Valorization of Cellulosic Waste from Artichoke for Incorporation into Biodegradable Polylactic Acid Matrices
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.1.1. Sample Preparation
2.1.2. Particle Size Measurement
2.1.3. Thermal Properties Measurement
2.1.4. Mechanical Properties Measurement
2.1.5. Electron Microscopy (SEM)
2.1.6. Colorimetry
2.1.7. Water Uptake Characterization
2.1.8. FTIR Analysis
3. Results and Discussion
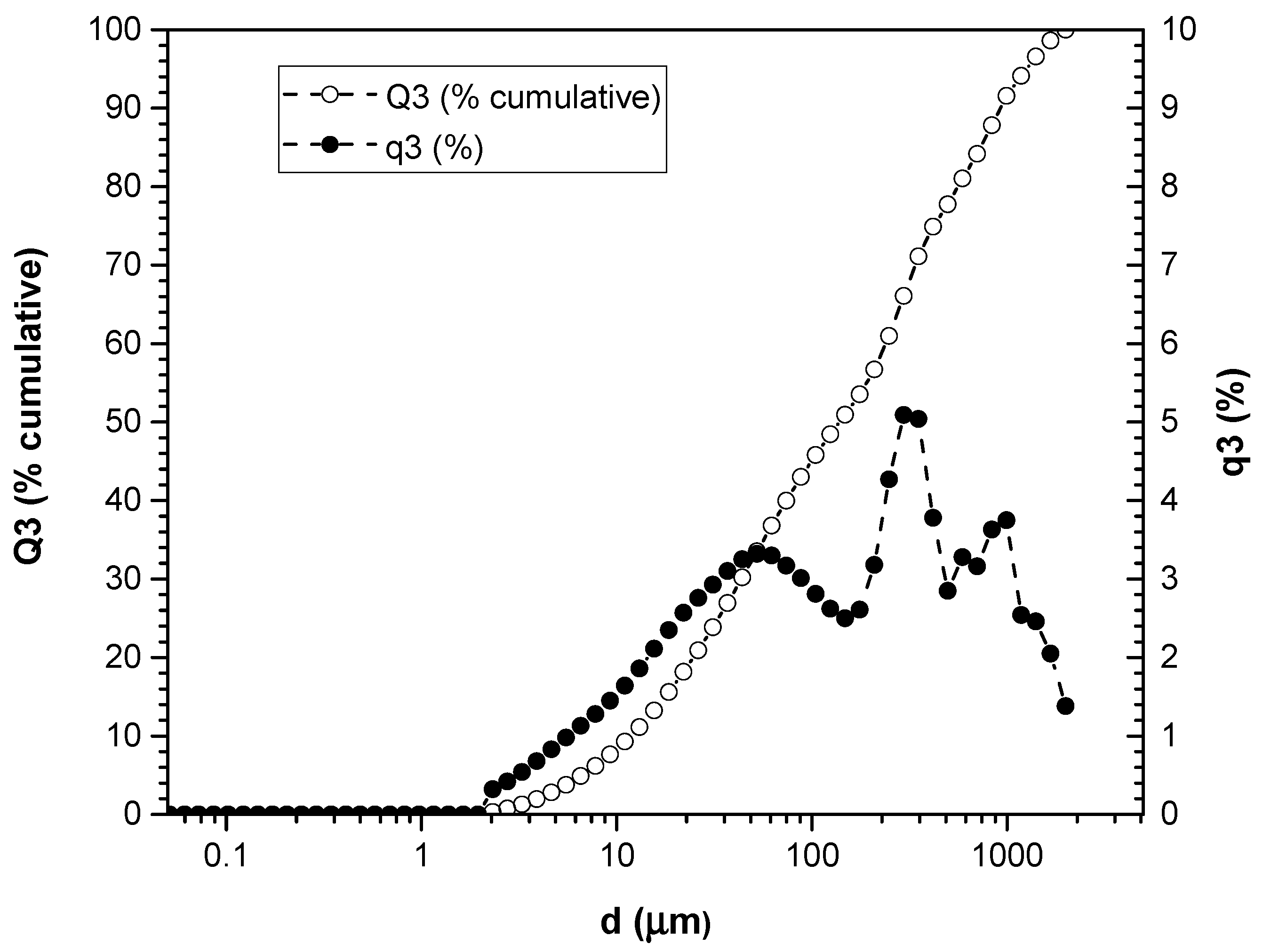
