Design and Study of Novel Composites Based on EPDM Rubber Containing Bismuth (III) Oxide and Graphene Nanoplatelets for Gamma Radiation Shielding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Preparation
2.3. Characterization
3. Results and Discussion
3.1. Curing Curves and Vulcanization Parameters
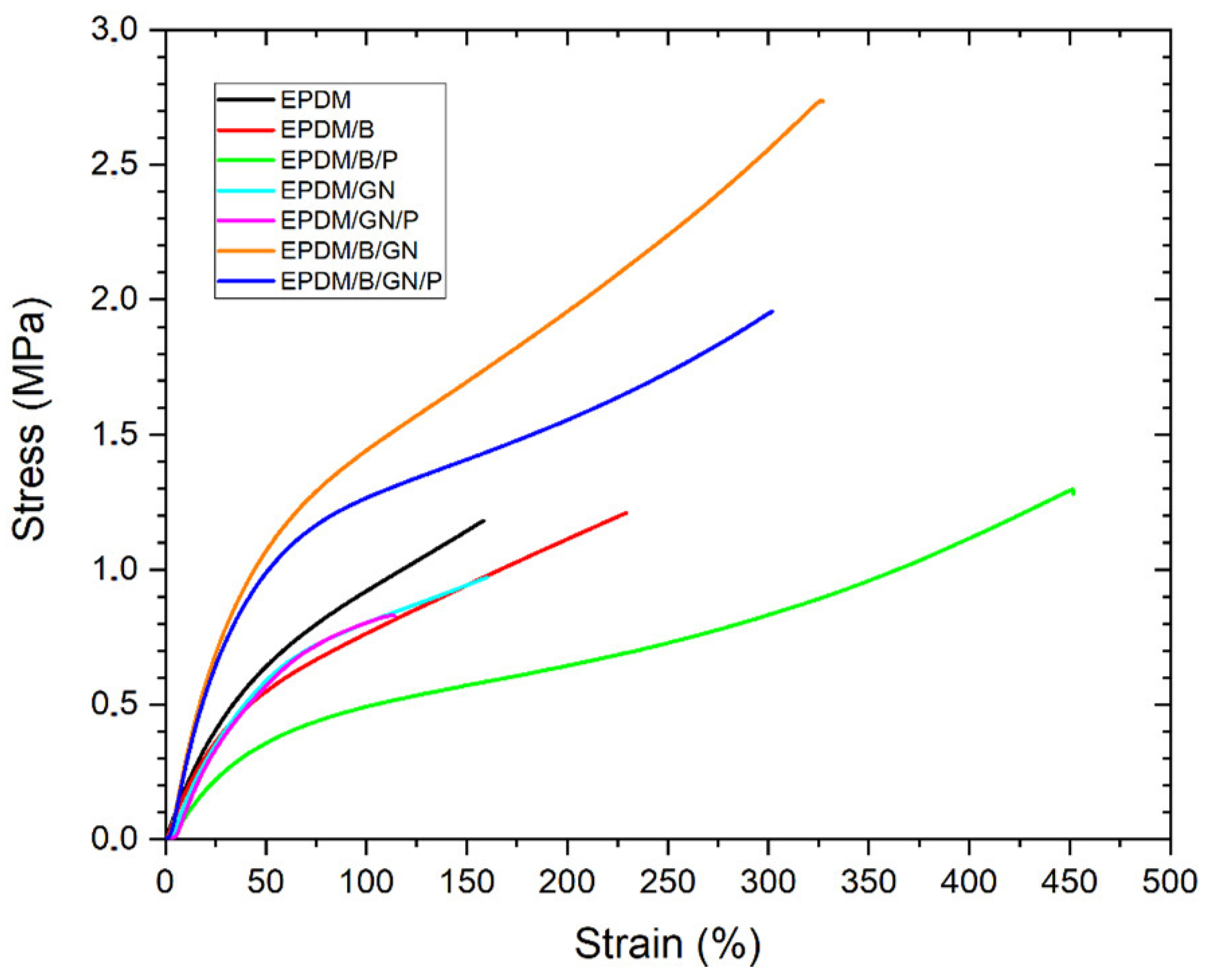
3.2. Mechanical Properties
3.3. FT-IR Spectroscopy Analysis
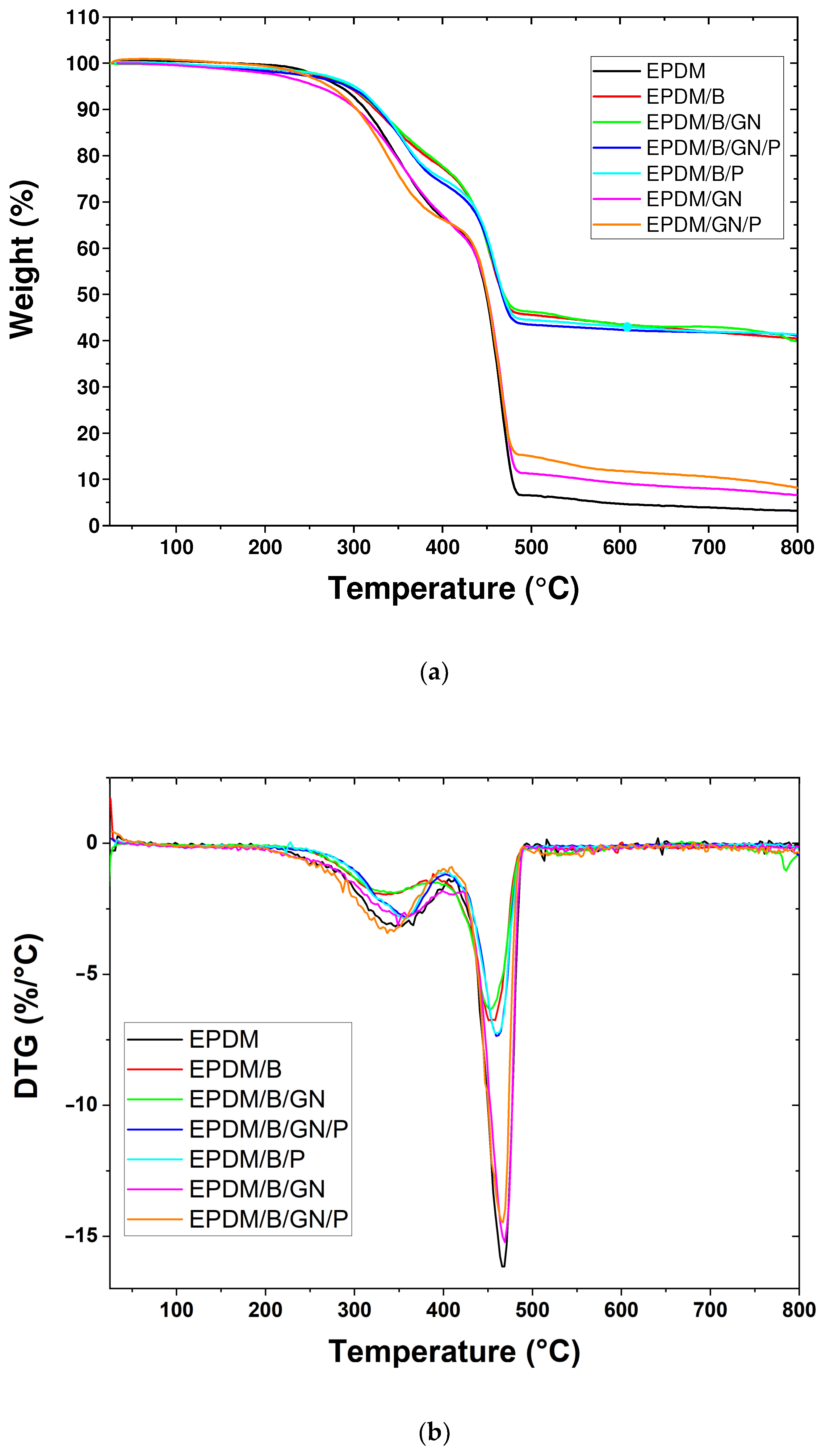
3.4. Thermal Properties
3.5. Morphology
3.6. High-Energy Electromagnetic Radiation Shielding Properties of EPDM-Based Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahamatifard, F.; Rodrigue, D.; Mighri, F. Thermal and mechanical properties of carbon-based rubber nanocomposites: A review. Plast. Rubber Compos. 2023, 52, 483–505. [Google Scholar] [CrossRef]
- Dieu, T.V.; Chuong, B.; Hung, D.V.; Tung, N.H.; Linh, N.P.D.; Oanh, D.T.Y. Review: Natural rubber—Improvement of properties. Vietnam J. Chem. 2023, 61, 269–283. [Google Scholar] [CrossRef]
- Mahmood, N.Q.; Marossy, K.; Baumli, P. Effects of nanocrystalline calcium oxide particles on mechanical, thermal, and electrical properties of EPDM rubber. Colloid Polym. Sci. 2021, 299, 1669–1682. [Google Scholar] [CrossRef]
- Chen, H.; Chen, N.; Ma, Q.; He, Y. Experimental study on preparation and properties of carbon nanotubes-, graphene –natural rubber composites. J. Thermoplast. Compos. Mater. 2023. [Google Scholar] [CrossRef]
- Saeed, A.; Abu-Raia, W.A. Silicone rubber composite reinforced by bismuth tungsten oxide as an effective gamma ray protective materials. J. Polym. Res. 2022, 29, 208. [Google Scholar] [CrossRef]
