Facile Synthesis of Bis-Diphenylphosphine Oxide as a Flame Retardant for Epoxy Resins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ODDPO
2.3. Preparation of Epoxy Resin Thermosets
2.4. Characterization
3. Results and Discussion
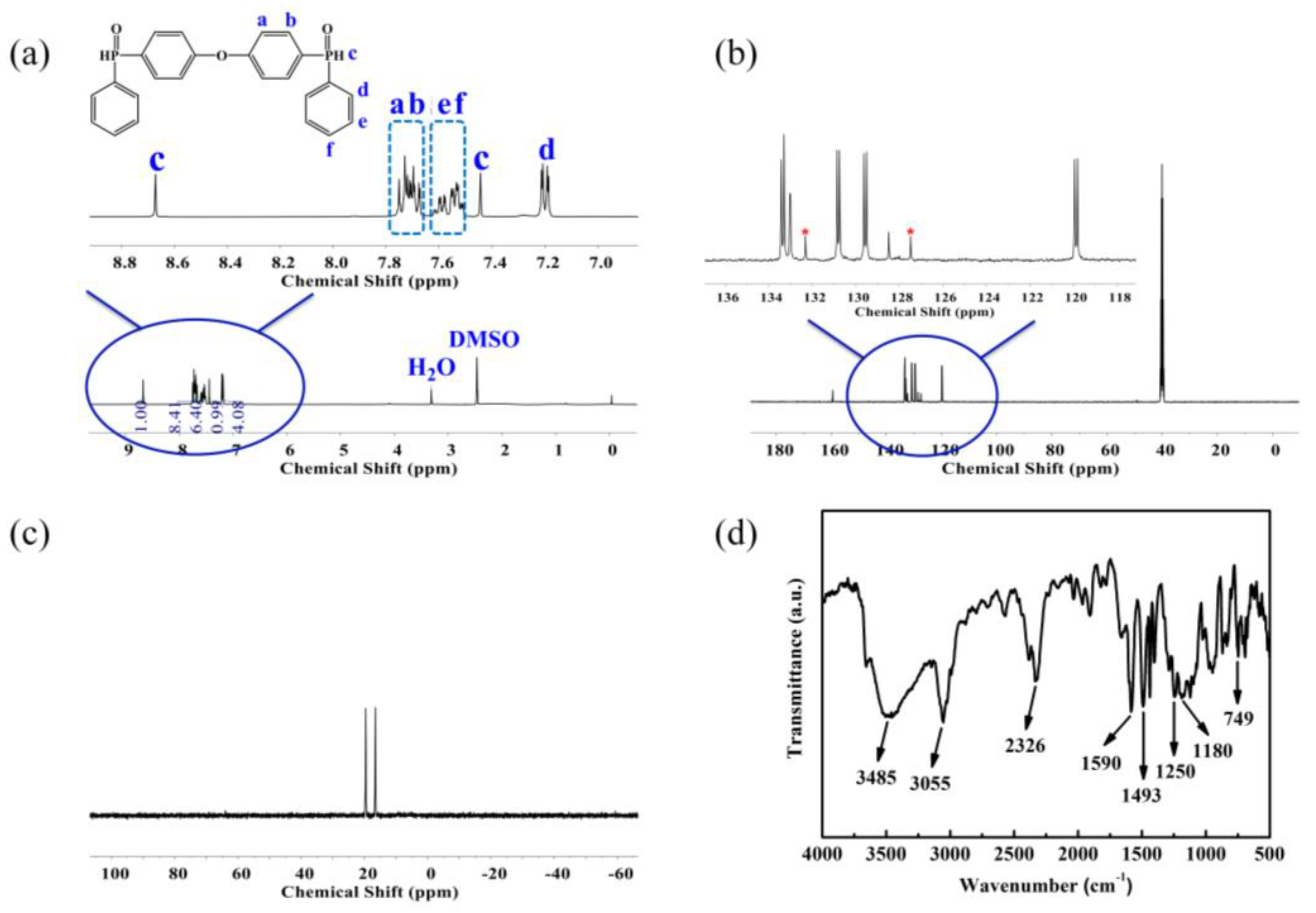
3.1. Characterization of ODDPO
3.2. Thermomechanical Properties of Epoxy Composites
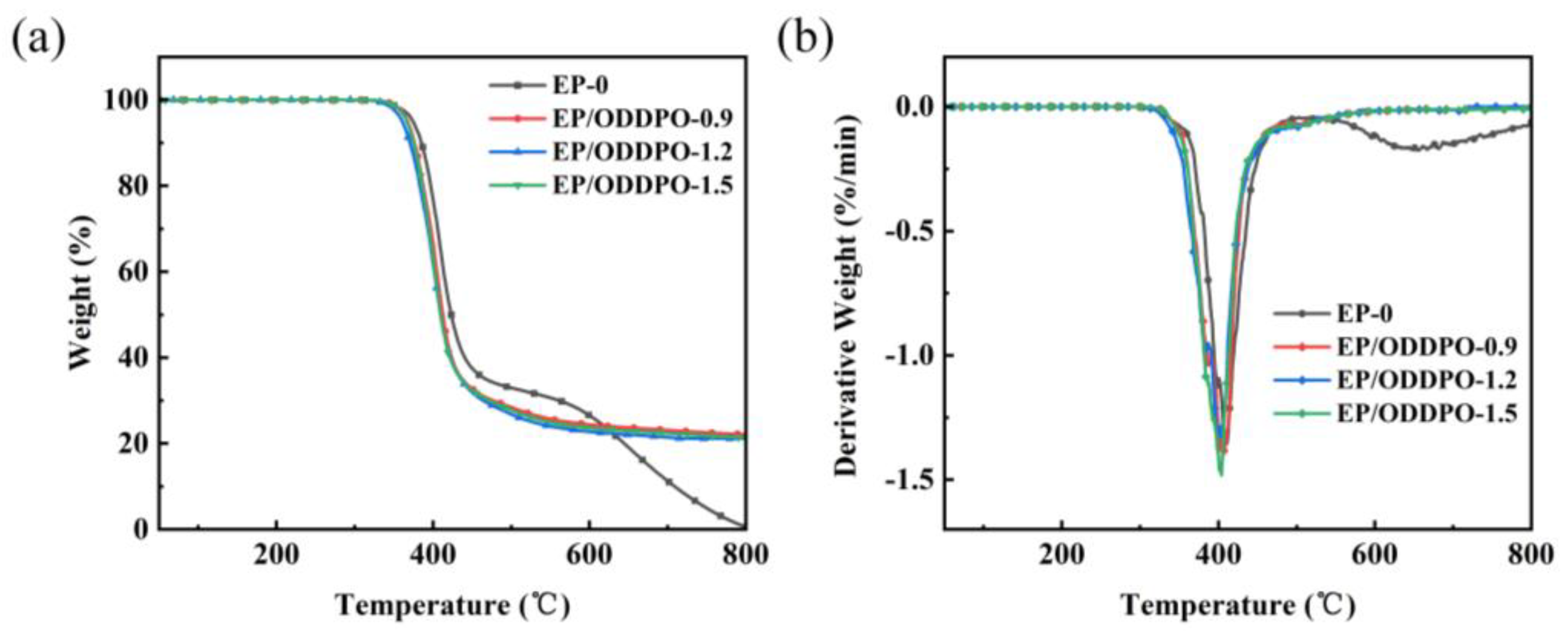
3.3. Thermal Stability of Epoxy Composites
3.4. Flame Retardancy of Epoxy Composites
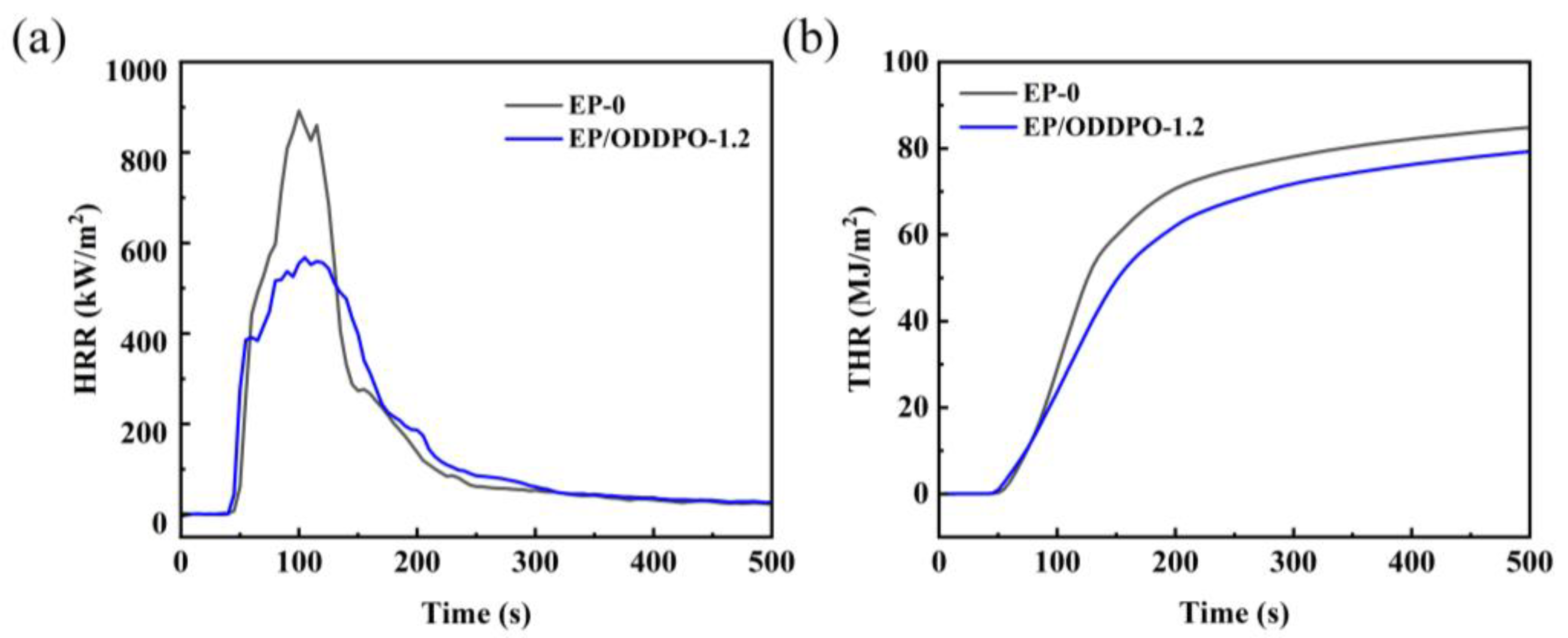
3.5. Combustion Behavior of Epoxy Composites
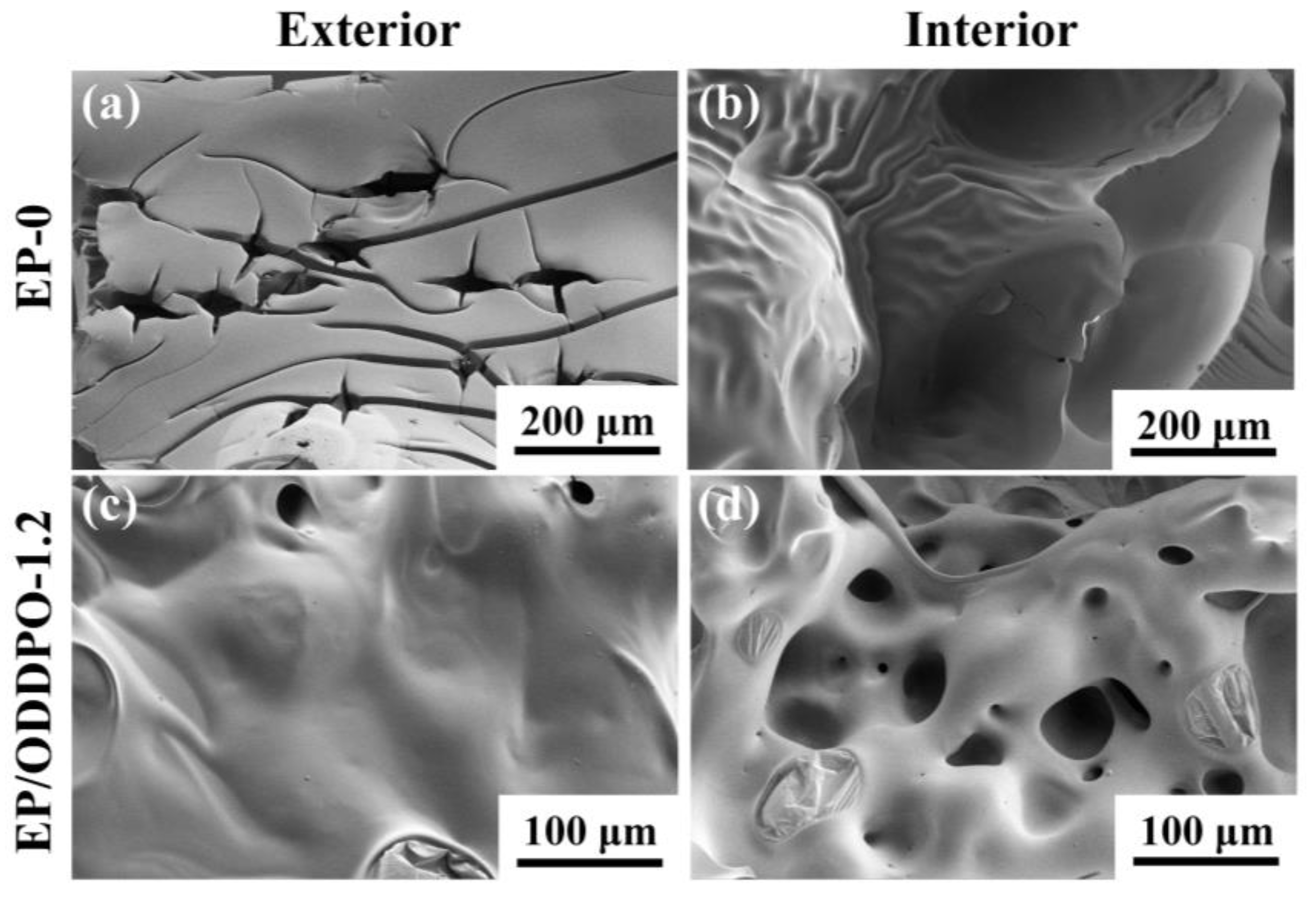
3.6. Flame Retardancy Mechanism
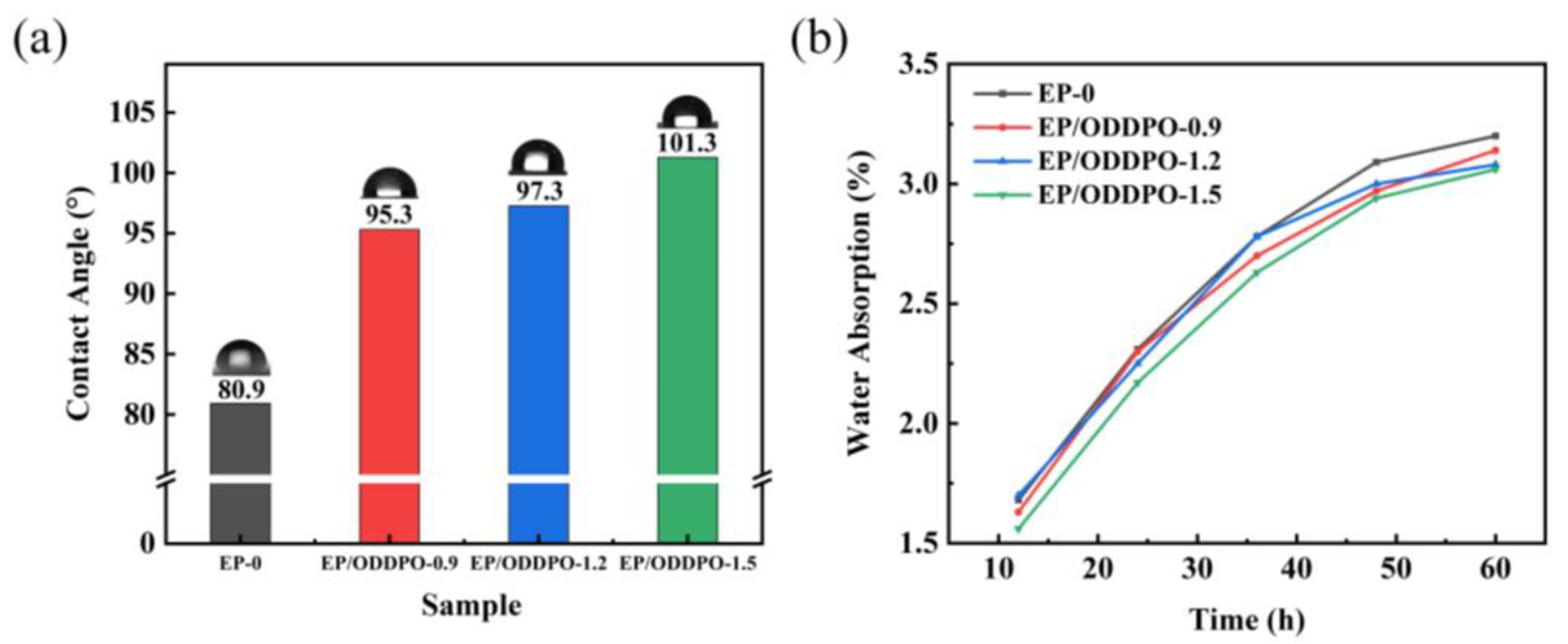
3.7. Wettability and Water Absorption Properties of Epoxy Composites
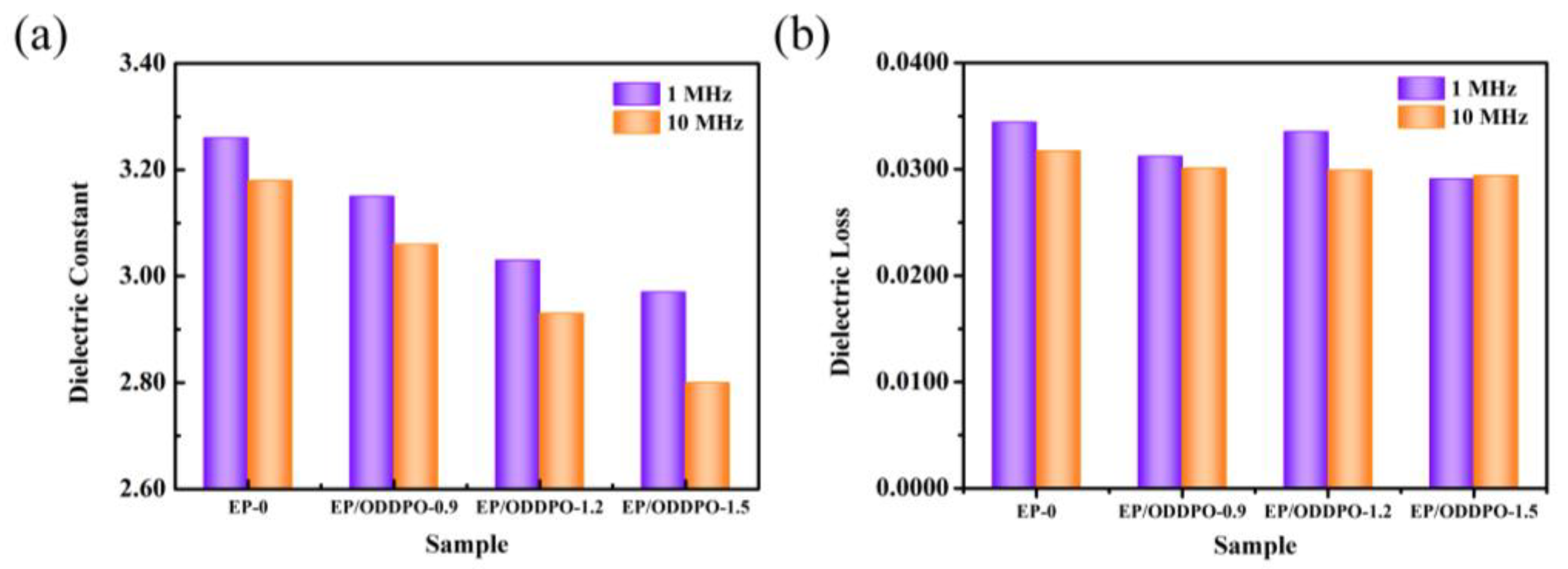
3.8. Dielectric Properties of Epoxy Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Memon, H.; Wei, Y.; Zhu, C. Recyclable and reformable epoxy resins based on dynamic covalent bonds–Present, past, and future. Polym. Test. 2022, 105, 107420. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Zhumabekova, A.; Serikbayeva, G.; Vikulova, M.; Svitkina, V. Effect of phosphorus and chlorine containing plasticizers on the physicochemical and mechanical properties of epoxy composites. J. Compos. Sci. 2023, 7, 178. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Facile synthesis of a novel zinc-triazole complex for simultaneous improvement in fire safety and mechanical properties of epoxy resins. Compos. Appl. Sci. Manuf. 2021, 143, 106284. [Google Scholar] [CrossRef]
