Synthesis of a Novel P/N-Triazine-Containing Ring Flame Retardant and Its Application in Epoxy Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
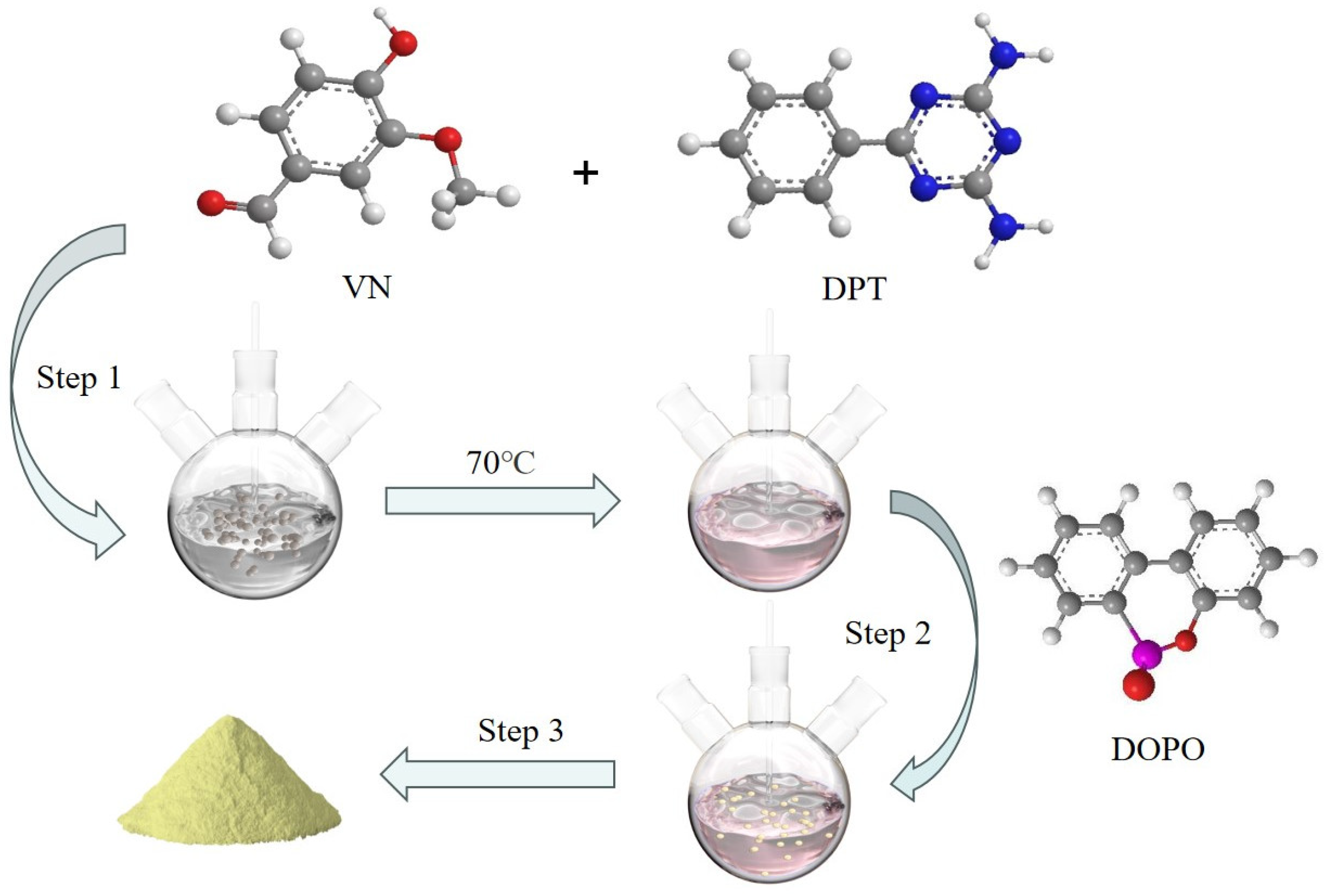
2.2. Synthesis of VDPD
2.3. Preparation of Flame-Retardant EP
2.4. Characterization
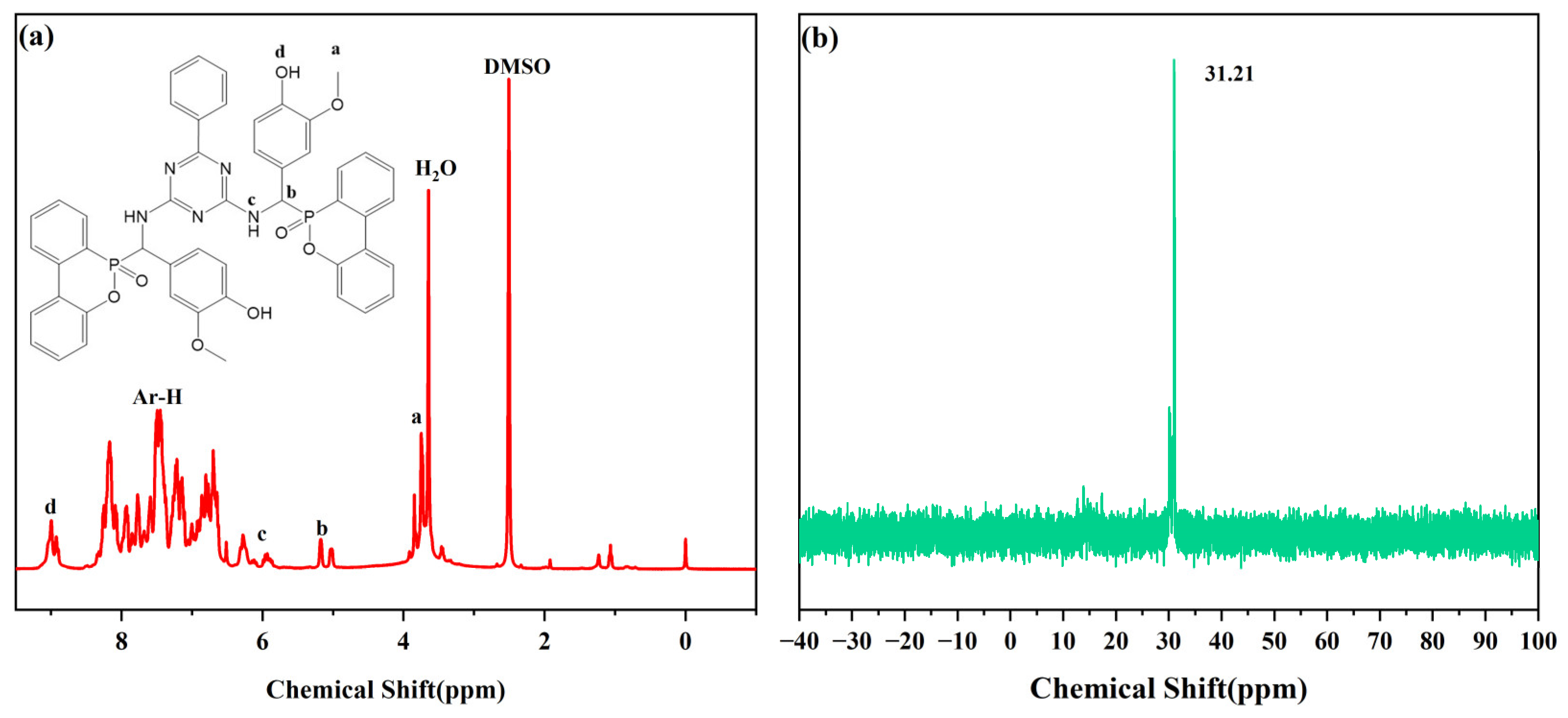
2.4.1. Structure Characterization of VDPD
2.4.2. Curing Behavior and Thermal Stability Analysis
2.4.3. Mechanical Properties Analysis
2.4.4. Flame Retardance and Combustion Behavior Analysis
2.4.5. Morphology and Chemical Analysis of Char Residues
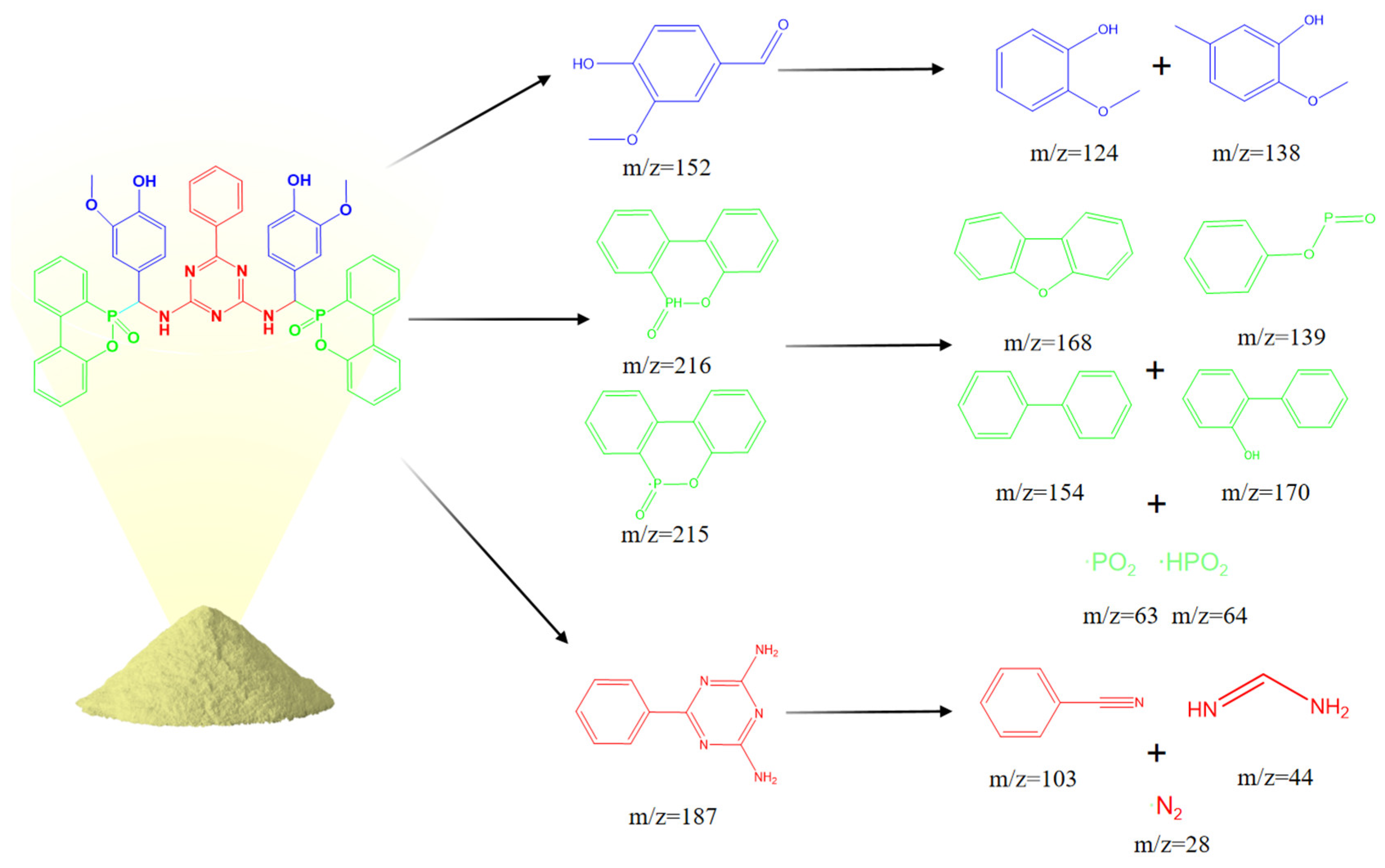
2.4.6. Analysis of Pyrolysis Behavior
3. Results and Discussion
3.1. Structural Characterization of VDPD
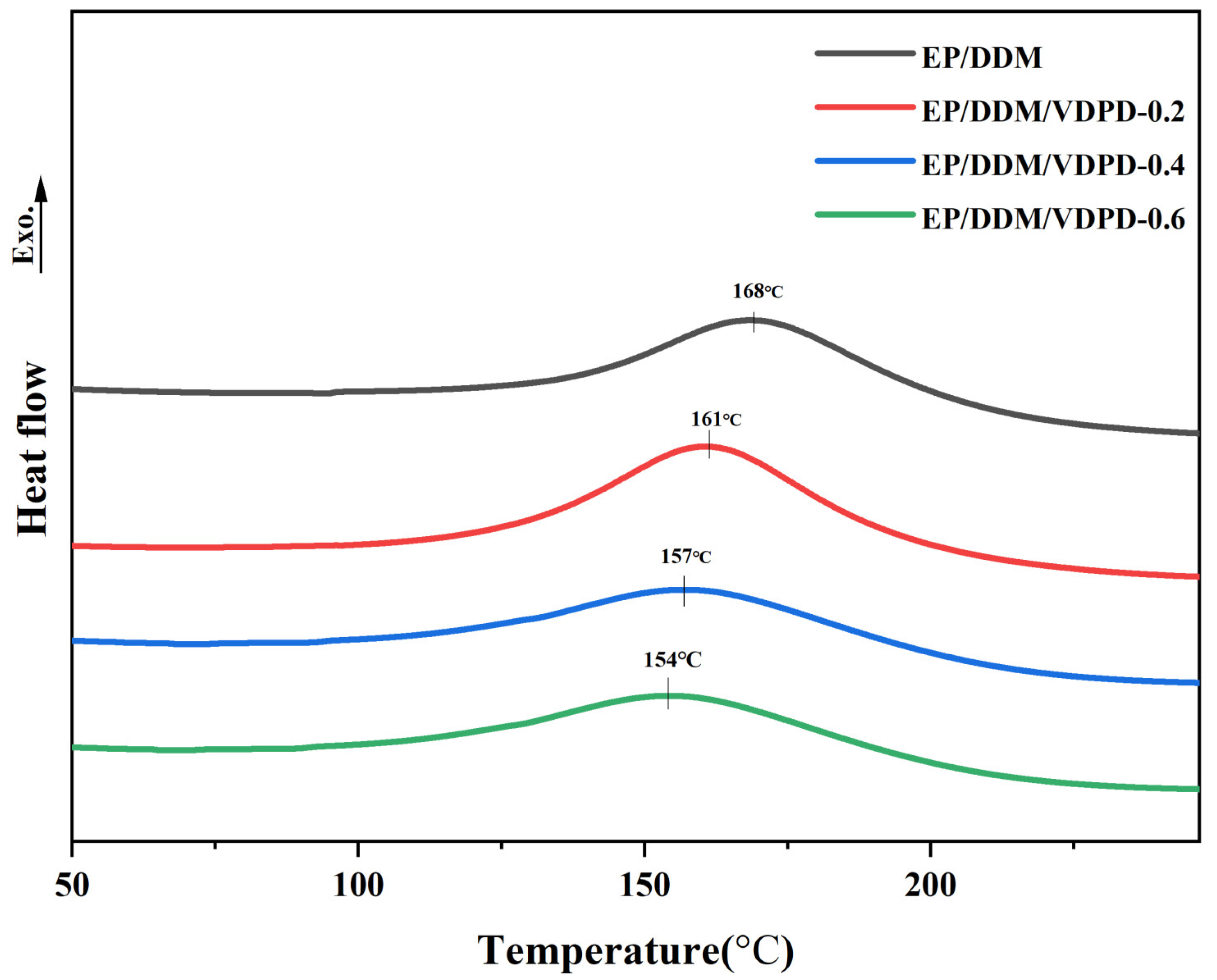
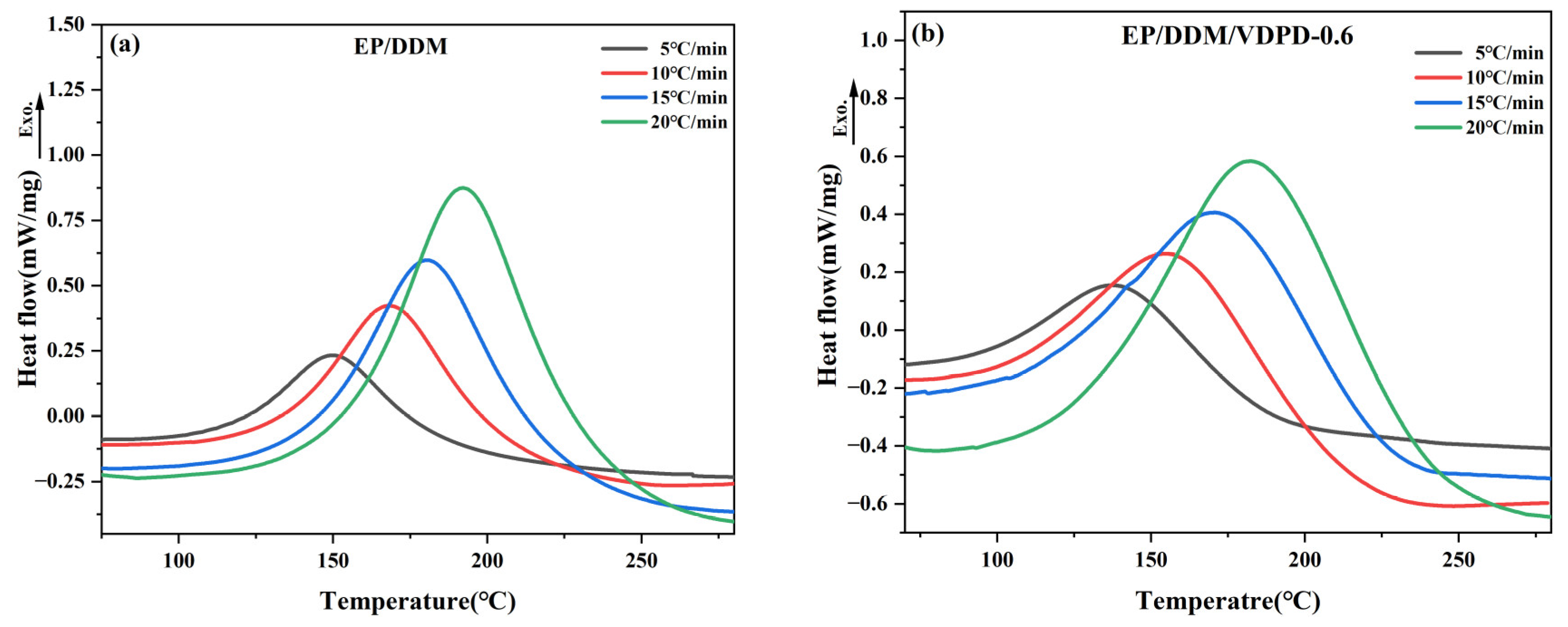
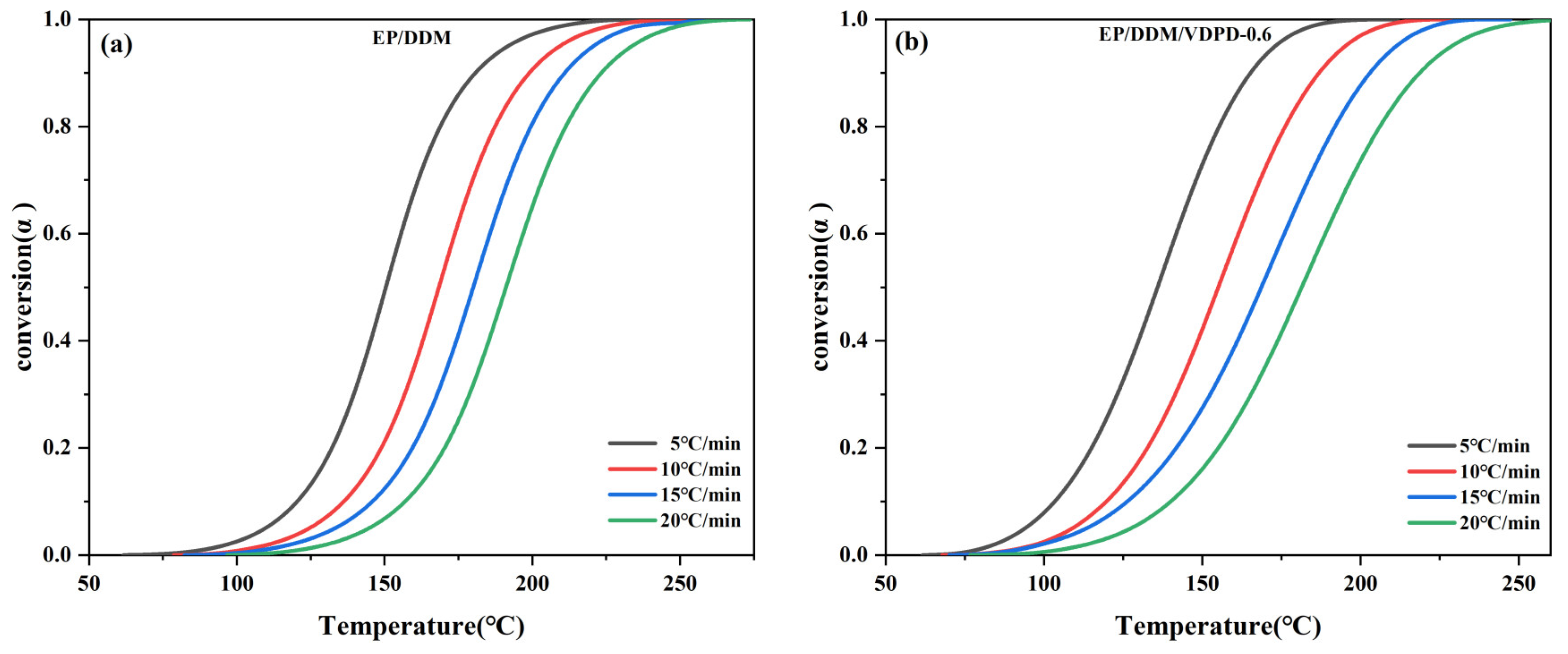
3.2. Analysis of Curing Behavior
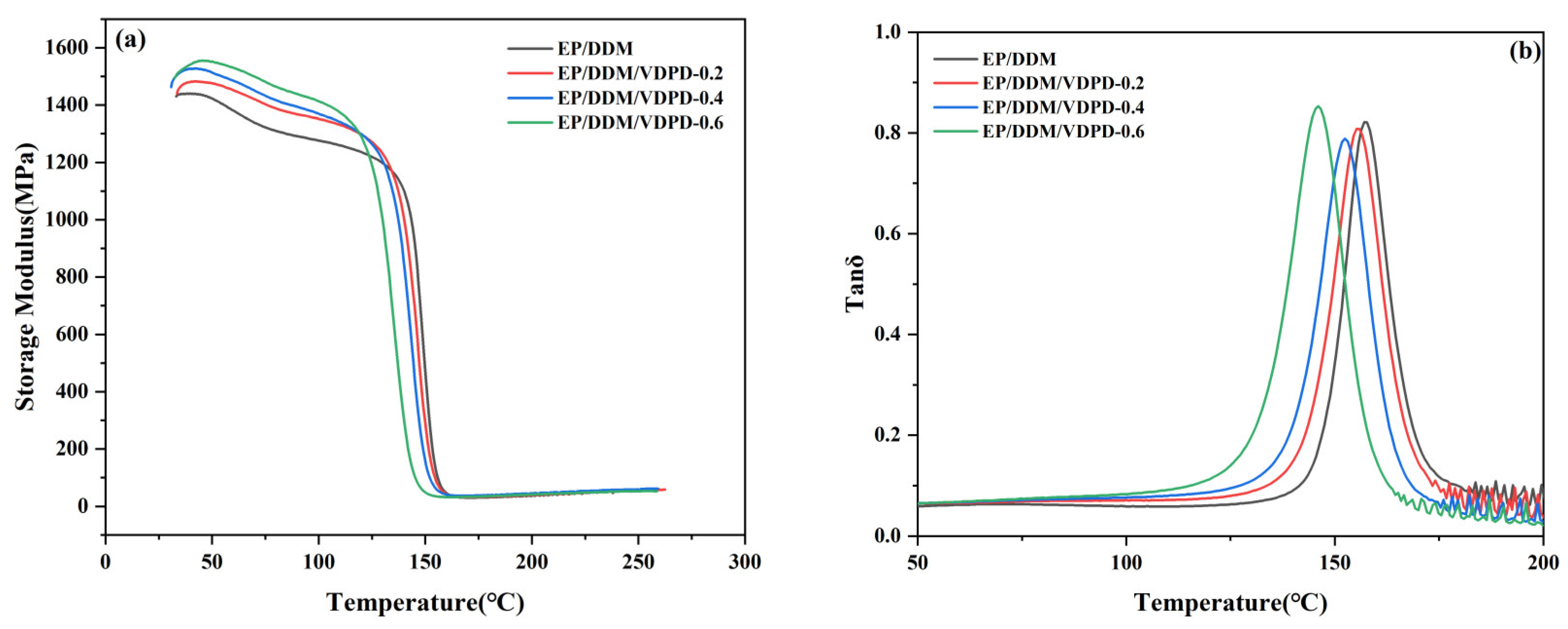
3.3. Dynamic Mechanical and Mechanical Properties
3.4. Thermal Stability
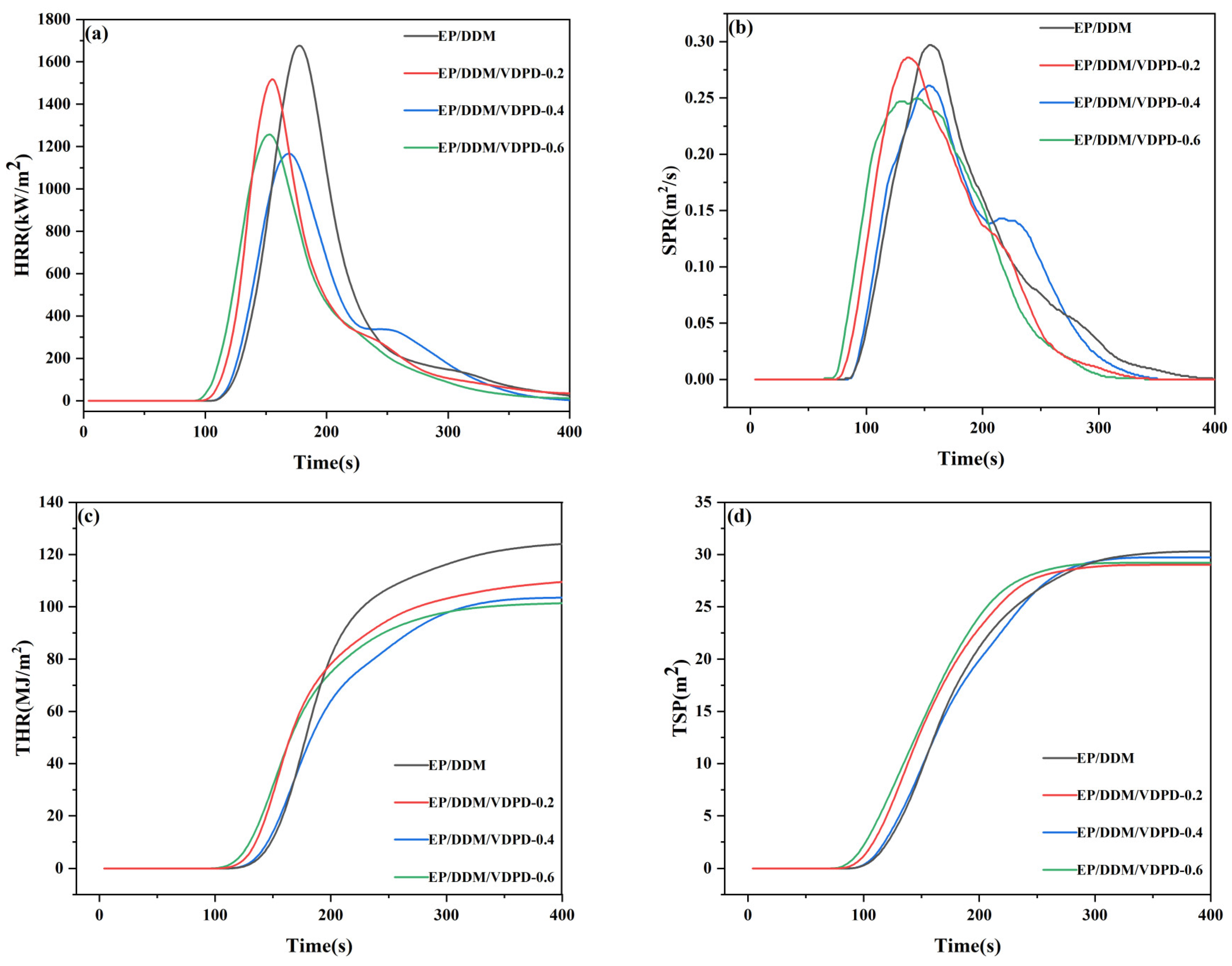
3.5. Flame Retardancy and Combustion Behavior
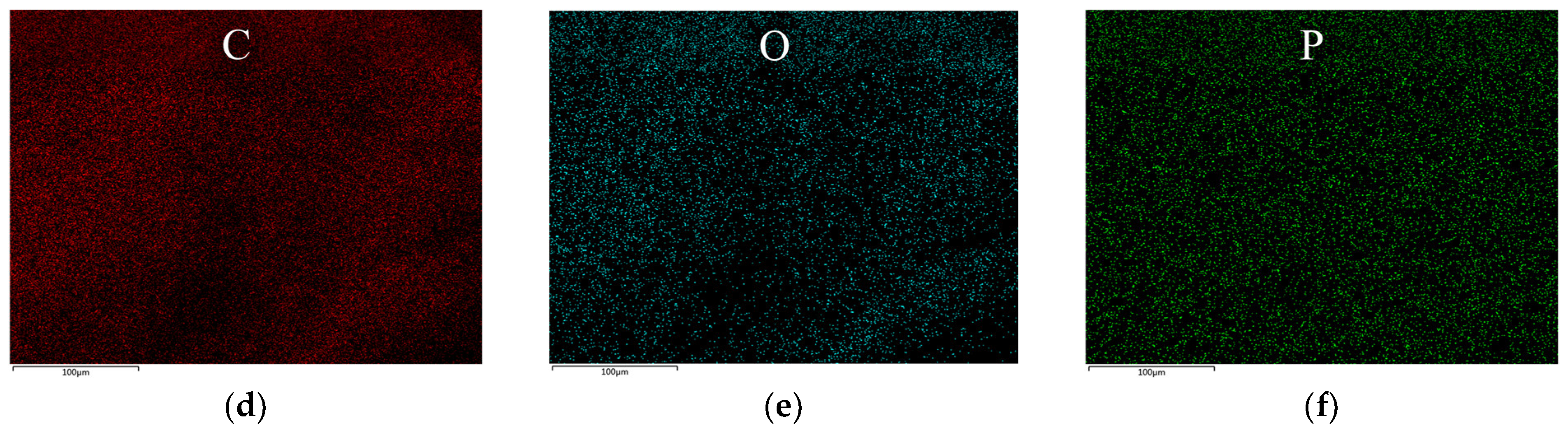
3.6. Morphology and Chemical Analysis of Char Residue
3.7. Analysis of Pyrolysis Behavior
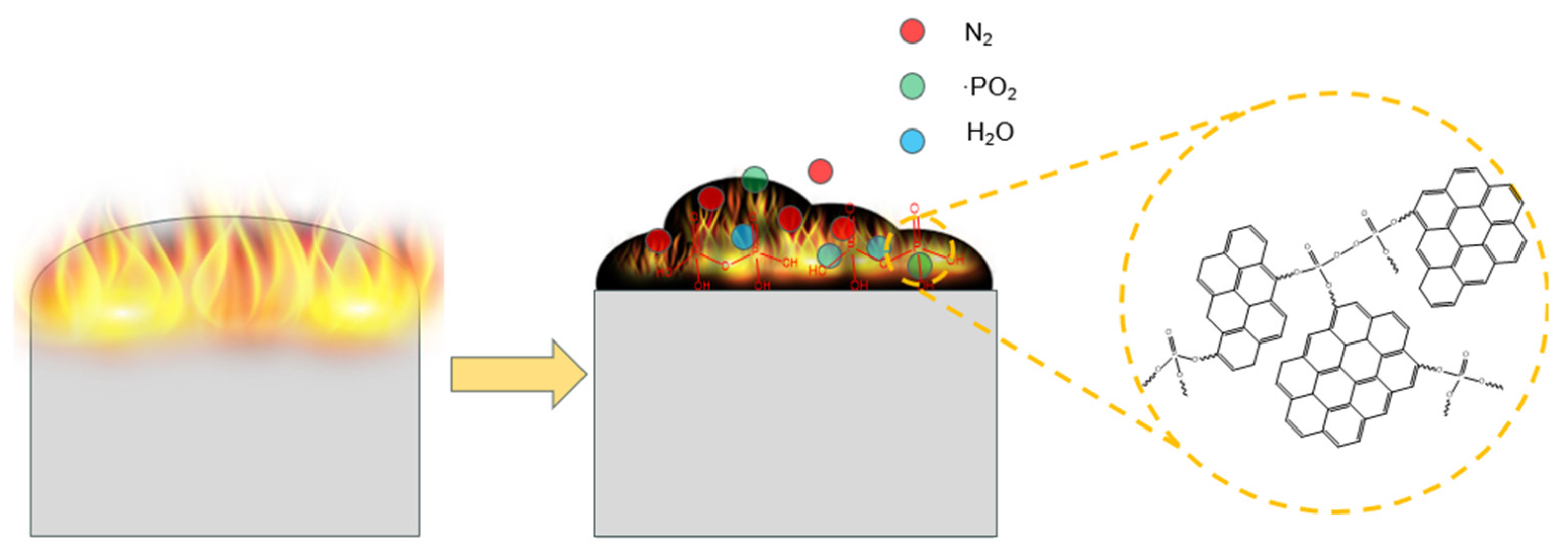
3.8. Flame-Retardant Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, G.; Huo, S.; Wang, C.; Song, P.; Fang, Z.; Wang, H.; Liu, Z. Durable flame-retardant, strong and tough epoxy resins with well-preserved thermal and optical properties via introducing a bio-based, phosphorus-phosphorus, hyperbranched oligomer. Polym. Degrad. Stab. 2023, 207, 110235. [Google Scholar] [CrossRef]
- Wang, S.; Lou, S.; Fan, P.; Ma, L.; Liu, J.; Tang, T. A novel aromatic imine-containing DOPO-based reactive flame retardant towards enhanced flame-retardant and mechanical properties of epoxy resin. Polym. Degrad. Stab. 2023, 213, 110364. [Google Scholar] [CrossRef]
