Avocado Seed Starch-Based Films Reinforced with Starch Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Avocado Starch Production
2.3. Avocado Starch Nanocrystal Elaboration
2.4. Avocado-Starch-Based Film Formation
2.5. Avocado Starch and Starch Nanocrystal Characterization
2.6. Film Characterization
3. Results and Discussion
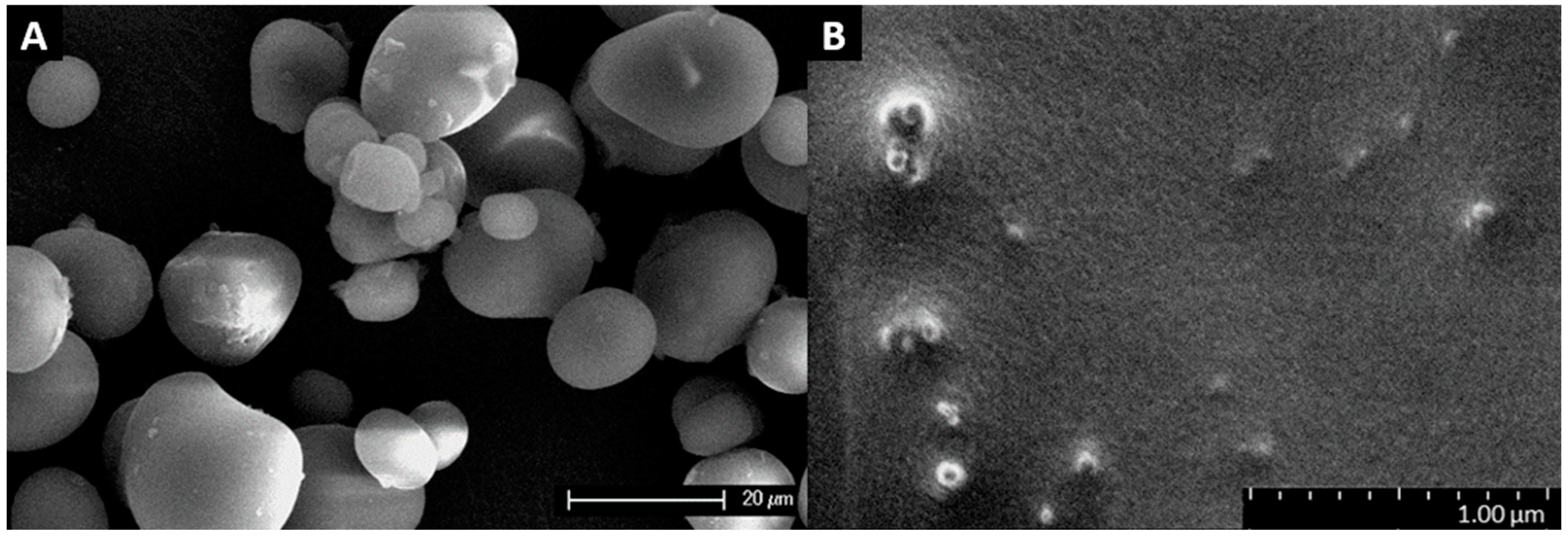
3.1. Starch Granule and Nanocrystal Characterization
3.2. Film Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, G.; Song, J.H. Biodegradable Packaging Based on Raw Materials from Crops and Their Impact on Waste Management. Ind. Crops Prod. 2006, 23, 147–161. [Google Scholar] [CrossRef]
- Europian Bioplatics December 2023 Bioplastics Market Development Update 2023. Available online: https://www.european-bioplastics.org/market/ (accessed on 4 March 2024).
