Study of Styrene Butadiene Rubber Reinforced by Polybutadiene Liquid Rubber-Modified Silica
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ITPB-Modified Silica
2.3. Preparation of Silica/SBR Composites
2.4. FTIR Spectroscopy Analysis
2.5. Thermogravimetric Analysis (TGA)
2.6. BET Surface Area Analysis
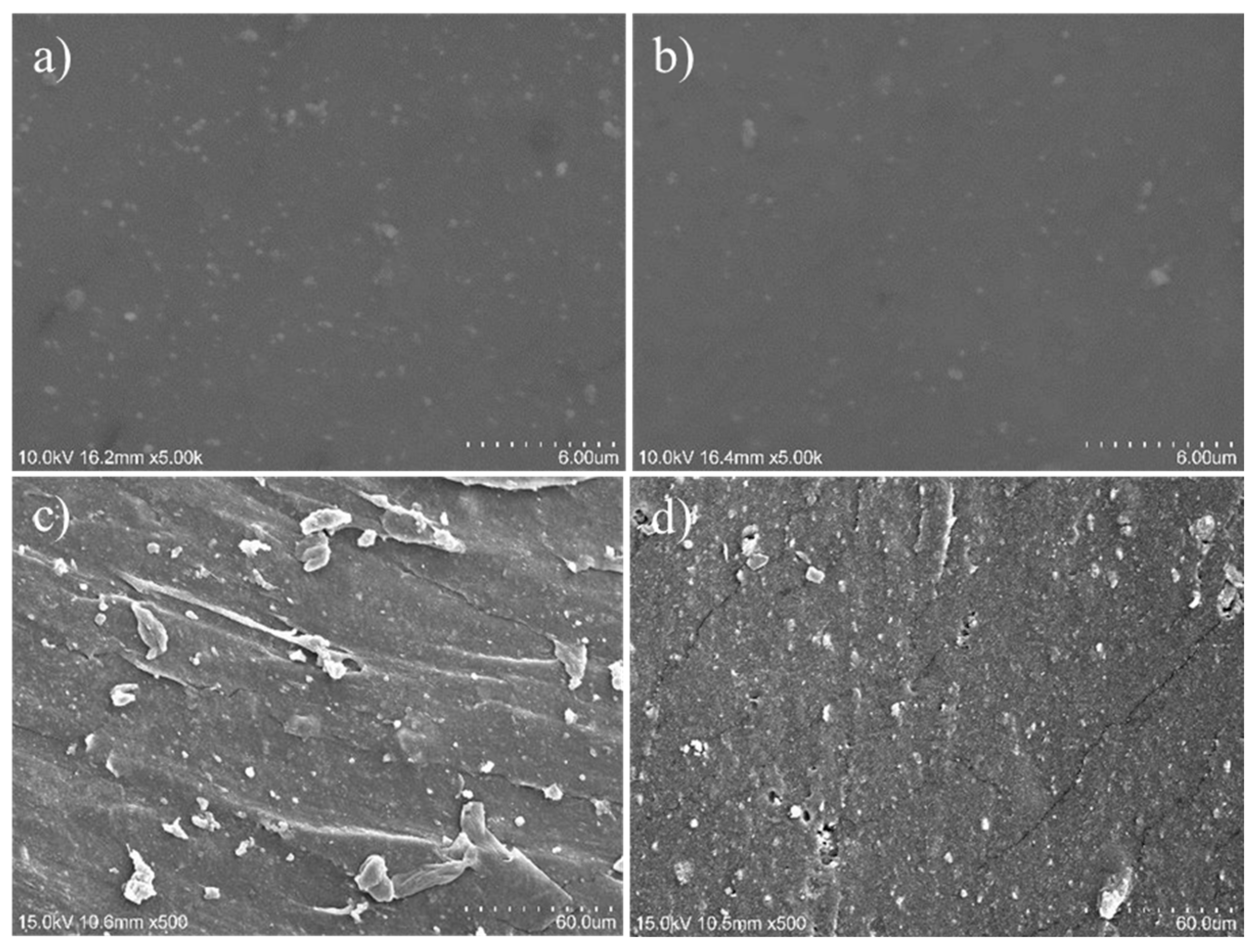
2.7. Scanning Electron Microscopy (SEM)
2.8. Analysis of Rubber Curing Characteristics
2.9. Crosslinking Density
2.10. Mechanical Properties
2.11. Dynamic Mechanical Analysis (DMA)
3. Results
3.1. Characterization of ITPB-Modified Silica
3.1.1. FTIR Analysis
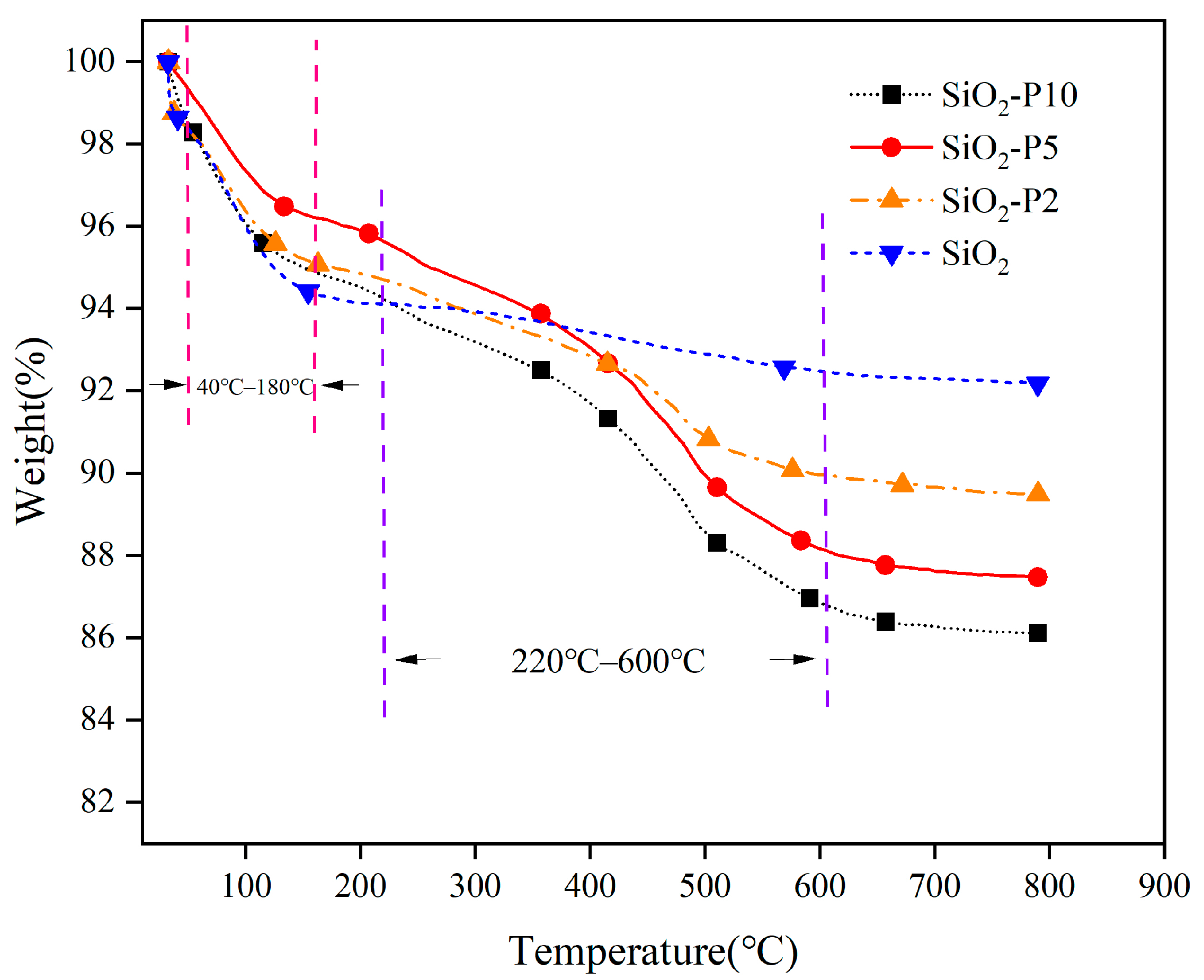
3.1.2. Thermogravimetric Analysis (TGA)
3.1.3. Surface Area Analysis
3.2. Tests of ITPB-Modified Silica for Enhanced SBR
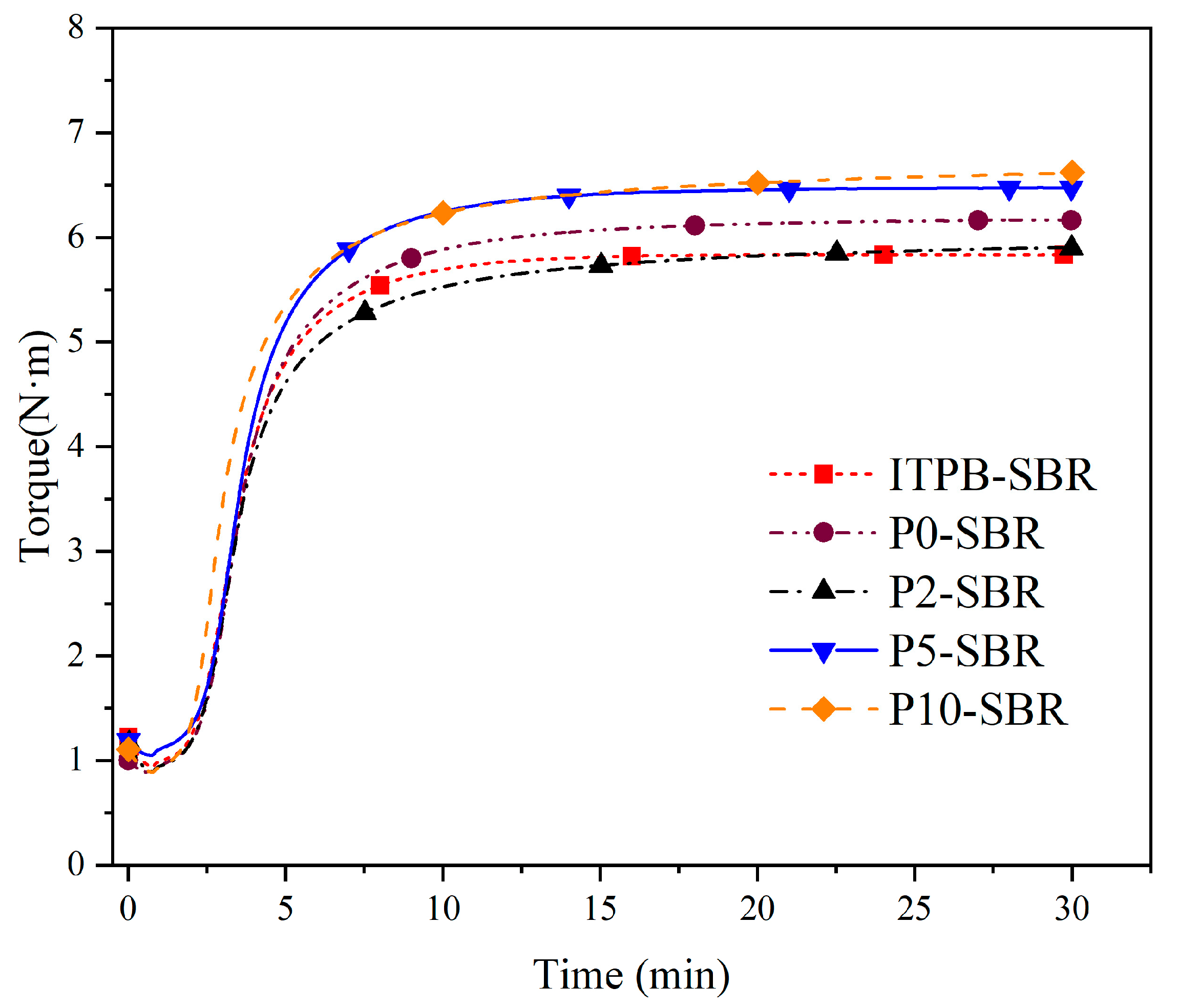
3.2.1. Curing Characteristics
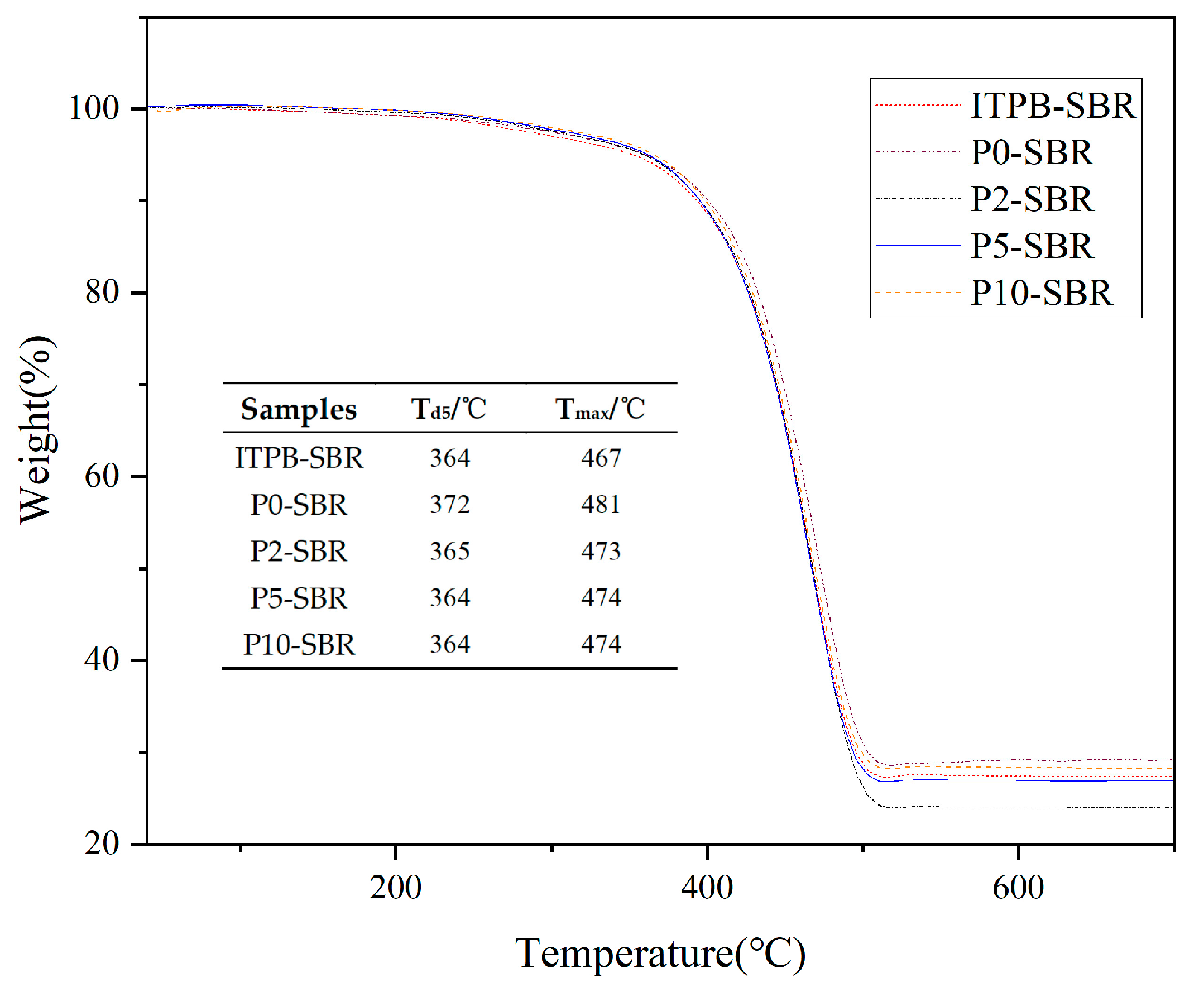
3.2.2. Thermal Stability Analysis
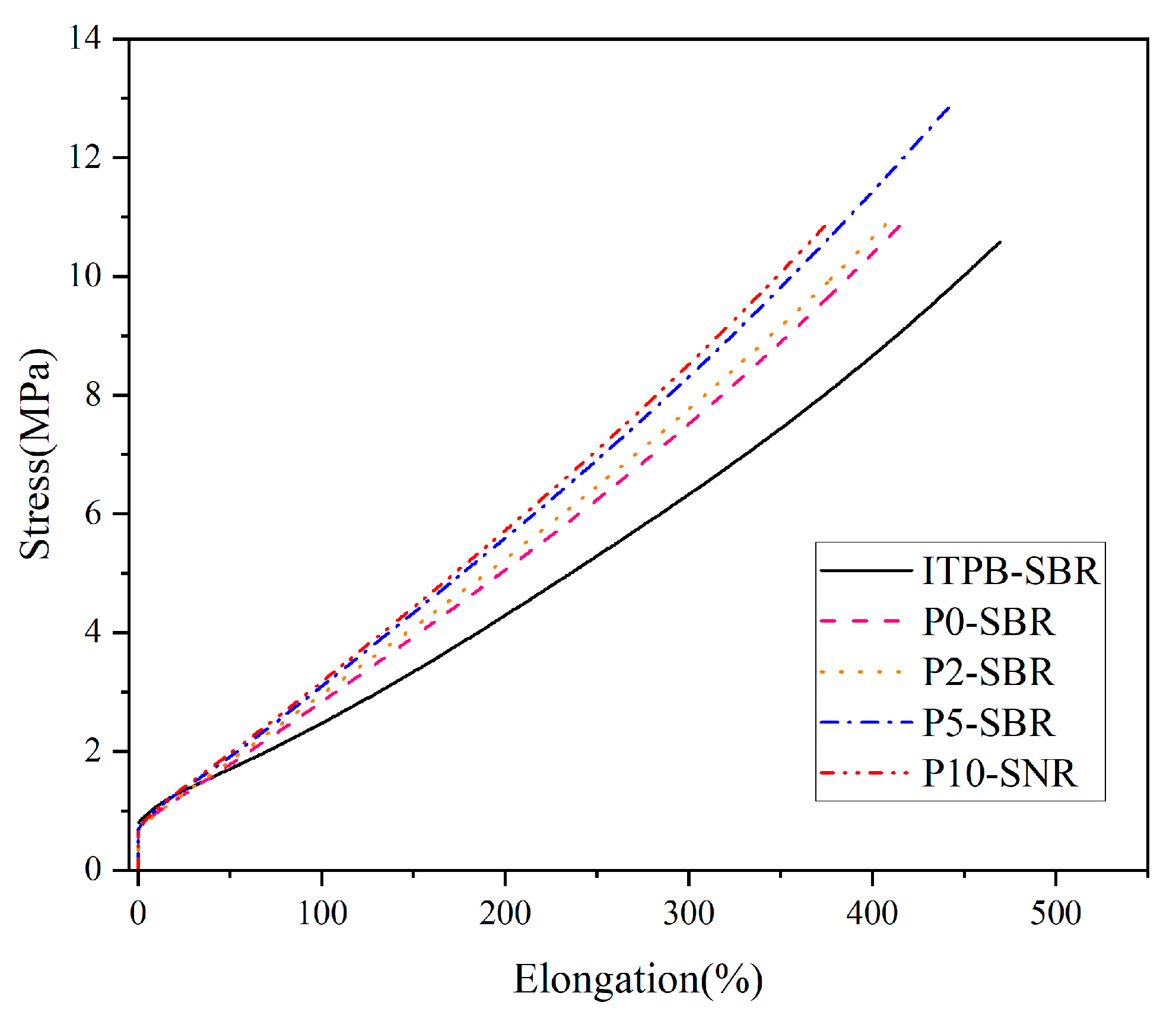
3.2.3. Mechanical Properties
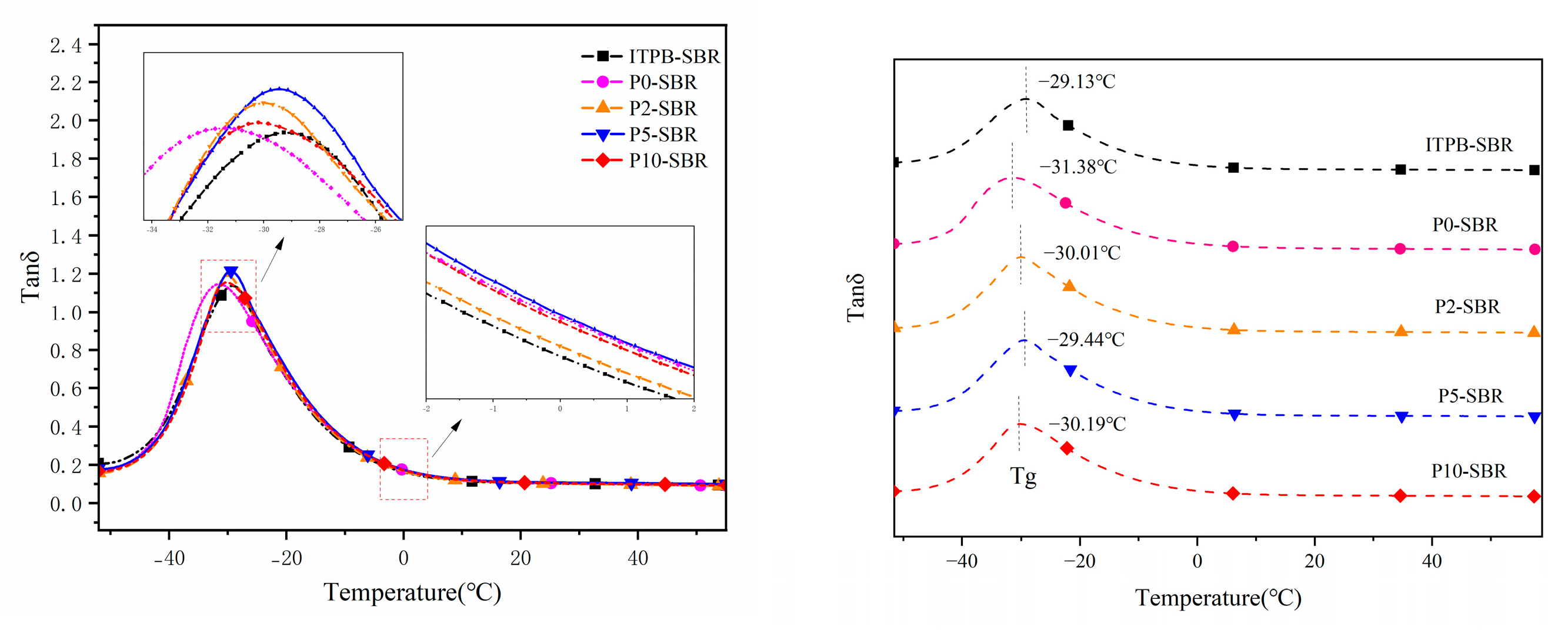
3.2.4. Dynamic Mechanical Thermal Performance Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, B.; Xu, Y.; Zhang, L.; Zhou, X.; Zhang, H.; Liu, L.; Zhao, J. Soft Feel Material Coatings on the Surface of Plastic Products and Their Application Prospects in the Popular Fields: A Review. Coatings 2024, 14, 748. [Google Scholar] [CrossRef]
- Qu, Y.-X.; Xia, Q.-Q.; Li, L.-T.; Cao, C.-F.; Zhang, G.-D.; Castignolles, P.; Bae, J.; Song, P.; Gao, J.-F.; Tang, L.-C. Rational Design of Oil-Resistant and Electrically Conductive Fluorosilicone Rubber Foam Nanocomposites for Sensitive Detectability in Complex Solvent Environments. ACS Nano 2024, 18, 22021–22033. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; He, Q.; Zhang, F.; Dai, F. Preparation and Analysis of Conductive and Superhydrophobic Silicone Rubber. Sens. Actuators A Phys. 2023, 350, 114123. [Google Scholar] [CrossRef]