- Memon, H.; Wei, Y.; Zhu, C. Correlating the thermomechanical properties of a novel bio-based epoxy vitrimer with its crosslink density. Mater. Today Commun. 2021, 29, 102814. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Y.; Wei, S.; Zhao, S.; Qian, L.; Chen, Y.; Shan, H.; Zhang, Q. A phosphorous-based bi-functional flame retardant based on phosphaphenanthrene and aluminum hypophosphite for an epoxy thermoset. Int. J. Mol. Sci. 2022, 23, 11256. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-H.; Xu, Y.-J.; Chen, L.; Chen, J.-H.; He, J.-H.; Li, Z.; Li, S.-L.; Wang, Y.-Z. Facile fabrication of intrinsically fire-safety epoxy resin cured with phosphorus-containing transition metal complexes for flame retardation, smoke suppression, and latent curing behavior. Chem. Eng. J. 2022, 442, 136097. [Google Scholar] [CrossRef]
- Gao, T.-Y.; Wang, F.-D.; Xu, Y.; Wei, C.-X.; Zhu, S.-E.; Yang, W.; Lu, H.-D. Luteolin-based epoxy resin with exceptional heat resistance, mechanical and flame retardant properties. Chem. Eng. J. 2022, 428, 131173. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Q.; Shen, L.; Cui, Z.; Kou, L.; Cheng, J.; Zhang, J. A renewable resveratrol-based epoxy resin with high Tg, excellent mechanical properties and low flammability. Chem. Eng. J. 2020, 383, 123124. [Google Scholar] [CrossRef]
- Xie, Z.; Xiao, D.; Yu, Q.; Wang, Y.; Liao, H.; Zhang, T.; Liu, P.; Xu, L. Fabrication of multifunctional silylated GO/FeSiAl epoxy composites: A heat conducting microwave absorber for 5G base station packaging. Materials 2023, 16, 7511. [Google Scholar] [CrossRef]