- Ai, Y.F.; Xia, L.; Pang, F.Q.; Xu, Y.L.; Zhao, H.B.; Jian, R.K. Mechanically strong and flame-retardant epoxy resins with anti-corrosion performance. Compos. Part B Eng. 2020, 193, 108019. [Google Scholar] [CrossRef]
- Peng, X.; Liu, Q.; Wang, D.; Liu, C.; Zhao, Y.; Wang, R.; Zheng, P. A hyperbranched structure formed by in-situ crosslinking of additive flame retardant endows epoxy resins with great flame retardancy improvement. Compos. Part B Eng. 2021, 224, 109162. [Google Scholar] [CrossRef]
- Dai, X.; Li, P.; Sui, Y.; Zhang, C. Thermal and flame-retardant properties of intrinsic flame-retardant epoxy resin containing biphenyl structures and phosphorus. Eur. Polym. J. 2021, 147, 110319. [Google Scholar] [CrossRef]
- Xie, W.; Huang, S.; Liu, S.; Zhao, J. Phosphorus-based triazine compound endowing epoxy thermosets with excellent flame retardancy and enhanced mechanical stiffness. Polym. Degrad. Stab. 2020, 180, 109293. [Google Scholar] [CrossRef]
- Huo, S.; Sai, T.; Ran, S.; Guo, Z.; Fang, Z.; Song, P.; Wang, H. A hyperbranched P/N/B-containing oligomer as multifunctional flame retardant for epoxy resins. Compos. Part B Eng. 2022, 234, 109701. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Cheng, L.; Wang, M. Preparation and flame retardancy of an intumescent flame-retardant epoxy resin system constructed by multiple flame-retardant compositions containing phosphorus and nitrogen heterocycle. Polym. Degrad. Stab. 2015, 119, 251–259. [Google Scholar] [CrossRef]
- Xu, M.J.; Xu, G.R.; Leng, Y.; Li, B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016, 123, 105–114. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Cheng, L. Synthesis of a phosphorus/nitrogen-containing additive with multifunctional groups and its flame-retardant effect in epoxy resin. Ind. Eng. Chem. Res. 2015, 54, 7777–7786. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.; Liu, Q.; He, Y. A comprehensive review of reactive flame-retardant epoxy resin: Fundamentals, recent developments, and perspectives. Polym. Degrad. Stab. 2022, 201, 109976. [Google Scholar] [CrossRef]
