Synergistic Modification of Polyformaldehyde by Biobased Calcium Magnesium Bi-Ionic Melamine Phytate with Intumescent Flame Retardant
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
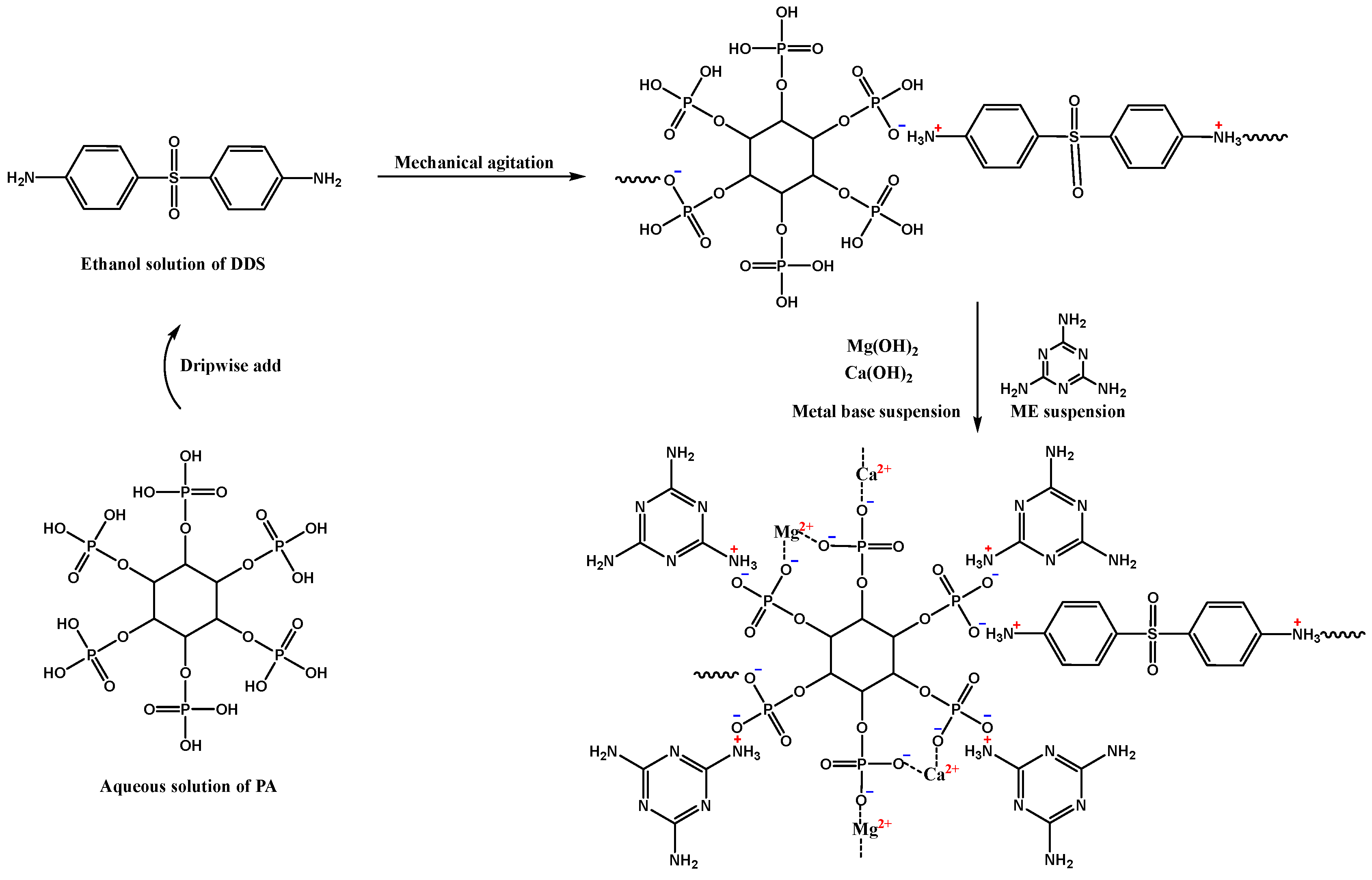
2.2. Preparation of DPM
2.3. Preparation of POM Composites
2.4. Characterization
3. Results and Discussion
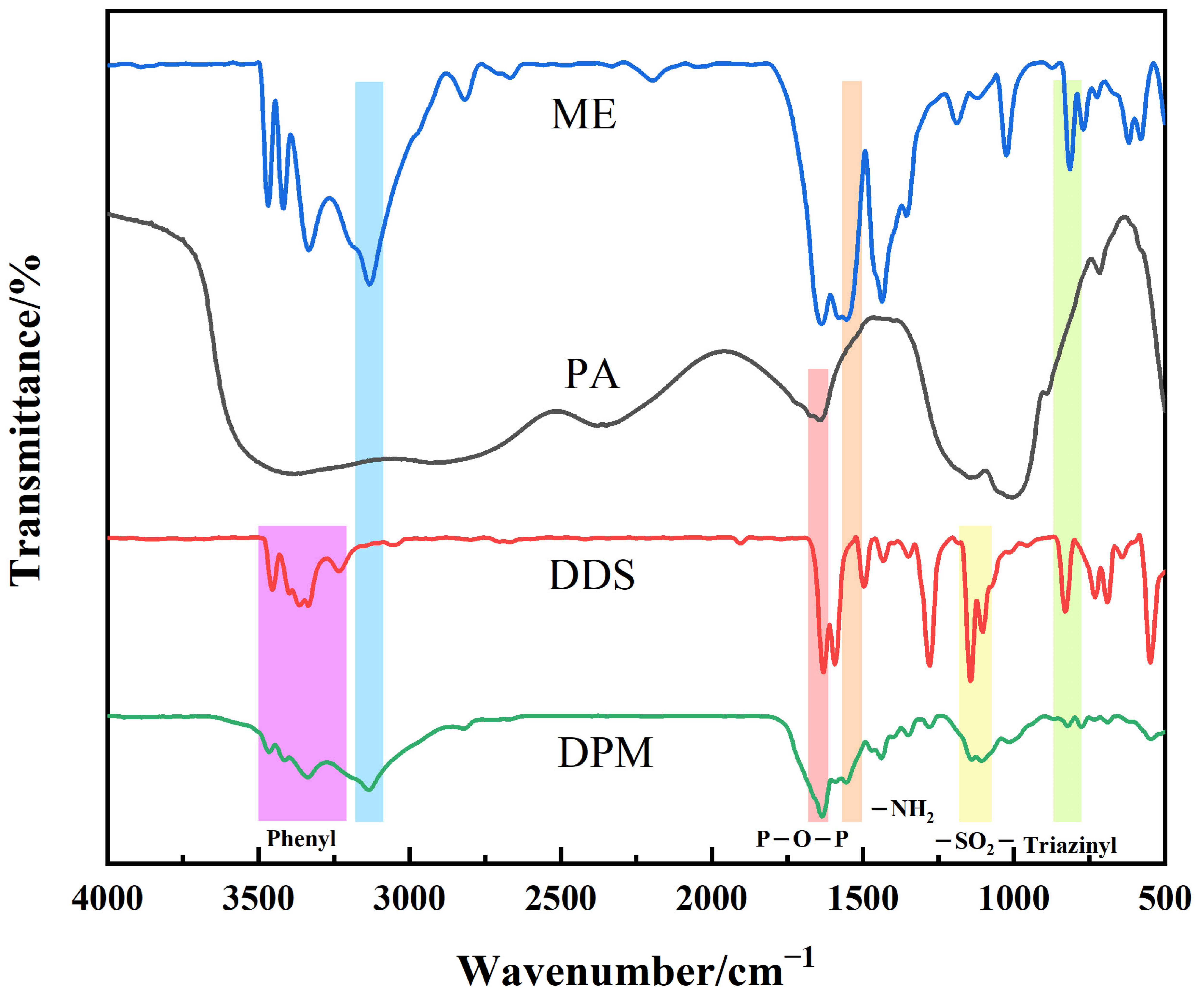
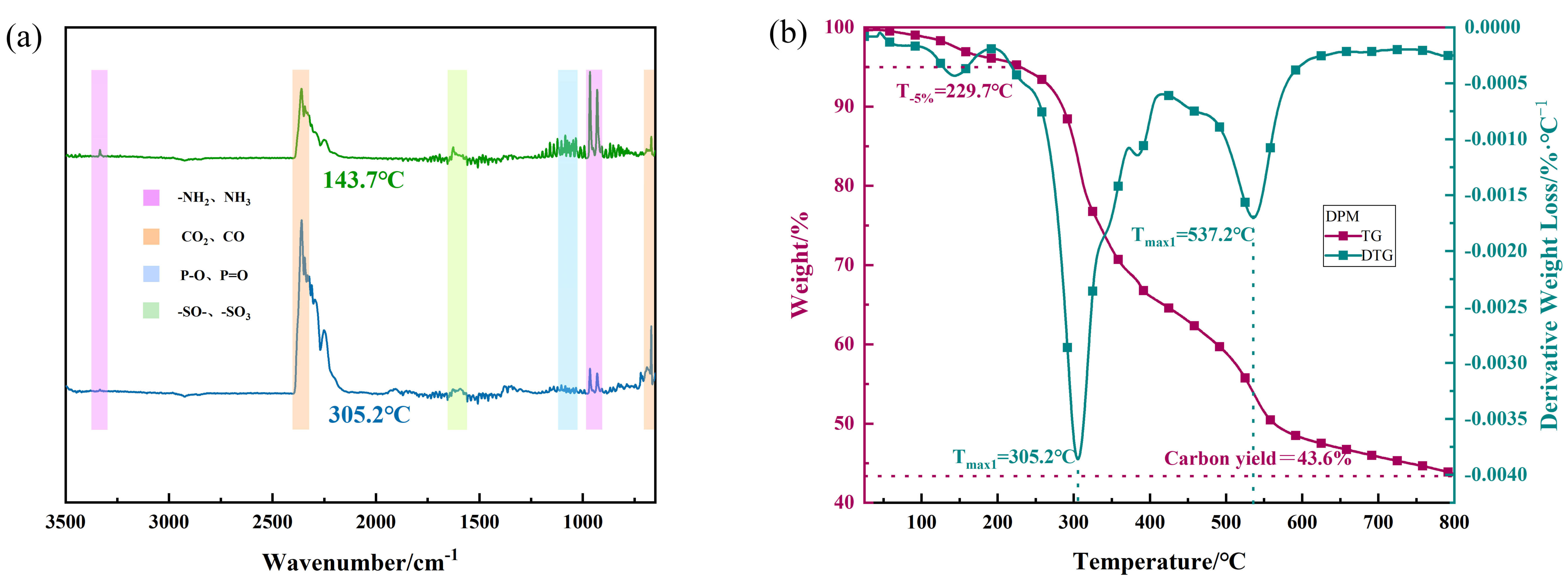
3.1. Characterization of DPM
3.2. Flame-Retardancy Analysis
3.3. Investigation of Combustion Behavior
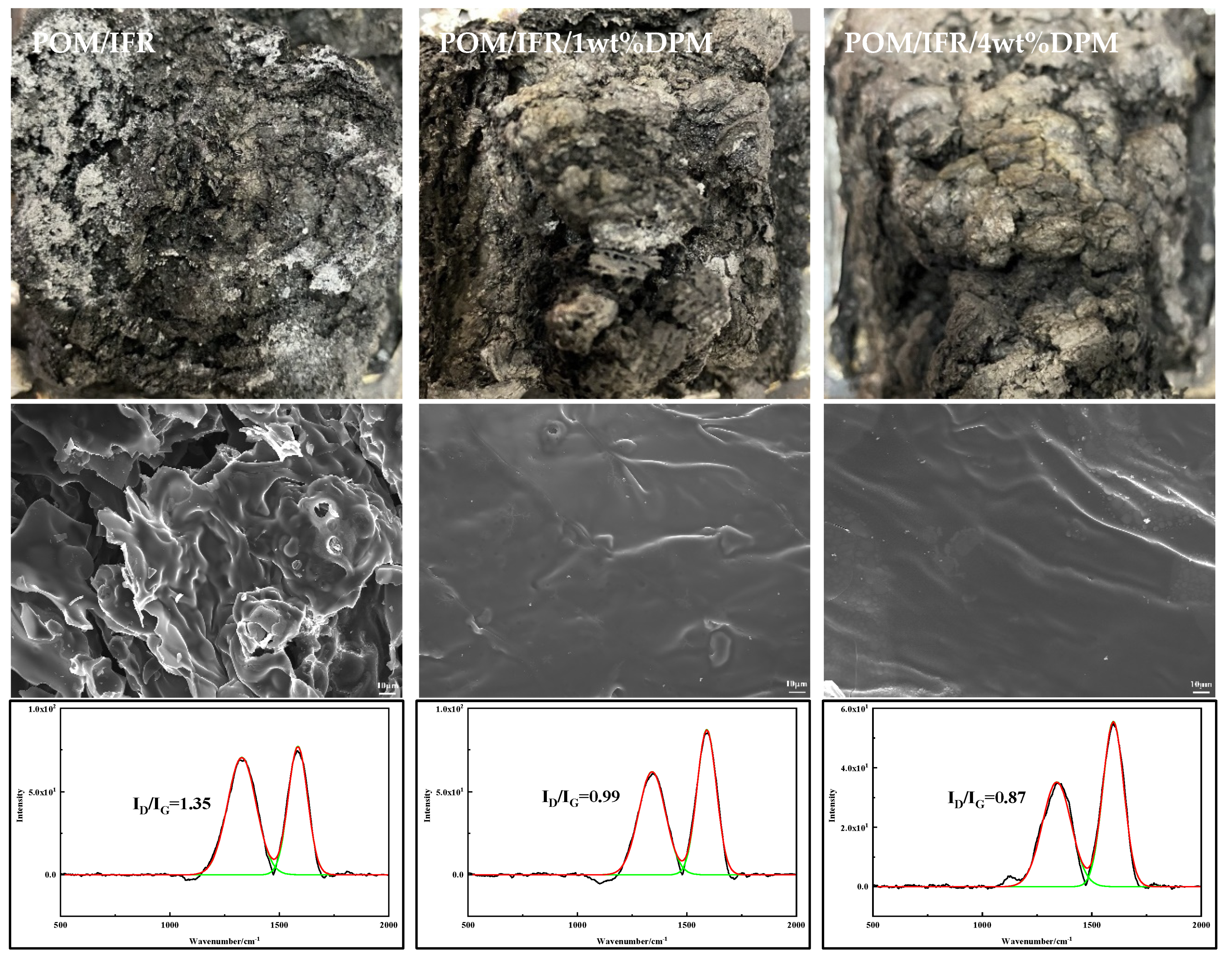
3.4. Carbon Residue Analysis
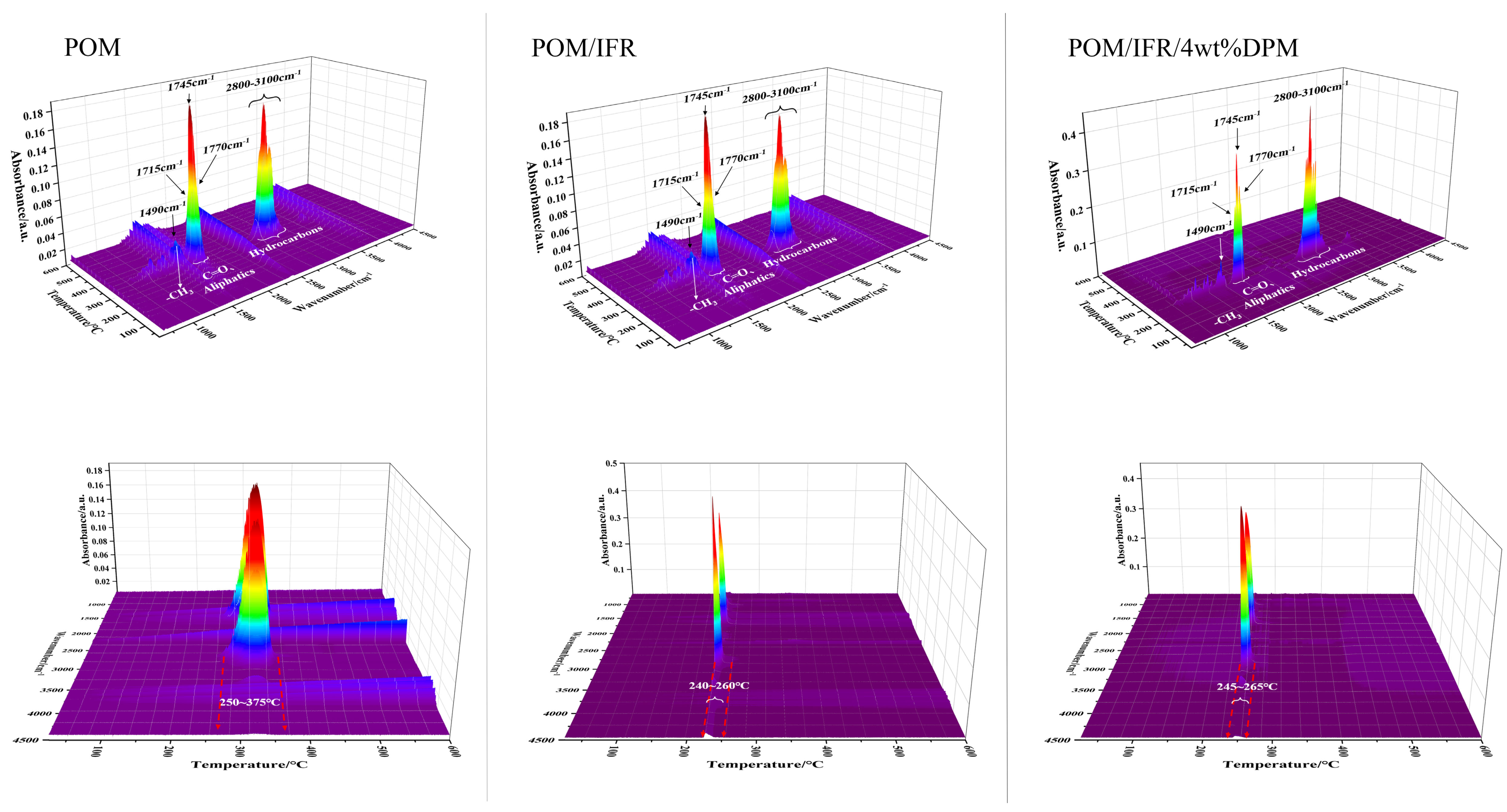
3.5. Thermogravimetric Infrared Analysis
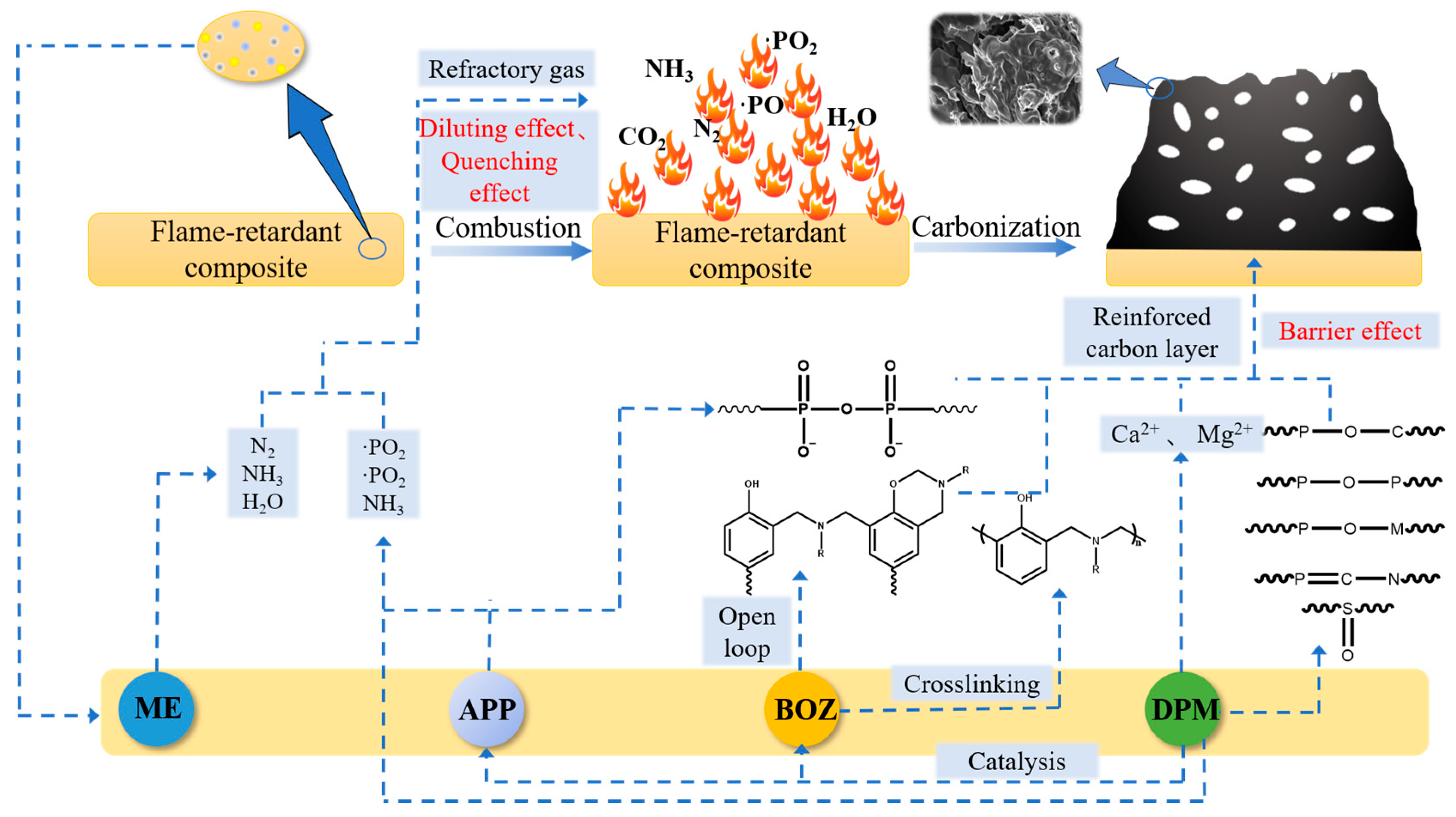
3.6. Flame-Retardant Mechanism Analysis
3.7. Mechanical Property Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, S.Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Z.; Qi, W. Effects of magnesium hydroxide and its synergistic systems on the flame retardance of polyformaldehyde. J. Appl. Polym. Sci. 2012, 125, 968–974. [Google Scholar]
- Andrzejewski, J.; Skórczewska, K.; Kloziński, A. Improving the toughness and thermal resistance of polyoxymethylene/poly(lactic acid) blends: Evaluation of structure-properties correlation for reactive processing. Polymers 2020, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Liu, Y.; Chen, N.; Feng, W.; Liu, B.; Xu, Y.; Li, J.; Ding, F.; Fang, X. Influence of modified ammonium polyphosphate on the fire behavior and mechanical properties of polyformaldehyde. J. Appl. Polym. Sci. 2021, 138, 50156. [Google Scholar] [CrossRef]