- Jia, X.-W.; Mu, W.-L.; Shao, Z.-B.; Xu, Y.-J. Flame-retardant cycloaliphatic epoxy systems with high dielectric performance for electronic packaging materials. Int. J. Mol. Sci. 2023, 24, 2301. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Deng, Y.; Ge, H. Preparation of fully epoxy resin microcapsules and their application in self-healing epoxy anti-corrosion coatings. Prog. Org. Coat. 2024, 188, 108247. [Google Scholar] [CrossRef]
- Ai, D.; Mo, R.; Wang, H.; Lai, Y.; Jiang, X.; Zhang, X. Preparation of waterborne epoxy dispersion and its application in 2K waterborne epoxy coatings. Prog. Org. Coat. 2019, 136, 105258. [Google Scholar] [CrossRef]
- Langer, E.; Waskiewicz, S.; Kuczynska, H. Application of new modified Schiff base epoxy resins as organic coatings. J. Coat. Technol. Res. 2019, 16, 1109–1120. [Google Scholar] [CrossRef]
- Cardoso, A.P.; de Sa, S.C.; Beraldo, C.H.M.; Hidalgo, G.E.N.; Ferreira, C.A. Intumescent coatings using epoxy, alkyd, acrylic, silicone, and silicone-epoxy hybrid resins for steel fire protection. J. Coat. Technol. Res. 2020, 17, 1471–1488. [Google Scholar] [CrossRef]
- Fu, Y.-X.; He, Z.-X.; Mo, D.-C.; Lu, S.-S. Thermal conductivity enhancement with different fillers for epoxy resin adhesives. Appl. Therm. Eng. 2014, 66, 493–498. [Google Scholar] [CrossRef]
- Lee, W.-J.; Cha, S.-H.; Kim, D.-H. Preparation and characterization of cardanol-based flame retardant for enhancing the flame retardancy of epoxy adhesives. Polymers 2022, 14, 5205. [Google Scholar] [CrossRef]