- Liu, C.; Duan, H.; Zhao, H.; Gao, Y.; Zhang, J.; Kang, J.; Ma, H. Facile synthesis of a P/N-containing heterocyclic compound for simultaneous enhancement of heat resistance, mechanical properties and fire safety of epoxy resin. React. Funct. Polym. 2023, 184, 105530. [Google Scholar] [CrossRef]
- Qi, Y.; Weng, Z.; Kou, Y.; Song, L.; Li, J.; Wang, J.; Zhang, S.; Liu, C.; Jian, X. Synthesize and introduce bio-based aromatic s-triazine in epoxy resin: Enabling extremely high thermal stability, mechanical properties, and flame retardancy to achieve high-performance sustainable polymers. Chem. Eng. J. 2021, 406, 126881. [Google Scholar] [CrossRef]
- Liu, T.; Peng, J.; Liu, J.; Hao, X.; Guo, C.; Ou, R.; Liu, Z.; Wang, Q. Fully recyclable, flame-retardant and high-performance carbon fiber composites based on vanillin-terminated cyclophosphazene polyimine thermosets. Compos. Part B Eng. 2021, 224, 109188. [Google Scholar] [CrossRef]
- Liu, J.; Dai, J.; Wang, S.; Peng, Y.; Cao, L.; Liu, X. Facile synthesis of bio-based reactive flame retardant from vanillin and guaiacol for epoxy resin. Compos. Part B Eng. 2020, 190, 107926. [Google Scholar] [CrossRef]
- Chi, Z.; Guo, Z.; Xu, Z.; Zhang, M.; Li, M.; Shang, L.; Ao, Y. A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polym. Degrad. Stab. 2020, 176, 109151. [Google Scholar] [CrossRef]
- He, X.; Qi, J.; Chen, M.; Lv, J.; Xiao, H.; Hu, J.; Zeng, K.; Yang, G. Preparation of novel bio-based imine-containing phthalonitrile resin through the nucleophilic reaction in green solvent. Polymer 2022, 253, 124973. [Google Scholar] [CrossRef]
