Abstract
Infections of agricultural crops caused by pathogen ic fungi are among the most widespread and harmful, as they not only reduce the quantity of the harvest but also significantly deteriorate its quality. This study aims to develop unique seed-coating formulations incorporating biopolymers (polyhydroxyalkanoate and pullulan) and beneficial microorganisms for plant protection against phytopathogens. A microbial association of biocompatible endophytic bacteria has been created, including Pseudomonas flavescens D5, Bacillus aerophilus A2, Serratia proteamaculans B5, and Pseudomonas putida D7. These strains exhibited agronomically valuable properties: synthesis of the phytohormone IAA (from 45.2 to 69.2 µg mL−1), antagonistic activity against Fusarium oxysporum and Fusarium solani (growth inhibition zones from 1.8 to 3.0 cm), halotolerance (5–15% NaCl), and PHA production (2.77–4.54 g L−1). A pullulan synthesized by Aureobasidium pullulans C7 showed a low viscosity rate (from 395 Pa·s to 598 Pa·s) depending on the concentration of polysaccharide solutions. Therefore, at 8.0%, w/v concentration, viscosity virtually remained unchanged with increasing shear rate, indicating that it exhibits Newtonian flow behavior. The effectiveness of various antifungal seed coating formulations has been demonstrated to enhance the tolerance of barley plants to phytopathogens.
1. Introduction
According to the estimates of the Food and Agriculture Organization (FAO), 20–40% of global crop losses are related to plant diseases, with 42% of those attributed to infections caused by pathogenic fungi. Fusarium fungi are common pathogens of cereals, including barley, and can cause diseases such as Fusarium head blight, seedling blight, root rot, and Fusarium crown rot throughout their life cycle. In addition, many species of Fusarium are capable of producing mycotoxins (deoxynivalenol, nivalenol, HT2/T2, zearalenone), even in some cases in the absence of severe disease symptoms [1].
The use of microorganisms and their metabolites as bio-control agents is one of the most promising methods for the effective and safe protection of plants. The widespread use of antibiotics in the food industry, agriculture, and medicine leads to an increase in antibiotic resistance of pathogenic microorganisms. In this regard, endophytes have advantages over other biocontrol agents, as they are producers of many biologically active metabolites, such as phenolic acids, alkaloids, quinones, steroids, saponins, tannins, and terpenoids. Microbiological strategies for protecting agricultural crops are based on the plant growth-promoting properties of these strains.
Biopolymers can also be used in the development of plant protection products against phytopathogens. They are non-toxic and biodegradable and can be obtained from renewable sources, making them suitable for use in organic farming. Additionally, they can interact with many hydrophobic and hydrophilic compounds in more complex formulations. Biopolymers play a protective role for plants against pathogenic fungi through several mechanisms [2]. Polymers can directly interact with fungi, suppressing spore germination and mycelium growth, as demonstrated, for example, with chitosan [3,4]. They can act as effective elicitors, inducing the plant immune system to fight pathogens [5]. They can also be used as carriers for active ingredients with controlled release [2].
Among biopolymers, the most used are carboxymethyl cellulose, chitosan, xanthan gum, gum arabic, polyvinyl alcohol, starch, gelatin, polyacrylamide, and alginates. These polymers are used for treating the seeds of turnips, tomatoes, chickpeas, corn, beans, eggplants, okra, chili peppers, guar, pumpkins, cucumbers, lupine, clover, soybeans, and wheat [6,7,8].
One of the promising microbial polymers for seed coating is pullulan. It is a water-soluble, low-viscosity polysaccharide that has the property of biodegrading under the action of microorganisms. Pullulan has oxygen barrier properties, excellent moisture retention, and also prevents the growth of pathogens. Additionally, pullulan is a prebiotic—a substance that stimulates the growth and development of microorganisms [9,10].
Polyhydroxyalkanoates (PHAs) are of great interest as well, as they are non-toxic, biodegradable, and biocompatible polymers [11]. Previous studies have reported the use of PHA with the addition of polycaprolactone to obtain biodegradable films for rice seed germination [12]. Furthermore, some PHAs exhibit antagonistic activity against bacteria [13,14]. In our previous studies, we demonstrated that PHA produced by the strain Pseudomonas fluorescens D5 has pronounced antifungal activity against Fusarium graminearum, Fusarium solani, Fusarium oxysporum [15], and Penicillium expansum [16].
However, there is no information about the use of a mixture of PHA with pullulan in the composition of seed coatings. Therefore, this work is aimed at developing unique compositions for seed treatment, including effective microorganisms and biopolymers (PHA and pullulan) as seed coating agents to enhance the microbially induced tolerance of plants to phytopathogenic fungi.
The main objectives of the present study are as follows: (1) formation of a microbial association with agronomically valuable properties, (2) investigation of the rheological properties of polysaccharide solutions, and (3) study of various seed coating types for barley tolerance to Fusarium.
This study is significant for a better understanding of the effect of seed coating on the microbially induced tolerance of barley to phytopathogens. In this research, a new opportunity is proposed for the use of pullulan as a seed coating agent, expanding the areas of application for microbial polymers. The excellent gelling and thickening properties, as well as the biodegradability and non-toxicity of the investigated biopolymers, pullulan, and PHA, make them promising for use in antifungal formulations for seed treatments.
2. Materials and Methods
The following strains were used in the present study:
- Bacillus aerophilus A2 (accession number OQ569360) isolated from leaves of peppermint (Mentha piperita);
- Pseudomonas flavescens D5 (accession number OP642636) isolated from flowers of common chicory (Cichórium intybus);
- Serratia proteamaculans B5 (accession number OR858823) isolated from the leaves of Iris;
- Bacillus simplex B9 (accession number OR864231) isolated from the roots of wormwood (Artemisia absinthium);
- Pseudomonas putida D7 (accession number OR863903) isolated from the roots of Echinacea (Echinacea purpurea);
- Aureobasidium pullulans C7 (accession number OR864236) isolated from dark chestnut soil;
- Bacillus thuringiensis C8 (accession number OR858828) isolated from the surface of apples.
2.1. Production of IAA
To determine the amount of indole-3-acetic acid (IAA) produced by microorganisms, a colorimetric method was employed. Isolates were cultivated in nutrient broth for 48 h at 28 °C. After incubation, the culture was centrifuged at 6000× g for 20 min. The supernatant, with a volume of 1 mL, was mixed with 2 mL of Salkowski reagent. The optical density was measured at 530 nm. The concentration of IAA was expressed in μg mL−1 [17].