- Xiang, Q.; Xiao, F. Applications of epoxy materials in pavement engineering. Constr. Build. Mater. 2020, 235, 117529. [Google Scholar] [CrossRef]
- Qi, B.; Gao, S.; Xu, P. The application of recycled epoxy plastic sheets waste to replace concrete in urban construction and building. Processes 2023, 11, 201. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Z.; Qiu, H.; Zhang, Y.; Yu, Z.; Gu, J. Significantly enhanced electromagnetic interference shielding performances of epoxy nanocomposites with long-range aligned lamellar structures. Nano-Micro Lett. 2022, 14, 224. [Google Scholar] [CrossRef]
- Rong, L.; Su, J.; Li, Z.; Liu, X.; Zhang, D.; Zhu, J.; Li, X.; Zhao, Y.; Mi, C.; Kong, X.; et al. Silicon hybridization for the preparation of room-temperature curing and high-temperature-resistant epoxy resin. Polymers 2024, 16, 634. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoon, M.; Seo, B.; Lim, C.-S. Effects of fatty acid modified epoxy resin on long-chain epoxy and its physical properties. J. Polym. Sci. 2023, 61, 2194–2202. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Qiu, Y.; Wei, Y. A one-component, fast-cure, and economical epoxy resin system suitable for liquid molding of automotive composite parts. Materials 2018, 11, 685. [Google Scholar] [CrossRef]
- Ji, Y.; Qiao, H.; Liang, Y.; Xu, G.; Wang, Y.; Hu, J. Preparation of water-based epoxy resin and its application as an automotive air filter paper binder. BioResources 2019, 14, 7148–7156. [Google Scholar] [CrossRef]
