Hydrothermal Carbonization of Biomass for Electrochemical Energy Storage: Parameters, Mechanisms, Electrochemical Performance, and the Incorporation of Transition Metal Dichalcogenide Nanoparticles
Abstract
1. Introduction
2. Insights into the Hydrothermal Carbonization of Biomass
2.1. Lignocellulosic Biomass
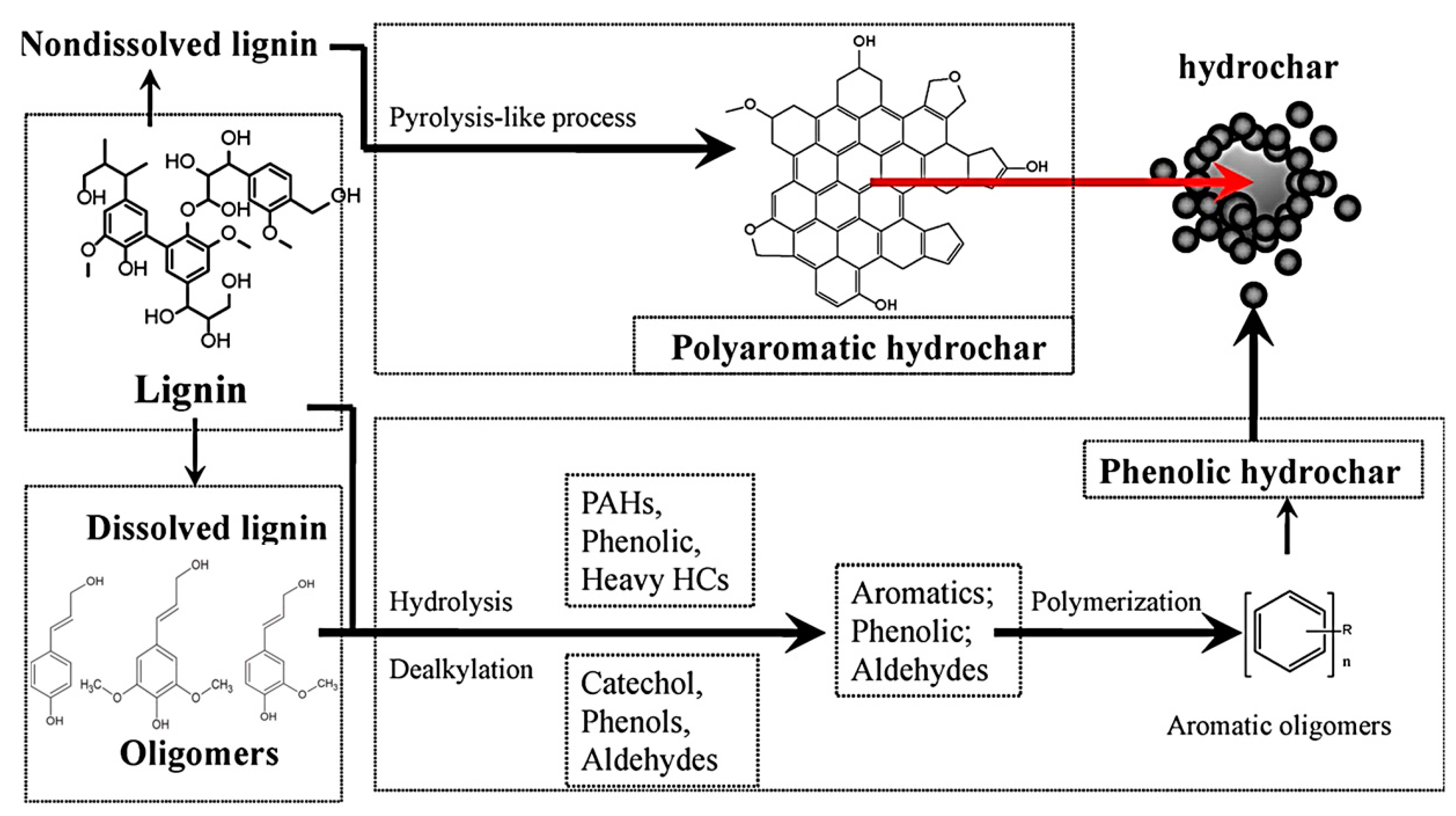
2.2. HTC Mechanism and Relation to Biomass Type
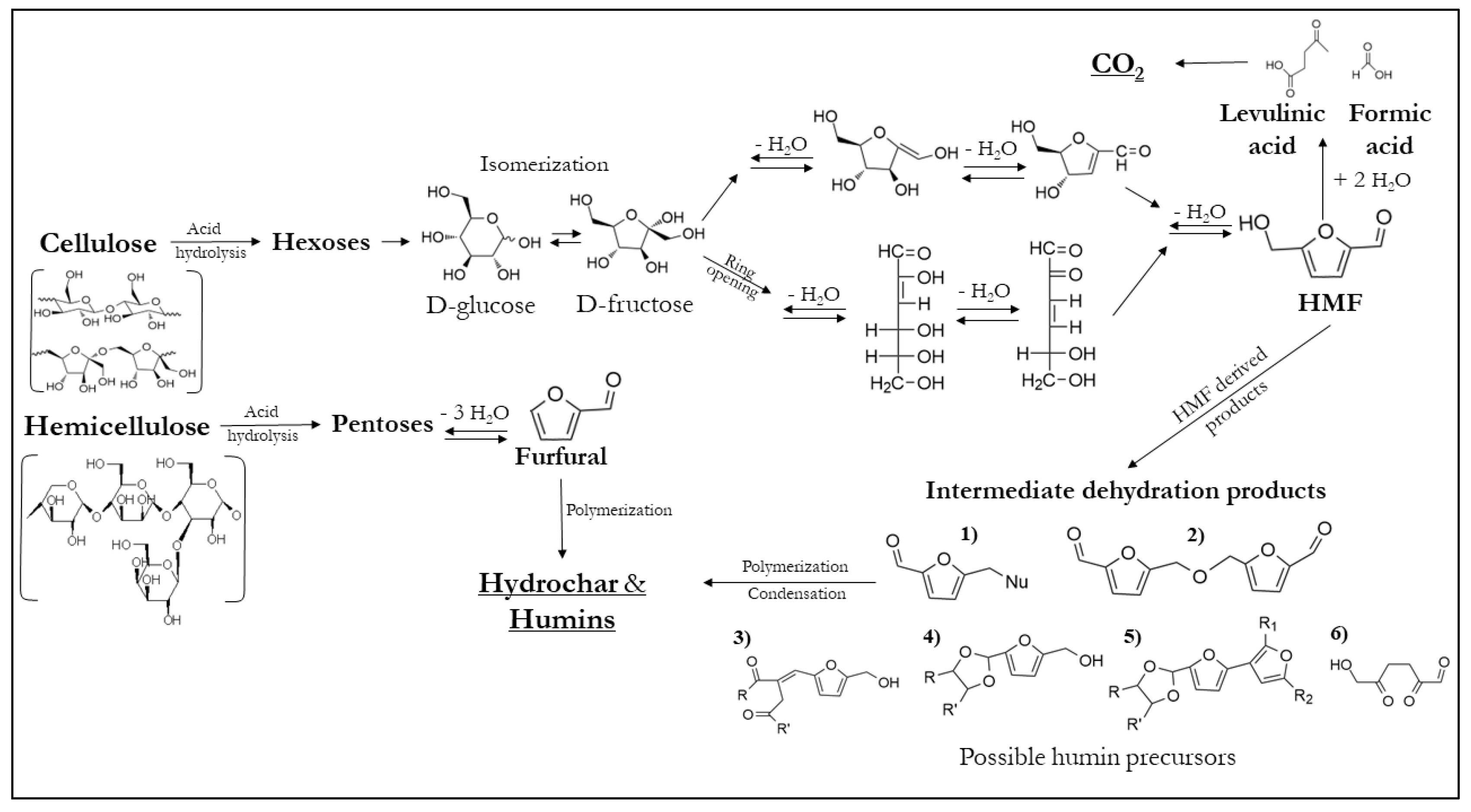
2.3. Parameters Governing the HTC Process
2.3.1. Temperature
2.3.2. Residence Time
2.3.3. Feedwater Acidity and Catalyst
2.3.4. Feedstock
2.3.5. Heating Rate
2.3.6. Pressure
2.4. Hydrochar Pore Formation
2.4.1. Physical Activation
2.4.2. Chemical Activation
2.4.3. Chemical or Physical Activation: Which Is Better When Targeting EDLC?
2.4.4. Why Is HTC a Beneficial Pre-Treatment to Activation?
3. Electrochemical Energy Storage—EDLCs
3.1. Overview
3.2. Electrode Properties
3.3. Biomass-Derived Electrode Materials for Supercapacitor Applications Prepared without HTC-Pretreatment
| Electrode Material | Synthesis Method | Electrolyte | Stability | Microporosity (%) | BET Area (m2/g) | Cap (F/g) 1 |