- Zhao, W.; Li, B.; Xu, M.; Yang, K.; Lin, L. Novel intumescent flame retardants: Synthesis and application in polycarbonate. Fire Mater. 2013, 37, 530–546. [Google Scholar] [CrossRef]
- Hu, X.M.; Wang, D.M. Enhanced fire behavior of rigid polyurethane foam by intumescent flame retardants. J. Appl. Polym. Sci. 2013, 129, 238–246. [Google Scholar] [CrossRef]
- Su, X.; Yi, Y.; Tao, J.; Qi, H. Synergistic effect of zinc hydroxystannate with intumescent flame retardants on fire retardancy and thermal behavior of polypropylene. Polym. Degrad. Stab. 2012, 97, 2128–2135. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Zhao, S.; Huang, R.Y.; Kong, X.J. A novel intumescent flame retardant imparts high flame retardancy to epoxy resin. Polym. Adv. Technol. 2020, 31, 932–940. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, L.; Fan, Z.; Yu, Y.; Liu, R. Bio-based coating of phytic acid, chitosan, and biochar for flame retardant cotton fabrics. Polym. Degrad. Stab. 2022, 199, 109898. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, B.; Shang, S.; Zhang, H.; Shi, Y.; Yu, B.; Qi, C.; Dong, H.; Chen, X.; Yang, X. Surface modification of ammonium polyphosphate by supramolecular assembly for enhancing fire safety properties of polypropylene. Compos. Part B Eng. 2020, 181, 107588. [Google Scholar] [CrossRef]
- Xiao, D.; Li, Z.; Gohs, U.; Wagenknecht, U.; Voit, B.; Wang, D.Y. Functionalized allylamine polyphosphate as a novel multifunctional highly efficient fire retardant for polypropylene. Polym. Chem. 2017, 8, 6309–6318. [Google Scholar] [CrossRef]