- Yoon, M.; Yoo, K.; Seo, B.; Ko, S.H.; Lim, C.-S. Development of low-shrink epoxy putty to solve appearance-quality defects of carbon-fiber-reinforced plastic automotive exterior parts. Materials 2021, 14, 6419. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, Y.; Wang, Q.; Cheng, L.; Zhang, C.; Zhang, T.; Shao, J.; Dai, F. Mechanical properties and thermal characteristics of three nano-filler/silk fiber reinforced hybrid composites: A comparative study using a ductile epoxy resin matrix. Polym. Test. 2024, 130, 108319. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, X.; Wu, J.; Wei, M.; Yu, Q.; Tian, J.; Wang, Z. Performance comparison of epoxy resins modified with diphenylphosphine oxide and DOPO. Fire Mater. 2019, 43, 892–902. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, X.; Huang, S.; Tian, X.; Song, L.; Yu, Q.; Wang, Z. Performance comparison of flame retardant epoxy resins modified by DPO–PHE and DOPO–PHE. Polym. Degrad. Stab. 2018, 156, 89–99. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Gao, M.; Han, L.-B. 2-(Hydroxypropan-2-yl)diphenylphosphine oxide (HDPO): A convenient precursor to diphenylphosphine oxide (DPO). Tetrahedron Lett. 2024, 135, 154889. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, M.; Li, B. Synthesis of a novel phosphorus-containing flame retardant curing agent and its application in epoxy resins. J. Nanosci. Nanotechnol. 2016, 16, 2811–2821. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, Z.; Zeng, P.; Gao, T.; Lv, Y.; Liu, X.; Ou, Y.; Chen, L. Behavior and mechanism of flame retardant epoxy resins with intrinsic phosphorus-nitrogen phenolic resin curing agents. Eur. Polym. J. 2024, 210, 112959. [Google Scholar] [CrossRef]