- Lalnunthari, C.; Devi, L.M.; Amami, E.; Badwaik, L.S. Valorisation of Pumpkin Seeds and Peels into Biodegradable Packaging Films. Food Bioprod. Process. 2019, 118, 58–66. [Google Scholar] [CrossRef]
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado Seed: A Comparative Study of Antioxidant Content and Capacity in Protecting Oil Models from Oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef]
- Tesfaye, T.; Ayele, M.; Gibril, M.; Ferede, E.; Limeneh, D.Y.; Kong, F. Beneficiation of Avocado Processing Industry By-Product: A Review on Future Prospect. Curr. Res. Green Sustain. Chem. 2022, 5, 100253. [Google Scholar] [CrossRef]
- Kringel, D.H.; Dias, A.R.G.; Zavareze, E.d.R.; Gandra, E.A. Fruit Wastes as Promising Sources of Starch: Extraction, Properties, and Applications. Starch/Staerke 2020, 72, 1900200. [Google Scholar] [CrossRef]
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An Untapped Resource for Food Packaging. Front. Microbiol. 2017, 8, 1735. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.E.; García, M.A.; Castillo, L.; Lopez, O.V.; Villar, M. Starch-Based Materials in Food Packaging: Processing, Characterization and Applications; Academic Press: Cambridge, MA, USA, 2017; Volume 4, ISBN 978-0-12-809439-6/0128094397. [Google Scholar]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of Glycerol Plasticizer Loading on the Physical, Mechanical, Thermal, and Barrier Properties of Arrowroot (Maranta Arundinacea) Starch Biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Ferreira, D.C.; Louzada, L.B.; Dos Santos, F.; Corrêa, A.C.; Moreira, F.K.V.; Mattoso, L.H. Starch-Based Edible Films and Coatings: An Eco-Friendly Alternative for Food Packaging; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128094402. [Google Scholar]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef]
- Guo, A.; Li, J.; Li, F.; Xu, J. Comparison of Single/Compound Plasticizer to Prepare Thermoplastic Starch in Starch-Based Packaging Composites. Medziagotyra 2019, 25, 183–189. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Staciwa, P.; Jedrzejewski, R.; Spychaj, T. Sugar Alcohol-Based Deep Eutectic Solvents as Potato Starch Plasticizers. Polymers 2019, 11, 1385. [Google Scholar] [CrossRef]
- Santhosh, R.; Ahmed, J.; Thakur, R.; Sarkar, P. Starch-Based Edible Packaging: Rheological, Thermal, Mechanical, Microstructural, and Barrier Properties—A Review. Sustain. Food Technol. 2024, 2, 307–330. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Oleyaei, S.A.; Almasi, H. Nanostructured Materials Utilized in Biopolymer-Based Plastics for Food Packaging Applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1699–1723. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Gimena, P.F.; Oliver-Cuenca, V.; Peponi, L.; López, D. A Review on Reinforcements and Additives in Starch-Based Composites for Food Packaging. Polymers 2023, 15, 2972. [Google Scholar] [CrossRef]
- Xie, F.; Pollet, E.; Halley, P.J.; Avérous, L. Starch-Based Nano-Biocomposites. Prog. Polym. Sci. 2013, 38, 1590–1628. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Basiura-Cembala, M.; Materska, M.; Łupina, K. Corn Starch and Methylcellulose Edible Films Incorporated with Fireweed (Chamaenerion angustifolium L.) Extract: Comparison of Physicochemical and Antioxidant Properties. Int. J. Biol. Macromol. 2021, 190, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, J.C.F.; Monteiro, A.R.G.; Scapim, M.R.S.; Monteiro, C.C.F.; Morais, D.R.; Claus, T.; Visentainer, J.V.; Yamashita, F. Development of an Active Biodegradable Film Containing Tocopherol and Avocado Peel Extract. Ital. J. Food Sci. 2015, 27, 468–475. [Google Scholar]
- Vianna, T.C.; Marinho, C.O.; Marangoni Júnior, L.; Ibrahim, S.A.; Vieira, R.P. Essential Oils as Additives in Active Starch-Based Food Packaging Films: A Review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]
- Bertling, I.; Tesfay, S.; Bower, J. Antioxidants in “Hass” Avocado. S. Afr. Avocado Grow. Asoc. 2007, 30, 17–19. [Google Scholar]
- Chel-Guerrero, L.; Barbosa-Martín, E.; Martínez-Antonio, A.; González-Mondragón, E.; Betancur-Ancona, D. Some Physicochemical and Rheological Properties of Starch Isolated from Avocado Seeds. Int. J. Biol. Macromol. 2016, 86, 302–308. [Google Scholar] [CrossRef]
- Angellier, H.; Choisnard, L.; Molina-Boisseau, S.; Ozil, P.; Dufresne, A. Optimization of the Preparation of Aqueous Suspensions of Waxy Maize Starch Nanocrystals Using a Response Surface Methodology. Biomacromolecules 2004, 5, 1545–1551. [Google Scholar] [CrossRef]
- Hoover, R.; Ratnayake, W.S. Determination of Total Amylose Content of Starch. Curr. Protoc. Food Anal. Chem. 2001, 1, E2–E3. [Google Scholar] [CrossRef]
- ASTM D3985-17; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. American Society for Testing and Materials: Philadelphia, PA, USA, 2024.