- Fan, M.; Feng, N.; Zhang, Y.; Wang, Z.; Qu, M.; Zhang, G. Synergistic effects of aluminium hypophosphite on the flame retardancy and thermal degradation behaviours of a novel intumescent flame retardant thermoplastic vulcanisate composite. Plast. Rubber Compos. 2019, 48, 270–280. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Chevali, V.S.; Wang, H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Zhou, Q.; Gong, K.; Zhou, K.; Zhao, S.; Shi, C. Synergistic effect between phosphorus tailings and aluminum hypophosphite in flame-retardant thermoplastic polyurethane composites. Polym. Adv. Technol. 2019, 30, 2480–2487. [Google Scholar] [CrossRef]
- Chen, K.; Cheng, J.; Wu, B.; Liu, C.; Guo, J. Synergistic effects of strontium carbonate on a novel intumescent flame-retardant polypropylene system. Polym. Adv. Technol. 2021, 32, 3018–3027. [Google Scholar] [CrossRef]
- Sheng, Y.; Chen, Y.; Bai, Y. Catalytically synergistic effects of novel LaMnO3 composite metal oxide in intumescent flame-retardant polypropylene system. Polym. Compos. 2014, 35, 2390–2400. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, X.; Ma, S.; Xiong, Z.; Zhang, C.; Jiang, Y.; Zhu, J. Syntheses of metallic cyclodextrins and their use as synergists in a poly (vinyl alcohol)/intumescent flame retardant system. Ind. Eng. Chem. Res. 2013, 52, 2784–2792. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H.; Hu, X.; Liu, Y.; Zhang, S.; Xie, C. Self-assembled bio-derived microporous nanosheet from phytic acid as efficient intumescent flame retardant for polylactide. Polym. Degrad. Stab. 2021, 191, 109664. [Google Scholar] [CrossRef]