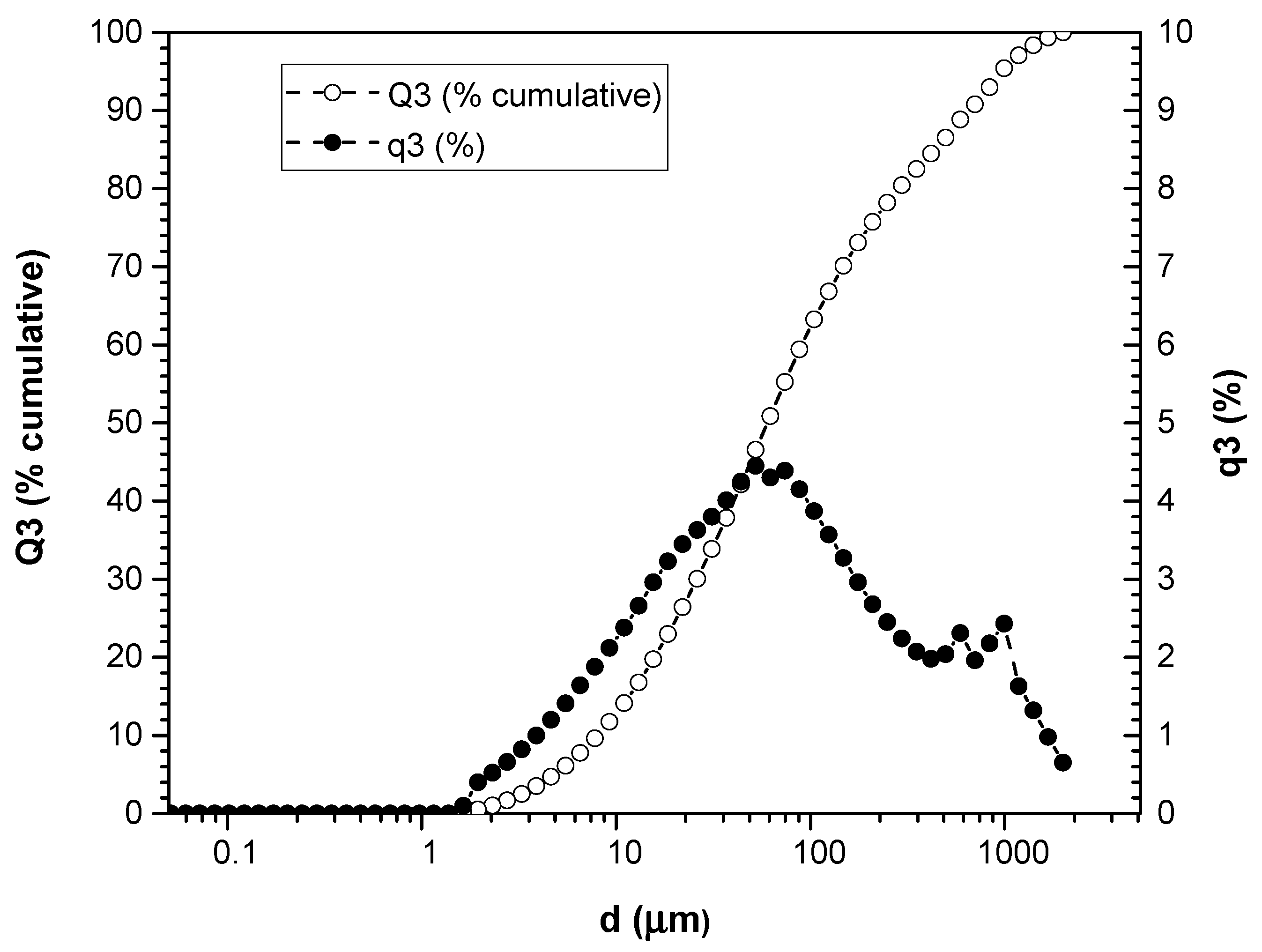
3.1. Particle Size Measurement
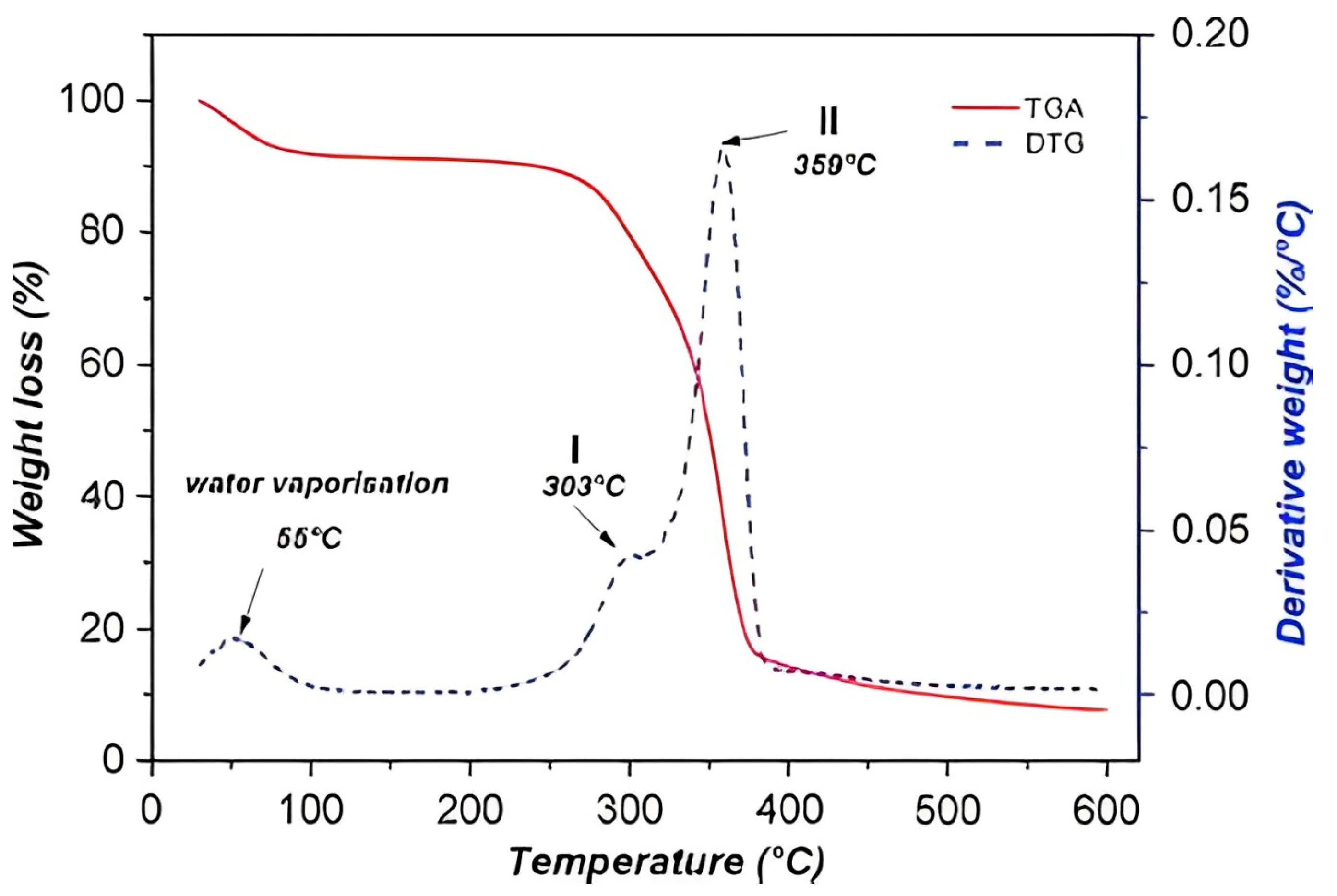
3.2. Thermal Properties
3.3. Mechanical Properties
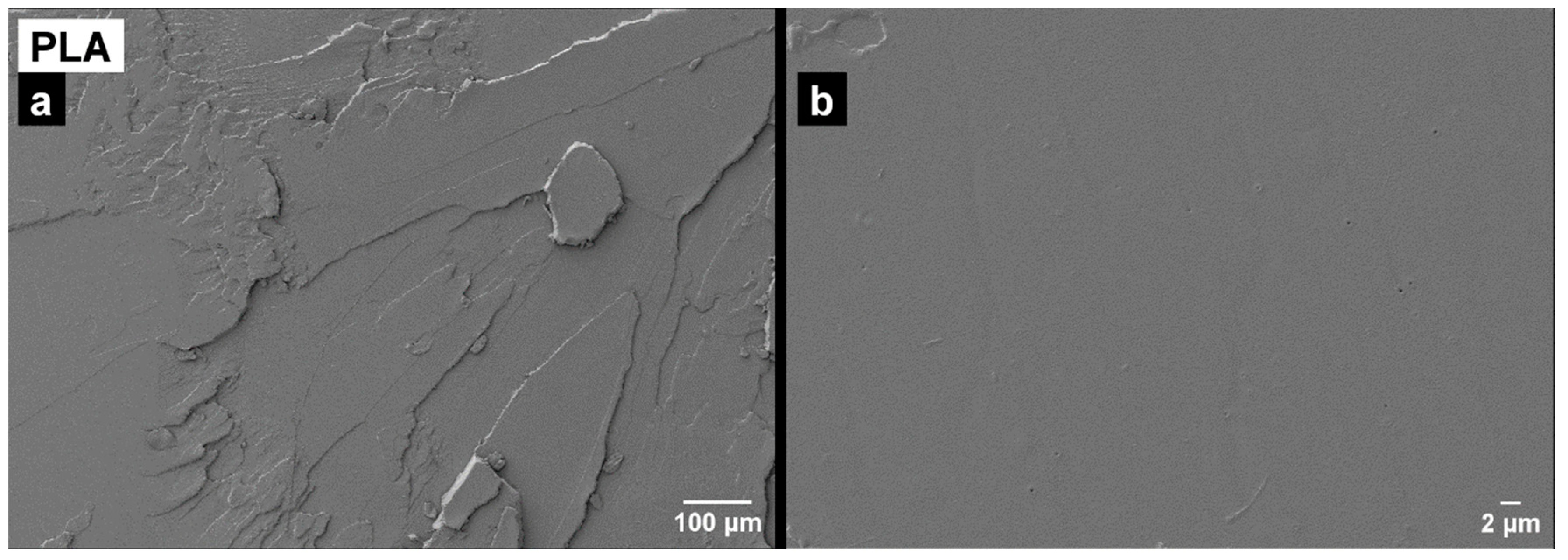
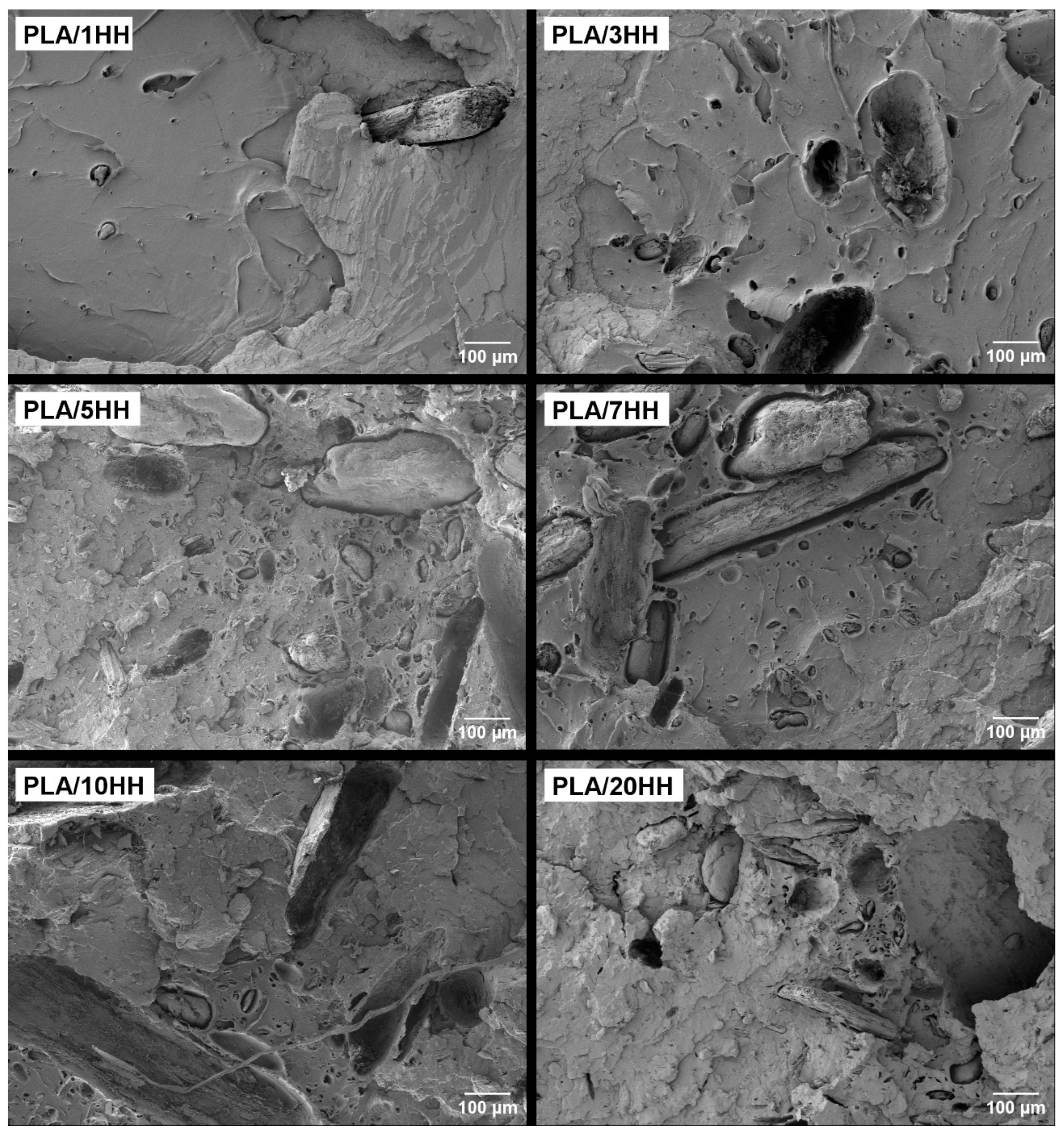