- European Commission. Commission Regulation (Eu) No. 10, 2011 of 14, on Plastic Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2011, L12, 1–89. [Google Scholar]
- ISO 20200:2015; Plastics—Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test. International Organization for Standardization: Geneva, Switzerland, 2015; pp. 1–8.
- Esquivel-Fajardo, E.A.; Martinez-Ascencio, E.U.; Oseguera-Toledo, M.E.; Londoño-Restrepo, S.M.; Rodriguez-García, M.E. Influence of Physicochemical Changes of the Avocado Starch throughout Its Pasting Profile: Combined Extraction. Carbohydr. Polym. 2022, 281, 119048. [Google Scholar] [CrossRef]
- Pires, J.B.; Santos, F.N.d.; Cruz, E.P.d.; Fonseca, L.M.; Siebeneichler, T.J.; Lemos, G.S.; Gandra, E.A.; Zavareze, E.d.R.; Dias, A.R.G. Starch Extraction from Avocado By-Product and Its Use for Encapsulation of Ginger Essential Oil by Electrospinning. Int. J. Biol. Macromol. 2024, 254, 127617. [Google Scholar] [CrossRef] [PubMed]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of Starch Type on the Physico-Chemical Properties of Edible Films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Montilla-Buitrago, C.E.; Gómez-López, R.A.; Solanilla-Duque, J.F.; Serna-Cock, L.; Villada-Castillo, H.S. Effect of Plasticizers on Properties, Retrogradation, and Processing of Extrusion-Obtained Thermoplastic Starch: A Review. Starch-Stärke 2021, 73, 2100060. [Google Scholar] [CrossRef]
- Silva, A.P.M.; Oliveira, A.V.; Pontes, S.M.A.; Pereira, A.L.S.; Souza Filho, M.d.s.M.; Rosa, M.F.; Azeredo, H.M.C. Mango Kernel Starch Films as Affected by Starch Nanocrystals and Cellulose Nanocrystals. Carbohydr. Polym. 2019, 211, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Castillo, L.E.; Leite, M.A.; Ditchfield, C.; Sobral, P.J.d.A.; Moraes, I.C.F. Quinoa Starch Nanocrystals Production by Acid Hydrolysis: Kinetics and Properties. Int. J. Biol. Macromol. 2020, 143, 93–101. [Google Scholar] [CrossRef]
- Velásquez-Castillo, L.E.; Leite, M.A.; Tisnado, V.J.A.; Ditchfield, C.; Sobral, P.J.d.A.; Moraes, I.C.F. Cassava Starch Films Containing Quinoa Starch Nanocrystals: Physical and Surface Properties. Foods 2023, 12, 576. [Google Scholar] [CrossRef]
- Yogananda, K.C.; Ramasamy, E.; Vasantha Kumar, S.; Rangappa, D. Synthesis, Characterization, and Dye-Sensitized Solar Cell Fabrication Using Potato Starch– and Potato Starch Nanocrystal–Based Gel Electrolytes. Ionics 2019, 25, 6035–6042. [Google Scholar] [CrossRef]
- LeCorre, D.; Bras, J.; Dufresne, A. Influence of Native Starch’s Properties on Starch Nanocrystals Thermal Properties. Carbohydr. Polym. 2012, 87, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Park, S.S.; Lim, S.T. Preparation, Characterization and Utilization of Starch Nanoparticles. Colloids Surf. B Biointerfaces 2015, 126, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.G.; Colman, T.A.D.; Bauab, T.; Da Silva Carvalho Filho, M.A.; Demiate, I.M.; De Vasconcelos, E.C.; Schnitzler, E. Thermal, Structural and Rheological Properties of Starch from Avocado Seeds (Persea Americana, Miller) Modified with Standard Sodium Hypochlorite Solutions. J. Therm. Anal. Calorim. 2014, 115, 1893–1899. [Google Scholar] [CrossRef]