- Gong, W.; Fan, M.; Luo, J.; Liang, J.; Meng, X. Effect of nickel phytate on flame retardancy of intumescent flame retardant polylactic acid. Polym. Adv. Technol. 2021, 32, 1548–1559. [Google Scholar] [CrossRef]
- Zhan, Y.; Yuan, B.; Shang, S. Synergistic effect of layered melamine-phytate and intumescent flame retardant on enhancing fire safety of polypropylene. J. Therm. Anal. Calorim. 2022, 147, 285–295. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Lu, S.; Wang, Z.; Li, J.; Liu, B.; Fang, X.; Ding, T.; Xu, Y. Synergistic Flame Retardant Properties of Polyoxymethylene with Surface Modified Intumescent Flame Retardant and Calcium Carbonate. Polymers 2023, 15, 537. [Google Scholar] [CrossRef]
- Lu, Z.; Feng, W.; Kang, X.; Wang, J.; Xu, H.; Li, J.; Wang, W.; Liu, B.; Fang, X. Flame retardant effect and mechanism of benzoxazine as synergist in intumescent flame-retardant polyoxymethylene. Polym. Adv. Technol. 2020, 31, 2512–2525. [Google Scholar] [CrossRef]
- Standardization Administration of the People’s Republic of China. GB/T 2408-2008; Plastics—Determination of Burning Characteristics—Horizontal and Vertical Test. Standards Press of China: Beijing, China, 2008; pp. 8–14.
- Standardization Administration of the People’s Republic of China. GB/T 2406.2-2009; Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. Standards Press of China: Beijing, China, 2009; pp. 6–15.
- International Organization for Standardization. ISO5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate. International Organization for Standardization: Geneva, Switzerland, 2015; pp. 3–31.