2.2. The Antifungal Properties of Microorganisms
The antifungal activity was determined using the agar disk diffusion method. Bacteria were cultured for 48 h in nutrient broth at 28 °C with aeration. Bacterial cultures were spread on the surface of nutrient agar as a continuous lawn in a volume of 100 µL, and after 48 h of growth, 5 mm diameter disks were cut. Disks with bacterial culture were placed on Petri dishes previously inoculated with a continuous lawn of phytopathogenic test cultures (Fusarium solani, Fusarium oxysporum) at a concentration of 106 spores mL−1. A nutrient agar disk served as a control. The Petri dishes were incubated at 28 °C for 72 h. The antifungal activity was assessed by measuring the diameter of the growth inhibition zone of the tested phytopathogens [18].
2.3. Determination of Microbial Halotolerance
To assess the halotolerance of bacteria, nutrient agar medium supplemented with NaCl at concentrations of 5%, 10%, 15%, and 25% was used. Microorganisms were inoculated using the streak method. Strains capable of cultivation at different salt concentrations were selected based on the research results.
2.4. PHA Production Assay
Strains producing PHA were cultivated in liquid MSM medium at 28 °C for 48 h at 150 rpm. The medium composition (g·L−1) was as follows: MgSO4·7H2O—0.1; KH2PO4—0.68; K2HPO4—1.73; NaCl—4.0; NH4NO3—1.0; FeSO4·7H2O—0.03; CaCl2·2H2O—0.02; and glucose—5.0 [19]. Subsequently, the suspension was centrifuged at 6000× g for 10 min, the supernatant was decanted, and PHA was extracted from the residue. Sodium hypochlorite and hot chloroform were added to the residue at a 1:1 ratio, and the mixture was kept at 30 °C for 1 h. The suspension was then centrifuged at 6000× g for 15 min, and the upper and middle layers were removed. The residue was precipitated with a 1:1 mixture of ethanol and acetone, dried at 35 °C, and weighed [20].
2.5. Microbe–Microbe In Vitro Compatibility Test
Five bacterial strains (Pseudomonas flavescens D5, Bacillus aerophilus A2, Serratia myotis B5, Bacillus simplex B9, and Pseudomonas putida D7) were used for in vitro compatibility test. The agar diffusion method was selected to determine biocompatibility. Bacterial strains were separately grown on nutrient agar at 28 °C for 24 h. Then, colonies were transferred to nutrient broth and incubated overnight at 28 °C at 160 rpm [21].
A volume of 100 µL of the test microorganism (0.5 McFarland) was spread on the surface of nutrient agar. Sterile filter paper disks (d = 5 mm) were inoculated with the overnight bacterial culture adjusted to a concentration of 0.5 McFarland. Inoculated disks were placed on Petri dishes (4 disks per each) with the test microorganism, and each was incubated in darkness at 28 °C for 4 days. Experiments were conducted with three replicates.
2.6. Extraction of Polysaccharide
For the extraction of polysaccharides, the 4-day-old culture of A pullulans C7 and 3-day-old culture of B. thuringiensis C8 were centrifuged for 15 min at 10,000× g. The fungal polysaccharide was precipitated with a double volume of 96% ethanol and the bacterial polysaccharide with a triple volume of alcohol. The yield coefficient for biomass (P/X) was calculated as a ratio of production of EPS to the dry biomass and expressed in percent. The yield coefficient for substrate (P/S) was calculated as a ratio of production of EPS to the utilized glucose and expressed in percent [22].
2.7. Measurement of Dynamic Viscosity
The dynamic viscosity of the polymer’s solution with different concentrations—2, 4, 6, 8, 10, and 12% w/v—was performed using a rotational Ametek Brookfield DVPlus viscometer with a ULA spindle, at different shear rates, ranging from 0.1 to 500 s−1 at 25 °C [23]. The viscosimetric analyses of the samples were performed at 25 °C.
2.8. Development of Various Options for Processing Barley Seeds
Various antifungal formulations for seed treatments were developed, including (1) bacterial strain suspension, (2) polymer mixture, and (3) bacterial strain suspension + polymer mixture.
Bacterial strains were separately cultured in nutrient broth for 48 h at 180 rpm and 28 °C. The cultures were then centrifuged at 6000× g for 10 min and resuspended in a phosphate-buffered saline (PBS (g L−1), 8.0 NaCl, 0.2 KCl, 1.44 Na2HPO4, and 0.24 KH2PO4). The optical density of each bacterial suspension was adjusted to 108 CFU mL−1 Strain suspensions were mixed in equal proportions.
The polymer mixture was prepared using PHA at a concentration of 0.05% and pullulan at a concentration of 2% (wt./vol.), incorporated into phosphate-buffered saline.
For coating of seeds simultaneously in a bacterial suspension and a polymer blend, PHA at a concentration of 0.05% and pullulan at a concentration of 2% (wt./vol.) were introduced into a mixture of bacterial suspensions.
2.9. Pot Experiments
To conduct the research, barley seeds sterilized in a 5% sodium hypochlorite solution were used. Subsequently, the seeds were rinsed with sterile water and sown on nutrient agar medium [22] to ensure the absence of bacteria on the seed surface.
Phytopathogenic load conditions were simulated by introducing a suspension of the phytopathogenic fungus F. oxysporum into the soil at a titer of 108 spores mL−1, with 2 mL of the suspension per 100 g of soil.
Experiment options:
- T1—Untreated seeds;
- T2—Untreated seeds + Fusarium oxysporum;
- T3—Seed treatment with bacterial suspension + Fusarium oxysporum;
- T4—Seed coating with polymeric mixture + Fusarium oxysporum;
- T5—Simultaneous seed coating with bacterial suspension and polymeric mixture of Fusarium oxysporum.
Pre-sterilized seeds were immersed in various antifungal formulations, followed by transferring the seeds to 0.1 M CaCl2. After coating, the seeds were dried for 20 min before planting.
In each pot containing 300 g of sterile soil, 10 barley seeds were planted. The experiment was conducted under sterile conditions with three replicates. The plants were grown for 12 days.
2.10. Determination of Free Proline Concentration
The content of free proline was determined using a non-heated acidic ninhydrin reagent prepared as follows: (1.25 g ninhydrin + 30 mL glacial acetic acid + 20 mL 6 M H3PO4). A portion of fresh plant tissue from a leaf blade (200 mg) was homogenized in 10 mL of a 3% aqueous solution of sulfosalicylic acid and left for 1 h in a water bath at 100 °C.
Subsequently, 1.5 mL of glacial acetic acid, 1.5 mL of ninhydrin reagent, and 1.5 mL of the prepared extract were poured into a clean test tube. The samples were incubated for 1 h in a water bath at 100 °C and then rapidly cooled to room temperature. After artificial cooling (using cold water or ice), the optical density of the reaction products was measured at a wavelength of 520 nm using a spectrophotometer. Proline content values were calculated using a calibration curve, constructed using chemically pure proline [24].