|---|---|---|---|---|---|---|
| AC from paulownia flower [177] | Pyrolysis at 600 °C, mix with KOH (3:1 KOH: carbon ratio) and carbonization at 800 °C | 1 M H2SO4 | 93% retention after 1000 cycles | 81% | 1159 | 297 |
| AC from wheat straw [178] | Pyrolysis at 800 °C, KOH (5:1) activ. ** at 800 °C | PVA/KOH | 97.6% after 5000 cycles | 62% | 2115 | 294/296 * |
| Ginger straw-based AC [175] | Carbonization at 700 °C | 6 M KOH | 88% after 6000 cycles | 65% | 720 | 243 |
| AC from rice husk [179] | Mix with ZnCl2 (4:1) followed by microwave heating (600 W). | 6 M KOH | 28% at 20 A/g | 15% | 1565 | 240 |
| Carbon nanosheets derived from silk [176] | Mix with ZnCl2 (2.5:1) followed by annealing at 900 °C | EMIMBF4 | 92% after 10,000 cycles | 18% | 2494 | 213 |
| Porous carbon from tissue papers [180] | KOH (2.5:1) activ. at 700 °C | 6 M KOH | 58 F/g at 100 mV/s | Mainly microporous | 1320 | 200 (at 1 mV/s) |
| AC from biomass waste [181] | Pyrolysis at 500 °C and KOH (3:1) activ. at 700 °C | 6 M KOH | 75% at 10 A/g | 85% | 1831 | 197/289 * |