- Wang, S.; Hou, M.; Ma, K.; Li, Z.; Geng, H.; Zhang, W.; Li, N. Research on the Influence of Extremely Cold Environment on the Performance of Silicone Rubber and Fluorinated Silicone Rubber. Polymers 2022, 14, 1898. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, B.; Wen, S.; Lu, Y.; Yang, H.; Zhang, L.; Liu, L. Effect of the Temperature on Surface Modification of Silica and Properties of Modified Silica Filled Rubber Composites. Compos. Part A Appl. Sci. Manuf. 2014, 62, 52–59. [Google Scholar] [CrossRef]
- Saramolee, P.; Sahakaro, K.; Lopattananon, N.; Dierkes, W.K.; Noordermeer, J.W.M. Comparative properties of silica- and carbon black-reinforced natural rubber in the presence of epoxidized low molecular weight polymer. Rubber Chem. Technol. 2014, 87, 320–339. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Q.; Zhang, L.; Wang, Y.; Wu, Y. Tailoring Silica–Rubber Interactions by Interface Modifiers with Multiple Functional Groups. RSC Adv. 2017, 7, 38915–38922. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Zimmerman, J.B.; De Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.H.; Lounsbury, A.W.; Mellor, K.E.; Janković, N.Z.; Tu, Q.; et al. The Green ChemisTREE: 20 Years after Taking Root with the 12 Principles. Green Chem. 2018, 20, 1929–1961. [Google Scholar] [CrossRef]
- Kim, K.; Lee, J.-Y.; Choi, B.-J.; Seo, B.; Kwag, G.-H.; Paik, H.-J.; Kim, W. Styrene-Butadiene-Glycidyl Methacrylate Terpolymer/Silica Composites: Dispersion of Silica Particles and Dynamic Mechanical Properties. Compos. Interfaces 2014, 21, 685–702. [Google Scholar] [CrossRef]
