Synthesis and Characteristic Valuation of a Thermoplastic Polyurethane Electrode Binder for In-Mold Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
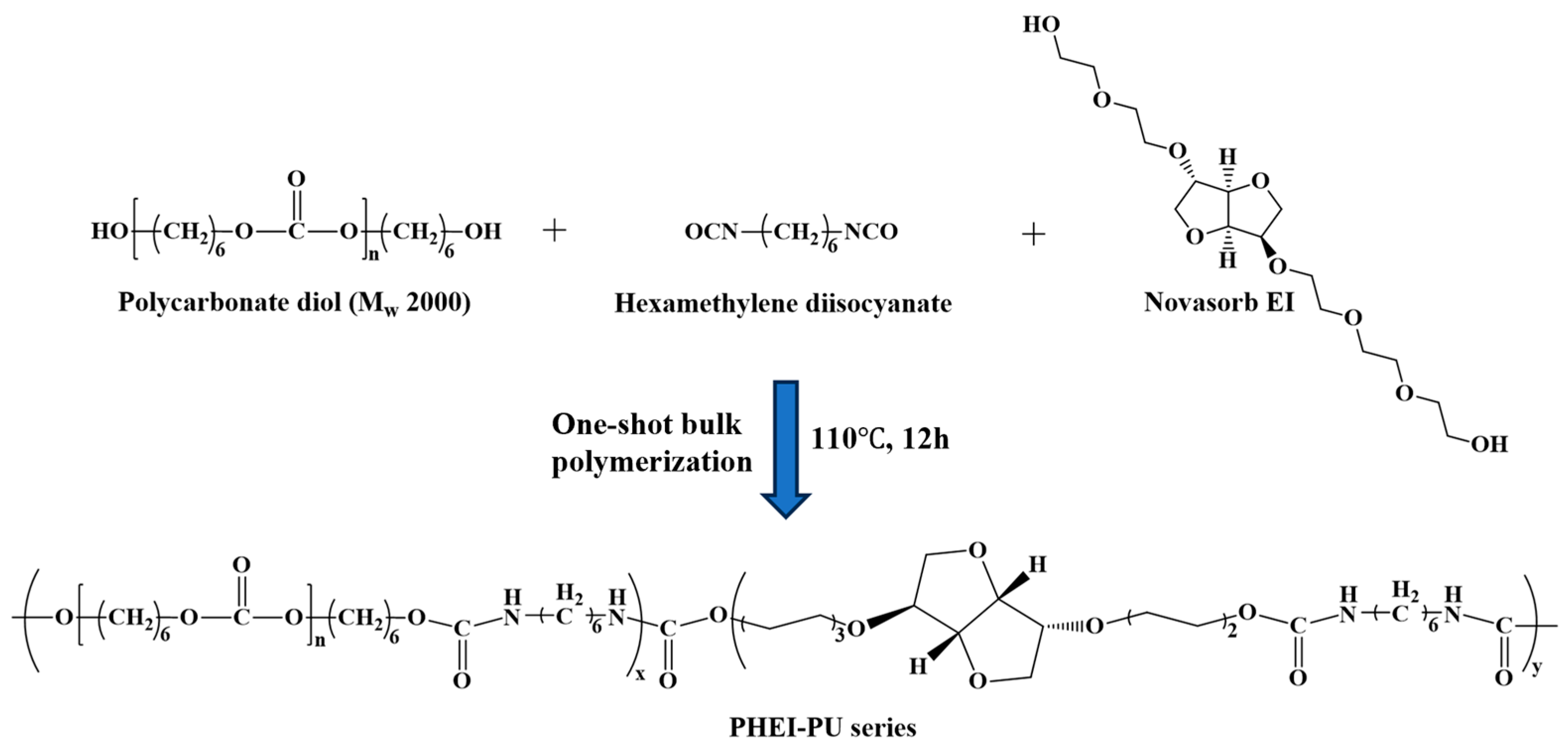
2.2. Synthesis of the PHEI-PU Series
2.3. Preparation of the PU/AGF Blend and Coating
2.4. Characterization of the PHEI-PU Series
2.5. Mechanical Properties
2.6. Thermal Properties
2.7. Electrical Properties
Sheet resistance × Thickness(cm) = Resistivity (Ω·cm)
2.8. Surface Dispersion Measurement
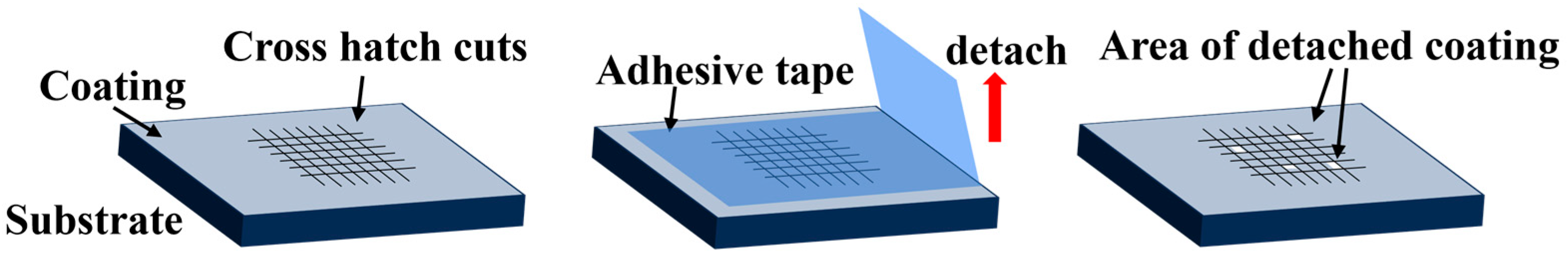
2.9. Adhesion Evaluation
3. Results and Discussion
3.1. Preparation of the PHEI-PU Series
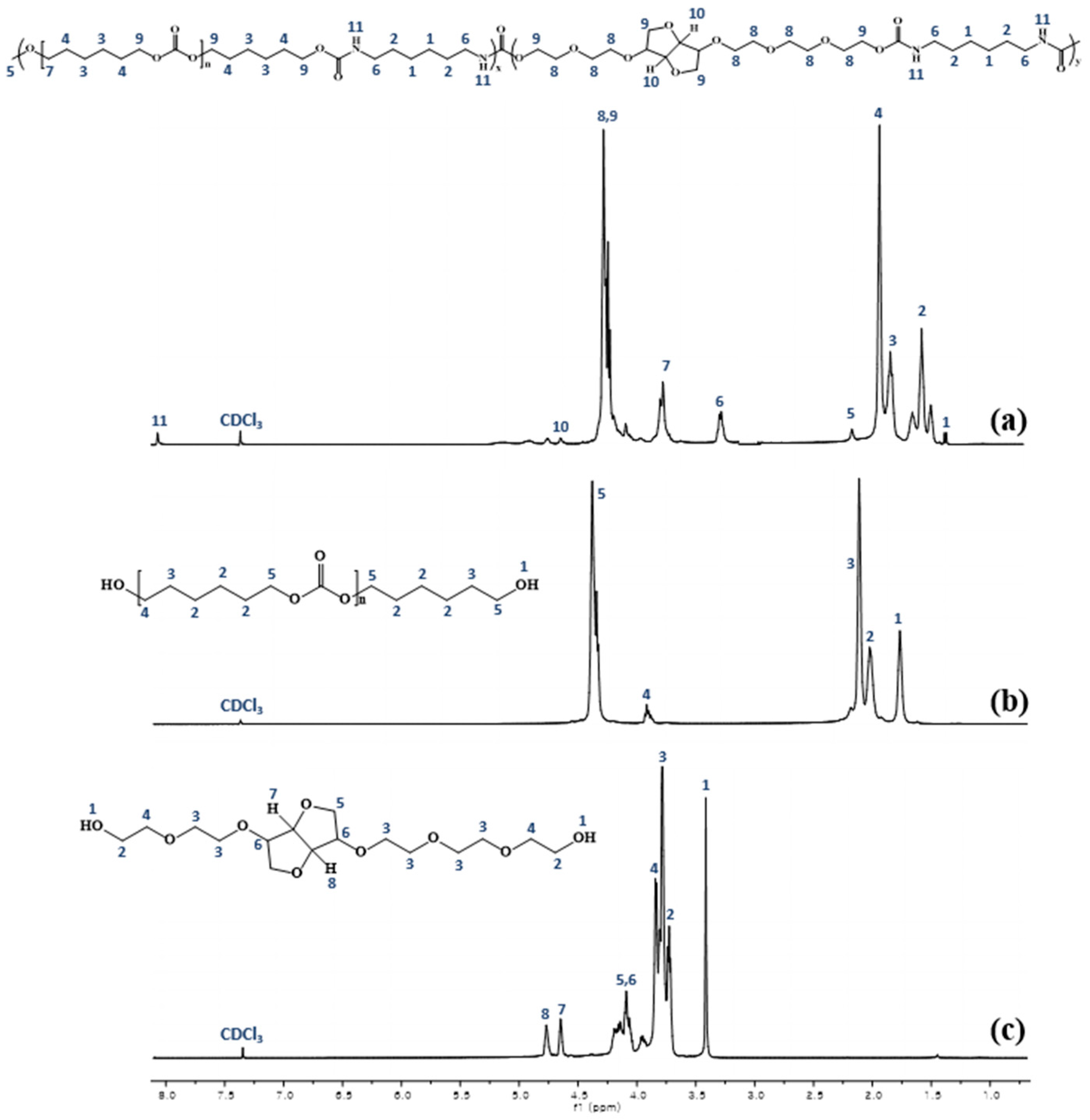
3.2. Characterization of PUs by 1H-NMR
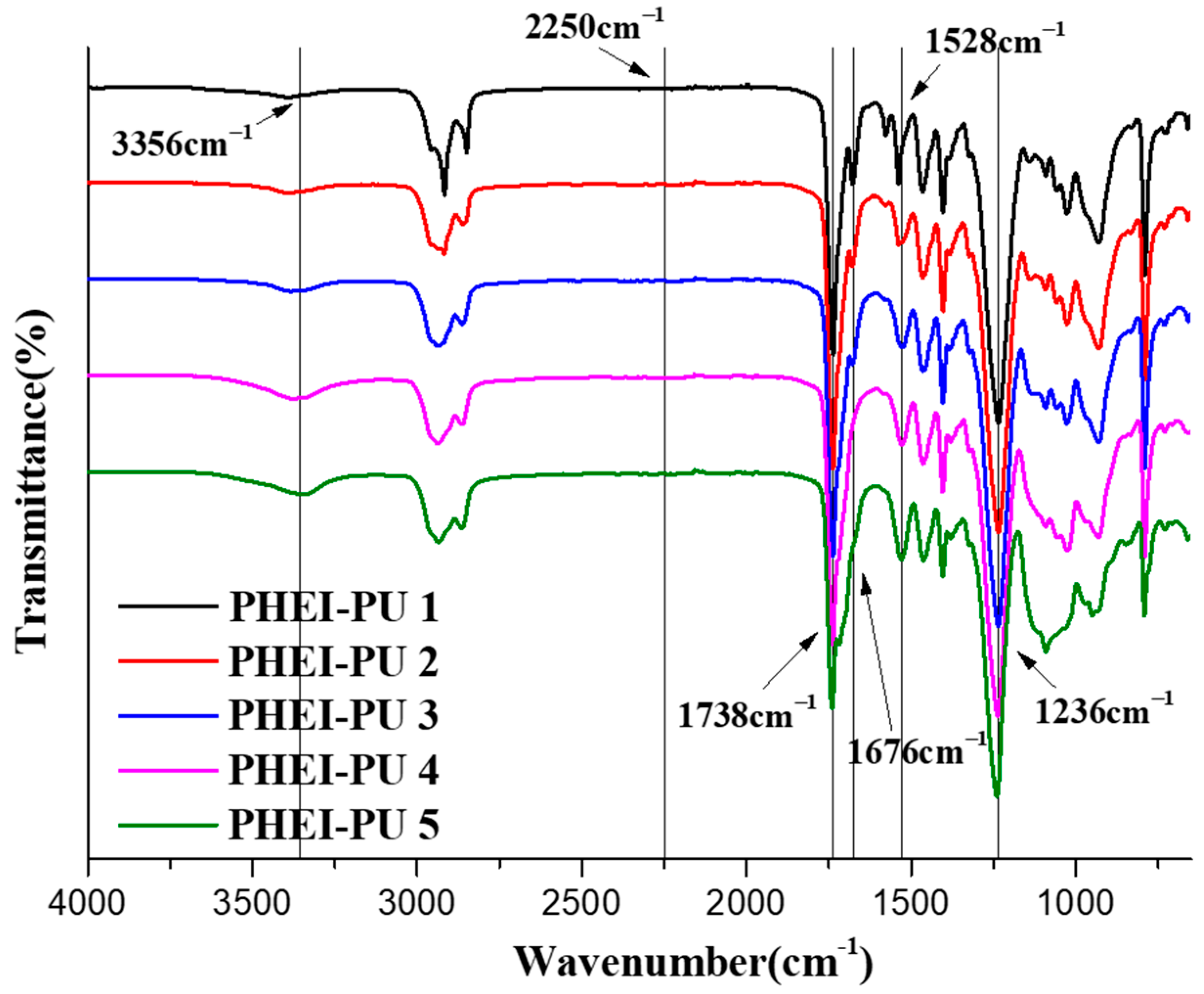
3.3. Characterization of PUs by FTIR Spectroscopy
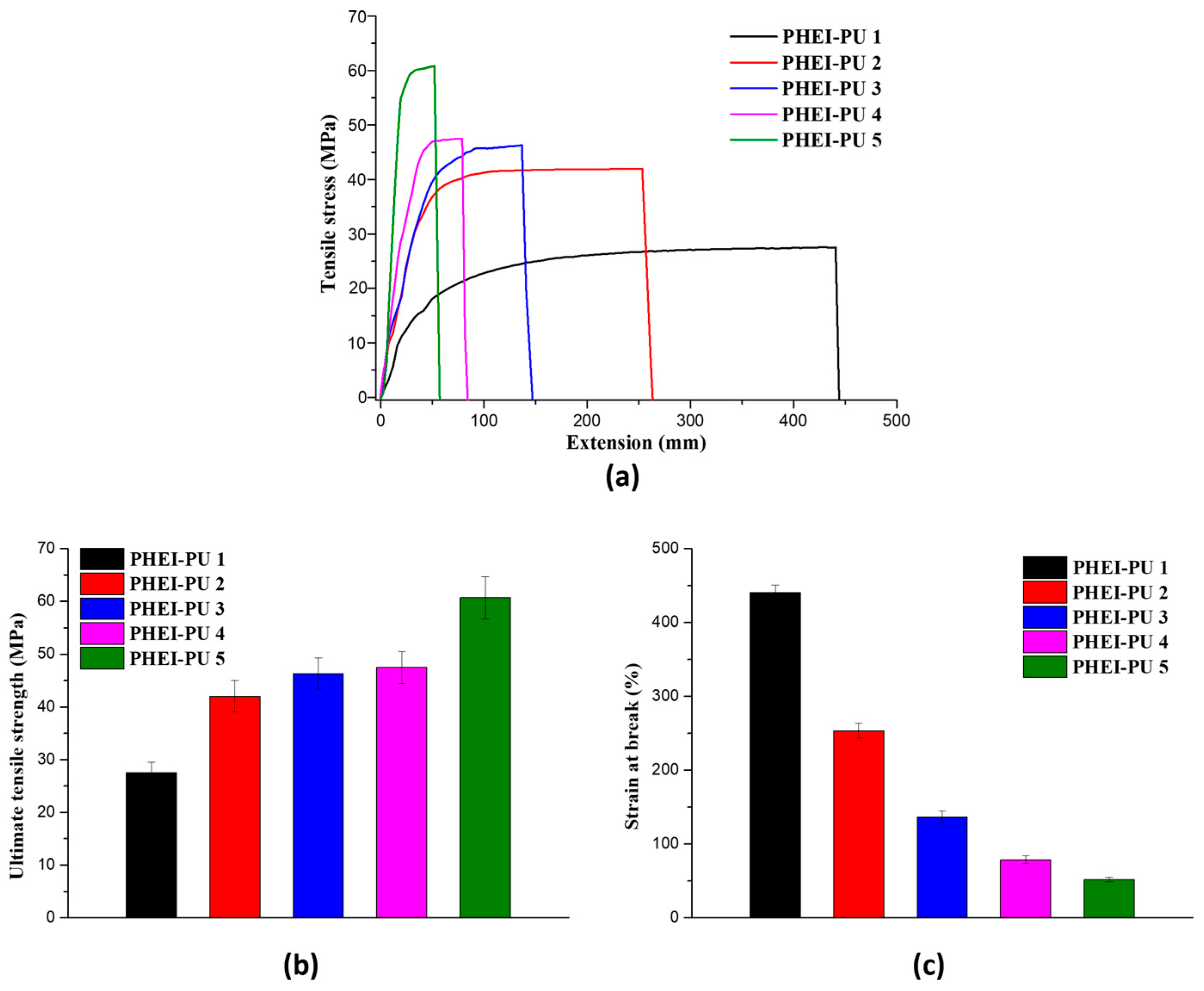
3.4. Mechanical Properties
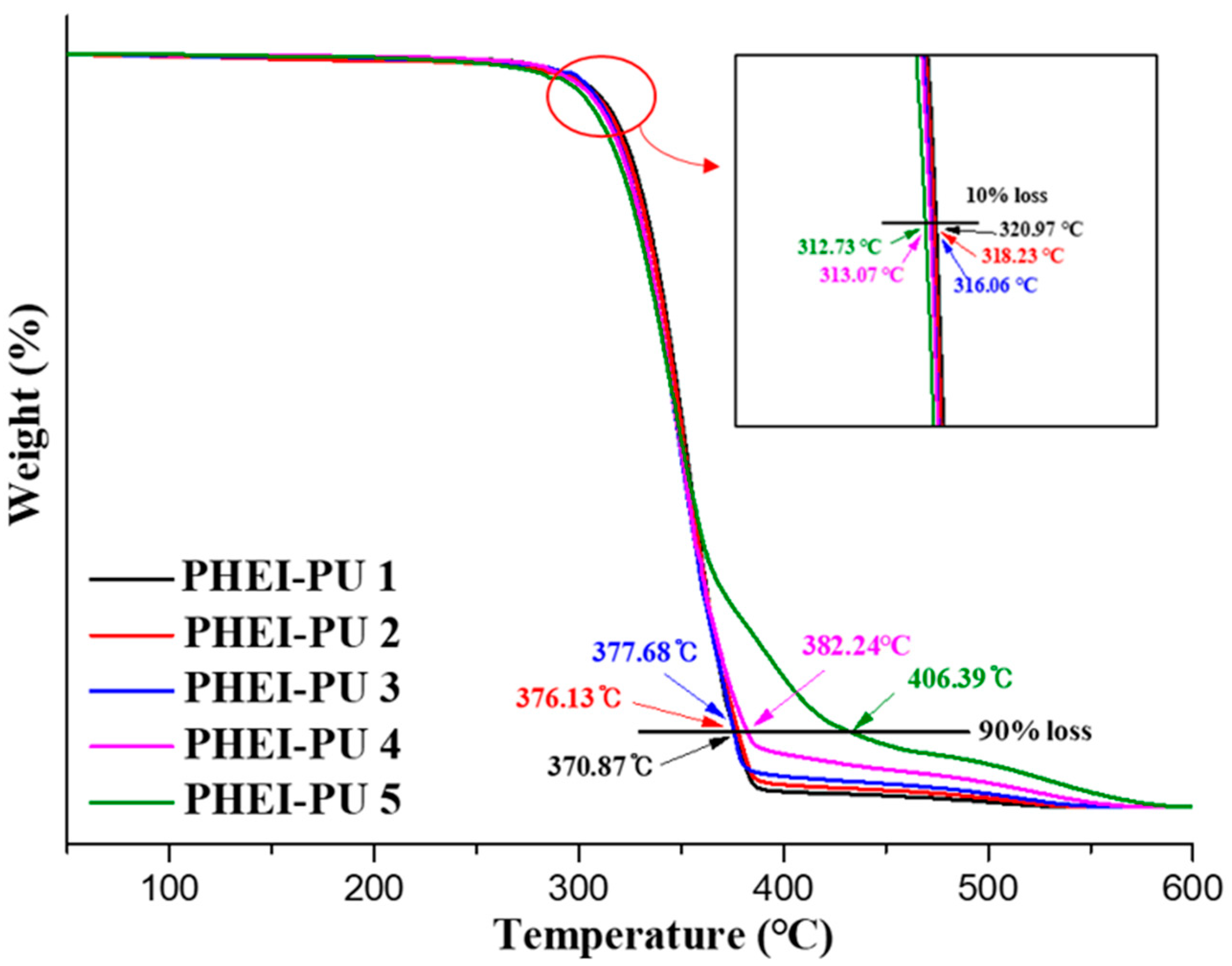
3.5. Thermal Properties
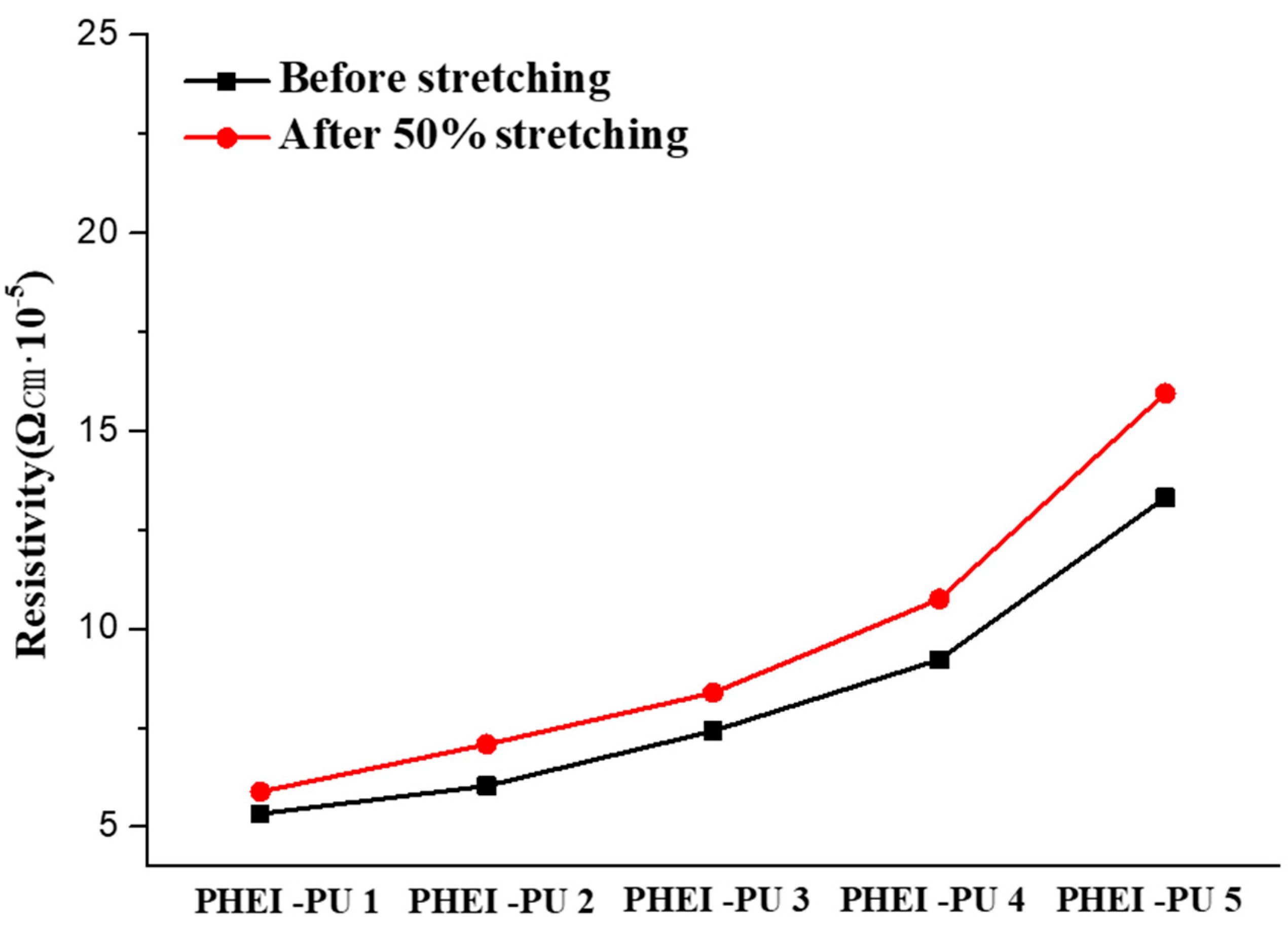
3.6. Electrical Properties
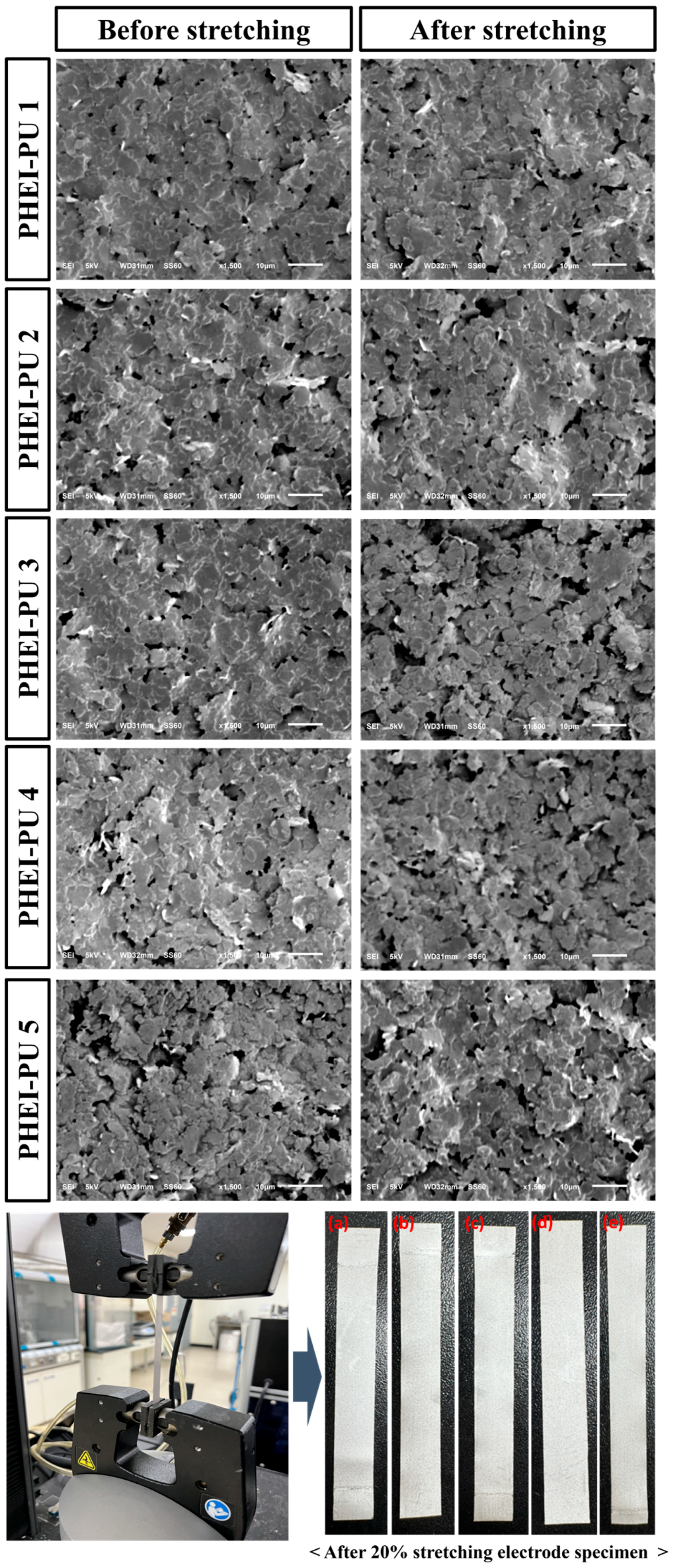
3.7. Surface Dispersion Measurement
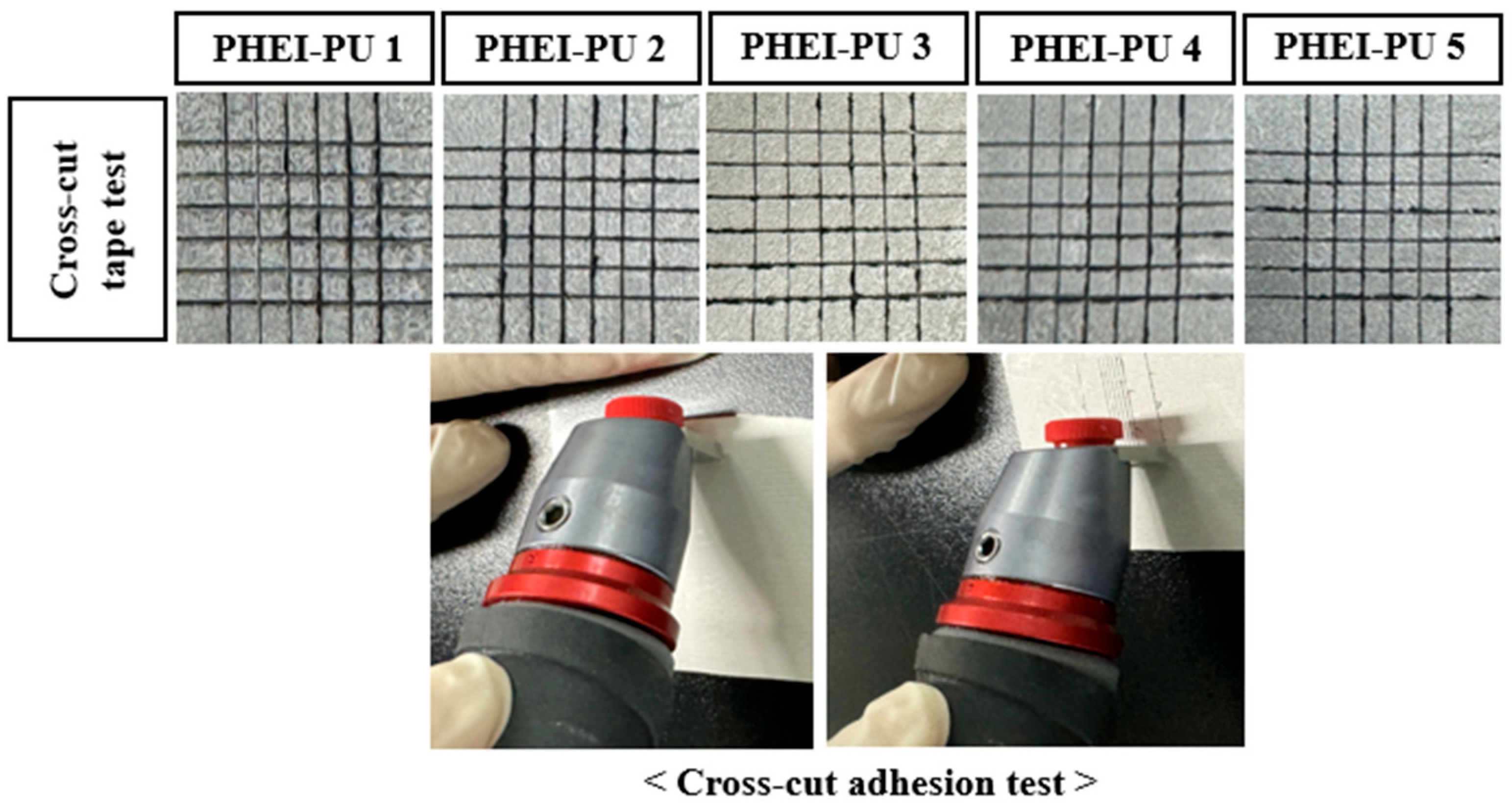
3.8. Adhesion Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czepiel, M.; Bankosz, M.; Sobczak-Kupiec, A. Advanced Injection Molding Methods: Review. Materials 2023, 16, 5802. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Song, Y.S. Analysis of in-mold coating process and ion beam irradiation. Prog. Org. Coat. 2019, 126, 28–34. [Google Scholar] [CrossRef]