| AC from peanut shell [179] | Mix with ZnCl2 (4:1) followed by microwave (MW) heating (600 W). | 6 M KOH | 52% at 20 A/g | 1% | 1552 | 188 |
| Glucose-derived graphene-based AC [174] | NH4Cl mix (1:1), heating at 400 °C, heating at 1100 °C, KOH (13:1) activ. at 800 °C. | EMIM-TFSI/AN | 90% after 10,000 cycles | Large micro and mesopore presence | 3657 | 175 |
| AC from bacterial cellulose 2 [182] | Freezing (liquid N2), heat at 900 °C, and KOH (1:1) activ. at 900 °C | 6 M KOH | Over 90% after 10,000 cycles | 32% | 491 | 167 |
| Porous carbon from starch 4 [39] | Graphite addition (20% w/w), MW heating (140 °C) and pyrolysis at 800 °C | 2 M H2SO4 | 85% after 10,000 cycles | 38% | 337 | 157 |
| Porous carbon from bamboo 3 [183] | Mix with KHCO3 (4:1) and carbonization at 400 °C | 6 M KOH | 98.4 after 10,000 cycles at 10 A/g | 56% | 1425 | 143 |
| Cashew nut husk derived AC [184] | Heating at 600 °C and KOH (4:1) activ. at 850 °C | 6 M KOH | Close to 100% after 4000 cycles | Mainly micro and small mesopores | 2742 | 125/305 * |
3.4. Hydrothermally Pretreated, Biomass-Derived Electrode Materials for Supercapacitor Applications
- Electrodes derived from biopolymers.