- Standardization Administration of the People’s Republic of China. GB/T 1040.1-2006; Plastics—Determination of Tensile Properties—Part 1: General Principles. Standards Press of China: Beijing, China, 2006; pp. 8–24.
- Standardization Administration of the People’s Republic of China. GB/T 9341-2008; Plastics—Determination of Flexural Properties. Standards Press of China: Beijing, China, 2008; pp. 8–14.
- Standardization Administration of the People’s Republic of China. GB/T 1043-2008; Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. Standards Press of China: Beijing, China, 2008; pp. 8–14.
- Yang, W.; Tawiah, B.; Yu, C.; Qian, Y.F.; Wang, L.L.; Yuen, A.C.Y.; Zhu, S.E.; Hu, E.Z.; Chen, T.B.Y.; Yu, B.; et al. Manufacturing, mechanical and flame retardant properties of poly(lactic acid) biocomposites based on calcium magnesium phytate and carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2018, 110, 227–236. [Google Scholar] [CrossRef]
- Li, W.X.; Zhang, H.J.; Hu, X.P.; Yang, W.X.; Cheng, Z.; Xie, C.Q. Highly efficient replacement of traditional intumescent flame retardants in polypropylene by manganese ions doped melamine phytate nanosheets. J. Hazard. Mater. 2020, 398, 123001. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Lu, Z.; Feng, W.; Wang, J.; Fang, X.; Xu, Y.; Wang, Y.; Liu, B.; Ding, T.; Ma, Y.; et al. A novel phosphorous and silicon-containing benzoxazine: Highly efficient multifunctional flame-retardant synergist for polyoxymethylene. Adv. Compos. Hybrid Mater. 2021, 4, 127–137. [Google Scholar] [CrossRef]