- Cai, K.; Zhang, D.; Straus, E.J.; Villarreal, M.G.; Castro, J.S. Improving processability for in-mold coating formulations. Polym. Eng. Sci. 2022, 62, 83–94. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.S. An Experimental Study of In-Mold Coating of Automotive Armrests. Trans. Korean Soc. Mech. Eng. A 2015, 39, 687–692. [Google Scholar] [CrossRef]
- Kang, C.; Song, Y.S. Enhancement in Surface Property via In-Mold Coating Process. Macromol. Res. 2021, 29, 185–190. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Lee, H.S. Effects of Coating Thickness on Cavity Pressure and Surface Characteristics in In-Mold Coating. Polym. Korea 2018, 42, 13–19. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, W.J.; Kwon, B.S.; Nam, S.Y.; Choa, S.H. Highly stretchable and conductive conductors based on Ag flakes and polyester composites. Microelectron. Eng. 2018, 199, 16–23. [Google Scholar] [CrossRef]
- Lee, Y.C.; Rhee, H.J. Studies on the Optimizing the Properties of Thermoset Polyurethane Resin for In-mold Coating. Polym. Korea 2022, 46, 484–490. [Google Scholar] [CrossRef]
- Deringer, T.; Drummer, D. The influence of mold temperature on thermoset in-mold forming. J. Polym. Eng. 2019, 40, 256–266. [Google Scholar] [CrossRef]