- Acevedo-Del-Castillo, A.; Águila-Toledo, E.; Maldonado-Magnere, S.; Aguilar-Bolados, H. A Brief Review on the High-Energy Electromagnetic Radiation-Shielding Materials Based on Polymer Nanocomposites. Int. J. Mol. Sci. 2021, 22, 9079. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, E.; Mesbahi, A.; Malekzadeh, R.; Mansouri, A. Shielding characteristics of nanocomposites for protection against X- and gamma rays in medical applications: Effect of particle size, photon energy and nano-particle concentration. Radiat. Environ. Biophys. 2020, 59, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, T.; Yuan, L.; Fang, Q.; Wang, X.; Guo, X.; Zhang, D.; Lai, C.; Wang, Q.; Liu, Y. A comparative study between pure bismuth/tungsten and the bismuth tungsten oxide for flexible shielding of gamma/X rays. Radiat. Phys. Chem. 2023, 208, 110906. [Google Scholar] [CrossRef]
- Seibert, J.A.; Boone, J.M. X-ray imaging physics for nuclear medicine technologists. Part 2: X-ray interactions and image formation. J. Nucl. Med. Technol. 2005, 33, 3–18. [Google Scholar] [PubMed]
- Toyen, D.; Rittirong, A.; Poltabtim, W.; Saenboonruang, K. Flexible, lead-free, gamma-shielding materials based on natural rubber/metal oxide composites. Iran. Polym. J. 2017, 27, 33–41. [Google Scholar] [CrossRef]
- Thumwong, A.; Wimolmala, E.; Markpin, T.; Sombatsompop, N.; Saenboonruang, K. Enhanced X-ray shielding properties of NRL gloves with nano-Bi2O3 and their mechanical properties under aging conditions. Radiat. Phys. Chem. 2021, 186, 109530. [Google Scholar] [CrossRef]
- Güngör, A.; Akbay, I.; Yaşar, D.; Özdemir, T. Flexible X/Gamma ray shielding composite material of EPDM rubber with bismuth trioxide: Mechanical, thermal investigations and attenuation tests. Prog. Nucl. Energy 2018, 106, 262–269. [Google Scholar] [CrossRef]