2.11. Determination of Chlorophyll Concentration
To obtain an ethanolic extract, 2 g of leaves were sliced and thoroughly ground in a mortar, gradually adding 96% ethanol in small portions (a total of 10 mL). The extract was centrifuged for 15 min at 6000× g [25]. Photocolorimetry was carried out using a spectrophotometer at wavelengths of 665 and 649 nm in a cuvette with an optical path length of 1 cm. The comparison cuvette was filled with 96% ethanol. The pigment concentration was determined using the following formula:
2.12. Preparation of the Extract for the Determination of Antioxidant Enzymes
Antioxidant enzyme activity was determined spectrophotometrically based on the rate of NADH oxidation using the method [26]. For this purpose, plant material (1.5–2 g) was homogenized with an extracting medium containing 50 mM K-phosphate buffer (pH 7.5), 1 mM EDTA, 0.3%, 1 mM ascorbic acid, filtered and centrifuged (15 min, 8000× g). The obtained supernatant was used to determine the activity of the enzymes.
2.12.1. Investigation of Catalase Activity
Catalase activity was determined using H2O2 according to the method [26]. The reaction mixture consisted of 15 mM H2O2, 100 mM K-phosphate buffer (pH 7.0), and 0.1 mL of the sample. Changes in optical density were measured at 240 nm, and activity was calculated using the extinction coefficient ε = 0.03 mM−1 cm−1. All experiments were conducted in triplicate and expressed in units per milligram of protein.
2.12.2. Investigation of Ascorbate Peroxidase Activity
The activity of ascorbate peroxidase was determined in a medium with the following composition: 50 mM K-phosphate buffer pH 7.0, 0.5 mM ascorbate, and 0.2 mM H2O2. The reaction was initiated by adding 0.1 mL of the sample [27]. Changes in optical density were measured at 290 nm. Enzyme activity was calculated using the extinction coefficient ε = 2.8 mM−1 cm−1 and expressed as 1 mmol of ascorbate min−1 per mg protein.
2.12.3. Investigation of Guaiacol Peroxidase Activity
Guaiacol peroxidase activity was determined using a spectrophotometric method, considering absorption due to guaiacol oxidation [28]. The reaction mixture consisted of 50 mM phosphate buffer (pH 7), 9 mM guaiacol, 10 mM H2O2, and 0.2 mL of the sample. Optical density was measured at 470 nm for 1 min, and enzyme activity was calculated using the extinction coefficient ε = 26.6 mM−1 cm−1, expressed as 1 mmol of ascorbate min−1 per mg protein.
2.13. Statistical Analysis
All the data are presented as the mean ± standard deviation (SD) of three replicates. The data were processed by the standard methods of one-way analysis of variance (ANOVA) using the software Statistica version 10.0 (TIBCO Software Inc., Palo Alto, CA, USA). Tukey’s honestly significant difference (HSD) test (p < 0.05) was performed for multiple comparisons to estimate significant differences between means.
3. Results
3.1. Characterization of the Biological Activity of Endophytic Bacteria
For the application of microorganisms both to enhance plant growth and to protect them from adverse factors, a crucial step is the selection of strains possessing a set of beneficial properties. In the first stage of the research, the agronomically valuable properties of five endophytic bacterial strains were investigated (Table 1).

Table 1.
Plant growth-promoting properties of bacterial strains.
One of the well-known mechanisms for improving and regulating plant growth by microorganisms is their ability to synthesize various phytohormones. The stimulation of plant growth resulting from the application of microorganisms is predominantly associated with their ability to synthesize auxins, primarily IAA [29]. All examined bacteria demonstrated the ability to produce IAA (Table 1), except for the Bacillus simplex B9 strain. The highest concentration of IAA was found in the Pseudomonas putida D7 strain (Table 1). The amount of produced IAA varied between 45.2 and 69.2 μg mL−1 depending on the strain, which is similar to or significantly higher than that observed in other endophytic bacterial strains [30,31].
The next criterion for assessing the biological activity of the strains was the evaluation of their resistance to adverse environmental factors.
Among the adverse factors of biotic nature, phytopathogenic microflora plays a key role. Infections of agricultural crops caused by pathogenic fungi are among the most widespread and harmful, as they not only reduce the quantity of the harvest but also significantly degrade its quality due to the accumulation of mycotoxins [32]. One of the positive effects of bacteria on crops is their ability to protect plants from phytopathogens through direct and indirect mechanisms [33].
The study of the antagonistic activity of bacterial strains against Fusarium solani and Fusarium oxysporum showed that three out of five strains inhibit the growth of phytopathogens (Figure 1). The zones of growth suppression ranged from 1.8 to 3.0 cm (Table 1).
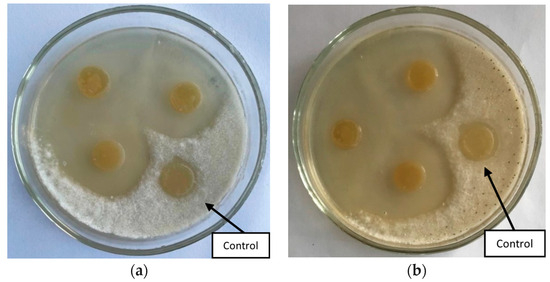
Figure 1.
Inhibition of the growth of the phytopathogenic fungus Fusarium solani by strains (a) Bacillus simplex B9 and (b) Serratia proteamaculans B5.
Salinization of soils is one of the most crucial abiotic stress factors that negatively impact plant privity [34]. The application of salt-tolerant growth-promoting bacteria may contribute to stress alleviation and enhance the resilience of crops grown in saline soils [34].
In the study of halotolerance, it was shown that all strains were resistant to a salt concentration of 5%, and one strain, Pseudomonas putida D7, demonstrated the ability to grow in a medium with 15% NaCl (Table 1, Figure 2). According to the classification, the investigated strains are moderately halophilic, exhibiting optimal growth at NaCl concentrations ranging from 3% to 15% (~0.5–2.7 M). Halophilic bacteria have several advantages compared to other microorganisms, as they possess high metabolic activity, allowing them to grow in extreme conditions and produce a variety of valuable biologically active compounds, including those with antimicrobial properties [35].