- Nam, S.Y.; Kwon, B.S.; Nam, H.J.; Nam, K.W.; Park, H.Z. Properties of Stretchable Electrode Pattern Printed on Urethane Film. J. Korean Soc. Power Syst. Eng. 2018, 22, 64–71. [Google Scholar] [CrossRef]
- Kim, N.K.; Park, G.W.; Yu, J.K.; Kim, H.J.; Hyun, K. Effect of EVA-g-MAH on Water Absorption Properties of TPU/EVA Blends. Polym. Korea 2022, 46, 251–256. [Google Scholar] [CrossRef]
- Andersson, R.; Hernandez, G.; See, J.D.; Flaim, T.; Brandell, D.; Mindemark, J. Designing Polyurethane Solid Polymer Electrolytes for High-Temperature Lithium Metal Batteries. ACS Appl. Energy Mater. 2022, 5, 407–418. [Google Scholar] [CrossRef]
- Kim, K.S.; Choi, S.B.; Kim, D.U.; Lee, C.R.; Kim, J.W. Photo-induced healing of stretchable transparent electrodes based on thermoplastic polyurethane with embedded metallic nanowires. J. Mater. Chem. A 2018, 6, 12420–12429. [Google Scholar] [CrossRef]
- Leitsch, E.K.; Heath, W.H.; Torkelson, J.M. Polyurethane/polyhydroxyurethane hybrid polymers and their applications as adhesive bonding agents Author links open overlay panel. Int. J. Adhes. Adhes. 2016, 64, 1–8. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, B.; Tian, M.; Ning, N.; Wang, W. Synthesis and applications of bio-based waterborne polyurethane, a review. Prog. Org. Coat. 2024, 186, 108095. [Google Scholar] [CrossRef]