- Bhowmick, A.K.; Stephens, H. (Eds.) Handbook of Elastomers; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780429177231. [Google Scholar]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Verdejo, R. Thermal, electrical, and sensing properties of rubber nanocomposites. In High-Performance Elastomeric Materials Reinforced by Nano-Carbons; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–175. [Google Scholar] [CrossRef]
- Lu, Z.; Hu, Y.; Zhang, B.; Zhang, G.; Guo, F.; Jiang, W. Anti-migration performance of EPDM composite improved by octadecylamine-functionalized graphene oxide. J. Appl. Polym. Sci. 2022, 139, e52713. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Lopez-Manchado, M.A.; Brasero, J.; Avilés, F.; Yazdani-Pedram, M. Effect of the morphology of thermally reduced graphite oxide on the mechanical and electrical properties of natural rubber nanocomposites. Compos. Part B Eng. 2016, 87, 350–356. [Google Scholar] [CrossRef]
- Sun, C.; Fan, C.; Kan, X.; Ma, Y.; Zhang, X.; Zhao, Y. Enhanced cross-linking performances and carbon black (CB) dispersion in solution styrene butadiene rubber (SSBR) filled with triazine-based graphdiyne (TGDY). Compos. Sci. Technol. 2022, 223, 109438. [Google Scholar] [CrossRef]
- Zhao, X.; Li, F.; Zhang, X.; Cao, J.; Wang, X. Rheological properties and viscosity reduction mechanism of aromatic/naphthenic oil pre-swelling crumb rubber modified asphalt. Constr. Build. Mater. 2023, 398, 132545. [Google Scholar] [CrossRef]
- Shijiazhuang City Horizon Chemical Industry Co. The Role of PEG-4000 in Rubber. Available online: https://www.horizonadmixtures.com/The-role-of-PEG-4000-in-rubber-id8557863.html (accessed on 15 December 2023).