| Electrode Material | Synthesis Method | Electrolyte | Stability | Microporosity (%) | BET Area (m2/g) | Cap (F/g) 1 |
|---|---|---|---|---|---|---|
| Biopolymers | ||||||
| AC from chitosan [192] | HTC (250 °C, 4 h), KHCO3 activ. ** (750 °C) | 1 M H2SO4 | 75% at 10 A/g | 36% | 2124 | 265/326 * |
| AC from cellulose and thiourea [189] | HTC (240 °C, 1 h) and KOH (3:1) activ. at 800 °C | 6 M KOH | Stable after 20,000 cycles | Mainly microporous | 952 | 224/236 * |
| Cellulose/AC/GO hydrogel [188] | Straw heating (500 °C) and KOH (3:1) activ. at 700 °C. HTC (180 °C, 1 h) of a cellulose, AC and GO mixture. | Lignin hydrogel | 88% after 10,000 cycles | Hierarchical structure (micro, meso and macro) | 762 | 208/565 * |
| Cellulose-based AC. [132] | HTC (250 °C cell; 230 °C starch, 2 h) and KOH (4:1) activ. at 700 °C | 1 M TEABF4/AN | 65% at 20 A/g. | 87% | 2457 | 170 |
| AC from starch. [132] | 65% at 20 A/g. | 87% | 2273 | 161 | ||
| Lignin derived AC [193] | HTC (220 °C, 14 h) in H2SO4 (aq.), KOH (1:1) activ. at 800 °C | 6 M KOH | 98% after 5000 cycles | 76% | 1337 | 110/255 * |
| Raw biomass | ||||||
| Wood sawdust derived AC. [194] | HTC (120 °C, 2 h) in KOH (aq.) and carbonization at 800 °C | 6 M KOH | 99% after 5000 cycles. | 74%. | 1185 | 244/302 * |
| AC from coconut shells. [134] | HTC (200 °C, 20 min) in H2O2 aq., HTC (275 °C, 12 h) in ZnCl2 aq. and CO2 activ. (800 °C) | 0.5 M H2SO4 | 88% after 2000 cycles. | Mesoporous structure | 2440 | 207 |
| AC from wood sawdust. [132] | HTC (250 °C, 2 h) and KOH (4:1) activ. at 800 °C | 1 M TEABF4/AN | 75% at 20 A/g. | 89% | 2967 | 197 |
| Enteromorpha Prolifera-based AC. [195] | HTC (180 °C, 24 h), heating at 450 °C, KOH (2:1) activ. at 700 °C | 6 M KOH | 90% after 10,000 cycles. | 88% | 1528 | 192 |
| AC from Spirulina platensis and glucose. [133] | HTC (180 °C, 24 h) and KOH (2:1) activ. at 700 °C | 6 M LiCl | 98% after 10,000 cycles. | 93% | 2130 | 177 |
| Jatropha derived AC. [196] | HTC (190 °C, 2 h) and KOH (1:1) activ. at 800 °C | 1 M KOH | 19% Increase after 5000 cycles. | Large micro and macropores. | 747 | 175 |
| AC from hemp fibers. [131] | HTC (180 °C, 24 h) in H2SO4 (aq.), KOH (1:1) activ. at 750 °C | BMPY TFSI | 90% at 100 A/g | 47% | 2287 | 160 |
| AC from corn straws. [136] | HTC (220 °C, 12 h) and KOH (1:1) activ. at 800 °C | 6 M KOH | 83% after 2000 cycles. | Mainly microporous. | 1229 | 66/271 * |
- Electrodes derived from raw biomass.
3.5. TMDCs: Energy Storage and Other Promising Applications, Enhancing Biomass-Derived Materials
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations Climate Change. Summary of Global Climate Action at COP 28. 2023. Available online: https://unfccc.int/documents/636485 (accessed on 14 September 2024).
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Global Energy Statistical Yearbook 2021. 2021. Available online: https://energydata.info/dataset/key-world-energy-statistics-enerdata (accessed on 14 September 2024).