- Teng, N.; Dai, J.; Wang, S.; Hu, J.; Yi, X.; Liu, X. Hyperbranched flame retardant to simultaneously improve the fire-safety, toughness and glass transition temperature of epoxy resin. Eur. Polym. J. 2021, 157, 110638. [Google Scholar] [CrossRef]
- Sun, J.; Wang, B.; Guo, Z.; Fang, Z.; Li, J. Flame retardant epoxy resin toughened and strengthened by a reactive compatibilizer. Polymer 2022, 248, 124798. [Google Scholar] [CrossRef]
- Li, M.; Hao, X.; Hu, M.; Huang, Y.; Qiu, Y.; Li, L. Synthesis of bio-based flame-retardant epoxy co-curing agent and application in wood surface coating. Prog. Org. Coat. 2022, 167, 106848. [Google Scholar] [CrossRef]
- Hua, Y.; Chen, J.; Liu, J.; Sun, J.; Gu, X.; Jiang, S.; Zhang, S. Fabrication of a transparent, flame retardant, and antimicrobial epoxy resin by a novel phosphorus-containing Schiff base molecule. Polym. Degrad. Stab. 2023, 209, 110274. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Li, C.; Molina-Aldareguia, J.; Kalali, E.N.; Wang, X.; Wang, D.-Y. A sustainable, eugenol-derived epoxy resin with high biobased content, modulus, hardness and low flammability: Synthesis, curing kinetics and structure–property relationship. Chem. Eng. J. 2016, 284, 1080–1093. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Wang, D.; Xiu, P.; Xu, C.; Lee, A.; Gu, X. Flame-retardant effect of a functional DOPO-based compound on lignin-based epoxy resins. Mater. Today Chem. 2021, 22, 100562. [Google Scholar] [CrossRef]
- Tian, Y.; Ke, M.; Wang, X.; Wu, G.; Zhang, J.; Cheng, J. A resveratrol-based epoxy resin with ultrahigh Tg and good processability. Eur. Polym. J. 2021, 147, 110282. [Google Scholar] [CrossRef]
- Chen, R.; Luo, Z.; Yu, X.J.; Tang, H.; Zhou, Y.; Zhou, H. Synthesis of chitosan-based flame retardant and its fire resistance in epoxy resin. Carbohydr. Polym. 2020, 245, 116530. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhou, Z.; Liu, J.; Luo, J.; Liu, R. A recyclable vanillin-based epoxy resin with high-performance that can compete with DGEBA. Eur. Polym. J. 2020, 140, 110053. [Google Scholar] [CrossRef]