- Shoul, B.; Marfavi, Y.; Sadeghi, B.; Kowsari, E.; Sadeghi, P.; Ramakrishna, S. Investigating the Potential of Sustainable Use of Green Silica in the Green Tire Industry: A Review. Env. Sci Pollut Res 2022, 29, 51298–51317. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.; Liu, L.; Zhang, F.; Zhang, L.; Wen, S.; Lu, Y.; Yang, H.; Shen, J. Surface Modification of Silica by Two-Step Method and Properties of Solution Styrene Butadiene Rubber (SSBR) Nanocomposites Filled with Modified Silica. Compos. Sci. Technol. 2013, 88, 69–75. [Google Scholar] [CrossRef]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.-D. The Effect of Filler–Filler and Filler–Elastomer Interaction on Rubber Reinforcement. Compos. Part A Appl. Sci. Manuf. 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Staropoli, M.; Rogé, V.; Moretto, E.; Didierjean, J.; Michel, M.; Duez, B.; Steiner, P.; Thielen, G.; Lenoble, D.; Thomann, J.-S. Hybrid Silica-Based Fillers in Nanocomposites: Influence of Isotropic/Isotropic and Isotropic/Anisotropic Fillers on Mechanical Properties of Styrene-Butadiene (SBR)-Based Rubber. Polymers 2021, 13, 2413. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, F.; Liu, Y.; Lu, A.; Song, Z.; Liu, W.; Xiong, Y.; He, H.; Li, S.; Zhao, X.; et al. Structural Analyses of the Bound Rubber in Silica-Filled Silicone Rubber Nanocomposites Reveal Mechanisms of Filler-Rubber Interaction. Compos. Sci. Technol. 2023, 233, 109905. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Luo, Y.; Jia, Z.; Chen, Y.; Jia, D. Inorganic and Organic Hybrid Nanoparticles as Multifunctional Crosslinkers for Rubber Vulcanization with High-Filler Rubber Interaction. Polymers 2018, 10, 1138. [Google Scholar] [CrossRef]