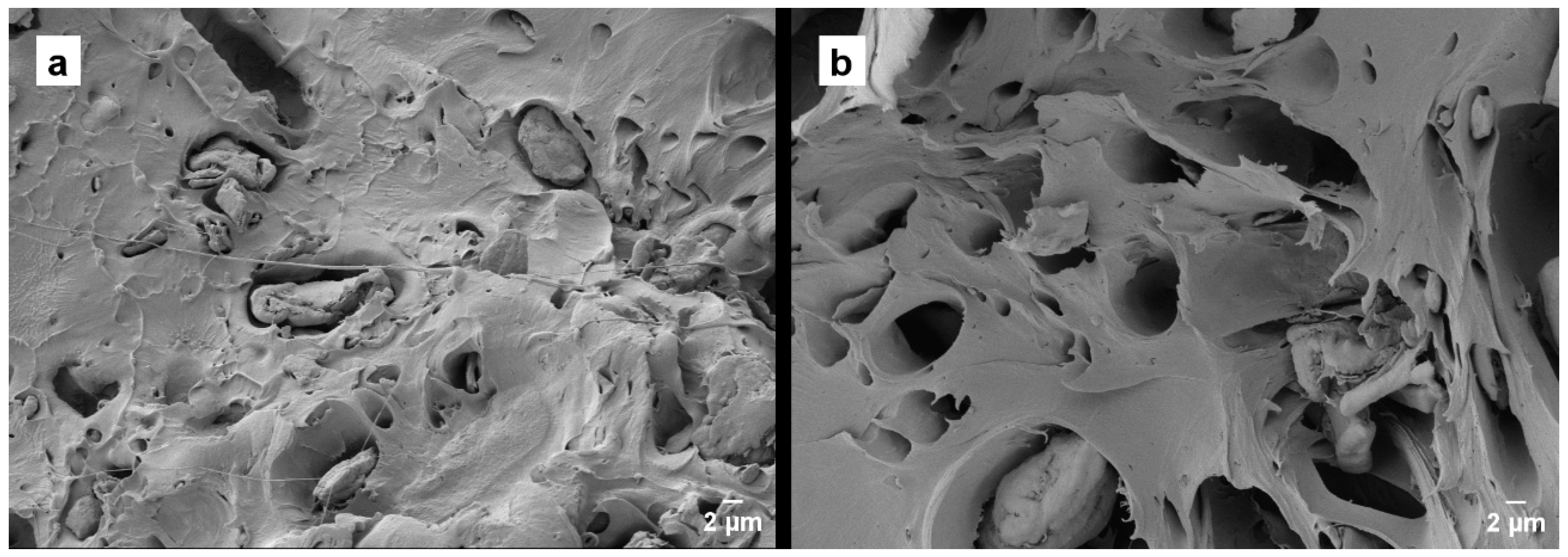
3.4. Morphology
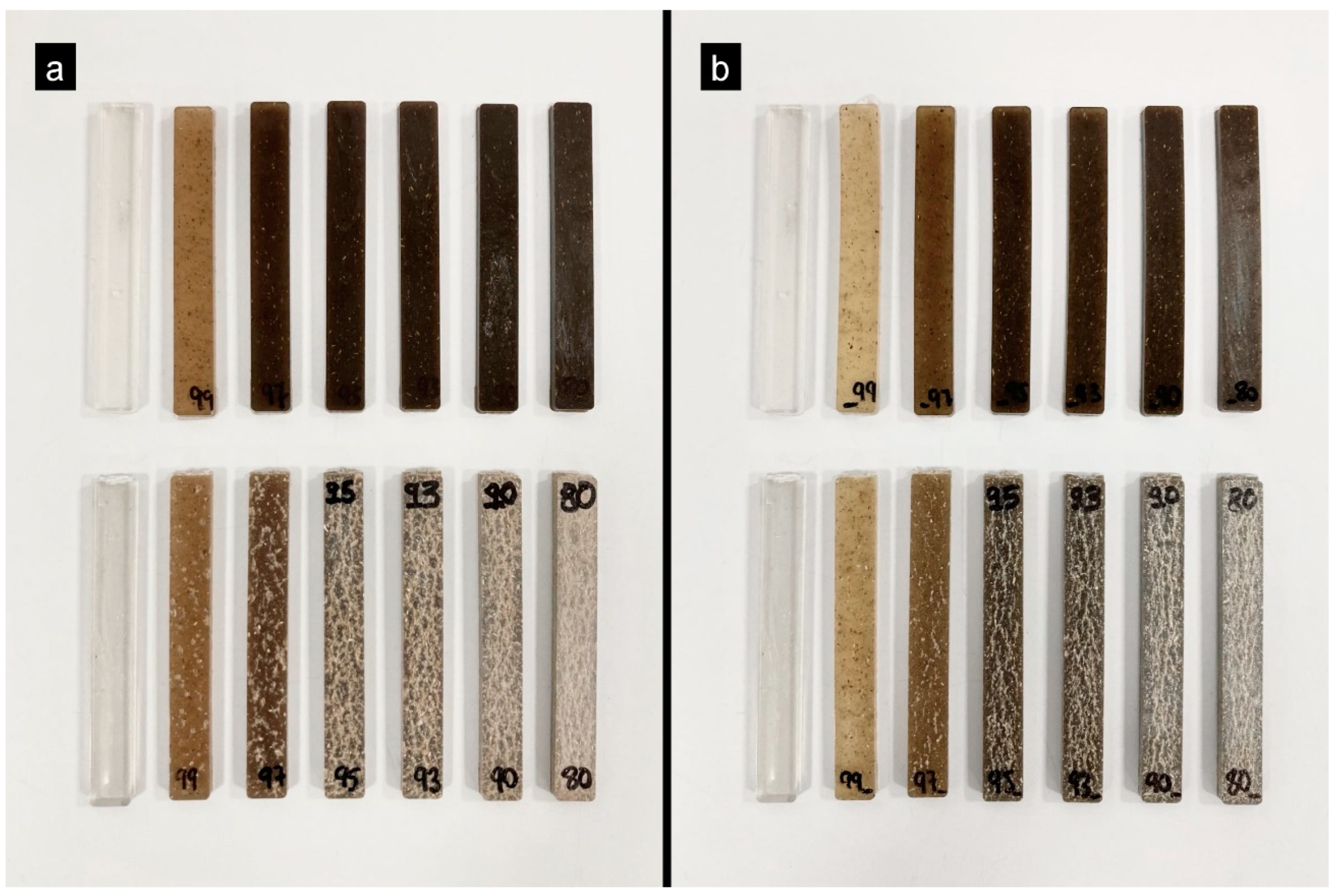
3.5. Color Tests
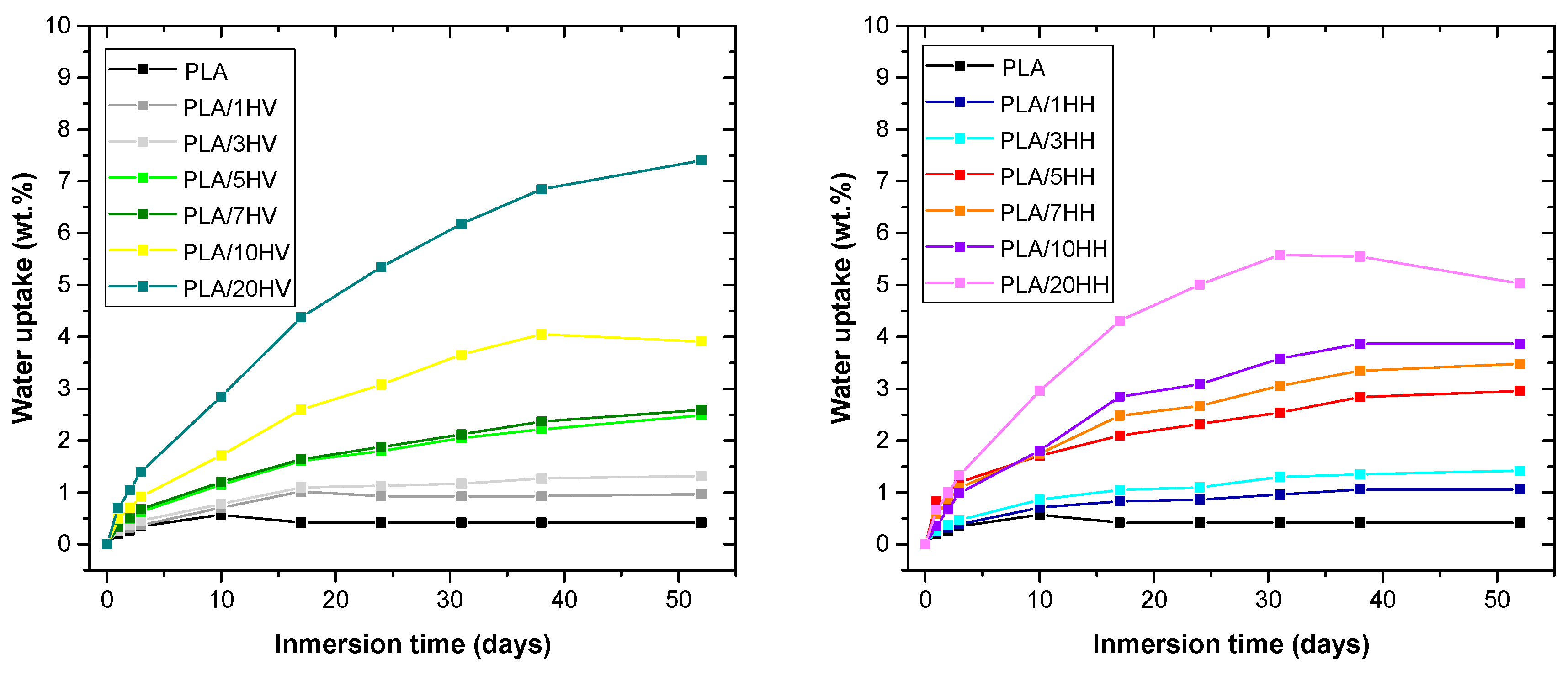
3.6. Water Absorption
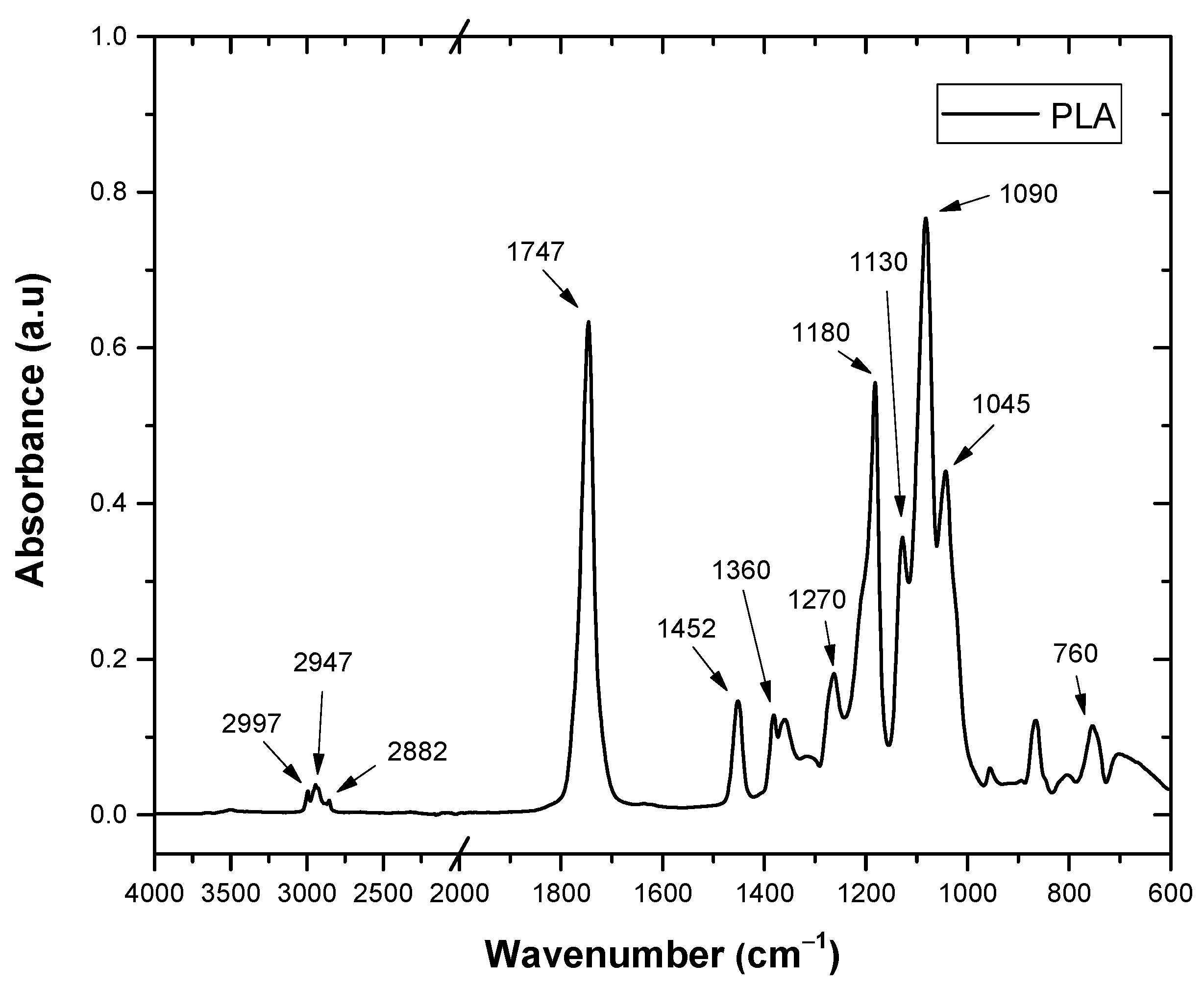
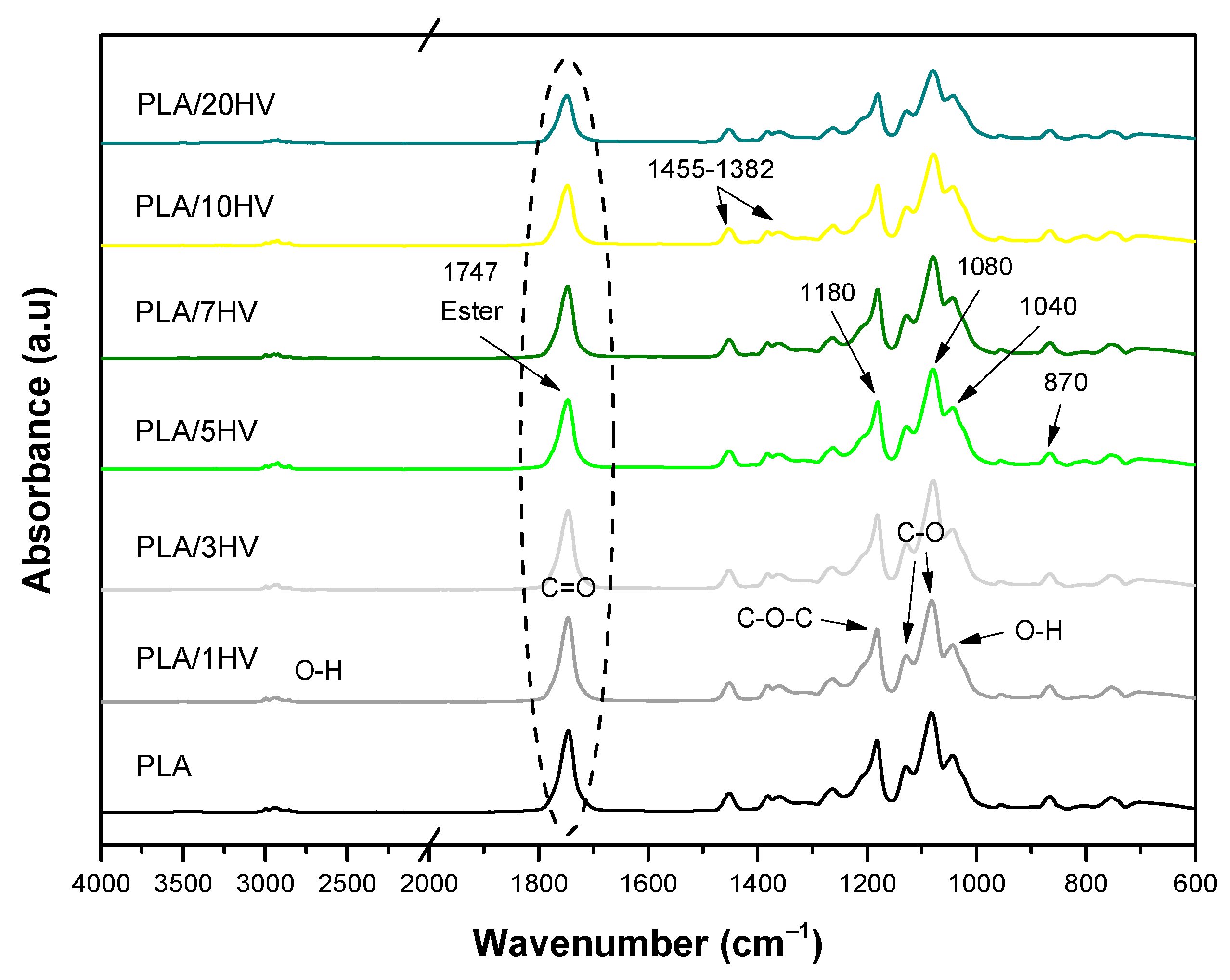
3.7. FTIR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PINELLI; PATRIZIA. Proceso Para Producir Extractos Nutracéuticos Refinados a Partir de Residuos de Alcachofa y Otras Plantas Del Género Cynara. 2 401 203. ES2401203T3 Patent 12 December 2012.