- Ha, Y.M.; Kim, Y.O.; Ahn, S.H.; Lee, S.K.; Lee, J.S.; Park, M.; Chung, J.W.; Jung, Y.C. Robust and stretchable self-healing polyurethane based on polycarbonate diol with different soft-segment molecular weight for flexible devices. Eur. Polym. J. 2019, 118, 36–44. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, C.W.; Chen, P.H.; Chen, Y.S.; Chuan, F.S.; Rewi, S.P. Characteristics of Polycarbonate Soft Segment-Based Thermoplastic Polyurethane. Appl. Sci. 2021, 11, 5359. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kang, M.S.; Knowles, J.C.; Gong, M.S. Synthesis of bio-based thermoplastic polyurethane elastomers containing isosorbide and polycarbonate diol and their biocompatible properties. J. Biomat Appl. 2015, 30, 327–337. [Google Scholar] [CrossRef]
- Lee, Y.H.; Moon, J.I.; Kim, H.J.; Lee, J.Y.; Noh, S.M.; Nam, J.H. Effect of Formability of Physical Properties of Polyester/Melamine Cured Coating Using Polycarbonate Diol with Various Molecular Weight. J. Adh. Interf. 2011, 12, 105–110. [Google Scholar]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Krol, P.; Uram, L.; Krol, B.; Pielichowska, K.; Sochacka-Pietal, M.; Walczak, M. Synthesis and property of polyurethane elastomer for biomedical applications based on nonaromatic isocyanates, polyesters, and ethylene glycol. Colloid Polym. Sci. 2020, 298, 1077–1093. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Podkoscielny, W. Studies on thermoplastic polyurethanes based on new diphenylethane-derivative diols. II. Synthesis and characterization of segmented polyurethanes from HDI and MDI. Eur. Polym. J. 2007, 43, 1402–1414. [Google Scholar] [CrossRef]
