, e54003. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Xu, L.; Qian, L. Phosphorus-Containing Silica Gel-Coated Ammonium Polyphosphate: Preparation, Characterization, and Its Effect on the Flame Retardancy of Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2018, 135, 46334. [Google Scholar] [CrossRef]
- Chang, C.; Liu, L.; Li, P.; Xu, G.; Xu, C. Preparation of Flame Retardant Polyurethane Foam from Crude Glycerol Based Liquefaction of Wheat Straw. Ind. Crops Prod. 2021, 160, 113098. [Google Scholar] [CrossRef]
- Rowell, R. The Chemistry of Solid Wood; American Chemical Society: Washington, DC, USA, 1984. [Google Scholar]
- Wise, L.E.; Maxine, M.; D’Addieco, A.A. Chlorite Holocellulose, Its Fractionation and Bearing on Summative Wood Analysis and on Studies on the Hemicelluloses. Tech. Assoc. Pulp Pap. Ind. 1946, 29, 210–218. [Google Scholar]
- da Silva, S.H.F.; Egüés, I.; Labidi, J. Liquefaction of Kraft Lignin Using Polyhydric Alcohols and Organic Acids as Catalysts for Sustainable Polyols Production. Ind. Crops Prod. 2019, 137, 687–693. [Google Scholar] [CrossRef]
- ASTM D4274-99; Standard Test Methods for Testing Polyurethane Raw Materials Determination of Hydroxyl Numbers of Polyols. ASTM International: West Conshohocken, PA, USA, 2017.
- UNE-EN 1602:2013; Thermal Insulating Products for Building Applications—Determination of the Apparent Density. UNE-Spanish Asociation of Standarization: Madrid, Spain, 2013.
- UNE-EN 826:2013; Thermal Insulating Products for Building Applications—Determination of Compression Behaviour. UNE-Spanish Asociation of Standarization: Madrid, Spain, 2013.
- UNE-EN ISO 11925-2:2021; Reaction to Fire Tests—Ignitability of Products Subjected to Direct Impingement of Flame—Part 2: Single-Flame Source Test. UNE-Spanish Asociation of Standarization: Madrid, Spain, 2021.
- ASTM D3801-10; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM-American Society for Testing and Materials: West Conshohocken, PA, USA, 2010.
- UNE-EN ISO 4589-2:2017; Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. UNE-Spanish Asociation of Standarization: Madrid, Spain, 2017.
- UNE-EN 12667:2002; Thermal Performance of Building Materials and Products. Determination of Thermal Resistance by Means of Guarded Hot Plate and Heat Flow Meter Methods. Products of High and Medium Thermal Resistance. UNE-Spanish Asociation of Standarization: Madrid, Spain, 2002.
- Fernandes, C.; Melro, E.; Magalhães, S.; Alves, L.; Craveiro, R.; Filipe, A.; Valente, A.J.M.; Martins, G.; Antunes, F.E.; Romano, A.; et al. New Deep Eutectic Solvent Assisted Extraction of Highly Pure Lignin from Maritime Pine Sawdust (Pinus Pinaster Ait.). Int. J. Biol. Macromol. 2021, 177, 294–305. [Google Scholar] [CrossRef]
- Mendes, C.V.T.; Moreira, R.; Portugal, A.; Graça, V.S.; Carvalho, M. Biorefining of Pinus Pinaster Stump Wood for Ethanol Production and Lignin Recovery. Chem. Eng. Technol. 2021, 44, 1043–1050. [Google Scholar] [CrossRef]
- Cruz, N.; Bustos, C.A.; Aguayo, M.G.; Cloutier, A.; Castillo, R. Impact of the Chemical Composition of Pinus Radiata Wood on Its Physical and Mechanical Properties Following Thermo-Hygromechanical Densification. Bioresources 2018, 13, 2268–2282. [Google Scholar] [CrossRef]