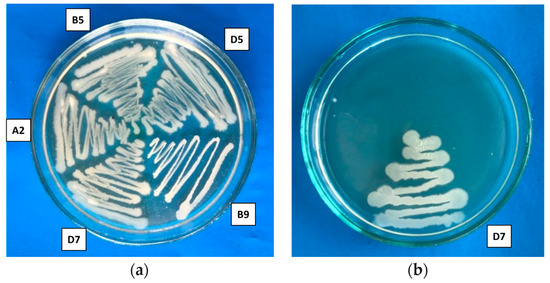
Figure 2.
Growth of bacterial strains on medium containing (a) 5% NaCl and (b) 15% NaCl. D5—Pseudomonas flavescens D5, A2—Bacillus aerophillus A2, B5—Serratia proteamaculans B5, B9—Bacillus simplex B9, D7—Pseudomonas putida D7.
PHA is a class of polyesters of various hydroxyalkanoic acids, which are synthesized by many Gram-positive and Gram-negative bacteria and accumulate intracellularly [33]. In the present study, the strains Ps. flavescens D5 and B. aerophillus A2 demonstrated the ability to produce PHA (Table 1).
3.2. Biocompatibility Assessment of Strains
Currently, the advantages of preparations based on microbial consortia over monocultures are convincingly confirmed, as the biotechnological potential of microorganisms in such preparations is more fully realized. There are several advantages of multi-component preparations: multiplicity of action, synergistic effect, increased stability and adaptability to different agro-climatic conditions, the ability to utilize inhomogeneous substrates in composition, and more complete utilization of the functional capabilities of microorganisms [36,37,38].
In the development of multi-strain inoculants, it is crucial to consider the type of relationships between microorganisms and the possibility of their combination. Therefore, the next stage of the research was the in vitro testing of the selected strains for compatibility during their co-cultivation on a solid nutrient medium (Table 2).

Table 2.
Pairwise compatibility among bacterial strains.
It was shown that during co-cultivation, four out of five strains did not suppress the growth and development of each other (Table 2), indicating their compatibility and the possibility of including them in the composition of a multi-strain inoculant. The identified biocompatibility of the studied strains indicates the absence of competition between them and insensitivity to the produced extracellular metabolites with antagonistic properties. An exception was the B.simplex B9 strain, which demonstrated pronounced incompatibility with most of the investigated bacterial strains (Table 2). Thus, for seed treatment in subsequent experiments, four out of five strains that showed compatibility were used.
3.3. Biosynthesis of Microbial Exopolysaccharides and Their Rheological Properties
In addition to plant-beneficial microorganisms, such ingredients of seed coating as binders that help to release a suitable amount of plant-beneficial microorganisms in physiologic conditions and ensure the adherence and cohesion of the material on the seed surface and keep the ingredients active are used [37,39,40]. The microbial polymer solution should be water-soluble with a low viscosity for complete atomization of the liquid onto seeds [40].
Earlier, we isolated strains Aureobasidium pullulans C7 [41] and Bacillus thuringiensis C8, which showed the ability to biosynthesize exopolysaccharide (EPS). The A. pullulans C7 strain synthesized 12.53 ± 0.48 g L−1 exoglycan on the 4th day of fermentation, and the yield coefficient for biomass was 349.02% (Table 3). This indicates that in this medium the substrate is utilized to a greater extent for the formation of EPS than for the formation of cell mass. The amount of polysaccharide accumulated by the studied strain is comparable with the data of other researchers [42,43].

Table 3.
Production of exopolysaccharides by strains A. pullulans C7 and B. thuringiensis C8 in presence of glucose.
The strain B. thuringiensis C8 produced 3.97 g L−1 of exoglycan (Table 3). The yield coefficient for bacterial biomass indicates the potential of this strain as a producer of EPS. The ability of strains of the genus Bacillus, including B. thuringiensis, to produce EPS is confirmed in the works of other researchers [44,45].
Further, measurements of dynamic viscosity were made for polymer solutions obtained by cultivation of A. pullulans C7 and B.thuringiensis C8.
The dynamic viscosity of each concentration solution produced by B. thuringiensis C8 obviously decreased with the increase in shear rate (Figure 3), showing a shear-thinning behavior, which means that this kind of polysaccharide solution belongs to non-Newtonian fluid (or pseudoplastic flow behavior). Solutions of polymers in water and at the same concentrations can sometimes have oppositional behaviors, i.e., Newtonian or non-Newtonian fluids, depending on their structures, molecular weights, and polymer microbial producers [46,47].
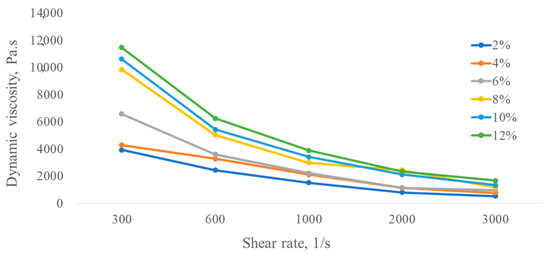
Figure 3.
Viscosity of exopolysaccharide solutions produced by B. thuringiensis C8 in different concentrations.
It was also noted that the viscosity increased with increasing polymer solution concentration; however, at lower concentrations, the rheological measurements became erratic. The shear-thinning phenomenon could be due to the rate of formation of new entanglements lower than the externally imposed disruption rate with an increase in shear rate. Another distinguishing feature is that the microbial solution showed comparatively high viscosity rates at all dilute concentrations.
The measurement of the dynamic viscosity of the polymer solution obtained by the cultivation of A. pullulans C7 showed the dependence on dynamic viscosity at the shear rate at different concentrations ranging from 2% to 12% (w/v) at 25 °C (Figure 4). The shear rate increased with increasing polymer concentration, thereby demonstrating that viscosity strongly depends on concentration.
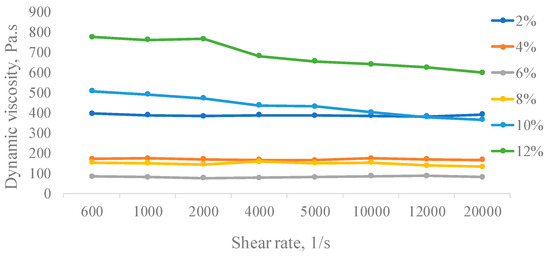
Figure 4.
Viscosity of pullulan solutions produced by A. pullulans C7 in different concentrations.