- Yoon, J.S.; Park, J.H.; Lee, G.M.; Choi, H.S.; Cho, Y.B.; Koh, S.B.; Cha, B.S. Gas Chromatographic Analysis of TDI, MDI and HDI Using 2-Chlorobenzyl Alcohol and 2,4-Dichlorobenzyl Alcohol Derivatives. J. Korean Soc. Occup. Environ. Hyg. 2006, 16, 222–232. [Google Scholar]
- Rogulska, M. The Influence of Diisocyanate Structure on Thermal Stability of Thermoplastic Polyurethane Elastomers Based on Diphenylmethane-Derivative Chain Extender with Sulfur Atoms. Materials 2023, 16, 2618. [Google Scholar] [CrossRef]
- Besse, V.; Auvergne, R.; Carlotti, S.; Boutevin, G.; Otazaghine, B.; Caillol, S.; Pascault, J.P.; Boutevin, B. Synthesis of isosorbide based polyurethanes: An isocyanate free method. React Funct. Polym. 2013, 73, 588–594. [Google Scholar] [CrossRef]
- Hong, S.M.; Cha, J.R.; Kim, J.G. Preparation of body-temperature-triggered shape-memory polyurethane with biocompatibility using isosorbide and castor oil. Polym. Test. 2020, 91, 106852. [Google Scholar] [CrossRef]
- Lee, C.H.; Takagi, H.; Okamoto, H.; Kato, M.; Usuki, A. Synthesis, characterization, and properties of polyurethanes containing 1,4:3,6-dianhydro-D-sorbitol. J. Polym. Sci. A Polym. Chem. 2009, 47, 6025–6031. [Google Scholar] [CrossRef]
- Oprea, S.; Potolinca, V.O.; Oprea, V. Synthesis and properties of new crosslinked polyurethane elastomers based on isosorbide. Eur. Polym. J. 2016, 83, 161–172. [Google Scholar] [CrossRef]