- Mun, S.Y.; Lee, S.Y.; Lim, H.M. Flame Retardant Properties of Basalt Fiber Reinforced Epoxy Composite with Inorganic Fillers. Compos. Res. 2019, 32, 368–374. [Google Scholar]
- Leng, Y.M.; Zhao, X.; Fu, T.; Wang, X.L.; Wang, Y.Z. Bio-based flame-retardant and smoke-suppressing wood plastic composites enabled by phytic acid tyramine salt. ACS Sustain. Chem. Eng. 2022, 10, 5055–5066. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Cui, Y.; Zhang, T.; Ji, P. Influence of the Chemical Structure on the Flame Retardant Mechanism and Mechanical Properties of Flame-Retardant Epoxy Resin Thermosets. Macromol. Mater. Eng. 2022, 307, 2200169. [Google Scholar] [CrossRef]





| Component | Weight/% | Element | Atomic Ratio (Relative to the P Element) * |
|---|---|---|---|
| P2O5 | 55.09 | P | 1.00 |
| SO3 | 17.88 | S | 0.29 |
| CaO | 17.64 | Ca | 0.41 |
| MgO | 9.10 | Mg | 0.29 |
| Cl | 0.17 | -- | -- |
| Rest | 0.16 | -- | -- |
| Sample | UL-94 (3.2 mm) | LOI/% | ||||
|---|---|---|---|---|---|---|
| t1/s | t2/s | TAll/s | Dripping | Grade | ||
| POM | -- | -- | -- | Yes | NR | 15 |
| POM/IFR | 12.4 | 221.3 | 233.7 | No | V-1 | 48.5 |
| POM/IFR/1 wt%DPM | 5 | 135.2 | 140.2 | No | V-1 | 53.2 |
| POM/IFR/2 wt%DPM | 0 | 75.2 | 75.2 | No | V-1 | 54.3 |
| POM/IFR/3 wt%DPM | 0 | 70.5 | 70.5 | No | V-1 | 55.5 |
| POM/IFR/4 wt%DPM | 0 | 36.2 | 36.2 | No | V-0 | 59.1 |
| POM/IFR/5 wt%DPM | 0 | 41.6 | 41.6 | No | V-0 | 57.6 |
| POM/IFR/6 wt%DPM | 0 | 110.2 | 110.2 | No | V-1 | 57.3 |
| POM/IFR/7 wt%DPM | 0 | 120.3 | 120.3 | No | V-1 | 56.9 |