- Leblanc, J.L. Rubber–Filler Interactions and Rheological Properties in Filled Compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Qu, L.; Yu, G.; Wang, L.; Li, C.; Zhao, Q.; Li, J. Effect of Filler–Elastomer Interactions on the Mechanical and Nonlinear Viscoelastic Behaviors of Chemically Modified Silica-Reinforced Solution-Polymerized Styrene Butadiene Rubber. J. Appl. Polym. Sci. 2012, 126, 116–126. [Google Scholar] [CrossRef]
- Sayfo, P.; Pölöskei, K.; Mészáros, L. Improving the Mechanical and Abrasion Properties of Silica-Reinforced Styrene-Butadiene Rubber Composites by Optimizing the Concentrations of Compatibilizers. Polym. Bull. 2024, 81, 12715–12731. [Google Scholar] [CrossRef]
- Ek, S.; Root, A.; Peussa, M.; Niinistö, L. Determination of the Hydroxyl Group Content in Silica by Thermogravimetry and a Comparison with 1H1H MAS NMR Results. Thermochim. Acta 2001, 379, 201–212. [Google Scholar] [CrossRef]
- Hussain, S.; Zhao, Z.; Song, Y.; Zhang, C. Effect of SiO Surface Modification on the Filler-Reinforced Interfaces in SiO-Filled Functional Styrene Butadiene Rubber Composites. J. Appl. Polym. Sci. 2023, 140, e54401. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Qin, X.; Liu, J.; Zhang, L. A Novel Strategy to Functionalize Styrene Butadiene Rubber toward Enhanced Interfacial Interaction with Silica. J. Appl. Polym. Sci. 2023, 140, e54476. [Google Scholar] [CrossRef]
- Pourhossaini, M.R.; Razzaghi-Kashani, M. Grafting Hydroxy-terminated Polybutadiene onto Nanosilica Surface for Styrene Butadiene Rubber Compounds. J. Appl. Polym. Sci. 2012, 124, 4721–4728. [Google Scholar] [CrossRef]
- Dubois, C.; Rajabian, M.; Rodrigue, D. Polymerization Compounding of Polyurethane-Fumed Silica Composites. Polym. Eng. Sci. 2006, 46, 360–371. [Google Scholar] [CrossRef]