- De Dios-Avila, N.; Tirado-Gallegos, J.M.; Rios-Velasco, C.; Luna-Esquivel, G.; Isiordia-Aquino, N.; Zamudio-Flores, P.B.; Estrada-Virgen, M.O.; Cambero-Campos, O.J. Physicochemical, Structural, Thermal and Rheological Properties of Flour and Starch Isolated from Avocado Seeds of Landrace and Hass Cultivars. Molecules 2022, 27, 910. [Google Scholar] [CrossRef]
- LeCorre, D.; Bras, J.; Dufresne, A. Influence of Botanic Origin and Amylose Content on the Morphology of Starch Nanocrystals. J. Nanopart. Res. 2011, 13, 7193–7208. [Google Scholar] [CrossRef]
- Bel Haaj, S.; Thielemans, W.; Magnin, A.; Boufi, S. Starch Nanocrystals and Starch Nanoparticles from Waxy Maize as Nanoreinforcement: A Comparative Study. Carbohydr. Polym. 2016, 143, 310–317. [Google Scholar] [CrossRef]
- Jiménez, R.; Sandoval-Flores, G.; Alvarado-Reyna, S.; Alemán-Castillo, S.E.; Santiago-Adame, R.; Velázquez, G. Extraction of Starch from Hass Avocado Seeds for the Preparation of Biofilms. Food Sci. Technol. 2022, 42, e56820. [Google Scholar] [CrossRef]
- García-Tejeda, Y.V.; Zamudio-Flores, P.B.; Bello-Pérez, L.A.; Romero-Bastida, C.A.; Solorza-Feria, J. Oxidación Del Almidón Nativo De Plátano Para Su Uso Potencial En La Fabricación De Materiales De Empaque Biodegradables: Caracterización Física, Química, Térmica Y Morfológica. Rev. Iberoam. Polim 2011, 12, 125–135. [Google Scholar]
- Tang, X.; Alavi, S.; Herald, T.J. Effects of Plasticizers on the Structure and Properties of Starch–Clay Nanocomposite Films. Carbohydr. Polym. 2008, 74, 552–558. [Google Scholar] [CrossRef]
- Zakaria, N.H.; Muhammad, N.; Abdullah, M.M.A.B. Effect of Glycerol Content on Mechanical, Microstructure and Physical Properties of Thermoplastic Potato Starch Film. AIP Conf. Proc. 2018, 2030, 020230. [Google Scholar] [CrossRef]
- Fan, H.; Ji, N.; Zhao, M.; Xiong, L.; Sun, Q. Characterization of Starch Films Impregnated with Starch Nanoparticles Prepared by 2,2,6,6-Tetramethylpiperidine-1-Oxyl (TEMPO)-Mediated Oxidation. Food Chem. 2016, 192, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Yadav, B.S.; Yadav, R. Development and Characterization of Mung Bean Starch–Based Composite Films Incorporated with Sweet Potato Starch Nanocrystals for Their Morphological and Thermo-Mechanical Properties. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Paluch, M.; Ostrowska, J.; Tyński, P.; Sadurski, W.; Konkol, M. Structural and Thermal Properties of Starch Plasticized with Glycerol/Urea Mixture. J. Polym. Environ. 2022, 30, 728–740. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable Polymer Blends Based on Corn Starch and Thermoplastic Chitosan Processed by Extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, C.; Ji, N.; Sun, C.; Xiong, L.; Sun, Q. Mechanical, Barrier and Morphological Properties of Starch Nanocrystals-Reinforced Pea Starch Films. Carbohydr. Polym. 2015, 121, 155–162. [Google Scholar] [CrossRef]
- Dai, M.; Xiong, X.; Cheng, A.; Zhao, Z.; Xiao, Q. Development of Pullulan-Based Nanocomposite Films Reinforced with Starch Nanocrystals for the Preservation of Fresh Beef. J. Sci. Food Agric. 2023, 103, 1981–1993. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Caturla, J.; Ivorra-Martinez, J.; Quiles-Carrillo, L.; Balart, R.; Garcia-Garcia, D.; Dominici, F.; Puglia, D.; Torre, L. Improvement of the Barrier and Mechanical Properties of Environmentally Friendly Mango Kernel Flour/Glycerol Films by Varying the Particle Size of Mango Kernel Flour. Ind. Crops Prod. 2022, 188, 115668. [Google Scholar] [CrossRef]