- Cao, J.; Duan, H.; Zou, J.; Zhang, J.; Ma, H. A bio-based phosphorus-containing co-curing agent towards excellent flame retardance and mechanical properties of epoxy resin. Polym. Degrad. Stab. 2021, 187, 109548. [Google Scholar] [CrossRef]
- Sun, J.; Qian, L.; Li, J. Toughening and strengthening epoxy resin with flame retardant molecular structure based on tyrosine. Polymer 2021, 230, 124045. [Google Scholar] [CrossRef]
- GB/T 1040.2-2006 (ISO 527-2:1993); National Standard of the People’s Republic of China. Plastics—Determination of Tensile Properties. Standards Press of China: Beijing, China, 2006.
- GB/T 1843-2008; Determination of Impact Strength of Plastic Cantilever Beams. National Quality Supervision Bureau: Beijing, China, 2008.
- ASTM D2863-19; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-like Combustion of Plastics (Oxygen Index). ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D3801-20a; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM International: West Conshohocken, PA, USA, 2020.
- ISO 5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- Ma, J.; Li, G.; Hua, X.; Liu, N.; Liu, Z.; Zhang, F.; Yu, L.; Chen, X.; Shang, L.; Ao, Y. Biodegradable epoxy resin from vanillin with excellent flame-retardant and outstanding mechanical properties. Polym. Degrad. Stab. 2022, 201, 109989. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, J.; Zhu, Z.; Yin, X.; Weng, Y.; Wang, Z.; Yang, F.; Zhan, J.; Wang, H.; Wang, L. High performance epoxy resin composites modified with multifunctional thiophene/phosphaphenanthrene-based flame retardant: Excellent flame retardance, strong mechanical property and high transparency. Compos. Part B Eng. 2021, 227, 109392. [Google Scholar] [CrossRef]