- García-Iruela, A.; Esteban, L.G.; García Fernández, F.; de Palacios, P.; Rodriguez-Navarro, A.B.; Martín-Sampedro, R.; Eugenio, M.E. Changes in Cell Wall Components and Hygroscopic Properties of Pinus Radiata Caused by Heat Treatment. Eur. J. Wood Wood Prod. 2021, 79, 851–861. [Google Scholar] [CrossRef]
- Dias, A.; Carvalho, A.; Silva, M.E.; Lima-Brito, J.; Gaspar, M.J.; Alves, A.; Rodrigues, J.C.; Pereira, F.; Morais, J.; Lousada, J.L. Physical, Chemical and Mechanical Wood Properties of Pinus Nigra Growing in Portugal. Ann. For. Sci. 2020, 77, 72. [Google Scholar] [CrossRef]
- Olszewski, A.; Kosmela, P.; Piszczyk, Ł. Synthesis and Characterization of Biopolyols through Biomass Liquefaction of Wood Shavings and Their Application in the Preparation of Polyurethane Wood Composites. Eur. J. Wood Wood Prod. 2022, 80, 57–74. [Google Scholar] [CrossRef]
- Amran, U.A.; Salleh, K.M.; Zakaria, S.; Roslan, R.; Chia, C.H.; Jaafar, S.N.S.; Sajab, M.S.; Mostapha, M. Production of Rigid Polyurethane Foams Using Polyol from Liquefied Oil Palm Biomass: Variation of Isocyanate Indexes. Polymers 2021, 13, 3072. [Google Scholar] [CrossRef] [PubMed]
- Sendijarevic, I.; Pietrzyk, K.W.; Schiffman, C.M.; Sendijarevic, V.; Kiziltas, A.; Mielewski, D. Polyol from Spent Coffee Grounds: Performance in a Model Pour-in-Place Rigid Polyurethane Foam System. J. Cell. Plast. 2020, 56, 630–645. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Formela, K.; Haponiuk, J.; Piszczyk, Ł. The Study on Application of Biopolyols Obtained by Cellulose Biomass Liquefaction Performed with Crude Glycerol for the Synthesis of Rigid Polyurethane Foams. J. Polym. Environ. 2018, 26, 2546–2554. [Google Scholar] [CrossRef]
- Jasiūnas, L.; Skvorčinskienė, R.; Miknius, L. Wet and Coarse: The Robustness of Two-Stage Crude Glycerol Mediated Solvothermal Liquefaction of Residual Biomass. Waste Biomass Valorization 2018, 11, 2171–2181. [Google Scholar] [CrossRef]
- Lee, Y.; Tran, M.H.; Lee, E.Y. Acid–Base-Catalyzed Two-Step Liquefaction of Empty Fruit Bunch Lignin Residue for Preparation of Biopolyol and High-Performance Biopolyurethanes. Wood Sci. Technol. 2021, 55, 315–330. [Google Scholar] [CrossRef]
- Fernandes, A.; Cruz-Lopes, L.; Dulyanska, Y.; Domingos, I.; Ferreira, J.; Evtuguin, D.; Esteves, B. Eco Valorization of Eucalyptus Globulus Bark and Branches through Liquefaction. Appl. Sci. 2022, 12, 3775. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Optimization of Agricultural Wastes Liquefaction Process and Preparing Bio-Based Polyurethane Foams by the Obtained Polyols. Ind. Crops Prod. 2019, 138, 111455. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Thermal and Time Regularities during Oilseed Rape Straw Liquefaction Process to Produce Bio-Polyol. J. Clean. Prod. 2020, 277, 124015. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Robles, E.; Khoukh, A.; Charrier-El Bouhtoury, F.; Labidi, J. Formulation of Multifunctional Materials Based on the Reaction of Glyoxalated Lignins and a Nanoclay/Nanosilicate. Biomacromolecules 2019, 20, 3535–3546. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman Spectroscopic Features of Plant Cuticles: A Review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Briones, R.; Rodriguez, J.; Labidi, J.; Cunningham, E.; Martin, P. Liquefaction of Corn Husks and Properties of Biodegradable Biopolyol Blends. J. Chem. Technol. Biotechnol. 2020, 95, 2973–2982. [Google Scholar] [CrossRef]