- Merino, D.; Bertolacci, L.; Paul, U.C.; Simonutti, R.; Athanassiou, A. Avocado Peels and Seeds: Processing Strategies for the Development of Highly Antioxidant Bioplastic Films. ACS Appl. Mater. Interfaces 2021, 13, 38688–38699. [Google Scholar] [CrossRef]
- Hu, H.; Yong, H.; Yao, X.; Yun, D.; Huang, J.; Liu, J. Highly Efficient Synthesis and Characterization of Starch Aldehyde-Catechin Conjugate with Potent Antioxidant Activity. Int. J. Biol. Macromol. 2021, 173, 13–25. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Andrade, M.J.; Kylli, P.; Estevez, M. Avocado (Persea Americana Mill.) Phenolics, in Vitro Antioxidant and Antimicrobial Activities, and Inhibition of Lipid and Protein Oxidation in Porcine Patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of Bioactive Compounds in Avocado (Persea Americana) and Their Health Benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, K.; Nabila, A.; Aktar, A.; Farahnaky, A. Bioactive Variability and in Vitro and in Vivo Antioxidant Activity of Unprocessed and Processed Flour of Nine Cultivars of Australian Lupin Species: A Comprehensive Substantiation. Antioxidants 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Plaza, L.; Sánchez-Moreno, C.; De Pascual-Teresa, S.; De Ancos, B.; Cano, M.P. Fatty Acids, Sterols, and Antioxidant Activity in Minimally Processed Avocados during Refrigerated Storage. J. Agric. Food Chem. 2009, 57, 3204–3209. [Google Scholar] [CrossRef] [PubMed]
- Lye, H.S.; Ong, M.K.; Teh, L.K.; Chang, C.C.; Wei, L.K. Avocado. In Valorization of Fruit Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–93. ISBN 9780128171066. [Google Scholar]
- Sessini, V.; Arrieta, M.P.; Kenny, J.M.; Peponi, L. Processing of Edible Films Based on Nanoreinforced Gelatinized Starch. Polym. Degrad. Stab. 2016, 132, 157–168. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, C.; Wu, D.; Xie, W.; Wang, Z. Crystallization of Green Poly(ε-Caprolactone) Nanocomposites with Starch Nanocrystal: The Nucleation Role Switching of Starch Nanocrystal with Its Surface Acetylation. Ind. Eng. Chem. Res. 2018, 57, 6257–6264. [Google Scholar] [CrossRef]







| Film | Water (g) | ASS (g) | Glycerol (g) | SNCs (g) |
|---|---|---|---|---|
| Gly0 | 30 | 0.3 | - | - |
| Gly15 | 30 | 0.3 | 0.045 | - |
| Gly25 | 30 | 0.3 | 0.075 | - |
| Gly35 | 30 | 0.3 | 0.105 | - |
| Gly45 | 30 | 0.3 | 0.135 | - |
| Gly35-1SNC | 30 | 0.3 | 0.105 | 0.003 |
| Gly35-3SNC | 30 | 0.3 | 0.105 | 0.009 |
| Gly35-5SNC | 30 | 0.3 | 0.105 | 0.015 |
| Sample | Thickness (µm) | L | a | b |
|---|---|---|---|---|
| Gly0 | 46.0 ± 2.0 | 84.62 ± 0.32 | 0.82 ± 0.34 | 21.38 ± 0.68 |
| Gly15 | 53.0 ± 1.2 | 84.70 ± 0.76 | 1.04 ± 0.33 | 21.77 ± 1.83 |
| Gly25 | 57.8 ± 0.4 | 84.38 ± 0.64 | 1.04 ± 0.21 | 21.29 ± 0.76 |
| Gly35 | 59.3 ± 1.7 | 84.37 ± 0.66 | 1.08 ± 0.28 | 21.96 ± 1.42 |
| Gly45 | 61.0 ± 4.0 | 84.98 ± 0.31 | 0.68 ± 0.22 | 21.44 ± 1.43 |
| Gly35-1SNC | 53.7 ± 3.7 | 83.70 ± 1.16 | 0.58 ± 0.26 | 20.06 ± 1.26 |
| Gly35-3SNC | 56.6 ± 1.0 | 83.37 ± 0.31 | 1.01 ± 0.19 | 20.46 ± 1.34 |
| Gly35-5SNC | 61.4 ± 4.7 | 82.80 ± 0.89 | 1.24 ± 0.13 | 21.01 ± 0.72 |