- Sampath, W.; Fernando, C.; Edirisinghe, D. Synthesis of polyethylene glycol-grafted graphite and effect of its loading on properties of natural rubber composites. J. Natl. Sci. Found. Sri Lanka 2022, 50, 785–798. [Google Scholar] [CrossRef]
- Siriwong, C.; Sae-Oui, P.; Sirisinha, C. Performance comparison of various surface modifying agents on properties of silica-filled chloroprene rubber. Rubber Chem. Technol. 2017, 90, 146–158. [Google Scholar] [CrossRef]
- Ratnayake, U.; Prematunga, D.E.; Peiris, C.; Karunaratne, V.; Amaratunga, G.A. Effect of polyethylene glycol-intercalated organoclay on vulcanization characteristics and reinforcement of natural rubber nanocomposites. J. Elastomers Plast. 2016, 48, 711–727. [Google Scholar] [CrossRef]
- Magnere, S.M.; Toledo, E.A.; Yazdani-Pedram, M.; Fuentealba, P.; Contreras-Soto, A.; Bascuñan-Heredia, A.; Alvarez-Cortes, G.; Zagal, A.; Molina, F.; Hernández-Santana, M.; et al. High performance fluoroelastomer composites filled with graphite and/or bismuth oxide for applications in gamma-ray shielding. Polym. Compos. 2024, in press. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, F.; Ke, Y.; Xiang, C.; Jia, X. Effect of vulcanization on deformation behavior of rubber seals: Thermal–mechanical–chemical coupling model, numerical studies, and experimental validation. Mater. Des. 2022, 224, 111314. [Google Scholar] [CrossRef]
- ASTM D1054-02; Standard Test Method for Rubber Property—Resilience Using a Goodyear-Healey Rebound Pendulum (Withdrawn 2010). ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM D2240; Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2015; pp. 1–13. [CrossRef]
- ASTM D412-16; Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension. ASTM: West Conshohocken, PA, USA, 2021.