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005; Volume 894, p. 274. [Google Scholar]
- Calvo, A. Determinación Experimental y Modelización de Isotermas de Absorción de Agua de Hojas de Alcachofa; Universitat Politècnica de València: Valencia, Spain, 2013. [Google Scholar]
- PlasticsEurope. Available online: https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf (accessed on 21 October 2022).
- Malinconico, M.; Cerruti, P.; Santagata, G.; Immirzi, B. Natural polymers and additives in commodity and specialty applications: A challenge for the chemistry of future. Macromol. Symp. 2014, 337, 124–133. [Google Scholar] [CrossRef]
- John, M.J.; Thomas, S. Biofibres and biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Vilpoux, O.; Averous, L. Starch-based plastics. Technol. Use Potentialities Lat. Am. Starchy Tubers 2004, 3, 521–553. Available online: http://averousl.free.fr/fichiers/Starch-based_Plastics.pdf (accessed on 20 September 2024).
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Oliveira, R.B. Polímeros na obtenção de sistemas de liberação de fármacos. Rev. Eletrônica De Farmácia 2006, 3. [Google Scholar] [CrossRef]
- Anugwom, I.; Lahtela, V.; Kallioinen, M.; Kärki, T. Lignin as a functional additive in a biocomposite: Influence on mechanical properties of polylactic acid composites. Ind. Crops Prod. 2019, 140, 111704. [Google Scholar] [CrossRef]
- Briassoulis, D. Una descripción general del comportamiento mecánico de las películas agrícolas biodegradables. J. Poli. Reinar 2004, 12, 65–81. [Google Scholar]
- VERT, M. Polymères de fermentation: Les poly (acide lactique) s et leurs précurseurs, les acides lactiques. L’Actualité Chim. (Paris. 1973) 2002, 11–12, 79–82. Available online: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=14372033 (accessed on 20 September 2024).
- Mochizuki, M.; Hirami, M. Efectos estructurales sobre la biodegradación de poliésteres alifáticos. Polimero. Adv. Tecnol. 1197, 8, 203. [Google Scholar] [CrossRef]
- Rutot, D.; Dubois, P. Les (bio) polymeres biodegradables: L’enjeu de demain? Chim. Nouv. 2004, 86, 66–74. Available online: http://pedagogie.ac-limoges.fr/physique-chimie/IMG/pdf/biopolymeres-2.pdf (accessed on 20 September 2024).
- Weng, Y.X.; Jin, Y.J.; Meng, Q.Y.; Wang, L.; Zhang, M.; Wang, Y.Z. Biodegradation behavior of poly (butylene adipate-co-terephthalate) (PBAT), poly (lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Sánchez-Acosta, D.; Rodriguez-Uribe, A.; Álvarez-Chávez, C.R.; Mohanty, A.K.; Misra, M.; López-Cervantes, J.; Madera-Santana, T.J. Physicochemical characterization and evaluation of pecan nutshell as biofiller in a matrix of poly (lactic acid). J. Polym. Environ. 2019, 27, 521–532. [Google Scholar] [CrossRef]
- Camacho-Muñoz, R.; Villada-Castillo, H.S.; Solanilla-Duque, J.F. Anaerobic biodegradation under slurry thermophilic conditions of poly (lactic acid)/starch blend compatibilized by maleic anhydride. Int. J. Biol. Macromol. 2020, 163, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Cruz Fabian, D.R.; Durpekova, S.; Dusankova, M.; Cisar, J.; Drohsler, P.; Elich, O.; Borkova, M.; Cechmankova, J.; Sedlarik, V. Renewable Poly(Lactic Acid)Lignocellulose Biocomposites for the Enhancement of the Water Retention Capacity of the Soil. Polymers 2023, 15, 2243. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani Chaboki, M.; Mohammadi-Rovshandeh, J.; Hemmati, F. Poly (Lactic Acid)/Thermoplasticized Rice Straw Biocomposites: Effects of Benzylated Lignocellulosic Filler and Nanoclay. Iran. Polym. J. 2019, 28, 777–788. [Google Scholar] [CrossRef]
- Ghanmi, I.; Slimani, F.; Ghanmi, S.; Guedri, M. Development and Characterization of a PLA Biocomposite reinforced with Date Palm Fibers. Eng. Technol. Appl. Sci. Res. 2024, 14, 13631–13636. [Google Scholar] [CrossRef]
- Carrión, F.J.; Avilés, M.D.; Nakano, K.; Tadokoro, C.; Nagamine, T.; Bermúdez, M.D. Diprotic ammonium palmitate ionic liquid crystal and nanodiamonds in aqueous lubrication. Film thickness and influence of sliding speed. Wear 2019, 418, 241–252. [Google Scholar] [CrossRef]
- De Rosa, I.M.; Kenny, J.M.; Puglia, D.; Santulli, C.; Sarasini, F. Morphological, Thermal and Mechanical Characterization of Okra (Abelmoschus Esculentus) Fibres as Potential Reinforcement in Polymer Composites. Compos. Sci. Technol. 2010, 70, 116–122. [Google Scholar] [CrossRef]
- Fiore, V.; Valenza, A.; Di Bella, G. Artichoke (Cynara Cardunculus L.) Fibres as Potential Reinforcement of Composite Structures. Compos. Sci. Technol. 2011, 71, 1138–1144. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Ouajai, S.; Shanks, R.A. Composition, Structure and Thermal Degradation of Hemp Cellulose after Chemical Treatments. Polym. Degrad. Stab. 2005, 89, 327–335. [Google Scholar] [CrossRef]