- Xiong, Y.; Jiang, Z.; Xie, Y.; Zhang, X.; Xu, W. Development of a DOPO-containing melamine epoxy hardeners and its thermal and flame-retardant properties of cured products. J. Appl. Polym. Sci. 2013, 127, 4352–4358. [Google Scholar] [CrossRef]
- Wang, P.; Cai, Z. Highly efficient flame-retardant epoxy resin with a novel DOPO-based triazole compound: Thermal stability, flame retardancy and mechanism. Polym. Degrad. Stab. 2017, 137, 138–150. [Google Scholar] [CrossRef]
- Zu, Y.; Zong, L.; Wang, J.; Jian, X. Kinetic analysis of the curing of branched phthalonitrile resin based on dynamic differential scanning calorimetry. Polym. Test. 2021, 96, 107062. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Zou, J.; Duan, H.; Chen, Y.; Ji, S.; Cao, J.; Ma, H. AP/N/S-containing high-efficiency flame retardant endowing epoxy resin with excellent flame retardance, mechanical properties and heat resistance. Compos. Part B Eng. 2020, 199, 108228. [Google Scholar] [CrossRef]
- Huo, S.; Zhou, Z.; Jiang, J.; Sai, T.; Ran, S.; Fang, Z.; Song, P.; Wang, H. Flame-retardant, transparent, mechanically-strong and tough epoxy resin enabled by high-efficiency multifunctional boron-based polyphosphonamide. Chem. Eng. J. 2022, 427, 131578. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, H.; Wan, C.; Liu, C.; Zhao, H.; Ma, H. Simultaneously improving the thermal stability, mechanical properties and flame retardancy of epoxy resin by a phosphorus/nitrogen/sulfur-containing reactive flame retardant. Mater. Today Commun. 2022, 30, 103108. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Rahman, M.Z.; Song, L.; Hu, Y. Improvement of the flame retardant and thermomechanical properties of epoxy resins by a vanillin-derived cyclotriphosphazene-cored triazole compound. Polym. Degrad. Stab. 2022, 204, 110088. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H.; Zhan, T. Nitrogen/sulfur-containing DOPO based oligomer for highly efficient flame-retardant epoxy resin. Polym. Degrad. Stab. 2020, 171, 109023. [Google Scholar] [CrossRef]