- Kurańska, M.; Malewska, E. Waste Cooking Oil as Starting Resource to Produce Bio-Polyol—Analysis of Transesteryfication Process Using Gel Permeation Chromatography. Ind. Crops Prod. 2021, 162, 113294. [Google Scholar] [CrossRef]
- Huo, S.; Wu, G.; Chen, J.; Liu, G.; Kong, Z. Constructing Polyurethane Foams of Strong Mechanical Property and Thermostability by Two Novel Environment Friendly Bio-Based Polyols. Korean J. Chem. Eng. 2016, 33, 1088–1094. [Google Scholar] [CrossRef]
- Li, Y.Y.; Luo, X.; Hu, S. Lignocellulosic Biomass-Based Polyols for Polyurethane Applications. In Bio-Based Polyols and Polyurethanes; Springer: Cham, Switzerland, 2015; pp. 1–79. ISBN 9783319215396. [Google Scholar]
- Yan, Y.; Pang, H.; Yang, X.; Zhang, R.; Liao, B. Preparation and Characterization of Water-Blown Polyurethane Foams from Liquefied Cornstalk Polyol. J. Appl. Polym. Sci. 2008, 110, 1099–1111. [Google Scholar] [CrossRef]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.B.; Lazzeri, A. Flexible Polyurethane Foams Green Production Employing Lignin or Oxypropylated Lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Vargas-Gutiérrez, C.V.; Castro-Salazar, H.T.; Ríos-Reyes, C.A.; Vargas-Gutiérrez, C.V.; Castro-Salazar, H.T.; Ríos-Reyes, C.A. Synthesis and Properties of Polyurethane Foams Obtained from Cassava Starch and Rice By-Products. J. Mex. Chem. Soc. 2018, 62, 1–8. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Mir Mohamad Sadeghi, G. Potential Use of Black Liquor as Lignin Source for Synthesis of Polyurethane Foam. J. Polym. Res. 2020, 27, 362. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, Ł.; Przybysz, M.; Ryl, J.; Saeb, M.R.; Piszczyk, Ł. Structural, Thermal and Physico-Mechanical Properties of Polyurethane/Brewers’ Spent Grain Composite Foams Modified with Ground Tire Rubber. Ind. Crops Prod. 2017, 108, 844–852. [Google Scholar] [CrossRef]
- Saffar, T.; Bouafif, H.; Braghiroli, F.L.; Magdouli, S.; Langlois, A.; Koubaa, A. Production of Bio-Based Polyol from Oxypropylated Pyrolytic Lignin for Rigid Polyurethane Foam Application. Waste Biomass Valorization 2020, 11, 6411–6427. [Google Scholar] [CrossRef]
- Burgaz, E.; Kendirlioglu, C. Thermomechanical Behavior and Thermal Stability of Polyurethane Rigid Nanocomposite Foams Containing Binary Nanoparticle Mixtures. Polym. Test. 2019, 77, 105930. [Google Scholar] [CrossRef]
- Laird, M.; Herrmann, N.; Ramsahye, N.; Totøe, Ø.; Carcel, C.; Unno, M.; Ohn, J.; Bartlett, R.; Wong, M.; Man, C. Large Polyhedral Oligomeric Silsesquioxane Cages: The Isolation of Functionalized POSS with an Unprecedented Si18O27 Core. Angew. Chemie 2021, 133, 3059–3064. [Google Scholar] [CrossRef]
- Rao, W.; Shi, J.; Yu, C.; Zhao, H.B.; Wang, Y.Z. Highly Efficient, Transparent, and Environment-Friendly Flame-Retardant Coating for Cotton Fabric. Chem. Eng. J. 2021, 424, 130556. [Google Scholar] [CrossRef]
- Cheng, H.H.; Whang, L.M. Resource Recovery from Lignocellulosic Wastes via Biological Technologies: Advancements and Prospects. Bioresour. Technol. 2022, 343, 126097. [Google Scholar] [CrossRef]