| POM/IFR/8 wt%DPM | 0 | 123.2 | 123.2 | No | V-1 | 56.3 |
| Sample | POM | POM/IFR | POM/IFR/1 wt%DPM | POM/IFR/4 wt%DPM |
|---|---|---|---|---|
| TTI (S) | 43 | 23 | 22 | 23 |
| PkHRR (kW/m2) | 335.55 | 163.17 | 132.79 | 129.17 |
| PFI | 7.8 | 7.1 | 6.0 | 5.6 |
| AvHRR (kW/m2) | 233.15 | 42.49 | 41.22 | 32.71 |
| THR (MJ/m2) | 133.08 | 89.47 | 95.45 | 64.90 |
| MeanEHC (MJ/kg) | 14.54 | 10.51 | 11.45 | 8.33 |
| SEA (m2/kg) | 0.00 | 86.30 | 159.40 | 109.31 |
| AvMLR (g/(m2·s)) | 19.71 | 4.13 | 3.87 | 3.7 |
| TSP (m2) | 0.00 | 7.57 | 9.2 | 6.8 |
| Residue (%) | 0.0 | 11.3 | 15.0 | 15.5 |
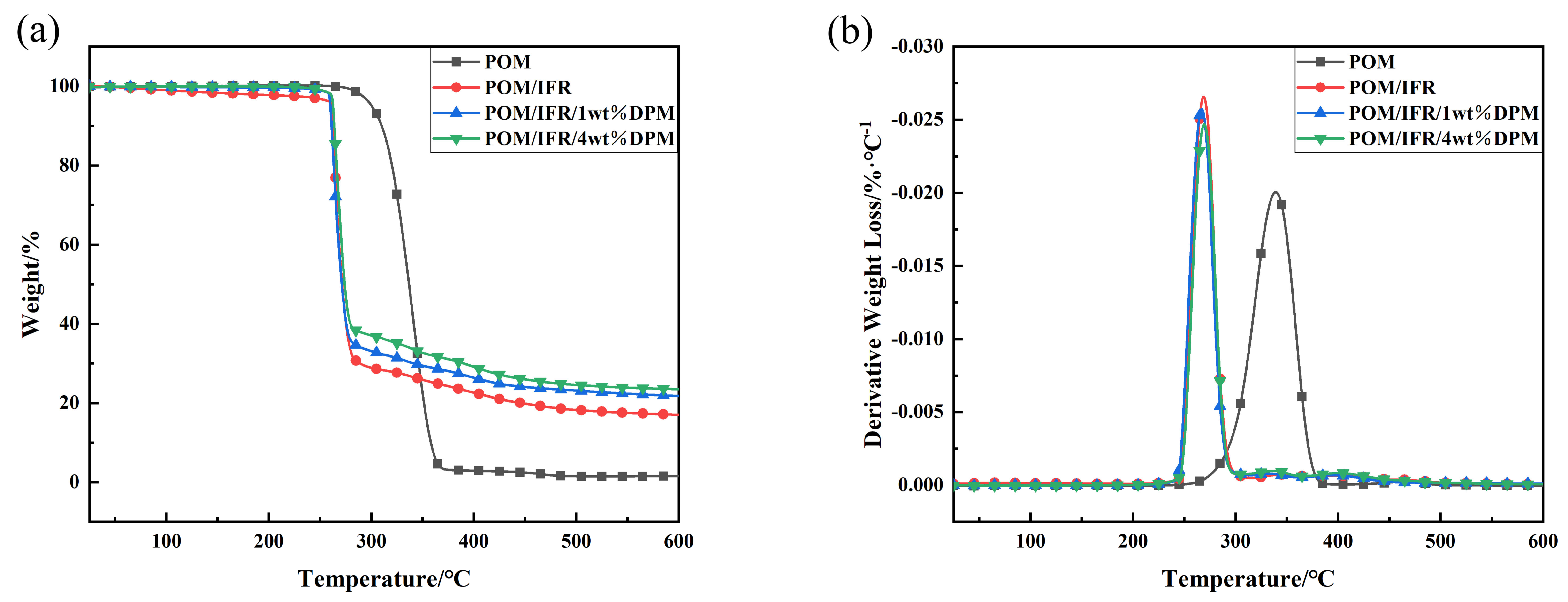
| Sample | T−5%/°C | T−10%/°C | T−50%/°C | Tmax/°C | Actual Carbon Residue (600 °C)/% |
|---|---|---|---|---|---|
| POM | 300.7 | 310.1 | 336.8 | 338.8 | 0.0 |
| POM/IFR | 261.7 | 263.1 | 271.5 | 269.1 | 17.0 |
| POM/IFR/1 wt%DPM | 260.1 | 261.2 | 271.1 | 266.8 | 21.8 (17.3 *) |
| POM/IFR/4 wt%DPM | 262.6 | 263.9 | 274.1 | 268.8 | 23.5 (18.6 *) |
| Sample | Notched Impact Strength (kJ/m2) | Bending Modulus (MPa) | Bending Strength (MPa) | Tensile Strength (MPa) |
|---|---|---|---|---|
| POM | 5.62 ± 0.06 | 2172.29 ± 22.57 | 70.37 ± 0.13 | 63.3.15 |
| POM/IFR | 2.78 ± 0.56 | 2707.04 ± 16.36 | 48.04 ± 0.34 | 36.20 ± 0.59 |
| POM/IFR/1 wt%DPM | 3.13 ± 0.21 | 2882.26 ± 17.23 | 48.25 ± 0.52 | 37.25 ± 0.32 |
| POM/IFR/2 wt% DPM | 3.25 ± 0.17 | 3058.51 ± 32.30 | 50.04 ± 0.63 | 37.87 ± 0.41 |
| POM/IFR/3 wt% DPM | 3.22 ± 0.05 | 2998.92 ± 42.26 | 50.32 ± 0.77 | 38.13 ± 0.33 |
| POM/IFR/4 wt% DPM | 3.28 ± 0.10 | 3067.59 ± 17.22 | 51.24 ± 0.11 | 38.68 ± 0.22 |