| Sample | E-51 (g) | DDM (g) | VDPD (g) | P (wt.%) |
|---|---|---|---|---|
| EP | 100 | 25 | 0 | 0 |
| EP/DDM/VDPD-0.2 | 100 | 25 | 3.79 | 0.2 |
| EP/DDM/VDPD-0.4 | 100 | 25 | 7.81 | 0.4 |
| EP/DDM/VDPD-0.6 | 100 | 25 | 12.13 | 0.6 |
| Element | C | N | H |
|---|---|---|---|
| Theory (%) | 66.29 | 7.89 | 4.43 |
| Test (%) | 66.26 | 7.74 | 4.58 |
| α | Eα of EP/DDM/(kJ/mol) | Eα of EP/DDM/VDPD-0.6 (kJ/mol) |
|---|---|---|
| 0.1 | 46.58 | 41.37 |
| 0.2 | 47.86 | 41.49 |
| 0.3 | 47.65 | 42.41 |
| 0.4 | 48.76 | 43.51 |
| 0.5 | 49.46 | 43.89 |
| 0.6 | 50.19 | 44.56 |
| 0.7 | 51.29 | 42.56 |
| 0.8 | 51.57 | 43.60 |
| 0.9 | 52.25 | 44.47 |
| Average value | 49.51 | 43.10 |
| Sample | Tg (°C) | E’ at 50 °C (MPa) | E’ at (Tg + 40) °C (MPa) | υe (mol/m3) |
|---|---|---|---|---|
| EP/DDM | 158 | 1410 | 42.3 | 3600 |
| EP/DDM/VDPD-0.2 | 155 | 1460 | 40.1 | 3435 |
| EP/DDM/VDPD-0.4 | 152 | 1500 | 37.9 | 3267 |
| EP/DDM/VDPD-0.6 | 146 | 1557 | 34.3 | 2996 |
| Samples | Tensile Strength (MPa) | Tensile Modulus (MPa) | Impact Strength (kJ/m2) |
|---|---|---|---|
| EP/DDM | 80.2 ± 1.4 | 3077 ± 81 | 22 ± 0.5 |
| EP/DDM/VDPD-0.2 | 78 ± 2 | 3360 ± 65 | 22 ± 0.4 |
| EP/DDM/VDPD-0.4 | 74.3 ± 1.6 | 3446 ± 51 | 21 ± 0.4 |
| EP/DDM/VDPD-0.6 | 68.8 ± 1.5 | 3490 ± 60 | 21 ± 0.2 |
| Sample | T5% (°C) | Tmax (°C) | Rmax (%/min) | Char Residue at 800 °C (%) |
|---|---|---|---|---|
| EP/DDM | 343 | 383 | 16.27 | 12.22 |
| EP/DDM/VDPD-0.2 | 328 | 378 | 11.17 | 13.92 |
| EP/DDM/VDPD-0.4 | 322 | 375 | 11.21 | 15.29 |
| EP/DDM/VDPD-0.6 | 324 | 374 | 9.99 | 21.20 |
| Sample | LOI (%) | UL-94 | Dripping | Level | |
|---|---|---|---|---|---|
| t1 (S) | t2 (S) | ||||
| EP/DDM | 24.4 | -- | -- | NO | -- |
| EP/DDM/VDPD-0.2 | 29.5 | 5.3 | 6.5 | NO | V1 |
| EP/DDM/VDPD-0.4 | 33.1 | 4.0 | 5.2 | NO | V0 |