- Hong, S.M.; Kwon, H.J.; Lee, C.W. Synthesizing Polyurethane Using Isosorbide in Primary Alcohol Form, and Its Biocompatibility Properties. Polymers 2023, 15, 418. [Google Scholar] [CrossRef]
- Kamaruzaman, M.R.; Jiang, X.X.; Hu, X.D.; Chin, S.Y. High yield of isosorbide production from sorbitol dehydration catalysed by Amberlyst 36 under mild condition. Chem. Eng. J. 2020, 388, 124186. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Hong, S.M.; Yoon, J.Y.; Cha, J.R.; Ahn, J.Y.; Mandakhbayar, N.; Park, J.H.; Im, J.S.; Jin, G.S.; Kim, M.Y.; Knowles, J.C.; et al. Hyperelastic, shape-memorable, and ultra-cell-adhesive degradable polycaprolactone-polyurethane copolymer for tissue regeneration. Bioeng. Transl. Med. 2022, 7, e10332. [Google Scholar] [CrossRef]
- Li, Y.M.; Patal, K.D.; Han, Y.K.; Hong, S.M.; Meng, Y.X.; Lee, H.H.; Park, J.H.; Knowles, J.C.; Hyun, J.K.; Lee, J.H.; et al. Electroconductive and mechano-competent PUCL@CNT nanohybrid scaffolds guiding neuronal specification of neural stem/progenitor cells. Chem. Eng. J. 2023, 466, 143125. [Google Scholar] [CrossRef]
- Mou, y.; Wang, H.; Peng, Y.; Liu, J.; Cheng, H.; Sun, Q.; Chen, M. Low temperature enhanced flexible conductive film by Ag flake/ion composite ink. Mater. Des. 2020, 186, 108339. [Google Scholar] [CrossRef]