- Tian, X.; Yin, Q.; Wang, Z. Synthesis of diphenylphosphine oxide derivative and its flame retardant application in epoxy resin. J. Photopolym. Sci. Technol. 2019, 32, 769–778. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, J.; Liu, Z.; Gu, Y.; Luan, G.; Sun, H.; Yu, Q.; Zhang, S.; Wang, Z. Effect of ethyl-bridged diphenylphosphine oxide on flame retardancy and thermal properties of epoxy resin. Polym. Adv. Technol. 2020, 31, 1426–1436. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, H.; Chen, J.; Wang, X.; Chen, L.; Ran, D.; Wang, Z.; Zeng, P. A new phosphorus flame-retard curing agent for epoxy resin/anhydride system. Polym. Adv. Technol. 2022, 33, 927–936. [Google Scholar] [CrossRef]
- Xiong, Y.-Q.; Zhang, X.-Y.; Liu, J.; Li, M.-M.; Guo, F.; Xia, X.-N.; Xu, W.-J. Synthesis of novel phosphorus-containing epoxy hardeners and thermal stability and flame-retardant properties of cured products. J. Appl. Polym. Sci. 2012, 125, 1219–1225. [Google Scholar] [CrossRef]
- Dong, J.; Sang, X.; Yin, W.; Chen, X. Preparation of fluorinated epoxy-phthalonitrile resins with excellent thermal stability and low dielectric constant. J. Appl. Polym. Sci. 2023, 140, e53641. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, H.; Dong, K.; Tang, S.; Zhao, C. Comparison of the properties of epoxy resins containing various trifluoromethyl groups with low dielectric constant. Polymers 2023, 15, 2853. [Google Scholar] [CrossRef]
- ISO 4589-2017; Plastics—Determination of Burning Behaviour by Oxygen Index. International Organization for Standardization: Geneva, Switzerland, 2017.