| EP/DDM/VDPD-0.6 | 34.5 | 1.2 | 3.3 | NO | V0 |
| Sample | PHRR (kW/m2) | THR (MJ/m2) | SPR (m2/s) | av-EHC (MJ/kg) | av-COY (kg/kg) | av-CO2 (kg/kg) | Residue (%) |
|---|---|---|---|---|---|---|---|
| EP/DDM | 1679 | 124.6 | 0.296 | 39.70 | 0.1356 | 3.42 | 10.3 |
| EP/DDM/VDPD-0.2 | 1514 | 111.4 | 0.285 | 36.64 | 0.1609 | 3.12 | 14.5 |
| EP/DDM/VDPD-0.4 | 1167 | 103.5 | 0.261 | 33.50 | 0.1887 | 3.07 | 15.0 |
| EP/DDM/VDPD-0.6 | 1265 | 101.8 | 0.249 | 33.29 | 0.2164 | 2.84 | 18.3 |
| Peak | Retention Time (min) | m/z | Assigned Structure |
|---|---|---|---|
| a | 3.72 | 91 |  |
| b | 7.06 | 103 |  |
| c | 8.69 | 124 | 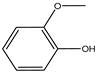 |
| d | 13.1 | 151 | 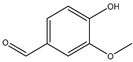 |
| e | 14.6 | 170 | 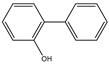 |
| f | 20.1 | 187 | 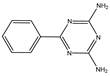 |
| g | 21.46 | 216 | 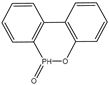 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Chen, J.; Ding, A.; Wang, C.; Wang, Y.; Yang, L. Synthesis of a Novel P/N-Triazine-Containing Ring Flame Retardant and Its Application in Epoxy Resin. Polymers 2024, 16, 871. https://doi.org/10.3390/polym16070871
Yu Y, Chen J, Ding A, Wang C, Wang Y, Yang L. Synthesis of a Novel P/N-Triazine-Containing Ring Flame Retardant and Its Application in Epoxy Resin. Polymers. 2024; 16(7):871. https://doi.org/10.3390/polym16070871
Chicago/Turabian StyleYu, Yi, Junlei Chen, Anxin Ding, Changzeng Wang, Yunfei Wang, and Ling Yang. 2024. "Synthesis of a Novel P/N-Triazine-Containing Ring Flame Retardant and Its Application in Epoxy Resin" Polymers 16, no. 7: 871. https://doi.org/10.3390/polym16070871
APA StyleYu, Y., Chen, J., Ding, A., Wang, C., Wang, Y., & Yang, L. (2024). Synthesis of a Novel P/N-Triazine-Containing Ring Flame Retardant and Its Application in Epoxy Resin. Polymers, 16(7), 871. https://doi.org/10.3390/polym16070871