- Moni, G.; Mayeen, A.; Abraham, J.; Jose, T.; Maya, M.G.; Bhowmik, R.; George, S.C. Flexible FKM/mRGO nanocomposites with excellent thermal, mechanical and electrical properties. Arab. J. Chem. 2018, 13, 2142–2152. [Google Scholar] [CrossRef]
- Al-Abbas, S.S.; Ghazi, R.A.; Al-Shammari, A.K.; Aldulaimi, N.R.; Abdulridha, A.R.; Al-Nesrawy, S.H.; Al-Bermany, E. Influence of the polymer molecular weights on the electrical properties of Poly(vinyl alcohol)–Poly(ethylene glycols)/Graphene oxide nanocomposites. Mater. Today Proc. 2021, 42, 2469–2474. [Google Scholar] [CrossRef]
- Saritha, D.; Salagram, M.; Bhikshamaiah, G. Physical and optical properties of Bi2O3-B2O3glasses. IOP Conf. Ser. Mater. Sci. Eng. 2009, 2, 012057. [Google Scholar] [CrossRef]
- Komatsu, T.; Dimitrov, V.; Tasheva, T.; Honma, T. Electronic polarizability in silicate glasses by comparison of experimental and theoretical optical basicities. Int. J. Appl. Glass Sci. 2021, 12, 424–442. [Google Scholar] [CrossRef]
- Aguila-Toledo, E.; Maldonado-Magnere, S.; Yazdani-Pedram, M.; Bascuñan-Heredia, A.; Dahrouch, M.R.; Molina, F.; Santana, M.H.; Verdejo, R.; Lopez-Manchado, M.A.; Aguilar-Bolados, H. Fluorosilicone Composites with Functionalized Graphene Oxide for Advanced Applications. ACS Appl. Polym. Mater. 2023, 5, 7755–7765. [Google Scholar] [CrossRef]
- Aziz, O.; Salama, E.; El-Nashar, D.E.; Bakry, A. Development of Sustainable Radiation-Shielding Blend Using Natural Rubber/NBR, and Bismuth Filler. Sustainability 2023, 15, 9679. [Google Scholar] [CrossRef]
- Mistonov, A.; Ermakov, R.; Iskhakova, L.; Zakharova, A.; Chumakova, A.; Kvashnina, K. Electronic structure studies of bismuth compounds using high energy resolution X-ray spectroscopy and ab initio calculations. J. Alloys Compd. 2018, 753, 646–654. [Google Scholar] [CrossRef]
- Liang, T.-T.; Guo, X.-G. Remarkably Facile Preparation of Superhydrophobic Functionalized Bismuth Trioxide (Bi2O3) Coatings. Appl. Sci. 2019, 9, 2653. [Google Scholar] [CrossRef]
- Khan, Y.; Al-Arainy, A.A.; Malik, N.H.; Qureshi, M.I.; Al-Ammar, A.E. Loss and Recovery of Hydrophobicity of EPDM Insulators in Simulated Arid Desert Environment. In Proceedings of the 2010 Asia-Pacific Power and Energy Engineering Conference, Chengdu, China, 28–31 March 2010; pp. 1–4. [Google Scholar]
- Murakami, L.M.S.; Diniz, M.F.; Silva, L.M.; Sanches, N.B.; Dutra, R.D. Off-line TLC-IR (UATR) characterization of additives in EPDM rubber. Polym. Test. 2019, 79, 106042. [Google Scholar] [CrossRef]
- Ruiz, J.-R.R.; Canals, T.; Cantero, R. Supervision of Ethylene Propylene Diene M-Class (EPDM) Rubber Vulcanization and Recovery Processes Using Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopy and Multivariate Analysis. Appl. Spectrosc. 2016, 71, 141–151. [Google Scholar] [CrossRef]
- Elalaily, N.A.; Abou-Hussien, E.M.; Saad, E.A. Bismuth silicate glass containing heavy metal oxide as a promising radiation shielding material. Radiat. Eff. Defects Solids 2016, 171, 840–854. [Google Scholar] [CrossRef]
- Labib, S. Preparation, characterization and photocatalytic properties of doped and undoped Bi2O3. J. Saudi Chem. Soc. 2017, 21, 664–672. [Google Scholar] [CrossRef]