- Dworakowska, S.; Cornille, A.; Bogdal, D.; Boutevin, B.; Caillol, S. Thiol-Ene Coupling of High Oleic Sunflower Oil towards Application in the Modification of Flexible Polyurethane Foams. Materials 2022, 15, 628. [Google Scholar] [CrossRef]
- Xue, B.L.; Wen, J.L.; Sun, R.C. Producing Lignin-Based Polyols through Microwave-Assisted Liquefaction for Rigid Polyurethane Foam Production. Materials 2015, 8, 586. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, N.; Feng, M. Polyurethane Foams Derived from Liquefied Mountain Pine Beetle-Infested Barks. J. Appl. Polym. Sci. 2012, 123, 2849–2858. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Chaleat, C.; Martin, D.J.; Annamalai, P.K. A Systematic Study Substituting Polyether Polyol with Palm Kernel Oil Based Polyester Polyol in Rigid Polyurethane Foam. Ind. Crops Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Yang, C.; Shao, S. Rigid Polyurethane Foams Containing Modified Ammonium Polyphosphate Having Outstanding Charring Ability and Increased Flame Retardancy. Front. Mater. 2021, 8, 249. [Google Scholar] [CrossRef]
- Lu, W.; Li, Q.; Zhang, Y.; Yu, H.; Hirose, S.; Hatakeyama, H.; Matsumoto, Y.; Jin, Z. Lignosulfonate/APP IFR and Its Flame Retardancy in Lignosulfonate-Based Rigid Polyurethane Foams. J. Wood Sci. 2018, 64, 287–293. [Google Scholar] [CrossRef]
- Wei, S.; Meng, L.; Liu, W.; Guo, S.; Zhang, X. Polyhedral Oligomeric Silsesquioxane (POSS) as Reinforcing Agent for Waterborne Polyurethane Coatings on Wood. Mater. Res. 2019, 22, 1–10. [Google Scholar] [CrossRef]
- Yang, W.; Han, Y.; Zhang, W.; Zhang, D. High-Strength and Low-Cost Biobased Polyurethane Foam Composites Enhanced by Poplar Wood Powder Liquefaction. Polymers 2021, 13, 2999. [Google Scholar] [CrossRef]
- Amran, U.A.; Zakaria, S.; Chia, C.H.; Roslan, R.; Jaafar, S.N.S.; Salleh, K.M. Polyols and Rigid Polyurethane Foams Derived from Liquefied Lignocellulosic and Cellulosic Biomass. Cellulose 2019, 26, 3231–3246. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Zhang, Y.; Cheng, X.; Yu, Y.; Jin, Y. Preparation of Bio-Polyols by Liquefaction of Hardwood Residue and Their Application in the Modification of Polyurethane Foams. J. Wuhan Univ. Technol. Sci. Ed. 2016, 31, 918–924. [Google Scholar] [CrossRef]
- Xing, W.; Yuan, H.; Zhang, P.; Yang, H.; Song, L.; Hu, Y. Functionalized Lignin for Halogen-Free Flame Retardant Rigid Polyurethane Foam: Preparation, Thermal Stability, Fire Performance and Mechanical Properties. J. Polym. Res. 2013, 20, 234. [Google Scholar] [CrossRef]
- Pang, X.Y.; Xin, Y.P.; Shi, X.Z.; Xu, J.Z. Effect of Different Size-Modified Expandable Graphite and Ammonium Polyphosphate on the Flame Retardancy, Thermal Stability, Physical, and Mechanical Properties of Rigid Polyurethane Foam. Polym. Eng. Sci. 2019, 59, 1381–1394. [Google Scholar] [CrossRef]
- Gurlek, G.; Altay, L.; Sarikanat, M. Thermal Conductivity and Flammability of Ulexite Filled Rigid Polyurethane Materials. Acta Phys. Pol. A 2019, 135, 825–828. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Wang, W.; Qian, L. Preparation and Characterization of Surface-Modified Ammonium Polyphosphate and Its Effect on the Flame Retardancy of Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2017, 134, 45369. [Google Scholar] [CrossRef]