- Silva, N.T.; Nascimento, N.F.; Cividanes, L.S.; Bertran, C.A.; Thim, G.P. Kinetics of Cordierite Crystallization from Diphasic Gels. J. Sol-Gel Sci. Technol. 2008, 47, 140–147. [Google Scholar] [CrossRef]
- Albano, C.; Gonzalez, J.; Ichazo, M.; Kaiser, D. Thermal stability of blends of polyolefins and sisal fiber. Polym. Degrad. Stab. 1999, 66, 179–190. [Google Scholar] [CrossRef]
- Wielage, B.; Lampke, T.; Marx, G.; Nestler, K.; Starke, D. Thermogravimetric and differential scanning calorimetric analysis of natural fibres and polypropylene. Thermochim. Acta 1999, 337, 169–177. [Google Scholar] [CrossRef]
- Spinacé, M.A.; Lambert, C.S.; Fermoselli, K.K.; De Paoli, M.A. Characterization of lignocellulosic curaua fibres. Carbohydr. Polym. 2009, 77, 47–53. [Google Scholar] [CrossRef]
- Kim, K.-W.; Lee, B.-H.; Kim, H.-J.; Sriroth, K.; Dorgan, J.R. Thermal and Mechanical Properties of Cassava and Pineapple Flours-Filled PLA Bio-Composites. J. Therm. Anal. Calorim. 2012, 108, 1131–1139. [Google Scholar] [CrossRef]
- Taşdemir, M. Effects of Olive Pit and Almond Shell Powder on Polypropylene. Key Eng. Mater. 2017, 733, 65–68. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Jorda-Vilaplana, A.; Balart, R.; Torres-Giner, S. A comparative study on the effect of different reactive compatibilizers on injection-molded pieces of bio-based high-density polyethylene/polylactide blends. J. Appl. Polym. Sci. 2019, 136, 47396. [Google Scholar] [CrossRef]
- Morreale, M.; Liga, A.; Mistretta, M.C.; Ascione, L.; Mantia, F.P. Mechanical, Thermomechanical and Reprocessing Behavior of Green Composites from Biodegradable Polymer and Wood Flour. Materials 2015, 8, 7536–7548. [Google Scholar] [CrossRef]
- Chaiwutthinan, P.; Chuayjuljit, S.; Srasomsub, S.; Boonmahitthisud, A. Composites of poly (lactic acid)/poly (butylene adipate-co-terephthalate) blend with wood fiber and wollastonite: Physical properties, morphology, and biodegradability. J. Appl. Polym. Sci. 2019, 136, 47543. [Google Scholar] [CrossRef]
- da Silva, W.A.; Luna, C.B.B.; de Melo, J.B.d.C.A.; Araújo, E.M.; Filho, E.A.d.S.; Duarte, R.N.C. Feasibility of Manufacturing Disposable Cups Using PLA/PCL Composites Reinforced with Wood Powder. J. Polym. Environ. 2021, 29, 2932–2951. [Google Scholar] [CrossRef]
- Liminana, P.; Quiles-Carrillo, L.; Boronat, T.; Balart, R.; Montanes, N. The Effect of Varying Almond Shell Flour (ASF) Loading in Composites with Poly (Butylene Succinate (PBS) Matrix Compatibilized with Maleinized Linseed Oil (MLO). Materials 2018, 11, 2179. [Google Scholar] [CrossRef]
- Lips, S.J.J.; Iñiguez de Heredia, G.M.; Op den Kamp, R.G.M.; van Dam, J.E.G. Water Absorption Characteristics of Kenaf Core to Use as Animal Bedding Material. Ind. Crops Prod. 2009, 29, 73–79. [Google Scholar] [CrossRef]
- Al Abdallah, H.; Abu-Jdayil, B.; Iqbal, M.Z. Improvement of Mechanical Properties and Water Resistance of Bio-Based Thermal Insulation Material via Silane Treatment. J. Clean. Prod. 2022, 346, 131242. [Google Scholar] [CrossRef]
- Ghanbari, S.; Niu, C.H. Characteristics of oat hull based biosorbent for natural gas dehydration in a PSA process. J. Nat. Gas Sci. Eng. 2019, 61, 320–332. Available online: https://www.sciencedirect.com/science/article/pii/S1875510018305079?casa_token=wFo0IaUsH7YAAAA:tylQM0TAlyodHd0-2qviXgCdSrzEuVogKwgTCfdu7OkfNO3u_vufmcuSPx_ybzivNbhgDl4CKk (accessed on 20 September 2024). [CrossRef]
- Sánchez, C. Desarrollo, Caracterización y Propiedades de Nanofases, Nanofluidos y Nanomaterials. Ph.D. Thesis, Universidad Politécnica de Cartagena, Cartagena, Spain, 2023. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Paiva, M.C.; Ammar, I.; Campos, A.R.; Cheikh, R.B.; Cunha, A.M. Alfa Fibres: Mechanical, Morphological and Interfacial Characterization. Compos. Sci. Technol. 2007, 67, 1132–1138. [Google Scholar] [CrossRef]
- Le Troëdec, M.; Dalmay, P.; Patapy, C.; Peyratout, C.; Smith, A.; Chotard, T. Mechanical Properties of Hemp-Lime Reinforced Mortars: Influence of the Chemical Treatment of Fibers. J. Compos. Mater. 2011, 45, 2347–2357. [Google Scholar] [CrossRef]