- International Energy Agency. Global Energy Review 2021, Assessing the Effects of Economic Recoveries on Global Enegy Demand and CO2 Emissions in 2021; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Electricity Prices in Europe–Who Pays the Most? Available online: https://strom-report.com/electricity-prices-europe/ (accessed on 1 June 2024).
- Gerland, P.; Raftery, A.E.; Ševčíková, H.; Li, N.; Gu, D.; Spoorenberg, T.; Alkema, L.; Fosdick, B.K.; Chunn, J.; Lalic, N.; et al. World population stabilization unlikely this century. Science 2014, 346, 234. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2014: Synthesis Report; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Renewable Power 2023. Available online: https://www.iea.org/reports/renewables-2023 (accessed on 14 September 2024).
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- García-Condado, S.; López-Lozano, R.; Panarello, L.; Cerrani, I.; Nisini, L.; Zucchini, A.; Van der Velde, M.; Baruth, B. Assessing lignocellulosic biomass production from crop residues in the European Union: Modelling, analysis of the current scenario and drivers of interannual variability. GCB Bioenergy 2019, 11, 809–831. [Google Scholar] [CrossRef]
- Kazmi, A.; Shuttleworth, P.S. The Economic Utilisation of Food Co-Products; The Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Li, T.; Remón, J.; Shuttleworth, P.S.; Jiang, Z.; Fan, J.; Clark, J.H.; Budarin, V.L. Controllable production of liquid and solid biofuels by doping-free, microwave-assisted, pressurised pyrolysis of hemicellulose. Energy Convers. Manag. 2017, 144, 104–113. [Google Scholar] [CrossRef]
- Berge, N.D.; Li, L.; Flora, J.R.V.; Ro, K.S. Assessing the environmental impact of energy production from hydrochar generated via hydrothermal carbonization of food wastes. Waste Manag. 2015, 43, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Owsianiak, M.; Ryberg, M.W.; Renz, M.; Hitzl, M.; Hauschild, M.Z. Environmental Performance of Hydrothermal Carbonization of Four Wet Biomass Waste Streams at Industry-Relevant Scales. ACS Sustain. Chem. Eng. 2016, 4, 6783–6791. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Guo, Z.; Xie, F.; Liu, H.; Yadegari, H.; Tebyetekerwa, M.; Ryan, M.P.; Hu, Y.-S.; Titirici, M.-M. The Role of Hydrothermal Carbonization in Sustainable Sodium-Ion Battery Anodes. Adv. Energy Mater. 2022, 12, 2200208. [Google Scholar] [CrossRef]
- Benavente, V.; Fullana, A.; Berge, N.D. Life cycle analysis of hydrothermal carbonization of olive mill waste: Comparison with current management approaches. J. Clean. Prod. 2017, 142, 2637–2648. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Z.; Guo, Z.; Feng, J.; Li, H.; Qiu, T.; Titirici, M. A life cycle assessment of hard carbon anodes for sodium-ion batteries. Philos. Trans. R. Soc. A 2021, 379, 20200340. [Google Scholar] [CrossRef]
- Baxter, L.L.; Miles, T.R.; Miles, T.R.; Jenkins, B.M.; Milne, T.; Dayton, D.; Bryers, R.W.; Oden, L.L. The behavior of inorganic material in biomass-fired power boilers: Field and laboratory experiences. Fuel Process. Technol. 1998, 54, 47–78. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Bevan, E.; Fu, J.; Zheng, Y. Challenges and opportunities of hydrothermal carbonisation in the UK; Case study in Chirnside. RSC Adv. 2021, 11, 34870–34897. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Bento, L.R.; Castro, A.J.R.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C.; Melo, C.A. Release of nutrients and organic carbon in different soil types from hydrochar obtained using sugarcane bagasse and vinasse. Geoderma 2019, 334, 24–32. [Google Scholar] [CrossRef]
- Sevilla, M.; Maciá-Agulló, J.A.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Wen, C.; Zhang, L.; Wang, Y.S. Preparation of carbon sphere from lactose by hydrothermal reaction and its performance in gas separation. Environ. Prog. Sustain. Energy 2014, 33, 581–587. [Google Scholar] [CrossRef]