- Maillard, D.; Osso, E.; Faye, A.; Li, H.; Ton-That, M.T.; Stoeffler, K. Influence of Lignin’s PH on Polyurethane Flexible Foam Formation and How to Control It. J. Appl. Polym. Sci. 2021, 138, 50319. [Google Scholar] [CrossRef]
- Strakowska, A.; Członka, S.; Strzelec, K. POSS Compounds as Modifiers for Rigid Polyurethane Foams (Composites). Polymers 2019, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Hebda, E.; Ozimek, J.; Raftopoulos, K.N.; Michałowski, S.; Pielichowski, J.; Jancia, M.; Pielichowski, K. Synthesis and Morphology of Rigid Polyurethane Foams with POSS as Pendant Groups or Chemical Crosslinks. Polym. Adv. Technol. 2015, 26, 932–940. [Google Scholar] [CrossRef]
- Michałowski, S.; Hebda, E.; Pielichowski, K. Thermal Stability and Flammability of Polyurethane Foams Chemically Reinforced with POSS. J. Therm. Anal. Calorim. 2017, 130, 155–163. [Google Scholar] [CrossRef]
- Huang, X.; De Hoop, C.F.; Xie, J.; Hse, C.Y.; Qi, J.; Hu, T. Characterization of Biobased Polyurethane Foams Employing Lignin Fractionated from Microwave Liquefied Switchgrass. Int. J. Polym. Sci. 2017, 2017, 4207367. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Chen, Y.; Yang, B. Preparation of Efficiently Intumescent-Flame-Retarded Polypropylene Composite: Synergistic Effect of Novel Phosphorus-Containing Polyhedral Oligomeric Silsesquioxane. Plast. Rubber Compos. 2021, 50, 464–476. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Z.; Tao, R.; Mao, L.; Zhan, J.; Xiao, J.; Yu, T. Combustion and Thermal Properties of Flame Retardant Polyurethane Foam with Ammonium Polyphosphate Synergized by Phosphomolybdic Acid. Front. Mater. 2022, 9, 400. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Liu, M.; Zhu, Q.; Liu, X.; Zhang, B.; Chen, D.; Liu, X.; Zhang, K.; Tang, G. Flame Retarded Rigid Polyurethane Foam Composites Based on Gel-Silica Microencapsulated Ammonium Polyphosphate. J. Sol-Gel Sci. Technol. 2021, 98, 212–223. [Google Scholar] [CrossRef]
- Hoang, C.N.; Pham, C.T.; Dang, T.M.; Hoang, D.Q.; Lee, P.C.; Kang, S.J.; Kim, J. Novel Oligo-Ester-Ether-Diol Prepared by Waste Poly(Ethylene Terephthalate) Glycolysis and Its Use in Preparing Thermally Stable and Flame Retardant Polyurethane Foam. Polymers 2019, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Qi, X.; Qian, L. The Pyrolysis Behaviors of Phosphorus-Containing Organosilicon Compound Modified APP with Different Polyether Segments and Their Flame Retardant Mechanism in Polyurethane Foam. Compos. Part B Eng. 2019, 173, 106784. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, S.; Zhu, J.; Li, G.; Peng, X. Establishment of a Highly Efficient Flame-Retardant System for Rigid Polyurethane Foams Based on Bi-Phase Flame-Retardant Actions. RSC Adv. 2018, 8, 9985–9995. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, Y.; Cheng, J.; Ma, Q.; Zhang, F.; Wang, Y.; Liu, M.; Wang, D.; Qu, W. Mechanical Property Improvement and Fire Hazard Reduction of Ammonium Polyphosphate Microencapsulated in Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2020, 137, 48307. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Wu, X.; Xu, B.; Qian, L. Bi-Phase Flame-Retardant Effect of Dimethyl Methylphosphonate and Modified Ammonium Polyphosphate on Rigid Polyurethane Foam. Polym. Adv. Technol. 2019, 30, 2721–2728. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z. High Performance Nano-Zinc Amino-Tris-(Methylenephosphonate) in Rigid Polyurethane Foam with Improved Mechanical Strength, Thermal Stability and Flame Retardancy. Polym. Degrad. Stab. 2018, 154, 62–72. [Google Scholar] [CrossRef]