- Pang, S.; Yu, Y.; Zhang, L.; Wu, Y. Adjusting Silica/Rubber Interfacial Interactions and Properties via the Click Reactions between Liquid Polybutadiene and Silane. Compos. Sci. Technol. 2021, 213, 108903. [Google Scholar] [CrossRef]
- Um, G.-Y.; Kwon, T.; Lee, S.H.; Kim, W.; Kim, J.; Yu, S.; Kim, K.; Lee, J.H. Effect of Functionality and Loading Procedure of Liquid Butadiene Rubber on Properties of Silica-Filled Tire Tread Compounds. Polym. Test. 2023, 129, 108283. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, J.; Qu, J.; Liu, L.; Sun, C.; Liu, J.; Liu, G.; Sun, L.; He, L. Synthesis and Application of Modified Low Molecular Weight Polyisoprene. Chem. J. Chin. Univ. 2022, 43, 20220066. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, T.; Qiao, Y.; Liu, H.; Zhang, C.; Zhang, X.; Sun, Y. Effects of Microstructures of Liquid Polyisoprene on the Properties of Styrene–Butadiene Rubber/Butadiene Rubber Compounds. Polym. Int. 2023, 72, 798–803. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Torbati-Fard, N.; Kiyani, H.; Razzaghi-Kashani, M. Comparative Role of Interface in Reinforcing Mechanisms of Nano Silica Modified by Silanes and Liquid Rubber in SBR Composites. J Polym Res 2016, 23, 203. [Google Scholar] [CrossRef]
- Chang, A.; Weng, G.; Fu, K.; Ding, Y.; Gong, D. Crack Growth of Natural Rubber Filled with Functionalized Silica Particles. J. Appl. Polym. Sci. 2016, 133, 42972. [Google Scholar] [CrossRef]
- Sun, C.; Wen, S.; Ma, H.; Li, Y.; Chen, L.; Wang, Z.; Yuan, B.; Liu, L. Improvement of Silica Dispersion in Solution Polymerized Styrene–Butadiene Rubber via Introducing Amino Functional Groups. Ind. Eng. Chem. Res. 2019, 58, 1454–1461. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, J.; Han, B.; Zhang, L.; Lu, J.; Ye, X. Designing Novel Epoxy-Terminated Polybutadiene to Construct Chemical Interface between Nanosilica and Rubbers with Green Nature. Compos. Part B Eng. 2019, 178, 107451. [Google Scholar] [CrossRef]