- Liu, W.; Mohanty, A.K.; Drzal, L.T.; Askel, P.; Misra, M. Effects of alkali treatment on the structure, morphology and thermal properties of native grass fibers as reinforcements for polymer matrix composites. J. Mater. Sci. 2004, 39, 1051–1054. Available online: https://www.academia.edu/45237217/Effects_of_alkali_treatment_on_the_structure_morphology_and_thermal_properties_of_native_grass_fibers_as_reinforcements_for_polymer_matrix_composites (accessed on 20 September 2024). [CrossRef]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Venkatesh, C.; Laurenti, M.; Bandeira, M.; Lanzagorta, E.; Lucherini, L.; Cauda, V.; Devine, D.M. Biodegradation and Antimicrobial Properties of Zinc Oxide–Polymer Composite Materials for Urinary Stent Applications. Coatings 2020, 10, 1002. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Z.-H.; Yang, W.; Yang, M.-B. Thermal and Mechanical Properties of Chemical Crosslinked Polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]






| Code | PLA (wt.%) | Artichoke (wt.%) |
|---|---|---|
| PLA | 100 | 0 |
| PLA/1HV | 99 | 1 |
| PLA/3HV | 97 | 3 |
| PLA/5HV | 95 | 5 |
| PLA/7HV | 93 | 7 |
| PLA/10HV | 90 | 10 |
| PLA/20HV | 80 | 20 |
| PLA/1HH | 99 | 1 |
| PLA/3HH | 97 | 3 |
| PLA/5HH | 95 | 5 |
| PLA/7HH | 93 | 7 |
| PLA/10HH | 90 | 10 |
| PLA/20HH | 80 | 20 |
| Material | Weight Loss (%) in a Temperature Range of 40–180 | I Degradation Stage | II Degradation Stage | Lignin Decomposition (% by Weight) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T5% (°C) | T90% (°C) | Tmax (°C) | Weight Loss (%) | T5% (°C) | T90% (°C) | Tmax (°C) | Weight Loss (%) | |||
| HV | 5.76 | 181.5 | 235.5 | 217.5 | 11.49 | 368.8 | 235.5 | 314.4 | 40.94 | 13.38 |
| HH | 3.50 | 181.7 | 265.1 | 222.7 | 17.38 | 373.5 | 262.7 | 309.4 | 37.04 | 11.49 |
| Code | τmax (MPa) | εb (%) | E (MPa) | Shore D Hardness | Impact Strength (kJ/m2) |
|---|---|---|---|---|---|
| PLA | 60.9 ± 2.2 | 6.9 ± 0.3 | 1133.7 ± 129.4 | 77.0 ± 0.5 | 25.6 ± 5.0 |
| PLA/1HV | 59.6 ± 0.55 | 6.9 ± 0.3 | 1176.2 ± 58.3 | 79.0 ± 0.0 | 20.5 ± 3.1 |
| PLA/3HV | 58.2 ± 1.62 | 7.0 ± 0.9 | 1141.2 ± 54.2 | 79.2 ± 0.4 | 16.4 ± 1.2 |
| PLA/5HV | 56.4 ± 0.87 | 6.3 ± 0.5 | 1153.2 ± 43.3 | 79.6 ± 0.5 | 15.7 ± 0.7 |
| PLA/7HV | 55.9 ± 0.76 | 5.8 ± 0.2 | 1201.8 ± 7.8 | 78.8 ± 0.5 | 14.8 ± 0.4 |
| PLA/10HV | 52.8 ± 0.71 | 5.8 ± 0.3 | 1120.0 ± 23.2 | 80.2 ± 0.5 | 12.1 ± 1.2 |
| PLA/20HV | 43.2 ± 2.96 | 4.4 ± 0.4 | 1125.4 ± 38.5 | 80.6 ± 0.9 | 11.5 ± 0.9 |
| PLA/1HH | 57.6 ± 0.6 | 6.4 ± 0.3 | 1159.5 ± 36.9 | 78.6 ± 0.4 | 19.2 ± 2.9 |
| PLA/3HH | 56.3 ± 1.6 | 6.1 ± 0.3 | 1172.2 ± 24.6 | 79.2 ± 0.5 | 17.4 ± 1.2 |
| PLA/5HH | 52.4 ± 0.9 | 6.3 ± 1.0 | 1133.9 ± 32.7 | 79.8 ± 0.0 | 16.0 ± 2.3 |
| PLA/7HH | 50.7 ± 0.8 | 5.7 ± 0.3 | 1133.9 ± 34.7 | 80.0 ± 0.0 | 15.6 ± 0.7 |
| PLA/10HH | 43.9 ± 0.7 | 5.0 ± 0.2 | 1085.0 ± 46.7 | 80.2 ± 0.4 | 13.5 ± 1.0 |
| PLA/20HH | 40.6 ± 2.9 | 4.5 ± 0.3 | 1075.7 ± 34.5 | 80.4 ± 0.5 | 13.1 ± 1.3 |
| Code | L* | a* | b* | ΔE (Control) |
|---|---|---|---|---|
| PLA | 30.9 | −0.4 | 3.8 | - |
| PLA/1HV | 25.6 | 0.4 | 3.0 | 5.4 |
| PLA/3HV | 24.6 | 1.5 | 3.5 | 6.6 |
| PLA/5HV | 25.2 | 2.4 | 3.4 | 6.4 |
| PLA/7HV | 25.4 | 1.7 | 3.9 | 5.9 |
| PLA/10HV | 26.9 | 1.9 | 3.3 | 4.6 |
| PLA/20HV | 29.4 | 2.4 | 3.5 | 3.2 |
| PLA/1HH | 25.1 | 1.9 | 5.4 | 6.4 |
| PLA/3HH | 27.6 | 1.7 | 5.0 | 4.1 |
| PLA/5HH | 27.9 | 2.1 | 4.7 | 4.0 |
| PLA/7HH | 27.8 | 2.1 | 4.6 | 4.1 |
| PLA/10HH | 28.9 | 2.6 | 4.6 | 3.7 |
| PLA/20HH | 28.2 | 2.9 | 4.1 | 4.5 |
| PLA | HV-HH | ||
|---|---|---|---|
| Assignment | Wavenumbers (cm−1) | Assignment | Wavenumbers (cm−1) |
| CH3 | 2997 | OH | 3500–3000 |
| -C-CH3 | 2947 | CH | 2918 |
| -CH, -CH3 | 2882 | (Carbonyl, ketone and ester) C=O | 1734 |
| C=O | 1747 | OH hydroxyls | 1645 |
| CH3 | 1452 | C=O | 1618 |
| -CH3, CH-CH3 | 1360 | CH | 1430–1407 |
| COC, -CO | 1180 | CH, polysaccharides | 1370 |
| COC, ras CH3 | 1130 | CO | 1318 |
| COC | 1090 | COC of phenol ether bond | 1239 |
| CC, COC | 1045 | CO | 1034 |
| CH3, C=O | 760 | CH | 896 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llidó Barragán, A.; Calle Salas, A.d.l.; Parres García, F.; Crespo Amorós, J.E. Valorization of Cellulosic Waste from Artichoke for Incorporation into Biodegradable Polylactic Acid Matrices. Polymers 2024, 16, 2778. https://doi.org/10.3390/polym16192778
Llidó Barragán A, Calle Salas Adl, Parres García F, Crespo Amorós JE. Valorization of Cellulosic Waste from Artichoke for Incorporation into Biodegradable Polylactic Acid Matrices. Polymers. 2024; 16(19):2778. https://doi.org/10.3390/polym16192778
Chicago/Turabian StyleLlidó Barragán, Alexandra, Alejandro de la Calle Salas, Francisco Parres García, and José Enrique Crespo Amorós. 2024. "Valorization of Cellulosic Waste from Artichoke for Incorporation into Biodegradable Polylactic Acid Matrices" Polymers 16, no. 19: 2778. https://doi.org/10.3390/polym16192778
APA StyleLlidó Barragán, A., Calle Salas, A. d. l., Parres García, F., & Crespo Amorós, J. E. (2024). Valorization of Cellulosic Waste from Artichoke for Incorporation into Biodegradable Polylactic Acid Matrices. Polymers, 16(19), 2778. https://doi.org/10.3390/polym16192778