- Turgut, G.; Dogan, M.; Tayfun, U.; Ozkoc, G. The Effects of POSS Particles on the Flame Retardancy of Intumescent Polypropylene Composites and the Structure-Property Relationship. Polym. Degrad. Stab. 2018, 149, 96–111. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, T.; Xiang, H.; Li, Z.; Xu, Z.; Kong, Q.; Zhang, J.; Li, Z.; Pan, Y.; Wang, D. Simultaneously Improving Flame Retardancy and Dynamic Mechanical Properties of Epoxy Resin Nanocomposites through Synergistic Effect of Zirconium Phenylphosphate and POSS. J. Therm. Anal. Calorim. 2019, 135, 2117–2124. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M. Environmentally-Benign Rigid Polyurethane Foam Produced from a Reactive and Phosphorus-Functionalized Biopolyol: Assessment of Physicomechanical and Flame-Retardant Properties. React. Funct. Polym. 2022, 177, 105320. [Google Scholar] [CrossRef]
- Estravís, S.; Tirado-Mediavilla, J.; Santiago-Calvo, M.; Ruiz-Herrero, J.L.; Villafañe, F.; Rodríguez-Pérez, M.Á. Rigid Polyurethane Foams with Infused Nanoclays: Relationship between Cellular Structure and Thermal Conductivity. Eur. Polym. J. 2016, 80, 1–15. [Google Scholar] [CrossRef]
- Akkoyun, M.; Akkoyun, S. Blast Furnace Slag or Fly Ash Filled Rigid Polyurethane Composite Foams: A Comprehensive Investigation. J. Appl. Polym. Sci. 2019, 136, 47433. [Google Scholar] [CrossRef]
- Do, T.V.V.; Le, V.H.V.; Thai, N.U.N.; Dai, H.N.; Grillet, A.C.; Thuc, C.N.H. The Influence of Nano-Silica on the Thermal Conductivity of Polyurethane Foam. J. Appl. Polym. Sci. 2021, 138, 50715. [Google Scholar] [CrossRef]
- Aydoğmuş, E.; Kamişli, F. New Commercial Polyurethane Synthesized with Biopolyol Obtained from Canola Oil: Optimization, Characterization, and Thermophysical Properties. J. Mol. Struct. 2022, 1256, 132495. [Google Scholar] [CrossRef]
- Dutta, A.S. Polyurethane Foam Chemistry. In Recycling of Polyurethane Foams; Sabu, T., Ajay, V.R., Krishnan, K., Abitha, V.K., Martin George, T., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 17–27. ISBN 9780323511346. [Google Scholar]
- Rezgar, H.; Taher, A.; Ali, D.; Richard, E.L. Thermal Conductivity of Low Density Polyethylene Foams Part I: Comprehensive Study of Theoretical Models. J. Therm. Sci. 2019, 28, 745–754. [Google Scholar] [CrossRef]
- Prociak, A.; Kurańska, M.; Cabulis, U.; Ryszkowska, J.; Leszczyńska, M.; Uram, K.; Kirpluks, M. Effect of Bio-Polyols with Different Chemical Structures on Foaming of Polyurethane Systems and Foam Properties. Ind. Crops Prod. 2018, 120, 262–270. [Google Scholar] [CrossRef]



| Formulation | Phase A (pbw a) | Phase B (pbw) | ||||||
|---|---|---|---|---|---|---|---|---|
| TEGOSTAB 847100 | Biopolyol | DBTDL | POLYCAT 5 | BA b | POSS | APP | Isocyanate | |
| F_REF | 20 | 100 | 16 | 2 | 1 | --- | --- | 120 |
| F_POSS | 12.5 | --- | ||||||
| F_APP | --- | 12.5 | ||||||
| Chemical Composition of Raw Material (%) | ||||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | Extractives | Ashes |
| 36.36 ± 0.06 | 16.17 ± 0.16 | 27.60 ± 0.34 | 5.84 ± 0.24 | 0.22 ± 0.11 |