- Braum, M.V.; Jacobi, M.A.M. Silica grafted with epoxidized liquid polybutadienes: Evidence for the mechanism of reinforcement. Rubber Chem. Technol. 2019, 92, 431–444. [Google Scholar] [CrossRef]
- Song, S.; Choi, H.; Jeong, J.; Kim, S.; Kwon, M.; Kim, M.; Kim, D.; Jeon, H.; Paik, H.; Chung, S.; et al. Optimized End Functionality of Silane-Terminated Liquid Butadiene Rubber for Silica-Filled Rubber Compounds. Polymers 2023, 15, 2583. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Nagappan, S.; Chung, I. Vulcanization Behavior and Mechanical Properties of Isoprene-Modified Silica Reinforced Butyl Rubber Composites. Mol. Cryst. Liq. Cryst. 2020, 707, 46–58. [Google Scholar] [CrossRef]
- Bernal-Ortega, P.; Anyszka, R.; Morishita, Y.; di Ronza, R.; Blume, A. Determination of the Crosslink Density of Silica-Filled Styrene Butadiene Rubber Compounds by Different Analytical Methods. Polym. Bull. 2024, 81, 995–1018. [Google Scholar] [CrossRef]
- Bala, P.; Samantaray, B.K.; Srivastava, S.K.; Nando, G.B. Organomodified Montmorillonite as Filler in Natural and Synthetic Rubber. J. Appl. Polym. Sci. 2004, 92, 3583–3592. [Google Scholar] [CrossRef]
- Robertson, C.G.; Lin, C.J.; Rackaitis, M.; Roland, C.M. Influence of Particle Size and Polymer−Filler Coupling on Viscoelastic Glass Transition of Particle-Reinforced Polymers. Macromolecules 2008, 41, 2727–2731. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Bhowmick, A.K. Dynamic Mechanical Properties of Styrene-Butadiene Rubber Vulcanizate Filled with Electron Beam Modified Surface-Treated Dual-Phase Filler. J. Appl. Polym. Sci. 2003, 88, 2992–3004. [Google Scholar] [CrossRef]