At lower concentrations (<8.0%, w/v), viscosity virtually remained unchanged with increasing shear rate, thus suggesting that the pullulan aqueous solution exhibits Newtonian flow behavior. It is known that Newtonian fluid viscosity is constant no matter the shear rate or applied shear stress experienced by the fluid. However, with increasing concentration to 8% w/v, the flow behavior was changed to pseudoplastic. Such flow behavior of pullulan can happen because of the separation of exopolysaccharides from each other or the alignment of them with the shear field and thereby a decrease in viscosity up to an approximately constant value [48]. Also, it is known that the viscosity is dependent on the structure and concentration of the polymer, its molecular weight and distribution, the conformation of macromolecules in the solution and its interaction with solvents, the type of intermolecular and intramolecular aggregation, and the flexibility of the chains with temperature. A similar change in flow behavior with increasing concentration was reported for pullulan and other polysaccharides [49,50].
The data obtained allow us to suggest the microbial polymer, pullulan, produced by A. pullulans C7 as a potential seed coating binder due to rheological characteristics. It is already known as an excellent film former and is functional for a variety of applications, including for use as an adhesive, binder, and thickener to modify or maintain the texture of food.
It has also been reported that pullulan has considerable mechanical strength and other functional properties such as adhesiveness, film and fiber formability, and enzymatically mediated degradability [51]. High flexibility and a lack of crystallinity provide pullulan with the capacity to form thin layers, electrospun nanofibers, nanoparticles, flexible coatings, stand-alone films, and three-dimensional objects [51,52]. Due to its peculiar characteristics, pullulan is extensively used in different sectors, the three main realms of application pertaining to the pharmaceutical, biomedical, and food fields.
3.4. The Use of Various Antifungal Formulations for Seed Treatments in Pot Experiments
Seed coating is a method that involves applying exogenous materials to the surface of seeds to enhance their properties and/or deliver active components (such as plant growth regulators, nutrients, and microbial inoculants). This process can protect seeds from phytopathogens, increase germination rates, improve plant resistance to stress factors, and enhance overall plant growth [37,52,53,54].
In the next stage of the research, various antifungal formulations for seed coating were developed, and their impact on barley growth under phytopathogenic conditions was assessed (Figure 5).
Figure 5.
Scheme of pot experiments.
As active components, a microbial inoculant consisting of a suspension of four compatible strains was used: Ps. flavescens D5, B. aerophilus A2, S. proteamaculans B5, and Ps. putida D7. As polymer components, PHA produced by the strain Ps. flavescens D5, and pullulan, produced by the yeast strain A. pullulans C7, were used. PHA was included in the mixture due to its antifungal properties, as previously identified in earlier studies [15,16].
Uniform seed emergence and early crop development are crucial aspects for achieving high crop yields. Seed coating is an effective method that improves seed-sowing qualities and activates the internal resources of the seed material [52]. In the conducted research, pre-sowing seed treatment, in most cases, enhanced their germination energy and germination capacity. The greatest effect was observed when applying a bacterial suspension in combination with a polymer mixture, where germination energy and germination capacity reached 95% and 97%, respectively (Figure 6).
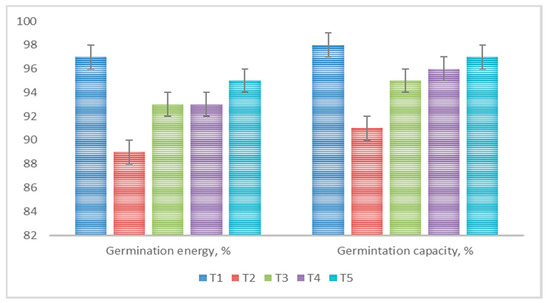
Figure 6.
Influence of various pre-sowing treatments on barley seed germination energy and germination capacity.
The pre-sowing treatment of seeds demonstrated a pronounced growth-stimulating effect on barley plants, as evidenced by a significant (p < 0.05) increase in morphometric indicators (Table 4).

Table 4.
Influence of various pre-sowing seed treatments on growth parameters of barley.
The barley’s response varied depending on the type of treatment. Root length is a crucial morphometric indicator as roots are in contact with soil and soil microflora, absorbing water with mineral compounds. The greatest root elongation was observed in variant T5 with the application of a bacterial suspension and a polymer mixture (1.6 times), followed by treatment T3, where root elongation was noted at 1.5 times. Stem length is also a significant characteristic when assessing the plant’s response to different pre-sowing seed treatments. Treated variants showed an increase in stem length by 20–53%. The greatest increase in stem length was observed in variants T3 and T5 (Figure 7). It is shown that the stem mass of treated plants was more than 1.5–1.8 times greater, and root mass was 1.1–1.6 times greater compared to the untreated control (Table 4).

Figure 7.
Barley growth under phytopathogenic stress with different seed treatment variants: (a) T2—untreated, (b) T3—treatment with bacterial suspension, (c) T5—seed coating in polymer mixture with bacterial suspension.
In the conducted research, the observed stimulating effect on the growth parameters of barley can be attributed to several reasons. One of the mechanisms of the positive influence on plants is the ability of the strains included in the composition to produce the phytohormone IAA, which regulates cell division and elongation, their proliferation and differentiation, as well as the development of vascular tissues and apical dominance [55]. Another mechanism for improving morphometric plant parameters under conditions of phytopathogenic stress is the biocontrol properties of strains and the protective role of biopolymers.
The state of the photosynthetic apparatus is an indicator of the physiological condition of plants. One of the primary characteristics of photosynthetic activity is the content of chlorophyll pigments [56]. In previous studies, fluorescence visualization analysis of chlorophyll was applied to assess the condition of the plant photosynthetic system under the influence of biotic [56,57,58] and abiotic [59,60,61] stress. High chlorophyll content may indicate potentially high agricultural productivity [56].
In the present study, under conditions of biotic stress induced by the phytopathogen F. oxysporum, the total chlorophyll content in barley leaves decreased by 2.7 times compared to the indicator for plants grown under normal conditions, reaching 1.03 ± 0.03 mg g−1 (Table 5). This likely indicates changes in the pigment–protein complexes of light-harvesting antennae and reaction centers of photosystems. Seed treatment had a positive effect on the photosynthetic activity of barley under phytopathogenic stress. This positive effect was to increase the content of chlorophyll a in leaves by 1.4–2.1 times, chlorophyll b by 2–2.4 times, and the total content of chlorophyll (a + b) by 1.6–2.2 times. The maximum effect was achieved in variant T5 with the application of a bacterial suspension and a polymer mixture (Table 5). The observed differences in pigment content may be associated with the production of certain compounds by the studied bacteria, influencing the biosynthesis and/or degradation processes of chlorophylls, as well as creating more favorable growth conditions for plants under stress.

Table 5.
Influence of different pre-sowing seed treatments on proline and chlorophyll content in barley.