- GB/T 2408-2008; Plastics—Vertical Burning Test. Standardization Administration of China: Beijing, China, 2008.
- ISO 5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method). International Organization for Standardization: Geneva, Switzerland, 2015.
- Janesko, B.G.; Fisher, H.C.; Bridle, M.J.; Montchamp, J.-L. P(=O)H to P−OH tautomerism: A theoretical and experimental study. J. Org. Chem. 2015, 80, 10025–10032. [Google Scholar] [CrossRef]
- Liu, D.; Ji, P.; Zhang, T.; Lv, J.; Cui, Y. A bi-DOPO type of flame retardancy epoxy prepolymer: Synthesis, properties and flame-retardant mechanism. Polym. Degrad. Stab. 2021, 190, 109629. [Google Scholar] [CrossRef]
- Tang, S.; Wachtendorf, V.; Klack, P.; Qian, L.; Dong, Y.; Schartel, B. Enhanced flame-retardant effect of a montmorillonite/phosphaphenanthrene compound in an epoxy thermoset. RSC Adv. 2017, 7, 720–728. [Google Scholar] [CrossRef]
- Wang, P.; Xia, L.; Jian, R.; Ai, Y.; Zheng, X.; Chen, G.; Wang, J. Flame-retarding epoxy resin with an efficient P/N/S-containing flame retardant: Preparation, thermal stability, and flame retardance. Polym. Degrad. Stab. 2018, 149, 69–77. [Google Scholar] [CrossRef]
- Huo, S.; Yang, S.; Wang, J.; Cheng, J.; Zhang, Q.; Hu, Y.; Ding, G.; Zhang, Q.; Song, P.; Wang, H. A liquid phosphaphenanthrene-derived imidazole for improved flame retardancy and smoke suppression of epoxy resin. ACS Appl. Polym. Mater. 2020, 2, 3566–3575. [Google Scholar] [CrossRef]
- Liu, J.; Dai, J.; Wang, S.; Peng, Y.; Cao, L.; Liu, X. Facile synthesis of bio-based reactive flame retardant from vanillin and guaiacol for epoxy resin. Compos. Part B-Eng. 2020, 190, 107926. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Luo, T.; Zhen, H.; Yang, X.; Yang, L.; Yan, Z. Synthesis of solid reactive organophosphorus-nitrogen flame retardant and its application in epoxy resin. J. Appl. Polym. Sci. 2023, 140, e54282. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, X.; Bu, M.; Lei, C. Toughening and strengthening epoxy resins with a new bi-DOPO biphenyl reactive flame retardant. Eur. Polym. J. 2022, 178, 111488. [Google Scholar] [CrossRef]
- Meng, J.; Zeng, Y.; Chen, P.; Zhang, J.; Yao, C.; Fang, Z.; Guo, K. New ultrastiff bio-furan epoxy networks with high Tg: Facile synthesis to excellent properties. Eur. Polym. J. 2019, 121, 109292. [Google Scholar] [CrossRef]
- Dong, C.; Wirasaputra, A.; Luo, Q.; Liu, S.; Yuan, Y.; Zhao, J.; Fu, Y. Intrinsic flame-retardant and thermally stable epoxy endowed by a highly efficient, multifunctional curing agent. Materials 2016, 9, 1008. [Google Scholar] [CrossRef]
- Qian, L.; Qiu, Y.; Sun, N.; Xu, M.; Xu, G.; Xin, F.; Chen, Y. Pyrolysis route of a novel flame retardant constructed by phosphaphenanthrene and triazine-trione groups and its flame-retardant effect on epoxy resin. Polym. Degrad. Stab. 2014, 107, 98–105. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H. Flame retardant effect and mechanism of a novel DOPO based tetrazole derivative on epoxy resin. J. Anal. Appl. Pyrol. 2019, 139, 104–113. [Google Scholar] [CrossRef]
- Huo, S.; Zhou, Z.; Jiang, J.; Sai, T.; Ran, S.; Fang, Z.; Song, P.; Wang, H. Flame-retardant, transparent, mechanically-strong and tough epoxy resin enabled by high-efficiency multifunctional boron-based polyphosphonamide. Chem. Eng. J. 2022, 427, 131578. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Li, Z.; Huang, G.; Wu, G. Efficient flame retardancy, good thermal stability, mechanical enhancement, and transparency of DOPO-conjugated structure compound on epoxy resin. Chem. Eng. J. 2022, 450, 138424. [Google Scholar] [CrossRef]