- Wang, S.; Cen, L.; Wu, Q. Maleated Glycidyl 3-Pentadecenyl Phenyl Ether with Styrene: Synthesis and Application as Compatibilizer in SBR/Silica Composite. Polym. Adv. Technol. 2015, 26, 953–959. [Google Scholar] [CrossRef]
- Kong, L.; Li, F.; Wang, F.; Miao, Y.; Huang, X.; Zhu, H.; Lu, Y. In Situ Assembly of SiO2 Nanodots/Layered Double Hydroxide Nanocomposite for the Reinforcement of Solution-Polymerized Butadiene Styrene Rubber/Butadiene Rubber. Compos. Sci. Technol. 2018, 158, 9–18. [Google Scholar] [CrossRef]
- Zhao, X.; Niu, K.; Xu, Y.; Peng, Z.; Jia, L.; Hui, D.; Zhang, L. Morphology and Performance of NR/NBR/ENR Ternary Rubber Composites. Compos. Part B Eng. 2016, 107, 106–112. [Google Scholar] [CrossRef]



| Sample | m(ITPB):m(Silica) |
|---|---|
| SiO2-P2 | 2:100 |
| SiO2-P5 | 5:100 |
| SiO2-P10 | 10:100 |
| Sample | ITPB-SBR | P0-SBR | P10-SBR | P5-SBR | P2-SBR |
|---|---|---|---|---|---|
| SBR | 100 | 100 | 100 | 100 | 100 |
| Silica | 25 | 25 | -- | -- | -- |
| ITPB | 1.5 | -- | -- | -- | -- |
| SiO2-P10 | -- | -- | 25 | -- | -- |
| SiO2-P5 | -- | -- | -- | 25 | -- |
| SiO2-P2 | -- | -- | -- | -- | 25 |
| ZnO | 5 | 5 | 5 | 5 | 5 |
| SAA | 2 | 2 | 2 | 2 | 2 |
| 774 | 20 | 20 | 20 | 20 | 20 |
| S | 1 | 1 | 1 | 1 | 1 |
| DM | 2 | 2 | 2 | 2 | 2 |
| TMTD | 1 | 1 | 1 | 1 | 1 |
| Sample | Grafting Ratio | Grafting Efficiency | SSA (cm2/g) |
|---|---|---|---|
| SiO2 | -- | -- | 170.7 ± 1.4 |
| SiO2-P2 | 1.71% | 85.5% | 137.3 ± 1.3 |
| SiO2-P5 | 3.63% | 72.6% | 129.7 ± 1.2 |
| SiO2-P10 | 6.30% | 63.0% | 123.3 ± 1.2 |
| Sample | tS2/min | t90/min | ML/N·m | MH/N·m | MH-ML/N·m |
|---|---|---|---|---|---|