It is known that stress factors lead to a disruption in the balance between the generation of reactive oxygen species (ROS) and their neutralization. Among the essential mechanisms of plant tolerance mediated by bacteria is the involvement of these microorganisms in detoxifying ROS through the modulation of the natural antioxidant defense systems of plants—both non-enzymatic (proline, ascorbic acid, glutathione, cysteine, flavonoids, carotenoids, and tocopherol) and enzymatic (superoxide dismutase, peroxidase, catalase, ascorbate peroxidase, guaiacol peroxidase, and glutathione reductase), all components of which are in complex functional interaction [62,63].
The increase in proline content is one of the characteristic responses of plants to various types of stress, including biotic stress, providing the first stage of plant adaptation. Proline serves multiple functions, including the regulation of cytosolic acidity, minimization of lipid peroxidation by scavenging free radicals, and stabilization of subcellular components and structures (proteins and membranes) [64]. A higher level of proline in barley leaves was observed when plants were grown in soil with an elevated infectious background compared to untreated plants in sterile soil (Table 5). In the untreated variant under phytopathogenic stress, the proline concentration was 1.7 mg g−1, exceeding this indicator in plants grown in favorable conditions by 1.8 times. In treated plants, the proline content was lower. The most noticeable decrease in proline was observed in the variant with simultaneous seed coating in a bacterial suspension and a polymer mixture (Table 5). The obtained results indicate a reduction in the stress experienced by plants due to the pre-sowing seed treatment. Similar to our findings, a reduction in proline levels in various plant species under the influence of microbial treatment has been demonstrated in several studies [65,66].
In the conducted studies, an increase in the activity of antioxidant enzymes was observed when untreated plants were grown under conditions of phytopathogenic stress compared to plants grown in stress-free conditions (Table 6). The obtained data indicate that in response to the action of stress factors, there is an activation of the plant’s defense system.

Table 6.
Influence of various pre-sowing seed treatments on the activity of antioxidant enzymes in barley.
The pre-sowing seed treatment (T3–T5) led to an increase in catalase activity by 1.3–1.5 times under stress conditions. For ascorbate peroxidase and guaiacol peroxidase, an increase in enzyme activity was observed under phytopathogenic stress in the seed treatment with the bacterial suspension and polymer mixture (T5)—by 2.4 times and 2.7 times, respectively (Table 6). Similarly to the obtained data, previous studies have reported an increase in the activity of antioxidant enzymes in plants when inoculated with bacteria as one of the defense mechanisms of plants when grown under stressful conditions [67,68,69].
Thus, it was shown that when seeds were treated with the T5 composition, plant growth parameters (weight and length of roots) significantly increased compared to the T3 variant with a bacterial suspension. In addition, the use of the T5 composition contributed more to the attenuation of plant stress caused by phytopathogens compared to the use of microorganisms only (Table 5 and Table 6). This indicates that the addition of biopolymers to formulations for seed treatments enhances microbe-induced plant tolerance to phytopathogens.
4. Conclusions
As a result of the research, a microbial association of bio-compatible endophytic bacteria has been created, possessing agronomically valuable properties such as the synthesis of the phytohormone IAA, antagonistic activity against Fusarium oxysporum and Fusarium solani, halotolerance, and PHA production. The study of the rheological properties of polysaccharide solutions showed that pullulan produced by Aureobasidium pullulans C7 can be used as seed coating binder at a low concentration of the polymer solution characterized by low viscosity ratio and exhibits Newtonian flow behavior. The effectiveness of various seed coating treatments including biopolymers (PHA and pullulan) and beneficial microorganisms in enhancing the resistance of barley plants to phytopathogens has been demonstrated. The innovative, eco-friendly antifungal seed treatments provide protection for barley against Fusarium diseases, significantly improving seed germination and plant growth in the field. In addition, these polymers will be a new progressive material with the possibility of use in medicine in the form of capsules for prolonged action of drugs, as absorbable suture threads, and dressings. In the form of a film material, the obtained microbial polymers can be used for packaging and storage of food products.
Author Contributions
Conceptualization, L.I. and Y.B.; Methodology, A.U., A.K. and I.S.; Software, A.O.; Validation, L.I. and Y.B.; Formal Analysis, Y.B., A.K. and I.S.; Investigation, L.I., A.O., Y.B., and A.U.; Resources, L.I. and A.O.; Data Curation, L.I., A.K. and I.S.; Writing—Original Draft Preparation, L.I., A.U., A.O. and Y.B.; Writing—Review and Editing, L.I. and Y.B.; Visualization, A.U. and A.O.; Supervision, L.I. and Y.B.; Project Administration, L.I.; Funding Acquisition, L.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan, grant number AP19679444.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.
Acknowledgments
The Institute of Polymer Materials and Technologies (Almaty, Kazakhstan) is greatly acknowledged for performing rheological studies of polymers.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight From a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef]
- Korbecka-Glinka, G.; Piekarska, K.; Wi’sniewska-Wrona, M. The Use of Carbohydrate Biopolymers in Plant Protection against Pathogenic Fungi. Polymers 2022, 14, 2854. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture. Agron. Sustain. Dev. 2015, 35, 569. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, L.; Zhang, P.F.; Zhou, J.Q.; Lu, X.F.; Tian, W. Carbohydrate polymers exhibit great potential as effective elicitors in organic agriculture: A review. Carbohydr. Polym. 2020, 230, 115637. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.M.; Lim, Y.Y.; Ting, A.S.Y. Biopolymers for biopriming of Brassica rapeseeds: A study on coating efficacy, bioagent viability and seed germination. J. Saudi Soc. Agric. Sci. 2021, 20, 198. [Google Scholar] [CrossRef]