- Shinzawa, H.; Uchimaru, T.; Mizukado, J.; Kazarian, S.G. Non-equilibrium behavior of polyethylene glycol (PEG)/polypropylene glycol (PPG) mixture studied by Fourier transform infrared (FTIR) spectroscopy. Vib. Spectrosc. 2017, 88, 49–55. [Google Scholar] [CrossRef]
- Murakami, L.M.S.; Azevedo, J.B.; Diniz, M.F.; Silva, L.M.; Dutra, R.D. Characterization of additives in NR formulations by TLC-IR (UATR). Polímeros 2018, 28, 205–214. [Google Scholar] [CrossRef]
- Kim, I.-W.; Jang, M.D.; Ryu, Y.K.; Cho, E.H.; Lee, Y.K.; Park, J.H. Dipolarity, Hydrogen-Bond Basicity and Hydrogen-Bond Acidity of Aqueous Poly(ethylene glycol) Solutions. Anal. Sci. 2002, 18, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Alamri, H.R.; El-Hadi, A.M.; Al-Qahtani, S.M.; Assaedi, H.S.; Alotaibi, A.S. Role of lubricant with a plasticizer to change the glass transition temperature as a result improving the mechanical properties of poly(lactic acid) PLLA. Mater. Res. Express 2020, 7, 025306. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2018, 128, 259–274. [Google Scholar] [CrossRef]
- Barala, S.S.; Manda, V.; Jodha, A.S.; Meghwal, L.R.; Gopalani, D. Ethylene-propylene diene monomer-based polymer composite for attenuation of high energy radiations. J. Appl. Polym. Sci. 2020, 138, 50334. [Google Scholar] [CrossRef]




| Compound | Function | Unfilled EPDM | Composite EPDM/B/P | Composite EPDM/GN/P | Composite EPDM/B | Composite EPDM/GN | Composite EPDM/B/GN/P | Composite EPDM/B/GN |
|---|---|---|---|---|---|---|---|---|
| EPDM | Polymer matrix | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| ZnO | Activator | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Stearic acid | Activator | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| CBS | Accelerator | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 |
| Graphene | Filler | - | - | 10 | - | 10 | 10 | 10 |
| Bi2O3 | Filler | - | 100 | - | 100 | - | 100 | 100 |
| PEG1500 | Dispersing agent | - | 10 | 1 | - | - | 11 | - |
| Sulfur powder | Crosslinking agent | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
| Material | ML (Nm) | MH (Nm) | ts2 (min) | t90 (min) |
|---|---|---|---|---|
| EPDM | 0.850 ± 0.001 | 0.735 ± 0.093 | 4.76 ± 0.70 | 11.46 ± 1.62 |
| EPDM/B/P | 0.950 ± 0.002 | 0.537 ± 0.090 | 1.92 ± 0.23 | 5.22 ± 2.63 |
| EPDM/B | 0.135 ± 0.099 | 0.625 ± 0.053 | 2.63 ± 0.01 | 4.06 ± 0.33 |
| EPDM/GN/P | 0.135 ± 0.029 | 0.824 ± 0.059 | 2.08 ± 0.31 | 6.30 ± 1.48 |
| EPDM/GN | 0.164 ± 0.027 | 0.819 ± 0.023 | 3.43 ± 0.45 | 7.58 ± 0.75 |
| EPDM/B/GN/P | 0.930 ± 0.003 | 0.618 ± 0.064 | 1.86 ± 0.28 | 3.58 ± 0.87 |
| EPDM/B/GN | 0.104 ± 0.001 | 0.750 ± 0.053 | 2.01 ± 0.20 | 6.83 ± 0.98 |
| Material | E 50 (MPa) | E 100 (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| EPDM | 0.63 ± 0.02 | 0.90 ± 0.03 | 1.19 ± 0.11 | 173 ± 16 |
| EPDM/B/P | 0.35 ± 0.01 | 0.50 ± 0.01 | 1.28 ± 0.13 | 440 ± 39 |
| EPDM/B | 0.58 ± 0.08 | 0.81 ± 0.12 | 1.27 ± 0.09 | 235 ± 25 |
| EPDM/GN/P | 1.27 ± 0.03 | 0.81 ± 0.01 | 0.84 ± 0.01 | 114 ± 1 |
| EPDM/GN | 0.54 ± 0.06 | 0.76 ± 0.06 | 0.91 ± 0.07 | 157 ± 6 |