| P0-SBR | 1:34 | 7:40 | 0.838 | 6.172 | 5.334 |
| ITPB-SBR | 1:44 | 6:46 | 0.933 | 5.845 | 4.912 |
| P2-SBR | 1:36 | 7:20 | 0.883 | 5.911 | 5.028 |
| P5-SBR | 1:38 | 7:15 | 1.006 | 6.484 | 5.478 |
| P10-SBR | 1:39 | 6:48 | 0.888 | 6.628 | 5.740 |
| Sample | Tensile Strength/MPa | Fracture Strength/N | Elongation at Break/% | Tensile Modulus/MPa | Shore Hardness | Crosslink Density/10−4 mol/cm3 |
|---|---|---|---|---|---|---|
| P0-SBR | 11.3 | 169.2 | 412.1 | 0.027 | 62 | 2.01 |
| ITPB-SBR | 10.8 | 135.6 | 469.5 | 0.023 | 64 | 1.70 |
| P2-SBR | 11.2 | 157.3 | 420.9 | 0.027 | 64 | 2.08 |
| P5-SBR | 12.6 | 185.0 | 441.5 | 0.028 | 65 | 2.15 |
| P10-SBR | 10.5 | 154.80 | 374.9 | 0.028 | 66 | 2.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Q.; Tang, X.; Tang, J.; Tang, J.; Xia, H.; Sheng, Z.; Zhou, J.; Niu, J. Study of Styrene Butadiene Rubber Reinforced by Polybutadiene Liquid Rubber-Modified Silica. Polymers 2024, 16, 2866. https://doi.org/10.3390/polym16202866
Liao Q, Tang X, Tang J, Tang J, Xia H, Sheng Z, Zhou J, Niu J. Study of Styrene Butadiene Rubber Reinforced by Polybutadiene Liquid Rubber-Modified Silica. Polymers. 2024; 16(20):2866. https://doi.org/10.3390/polym16202866
Chicago/Turabian StyleLiao, Qing, Xiao Tang, Jiao Tang, Jiaxiang Tang, Housheng Xia, Zhongyi Sheng, Jianping Zhou, and Junfeng Niu. 2024. "Study of Styrene Butadiene Rubber Reinforced by Polybutadiene Liquid Rubber-Modified Silica" Polymers 16, no. 20: 2866. https://doi.org/10.3390/polym16202866
APA StyleLiao, Q., Tang, X., Tang, J., Tang, J., Xia, H., Sheng, Z., Zhou, J., & Niu, J. (2024). Study of Styrene Butadiene Rubber Reinforced by Polybutadiene Liquid Rubber-Modified Silica. Polymers, 16(20), 2866. https://doi.org/10.3390/polym16202866