- Jurado, M.M.; Suárez-Estrella, F.; Toribio, A.J.; Martínez-Gallardo, M.R.; Estrella-González, M.J.; López-González, J.A.; López, M.J. Biopriming of cucumber seeds using actinobacterial formulas as a novel protection strategy against Botrytis cinerea. Front. Sustain. Food Syst. 2023, 7, 1158722. [Google Scholar] [CrossRef]
- Ren, X.X.; Chen, C.; Ye, Z.H.; Su, X.Y.; Xiao, J.J.; Liao, M.; Cao, H.Q. Development and Application of Seed Coating Agent for the Control of Major Soil-Borne Diseases Infecting Wheat. Agronomy 2019, 9, 413. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulanproductionfromagro-industrialwasteanditsapplicationsinfoodindustry: A review. Carbohydr. Polym. 2019, 217, 46. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent Advancements in Microbial Polysaccharides: Synthesis and Applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef]
- Behera, S.; Priyadarshanee, M.; Vandana, D.S. Polyhydroxyalkanoates, the bioplastics of microbial origin. Properties. Biochemical synthesis and their applications. Chemosphere 2022, 294, 133723. [Google Scholar] [CrossRef]
- Nor Azillah, F.O.; Sarala, S.; Noriaki, S. Biodegradable dual-layer Polyhydroxyalkanoate (pha)/Polycaprolactone (pcl) mulch film for agriculture. Energy Nexus 2022, 8, 100137. [Google Scholar]
- Ma, L.; Zhang, Z.; Li, J.; Yang, X.; Fei, B.; Leung, P.H.M.; Tao, X.M. A New Antimicrobial Agent: Poly (3-Hydroxybutyric Acid) Oligomer. Macromol. Biosci. 2019, 19, 1800432. [Google Scholar] [CrossRef]
- Abou-Aiad, T.H.M. Morphology and Dielectric Properties of Polyhydroxybutyrate (PHB)/Poly(Methylmethacrylate)(PMMA) Blends with Some Antimicrobial Applications. Polym. Plast. Technol. Eng. 2007, 46, 435. [Google Scholar] [CrossRef]
- Ignatova, L.; Usmanova, A.; Brazhnikova, Y.; Omirbekova, A.; Egamberdieva, D.; Mukasheva, T.; Kistaubayeva, A.; Savitskaya, I.; Karpenyuk, T.; Goncharova, A. Plant Probiotic Endophytic Pseudomonas flavescens D5 Strain for Protection of Barley Plants from Salt Stress. Sustainability 2022, 14, 15881. [Google Scholar] [CrossRef]
- Ignatova, L.; Brazhnikova, Y.; Omirbekova, A.; Usmanova, A. Polyhydroxyalkanoates (PHAs) from Endophytic Bacterial Strains as Potential Biocontrol Agents against Postharvest Diseases of Apples. Polymers 2023, 15, 2184. [Google Scholar] [CrossRef] [PubMed]
- Abulfaraj, A.; Jalal, R. Use of plant growth-promoting bacteria to enhance salinity stress in soybean (Glycine max L.) plants. Saudi J. Biol. Sci. 2021, 28, 3823. [Google Scholar] [CrossRef]
- Sharma, N. Polyhydroxybutyrate (PHB) Production by Bacteria and Its Application as Biodegradable Plastic in Various Industries. Acad. J. Polym. Sci. 2019, 2, 3. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Alrumman, S.A.; Otaif, K.A.; Alamri, S.A.; Mostafa, M.S.; Sahlabji, T. Production and Characterization of Bioplastic by Polyhydroxybutyrate Accumulating Erythrobacter aquimaris Isolated from Mangrove Rhizosphere. Molecules 2020, 25, 179. [Google Scholar] [CrossRef]
- Mahitha, G.; Jaya, M.R. Microbial polyhydroxybutyrate production by using cheap raw materials as substrates. Indian J. Pharm. Biol. Res. 2016, 4, 57. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S. Combination of Pseudomonas Aeruginosa and Pochonia Chlamydosporia for Control of Root- Infecting Fungi in Tomato. J. Phytopathol. 2003, 151, 215. [Google Scholar] [CrossRef]
- Goksungur, Y.; Uzunogullari, P.; Dagbagli, S. Optimization of pullulan production from hydrolysed potato starch waste by response surface methodology. Carbohydr. Polym. 2011, 83, 1330. [Google Scholar] [CrossRef]
- Oliveira, V.C. Study of the Molecular Weight of Pullulan Produced by Aureobasidium pullulans from Industrial Waste. Mater. Res. 2023, 26, e20230060. [Google Scholar] [CrossRef]
- Bates, L.E.; Waldre, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205. [Google Scholar] [CrossRef]
- Lichtestaller, H.K. Determination of total carotenoids and chlorophylls a and b of leaves extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591. [Google Scholar]
- Al-Taweel, K.; Iwaki, T.; Yabuta, Y. A bacterial transgene for catalase protects translation of D1 protein during exposure of salt-stressed tobacco leaves to strong light. Plant Physiol. 2007, 145, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Khor, S.; Rahmad, Z.; Sreeramanan, S. Ascorbate Peroxidase Activity of Aranda Broga Blue Orchid Protocorm-like Bodies (PLBs) In Response to PVS2 Cryopreservation Method. Trop. Life Sci. Res. 2016, 1, 139. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Lüthje, S. Properties of Guaiacol Peroxidase Activities Isolated from Corn Root Plasma Membranes. Plant Physiol. 2003, 132, 1489. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant Beneficial Endophytic Bacteria: Mechanisms, Diversity, Host Range and Genetic Determinants. Microbiol. Res. 2019, 221, 36. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, S.A.; Itoh, K. Effects of Co-Inoculation of Indole-3-Acetic Acid-Producing and -Degrading Bacterial Endophytes on Plant Growth. Horticulturae 2019, 5, 17. [Google Scholar] [CrossRef]
- Khianngam, S.; Meetum, P.; Chiangmai, P.N.; Tanasupawat, S. Identification and Optimisation of Indole-3-Acetic Acid Production of Endophytic Bacteria and Their Effects on Plant Growth. Trop. Life Sci. Res. 2023, 34, 219. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pedroso, I.R. Mycotoxins in Cereal-Based Products and Their Impacts on the Health of Humans. Livestock Animals and Pets. Toxins 2023, 15, 480. [Google Scholar] [CrossRef] [PubMed]
- Newitt, J.T.; Prudence, S.M.M.; Hutchings, M.I.; Worsley, S.F. Biocontrol of Cereal Crop Diseases Using Streptomycetes. Pathogens 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Behera, T.K.; Krishna, R.; Ansari, W.A.; Aamir, M.; Kumar, P.; Kashyap, S.P.; Pandey, S.; Kole, C. Approaches Involved in the Vegetable Crops Salt Stress Tolerance Improvement: Present Status and Way Ahead. Front. Plant Sci. 2022, 12, 787292. [Google Scholar] [CrossRef] [PubMed]