| Biopolyols | Density (g∙cm−3) | Viscosity (mPa∙s) | IOH a (mg KOH∙g−1) | Functionality | Conversion of Liquefaction (%) |
|---|---|---|---|---|---|
| 75:25 | 1.15 ± 0.01 | 394.20 ± 21.49 | 295.47 ± 46.71 | 2.74 ± 0.54 | 78.97 ± 4.35 |
| 70:30 | 1.17 ± 0.01 | 438.75 ± 22.98 | 360.29 ± 27.73 | 3.23 ± 0.25 | 81.02 ± 0.54 |
| 60:40 | 1.19 ± 0.01 | 578.3 ± 37.6 | 546.6 ± 22.32 | 3.30 ± 0.13 | 89.15 ± 3.68 |
| Polyols | Molecular Weights (g∙mol−1) | Equivalent Weight(g∙mol−1) | ||
|---|---|---|---|---|
| Mw | Mn | Polydispersity Index (PI) | ||
| BP-75:25 | 3148 | 520 | 5.55 | 189.87 ± 10.78 |
| BP-70:30 | 2887 | 501 | 6.28 | 155.71 ± 6.71 |
| BP-60:40 | 2269 | 339 | 7.32 | 102.63 ± 5.11 |
| Industrial | 857 | 524 | 1.6 | 280.5 |
| Polyols | Density (g∙cm−3) | Viscosity (mPa∙s) | IOH a (mg KOH∙g−1) | Functionality | Equivalent Weight (g∙mol−1) | Molecular Weights (g∙mol−1) | ||
|---|---|---|---|---|---|---|---|---|
| Mw | Mn | PI b | ||||||
| BP | 1.2 ± 0 | 578.3 ± 37.6 | 546.6 ± 22.3 | 3.3 ± 0.1 | 102.6 ± 5.1 | 2269 | 339 | 7.3 |
| IP | 1.1 | 300–500 | 200 | 1.9 | 280.5 | 857 | 524 | 1.6 |
| Formulation | T5% (°C) | T50% (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|---|
| F_REF a | 189.7 | 367.0 | 309.2 | 16.1 |
| F_POSS b | 185.4 | 379.7 | 397.8 | 16.2 |
| F_APP c | 197.3 | 396.0 | 401.2 | 22.9 |
| F_IND d | 187.9 | 377.4 | 324.4 | 16.3 |
| Samples | UL-94 Vertical Burning Test | LOI [%] | |||||
|---|---|---|---|---|---|---|---|
| t1 [s] | t2 [s] | Length Burnt [mm] | Dripping Particles | Flammable Droplets | Rating | ||
| F_REF | 27.0 ± 3.5 | --- * | 100 ± 0 | Yes | Yes | V-2 | 19.9 |
| F_POSS | 6.4 ± 2.6 | 0 | 100 ± 0 | No | No | V-0 | 21.7 |
| F_APP | 3.3 ± 1.3 | 1 ± 0 | 100 ± 0 | No | No | V-0 | 22.4 |
| F_IND | 4.8 ± 1.3 | 1 ± 0 | 97.4 ± 2.2 | No | No | V-0 | 23.8 |
| Formulation | Mass Remaining after Analysis a [%] | Length Burnt [mm] | Dripping Particles | Flammable Droplets |
|---|---|---|---|---|
| F_REF | 45.9 ± 0.9 | 125 ± 0 | Yes | Yes |
| F_POSS | 79.2 ± 2.1 | 125 ± 0 | No | No |
| F_APP | 79.8 ± 2.6 | 125 ± 0 | No | No |
| F_IND | 91.3 ± 1.6 | 63.8 ± 4.2 | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Hoyos-Martinez, P.L.; Mendez, S.B.; Martinez, E.C.; Wang, D.-Y.; Labidi, J. Elaboration of Thermally Performing Polyurethane Foams, Based on Biopolyols, with Thermal Insulating Applications. Polymers 2024, 16, 258. https://doi.org/10.3390/polym16020258
De Hoyos-Martinez PL, Mendez SB, Martinez EC, Wang D-Y, Labidi J. Elaboration of Thermally Performing Polyurethane Foams, Based on Biopolyols, with Thermal Insulating Applications. Polymers. 2024; 16(2):258. https://doi.org/10.3390/polym16020258
Chicago/Turabian StyleDe Hoyos-Martinez, Pedro Luis, Sebastian Barriga Mendez, Eriz Corro Martinez, De-Yi Wang, and Jalel Labidi. 2024. "Elaboration of Thermally Performing Polyurethane Foams, Based on Biopolyols, with Thermal Insulating Applications" Polymers 16, no. 2: 258. https://doi.org/10.3390/polym16020258
APA StyleDe Hoyos-Martinez, P. L., Mendez, S. B., Martinez, E. C., Wang, D.-Y., & Labidi, J. (2024). Elaboration of Thermally Performing Polyurethane Foams, Based on Biopolyols, with Thermal Insulating Applications. Polymers, 16(2), 258. https://doi.org/10.3390/polym16020258