| POM/IFR/5 wt% DPM | 3.31 ± 0.08 | 3079.59 ± 25.21 | 52.39 ± 0.21 | 39.98 ± 0.15 |
| POM/IFR/6 wt% DPM | 3.32 ± 0.06 | 2968.26 ± 32.18 | 53.39 ± 0.54 | 40.03 ± 0.12 |
| POM/IFR/7 wt% DPM | 3.27 ± 0.12 | 3033.28 ± 32.09 | 53.44 ± 0.82 | 40.55 ± 0.52 |
| POM/IFR/8 wt% DPM | 3.28 ± 0.03 | 3200.23 ± 24.86 | 53.84 ± 1.85 | 41.00 ± 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Chen, X.; Zhang, B.; Lu, Z.; Jiang, W.; Fang, X.; Li, J.; Liu, B.; Ding, T.; Xu, Y. Synergistic Modification of Polyformaldehyde by Biobased Calcium Magnesium Bi-Ionic Melamine Phytate with Intumescent Flame Retardant. Polymers 2024, 16, 614. https://doi.org/10.3390/polym16050614
Lu S, Chen X, Zhang B, Lu Z, Jiang W, Fang X, Li J, Liu B, Ding T, Xu Y. Synergistic Modification of Polyformaldehyde by Biobased Calcium Magnesium Bi-Ionic Melamine Phytate with Intumescent Flame Retardant. Polymers. 2024; 16(5):614. https://doi.org/10.3390/polym16050614
Chicago/Turabian StyleLu, Shike, Xueting Chen, Bin Zhang, Zhehong Lu, Wei Jiang, Xiaomin Fang, Jiantong Li, Baoying Liu, Tao Ding, and Yuanqing Xu. 2024. "Synergistic Modification of Polyformaldehyde by Biobased Calcium Magnesium Bi-Ionic Melamine Phytate with Intumescent Flame Retardant" Polymers 16, no. 5: 614. https://doi.org/10.3390/polym16050614
APA StyleLu, S., Chen, X., Zhang, B., Lu, Z., Jiang, W., Fang, X., Li, J., Liu, B., Ding, T., & Xu, Y. (2024). Synergistic Modification of Polyformaldehyde by Biobased Calcium Magnesium Bi-Ionic Melamine Phytate with Intumescent Flame Retardant. Polymers, 16(5), 614. https://doi.org/10.3390/polym16050614