- Guo, L.; Gu, C.; Feng, J.; Guo, Y.; Jin, Y.; Tu, J. Hydrophobic epoxy resin coating with ionic liquid conversion pretreatment on magnesium alloy for promoting corrosion resistance. J. Mater. Sci. Technol. 2020, 37, 9–18. [Google Scholar] [CrossRef]
- Duan, X.; Li, R.; Li, B.; Feng, Q.; Xu, P.; Wan, J. Thymol-derived trifunctional epoxy resin with outstanding thermomechanical properties and low dielectric constant: From high-throughput synthesis to high-performance thermoset. ACS Appl. Polym. Mater. 2024, 6, 3481–3492. [Google Scholar] [CrossRef]
- Fu, F.; Zhou, X.; Shen, M.; Wang, D.; Xu, X.; Shang, S.; Song, Z.; Song, J. Preparation of hydrophobic low-k epoxy resins with high adhesion using a benzocyclobutene-rosin modifier. ACS Sustain. Chem. Eng. 2023, 11, 5973–5985. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Li, W.-D.; Zhao, X.; Meng, F.-B.; Sun, P.; Wang, C.; Guo, G.-Z.; Li, W.-R.; Zhang, G.-J. Epoxy-based high-k composite vitrimer: With low dielectric loss, high breakdown strength and surface electrical damage repairability. Chem. Eng. J. 2023, 473, 145199. [Google Scholar] [CrossRef]



| Samples | DGEBA (g) | DDS (g) | ODDPO (g) | P (wt%) |
|---|---|---|---|---|
| EP-0 | 100 | 28.55 | 0 | 0 |
| EP/ODDPO-0.9 | 100 | 27.34 | 8.16 | 0.9 |
| EP/ODDPO-1.2 | 100 | 26.91 | 11.04 | 1.2 |
| EP/ODDPO-1.5 | 100 | 26.47 | 14.01 | 1.5 |
| Samples | Tg (°C) | νe (×103 mol/m3) | T5% (°C) | T10% (°C) | Tmax (°C) | MMLR (%/min) | Char Residue at 800 °C (wt%) | References |
|---|---|---|---|---|---|---|---|---|
| EP-0 | 184.3 | 5.06 | 370.4 | 379.6 | 408.3 | 1.36 | 0.7 | This work |
| EP/ODDPO-0.9 | 175.2 | 3.56 | 367.3 | 376.1 | 402.7 | 1.38 | 20.4 | |
| EP/ODDPO-1.2 | 176.7 | 3.68 | 360.4 | 370.5 | 399.2 | 1.31 | 21.5 | |
| EP/ODDPO-1.5 | 171.5 | 2.40 | 366.5 | 375.1 | 403.9 | 1.41 | 22.6 | |
| EP/DPO-1.2 | 154 | - | 351 | 369 | 402 | - | - | [27] |
| EP/DOPO-1.2 | 148 | - | 350 | 359 | 374 | - | - |
| Samples | LOI (%) | UL-94 (3 mm) | References | ||
|---|---|---|---|---|---|
| Dripping | Igniting Cotton | Rating | |||
| EP-0 | 23.2 | Yes | Yes | NR | This work |
| EP/ODDPO-0.9 | 28.1 | No | No | V-1 | |
| EP/ODDPO-1.2 | 29.2 | No | No | V-0 | |
| EP/ODDPO-1.5 | 29.9 | No | No | V-0 | |
| EP/DPO-1.2 | 30.8 | - | - | V-0 | [27] |
| EP/DOPO-1.2 | 30.0 | - | - | V-0 | |
| Samples | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | av-EHC (MJ/kg) | av-CO (MJ/kg) | av-CO2 (MJ/kg) | TML (%) | Residue (wt%) |
|---|---|---|---|---|---|---|---|---|
| EP-0 | 38 | 891.85 | 88.20 | 22.12 | 0.10 | 1.60 | 97.58 | 2.42 |
| EP/ODDPO-1.2 | 34 | 567.60 | 83.94 | 20.82 | 0.12 | 1.39 | 90.84 | 9.16 |
| Samples | Flame Inhibition Effect | Charring Effect | Barrier Effect |
|---|---|---|---|
| EP-0 | - | - | - |
| EP/ODDPO-1.2 | 5.88% | 6.91% | 33.12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tian, C.; Cheng, G.; Li, C.; Wang, Z. Facile Synthesis of Bis-Diphenylphosphine Oxide as a Flame Retardant for Epoxy Resins. Polymers 2024, 16, 2635. https://doi.org/10.3390/polym16182635
Li Y, Tian C, Cheng G, Li C, Wang Z. Facile Synthesis of Bis-Diphenylphosphine Oxide as a Flame Retardant for Epoxy Resins. Polymers. 2024; 16(18):2635. https://doi.org/10.3390/polym16182635
Chicago/Turabian StyleLi, Yan, Chong Tian, Guiqing Cheng, Chunhui Li, and Zhongwei Wang. 2024. "Facile Synthesis of Bis-Diphenylphosphine Oxide as a Flame Retardant for Epoxy Resins" Polymers 16, no. 18: 2635. https://doi.org/10.3390/polym16182635
APA StyleLi, Y., Tian, C., Cheng, G., Li, C., & Wang, Z. (2024). Facile Synthesis of Bis-Diphenylphosphine Oxide as a Flame Retardant for Epoxy Resins. Polymers, 16(18), 2635. https://doi.org/10.3390/polym16182635