- Kim, S.H.; Jung, S.M.; Yoon, I.S.; Lee, C.H.; Oh, Y.S.; Hong, J.M. Ultrastretchable Conductor Fabricated on Skin-Like Hydrogel–Elastomer Hybrid Substrates for Skin Electronics. Adv. Mater. 2018, 30, 1800109. [Google Scholar] [CrossRef] [PubMed]
- Matshjisa, N.; Inoue, D.; Zalar, P.; Jin, H.; Matsuba, Y.; Itoh, A.; Yokota, T.; Hashizume, D.; Someya, T. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater. 2017, 16, 834–840. [Google Scholar] [CrossRef]
- Shahabadi, S.I.S.; Tan, J.M.R.; Magdassi, S. Thermoformable Conductive Compositions for Printed Electronics. Coatings 2023, 13, 1548. [Google Scholar] [CrossRef]
- Kang, S.Y.; Park, M.Y.; Jang, D.Y. Electro-mechanical Properties of Stretchable Ag Paste by the Difference of Ag Particles. J. Korean Soc. Manuf. Technol. Eng. 2019, 28, 188–192. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jang, S.H.; Lee, H.K.; Kim, J.S.; Lee, S.K.; Song, H.J.; Jung, J.W.; Yoo, E.S.; Choi, J. The development and investigation of highly stretchable conductive inks for 3-dimensional printed in-mold electronics. Org. Electron. 2020, 85, 105881. [Google Scholar] [CrossRef]
- Nam, H.M.; Sea, M.H.; Nam, S.Y. Stretchable Electrode Properties Study According to Particle Size of Flake-type Ag Powders. J. Microelectron. Packag. Soc. 2022, 29, 35–40. [Google Scholar]
- Standard EN ISO 2409; Paints and Varnishes-Cross Cut Test. 1997. Available online: https://www.iso.org/standard/76041.html (accessed on 5 January 2021).