| EPDM/B/GN/P | 0.95 ± 0.04 | 1.23 ± 0.04 | 1.90 ± 0.24 | 299 ± 40 |
| EPDM/B/GN | 1.03 ± 0.04 | 1.41 ± 0.04 | 2.63 ± 0.14 | 325 ± 24 |
| Material | Hardness (Shore A) | ARI (%) |
|---|---|---|
| EPDM | 39.8 ± 0.76 | 39.08 |
| EPDM/B/P | 38.8 ± 0.29 | 67.21 |
| EPDM/B | 44.0 ± 0.58 | 88.37 |
| EPDM/GN/P | 44.5 ± 0.29 | 59.29 |
| EPDM/GN | 44.5 ± 0.29 | 44.02 |
| EPDM/B/GN/P | 44.3 ± 0.76 | 47.46 |
| EPDM/B/GN | 47.3 ± 0.29 | 75.46 |
| Composites | Tid (°C) | Tmax (°C) | Residual Mass at 800 °C (%) | Tg (°C) |
|---|---|---|---|---|
| EPDM | 345.4 | 467.1 | 3.18 | −52.45 |
| EPDM/B | 335.3 | 452.1 | 40.45 | −53.06 |
| EPDM/GN | 348.5 | 468.0 | 6.55 | −52.45 |
| EPDM/B/P | 356.1 | 458.8 | 41.26 | −52.45 |
| EPDM/GN/P | 337.0 | 466.1 | 7.74 | −52.07 |
| EPDM/B/GN | 344.0 | 452.6 | 39.78 | −52.08 |
| EPDM/B/GN/P | 356.8 | 459.7 | 41.02 | −52.45 |
| Sample | ρ (g/cm3) | µ (cm−1) | µ Increase (%) | MFP (cm) | TVL (cm) | HVL (cm) | TF | RPE (%) | µmass (g/cm2) | µmass Increase (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EPDM | 1.014 | 0.0844 | 11.8 | 8.21 | 27.3 | 0.907 | 9.25 | 0.0832 | 3.11 | ||
| EPDM/B | 1.516 | 0.147 | 75 | 6.80 | 4.71 | 15.6 | 0.846 | 15.4 | 0.0972 | 17 | 4.85 |
| EPDM/GN | 0.939 | 0.0612 | −27 | 16.3 | 11.33 | 37.6 | 0.933 | 6.75 | 0.0652 | −22 | 3.22 |
| EPDM/B/GN | 1.664 | 0.131 | 55 | 7.63 | 5.29 | 17.6 | 0.866 | 13.4 | 0.0787 | −5 | 4.89 |
| EPDM/B/P | 1.418 | 0.129 | 53 | 7.75 | 5.37 | 17.8 | 0.871 | 12.9 | 0.0910 | 9 | 4.77 |
| EPDM/GN/P | 0.955 | 0.0837 | −1 | 11.9 | 8.28 | 27.5 | 0.911 | 8.92 | 0.0876 | 5 | 3.22 |
| EPDM/B/GN/P | 1.500 | 0.136 | 61 | 7.35 | 5.10 | 16.9 | 0.867 | 13.3 | 0.0907 | 9 | 4.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Cortez, G.; Molina, F.; Urbano, B.F.; Dahrouch, M.; Hernández Santana, M.; Manchado, M.A.L.; Verdejo, R.; Aguilar Bolados, H. Design and Study of Novel Composites Based on EPDM Rubber Containing Bismuth (III) Oxide and Graphene Nanoplatelets for Gamma Radiation Shielding. Polymers 2024, 16, 633. https://doi.org/10.3390/polym16050633
Álvarez-Cortez G, Molina F, Urbano BF, Dahrouch M, Hernández Santana M, Manchado MAL, Verdejo R, Aguilar Bolados H. Design and Study of Novel Composites Based on EPDM Rubber Containing Bismuth (III) Oxide and Graphene Nanoplatelets for Gamma Radiation Shielding. Polymers. 2024; 16(5):633. https://doi.org/10.3390/polym16050633
Chicago/Turabian StyleÁlvarez-Cortez, Gabriela, Francisco Molina, Bruno F. Urbano, Mohamed Dahrouch, Marianella Hernández Santana, Miguel A. Lopez Manchado, Raquel Verdejo, and Héctor Aguilar Bolados. 2024. "Design and Study of Novel Composites Based on EPDM Rubber Containing Bismuth (III) Oxide and Graphene Nanoplatelets for Gamma Radiation Shielding" Polymers 16, no. 5: 633. https://doi.org/10.3390/polym16050633
APA StyleÁlvarez-Cortez, G., Molina, F., Urbano, B. F., Dahrouch, M., Hernández Santana, M., Manchado, M. A. L., Verdejo, R., & Aguilar Bolados, H. (2024). Design and Study of Novel Composites Based on EPDM Rubber Containing Bismuth (III) Oxide and Graphene Nanoplatelets for Gamma Radiation Shielding. Polymers, 16(5), 633. https://doi.org/10.3390/polym16050633