- Corral, P.; Amoozegar, M.A.; Ventosa, A. Halophiles and Their Biomolecules. Recent Advances and Future Applications in Biomedicine. Mar. Drugs 2019, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Thomloudi, E.E.; Tsalgatidou, P.C.; Douka, D.; Spantidos, T.N.; Dimou, M.; Venieraki, A.; Katinakis, P. Multistrainversus Single-Strain Plant Growth Promoting Microbial Inoculants. The Compatibility Issue. Hell. Plant Prot. J. 2019, 12, 61. [Google Scholar]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial seed coating: An attractive tool for sustainable agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Zhubanova, A.A.; Ernazarova, A.K.; Kaiyrmanova, G.K.; Zayadan, B.K.; Savitskaya, I.S.; Abdieva, G.Z.; Kistaubaeva, A.S.; Akimbekov, N.S. Construction of cyanobacterial-bacterial consortium on the basis of axenic cyanobacterial cultures and heterotrophic bacteria cultures for bioremediation of oil-contaminated soils and water ponds. Russ. J. Plant Physiol. 2013, 60, 555. [Google Scholar] [CrossRef]
- Ma, Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef]
- Sohail, M.N.; Tahira, P.; Charles, H. Recent Advances in Seed Coating Technologies: Transitioning Toward Sustainable Agriculture. Green Chem. 2022, 5, 225. [Google Scholar] [CrossRef]
- Brazhnikova, Y.V.; Mukasheva, T.D.; Ignatova, L.V. Shtamm Drojjepodobnogo Griba Aureobasidium pullulans C7—Producent Ekzopolisaharida i Indoliluksusnoi Kisloti [Strain of Yeast-like Fungus Aureobasidium pullulans C7—PRODUCER of Exopolysaccharide and Indolylacetic Acid]. RK Patent No. 32992, 6 August 2018. [Google Scholar]
- Duan, X.; Chi, Z.; Li, H. High pullulan yield is related to low UDP-glucose level and high pullulan-related synthases activity in Aureobasidium pullulans Y68. Ann. Microbiol. 2007, 57, 243. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, F.; Chi, Z.; Yue, L.; Liu, G.; Zhang, T. Bioproducts from Aureobasidium pullulans, a biotechnologically important yeast. Appl. Microbiol. Biotechnol. 2009, 82, 793. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sheng, J.; Tian, X.; Wu, T.; Liu, W.; Shen, L. Optimization of the production of exopolysaccharides by Bacillus thuringiensis 27 in sand biological soil crusts and its bioflocculant activity. Afr. J. Microbiol. Res. 2011, 5, 207. [Google Scholar]
- Malick, A.; Khodaei, N.; Benkerroum, N.; Karboune, S. Production of exopolysaccharides by selected Bacillus strains: Optimization of media composition to maximize the yield and structural characterization. Int. J. Biol. Macromol. 2017, 102, 539. [Google Scholar] [CrossRef]
- Lee, Y.K.; Jung, S.K.; Chang, Y.H. Rheological properties of a neutral polysaccharide extracted from maca (Lepidium meyenii Walp.) roots with prebiotic and anti-inflammatory activities. Int. J. Biol. Macromol. 2020, 152, 757. [Google Scholar] [CrossRef]
- Hosseini, E.; Mozafari, H.; Hojjatoleslamy, M.; Rousta, E. Influence of temperature, pH and salts on rheological properties of bitter almond gum. Food Sci. Technol. 2017, 37, 437–443. [Google Scholar] [CrossRef]
- Haghighatpanah, N.M. Optimization and characterization of pullulan produced by a newly identified strain of Aureobasidium pullulans. Int. J. Biol. Macromol. 2020, 152, 305–313. [Google Scholar] [CrossRef]
- TeránHilares, R.; Resende, J.; Orsi, C.A.; Ahmed, M.A.; Lacerda, T.M.; Santos, J.C. Exopolysaccharide (pullulan) production from sugarcane bagasse hydrolysate aiming to favor the development of biorefineries. Int. J. Biol. Macromol. 2019, 15, 169. [Google Scholar]
- Farris, S.; Unalan, I.U.; Introzzi, L.; Fuentes-Alventosa, J.M.; Cozzolino, C.A. Pullulan-based films and coatings for food packaging: Present applications, emerging opportunities, and future challenges. J. Appl. Polym. Sci. 2014, 131, 40539. [Google Scholar] [CrossRef]
- Li, R. Electrospinning pullulan fibers from salt solutions. Polymers 2017, 9, 32. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Mpai, S.; Sivakumar, D. Extension of avocado fruit postharvest 591 quality using non-chemical treatments. Agronomy 2020, 10, 212. [Google Scholar] [CrossRef]
- Pirzada, T.; Farias, B.V.; Mathew, R.; Guenther, R.H.; Byrd, M.V.; Sit, T.L.; Pal, L.; Opperman, C.H.; Khan, S.A. Recent Advances in Biodegradable Matrices for Active Ingredient Release inCrop Protection: Towards Attaining Sustainability in Agriculture. Curr. Opin. Colloid Interface Sci. 2020, 48, 121. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Moustakas, M. Early-Stage Detection of Biotic and Abiotic Stress on Plants by Chlorophyll Fluorescence Imaging Analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Stamelou, M.L.; Sperdouli, I.; Pyrri, I.; Adamakis, I.D.S.; Moustakas, M. Hormetic responses of photosystem II in tomato to Botrytis cinerea. Plants 2021, 10, 521. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.C.; Vanegas, J.I.; Contreras, A.T.; Anzola, J.A.; Urban, M.O.; Beebe, S.E.; Rao, I.M. Chlorophyll fluorescence imaging as a tool for evaluating disease resistance of common bean lines in the western Amazon region of Colombia. Plants 2022, 11, 1371. [Google Scholar] [CrossRef]
- Legendre, R.; Basinger, N.T.; van Iersel, M.W. Low-cost chlorophyll fluorescence imaging for stress detection. Sensors 2021, 21, 2055. [Google Scholar] [CrossRef]
- Asfi, M.; Ouzounidou, G.; Panajiotidis, S.; Therios, I.; Moustakas, M. Toxicity effects of olive-mill wastewater on growth, photosynthesis and pollen morphology of spinach plants. Ecotoxicol. Environ. Saf. 2012, 80, 69. [Google Scholar] [CrossRef]
- Guidi, L.; Calatayud, A. Non-invasive tool to estimate stress-induced changes in photosynthetic performance in plants inhabiting Mediterranean areas. Environ. Exp. Bot. 2014, 103, 42. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J. Hazard. Mater. 2019, 379, 120813. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The PGPR Stenotrophomona smaltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 2017, 8, 1945. [Google Scholar] [CrossRef]
- Piri, R.; Moradi, A.; Balouchi, H.; Salehi, A. Improvement of cumin (Cuminum cyminum) seed performance under drought stress by seed coating and biopriming. Sci. Hortic. 2019, 257, 21. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Banerjee, S.; Acharya, U. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling. Sci. Rep. 2020, 10, 15536. [Google Scholar] [CrossRef]
- Neshat, M.; Abbasi, A.; Hosseinzadeh, A.; Sarikhani, M.R.; Dadashi Chavan, D.; Rasoulnia, A. Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol. Mol. Biol. Plants. 2022, 28, 347. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).