- Yu, D.H.; Zhao, J.; Wang, W.J.; Qi, J.J.; Hu, Y. Mono-acrylated isosorbide as a bio-based monomer for the improvement of thermal and mechanical properties of poly(methyl methacrylate). RSC Adv. 2019, 9, 35532–35538. [Google Scholar] [CrossRef]
- Park, H.S.; Gong, M.S.; Knowles, J.C. Catalyst-free synthesis of high elongation degradable polyurethanes containing varying ratios of isosorbide and polycaprolactone: Physical properties and biocompatibility. J. Mater. Sci. Mater. Med. 2013, 24, 281–294. [Google Scholar] [CrossRef]
- Mayer-Trzaskowska, P.; Robakowska, M.; Gierz, L.; Pach, J.; Mazur, E. Observation of the Effect of Aging on the Structural Changes of Polyurethane/Polyurea Coatings. Polymers 2024, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Yang, J.C.; Gupta, A.S.; Lopina, S.T. Synthesis and characterization of L-tyrosine based polyurethanes for biomaterial applications. J. Biomed. Mater. Res. A 2009, 90, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Park, S.A.; Park, S.B.; Kwak, H.J.; Oh, D.X.; Hwang, D.S.; Jeon, H.Y.; Koo, J.M.; Park, J.Y. Biobased super engineering plastic nanocomposite of cellulose nanofibers and isosorbide. Polym. Degrad. Stab. 2023, 215, 110445. [Google Scholar] [CrossRef]
- Javni, I.; Bilic, O.; Bilic, N.; Petrovic, Z.S.; Eastwood, E.A.; Zhang, F.; Ilacsky, J. Thermoplastic polyurethanes with isosorbide chain extender. J. Appl. Polym. 2015, 132, 42830. [Google Scholar] [CrossRef]
- Chen, Z.; Hadjichristidis, N.; Feng, X.; Gnaniu, Y. Poly(urethane−carbonate)s from Carbon Dioxide. Macromolecules 2017, 50, 2320–2328. [Google Scholar] [CrossRef]
- Li, R.; Wu, Y.; Bai, Z.; Guo, J.; Chen, X. Effect of molecular weight of polyethylene glycol on crystallization behaviors, thermal properties and tensile performance of polylactic acid stereocomplexes. RSC Adv. 2020, 10, 42120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hosseinpourpia, R.; Biziks, V.; Ahmed, S.A.; Militz, H.; Adamopoulos, S. Preparation of Polyurethane Adhesives from Crude and Purified Liquefied Wood Sawdust. Polymers 2021, 13, 3267. [Google Scholar] [CrossRef]

| PU | HDI (g) | PCD (Mw 2000) (g) | ISBD (g) | Yield (%) |
|---|---|---|---|---|
| Mole Ratio/Weight (g) | ||||
| PHEI-PU 1 | 9/(9) | 9/(107.0) | 1/(2.2) | 95 |
| PHEI-PU 2 | 9/(9) | 7/(83.2) | 3/(6.5) | 93 |
| PHEI-PU 3 | 9/(9) | 5/(59.5) | 5/(10.9) | 94 |
| PHEI-PU 4 | 9/(9) | 3/(35.7) | 7/(15.3) | 92 |
| PHEI-PU 5 | 9/(9) | 1/(11.9) | 9/(19.6) | 92 |
| PU | Ag Flake | Binder | Solvent |
|---|---|---|---|
| PU/AGF blend | 70 wt% | 9 wt% | 21 wt% |
| Classification | 5B | 4B | 3B | 2B | 1B | 0B |
|---|---|---|---|---|---|---|
| % of Area Removed | 0% | Less than 5% | 5–15% | 15–35% | 35–65% | Greater than 65% |
| PU | GPC | ||
|---|---|---|---|
| Mw | Mn | PDI | |
| PHEI-PU 1 | 82,800 | 54,000 | 1.524 |
| PHEI-PU 2 | 72,600 | 45,520 | 1.595 |
| PHEI-PU 3 | 61,500 | 38,850 | 1.583 |
| PHEI-PU 4 | 53,000 | 33,440 | 1.585 |
| PHEI-PU 5 | 44,200 | 27,900 | 1.584 |
| PU | Viscosity |
|---|---|
| cP (mPa·s) | |
| PHEI-PU 1 | 7300 |
| PHEI-PU 2 | 5700 |
| PHEI-PU 3 | 3420 |
| PHEI-PU 4 | 2570 |
| PHEI-PU 5 | 1930 |
| PU | Before Stretching | After Stretching (50%) | Change Rate |
|---|---|---|---|
| PHEI-PU 1 | 5.33 × 10−5 Ωcm | 5.88 × 10−5 Ωcm | 10.32% |
| PHEI-PU 2 | 6.03 × 10−5 Ωcm | 7.08 × 10−5 Ωcm | 11.59% |
| PHEI-PU 3 | 7.42 × 10−5 Ωcm | 8.38 × 10−5 Ωcm | 12.94% |
| PHEI-PU 4 | 9.22 × 10−5 Ωcm | 10.75 × 10−5 Ωcm | 16.60% |
| PHEI-PU 5 | 13.32 × 10−5 Ωcm | 15.95 × 10−5 Ωcm | 19.75% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-M.; Kwon, H.-J.; Sun, J.-M.; Lee, C.W. Synthesis and Characteristic Valuation of a Thermoplastic Polyurethane Electrode Binder for In-Mold Coating. Polymers 2024, 16, 375. https://doi.org/10.3390/polym16030375
Hong S-M, Kwon H-J, Sun J-M, Lee CW. Synthesis and Characteristic Valuation of a Thermoplastic Polyurethane Electrode Binder for In-Mold Coating. Polymers. 2024; 16(3):375. https://doi.org/10.3390/polym16030375
Chicago/Turabian StyleHong, Suk-Min, Hyuck-Jin Kwon, Jung-Min Sun, and Chil Won Lee. 2024. "Synthesis and Characteristic Valuation of a Thermoplastic Polyurethane Electrode Binder for In-Mold Coating" Polymers 16, no. 3: 375. https://doi.org/10.3390/polym16030375
APA StyleHong, S.-M., Kwon, H.-J., Sun, J.-M., & Lee, C. W. (2024). Synthesis and Characteristic Valuation of a Thermoplastic Polyurethane Electrode Binder for In-Mold Coating. Polymers, 16(3), 375. https://doi.org/10.3390/polym16030375