| Film | Elastic Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| (a) Mechanical properties of avocado seed starch (ASS)-based films based on the effect of glycerol. | |||
| Gly15 | 1246.8 ± 172.8 a | 17.5 ± 1.1 a | 1.3 ± 0.7 a |
| Gly25 | 420.1 ± 269.8 b | 4.8 ± 1.1 b | 15.0 ± 7.5 b |
| Gly35 | 177.5 ± 42.7 c | 4.5 ± 0.3 b | 24.9 ± 1.7 c |
| Gly45 | 109.3 ± 89.9 c | 3.7 ± 1.1 b | 22.5 ± 5.9 c |
| F ratio | 6.026 | 2.535 | 8.691 |
| p-value | 0.004 * | 0.091 * | 0.0005 * |
| (b) Mechanical properties of avocado seed starch (ASS)-based films based on the effect of starch nanocrystals (SNCs). | |||
| Gly35 | 177.5 ± 42.7 a | 4.5 ± 0.3 a,b | 24.9 ± 1.7 a |
| Gly35-1SNC | 376.6 ± 126.9 b | 6.4 ± 1.4 a | 16.7 ± 1.7 b |
| Gly35-3SNC | 209.0 ± 113.6 a | 4.8 ± 1.4 a,b | 24.9 ± 4.2 a |
| Gly35-5SNC | 133.4 ± 69.8 a | 4.1 ± 1.3 b | 24.9 ± 3.8 a |
| F ratio | 1.264 | 4.014 | 2.97 |
| p-value | 0.312 * | 0.021 * | 0.065 * |
| Reference | Gly0 | Gly15 | Gly25 | Gly35 | Gly45 | Gly35-1SNC | Gly35-3SNC | Gly35-5SNC |
|---|---|---|---|---|---|---|---|---|
| WVP (g/mm.d.KPa) | - | 5.1 ± 0.2 | 9.1 ± 1.7 | 13.5 ± 1.7 | 15.1 ± 0.8 | 8.8 ± 1.8 | 7.3 ± 1.2 | 10 ± 0.7 |
| OTR(mL/m2 day) | >4000 * | >4000 * | >4000 * | 11.0 ± 4.3 | 18.7 ± 2.6 | 9.8± 0.8 | 8.2 ± 3.0 | 12.2 ± 3.1 |
| tr (Min) | Proposed Compound | m/z | Molecular Formula |
|---|---|---|---|
| 9.53 | Avocadynofuran | 248.10 | C17H26O |
| 10.01 | (E)-Avocadynofuran | 246.20 | C17H26O |
| 10.39 | Avocadynofuran | 244.18 | C17H24O |
| 11.29 | n-Hexadecenoic acid | 256.24 | C16H32O2 |
| 11.55 | 2-(Pentadec-14-yn-1-yl)furan | 274.23 | C19H30O |
| 11.59 | (E)-2-(Pentadec-2-en-1-yl)furan | 276.25 | C19H32O |
| 11.65 | 2-Pentadecylfuran | 278.26 | C19H34O |
| 12.40 | (E)-2-(Pentadeca-1,14-dien-1-yl)furan | 274.23 | C19H30O |
| 12.46 | (Z)-2-(Pentadec-1-en-1-yl)furan | 276.25 | C19H32O |
| 13.18 | 9,12-Octadecadienoic acid (Z,Z)- | 280.24 | C18H32O2 |
| 13.50 | 2-((8Z,11Z)-Heptadeca-8,11-dien-1-yl)furan | 302.26 | C21H34O |
| 14.22 | 2-((1E,8Z,11Z)-Heptadeca-1,8,11-trien-1-yl)furan | 300.25 | C21H32O |
| 23.12 | β-Sitosterol | 414.39 | C29H50O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Gimena, P.F.; Aragón-Gutiérrez, A.; Blázquez-Blázquez, E.; Arrieta, M.P.; Rodríguez, G.; Peponi, L.; López, D. Avocado Seed Starch-Based Films Reinforced with Starch Nanocrystals. Polymers 2024, 16, 2868. https://doi.org/10.3390/polym16202868
Muñoz-Gimena PF, Aragón-Gutiérrez A, Blázquez-Blázquez E, Arrieta MP, Rodríguez G, Peponi L, López D. Avocado Seed Starch-Based Films Reinforced with Starch Nanocrystals. Polymers. 2024; 16(20):2868. https://doi.org/10.3390/polym16202868
Chicago/Turabian StyleMuñoz-Gimena, Pedro Francisco, Alejandro Aragón-Gutiérrez, Enrique Blázquez-Blázquez, Marina Patricia Arrieta, Gema Rodríguez, Laura Peponi, and Daniel López. 2024. "Avocado Seed Starch-Based Films Reinforced with Starch Nanocrystals" Polymers 16, no. 20: 2868. https://doi.org/10.3390/polym16202868
APA StyleMuñoz-Gimena, P. F., Aragón-Gutiérrez, A., Blázquez-Blázquez, E., Arrieta, M. P., Rodríguez, G., Peponi, L., & López, D. (2024). Avocado Seed Starch-Based Films Reinforced with Starch Nanocrystals. Polymers, 16(20), 2868. https://doi.org/10.3390/polym16202868