- Román, S.; Valente Nabais, J.M.; Ledesma, B.; González, J.F.; Laginhas, C.; Titirici, M.M. Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater. 2013, 165, 127–133. [Google Scholar] [CrossRef]
- Tan, T.; Yan, L.; Lian, Y.; Qi, X. Adsorption of reactive brilliant red K-2BP on amino-functionalized carbon materials prepared from hydrothermal carbonization of glucose. Chin. J. Environ. Eng. 2014, 8, 4122–4128. [Google Scholar]
- Mochidzuki, K.; Sato, N.; Sakoda, A. Production and characterization of carbonaceous adsorbents from biomass wastes by aqueous phase carbonization. Adsorption 2005, 11, 669–673. [Google Scholar] [CrossRef]
- Babeker, T.M.A.; Chen, Q. Heavy Metal Removal from Wastewater by Adsorption with Hydrochar Derived from Biomass: Current Applications and Research Trends. Curr. Pollut. Rep. 2021, 7, 54–71. [Google Scholar] [CrossRef]
- Li, Y.; Tsend, N.; Li, T.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Łoczechin, A.; Séron, K.; Barras, A.; Giovanelli, E.; Belouzard, S.; Chen, Y.-T.; Metzler-Nolte, N.; Boukherroub, R.; Dubuisson, J.; Szunerits, S. Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus. ACS Appl. Mater. Interfaces 2019, 11, 42964–42974. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.; Park, K.Y. Upgrading the fuel properties of sludge and low rank coal mixed fuel through hydrothermal carbonization. Energy 2017, 141, 598–602. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Upgrading the characteristics of biochar from cellulose, lignin, and xylan for solid biofuel production from biomass by hydrothermal carbonization. J. Ind. Eng. Chem. 2016, 42, 95–100. [Google Scholar] [CrossRef]
- Sengupta, A.N. An assessment of grindability index of coal. Fuel Process. Technol. 2002, 76, 1–10. [Google Scholar] [CrossRef]
- Mbarki, F.; Selmi, T.; Kesraoui, A.; Seffen, M.; Gadonneix, P.; Celzard, A.; Fierro, V. Hydrothermal pre-treatment, an efficient tool to improve activated carbon performances. Ind. Crops Prod. 2019, 140, 111717. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Garcia, A.M.; Budarin, V.L.; Zhou, Y.; De Bruyn, M.; Hunt, A.J.; Lari, L.; Lazarov, V.K.; Salavagione, H.J.; Morales, E.; Ellis, G.J.; et al. Monolithic mesoporous graphitic composites as super capacitors: From Starbons to Starenes. J. Mater. Chem. A 2018, 6, 1119–1127. [Google Scholar] [CrossRef]
- Research, P. Supercapacitors Market-Global Industry Analysis, Market Size, Share, Growth, Trends, Regional Outlook and Segment Forecasts 2020–2027. Available online: https://www.precedenceresearch.com/supercapacitors-market (accessed on 14 September 2024).
- Environmental and Energy Study Institute. Fact Sheet: Energy Storage (2019). Available online: https://www.eesi.org/papers/view/energy-storage-2019 (accessed on 14 September 2024).
- Pumped Hydro Storage Market-Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/pumped-hydro-storage-market (accessed on 14 September 2024).
- Butnoi, P.; Pangon, A.; Berger, R.; Butt, H.-J.; Intasanta, V. Electrospun nanocomposite fibers from lignin and iron oxide as supercapacitor material. J. Mater. Res. Technol. 2021, 12, 2153–2167. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. MnO2 and biomass-derived 3D porous carbon composites electrodes for high performance supercapacitor applications. J. Alloys Compd. 2018, 741, 360–367. [Google Scholar] [CrossRef]
- Lesbayev, B.; Auyelkhankyzy, M.; Ustayeva, G.; Yeleuov, M.; Rakhymzhan, N.; Maral, Y.; Tolynbekov, A. Modification of Biomass-Derived Nanoporous Carbon with Nickel Oxide Nanoparticles for Supercapacitor Application. J. Compos. Sci. 2023, 7, 20. [Google Scholar] [CrossRef]
- Panith, P.; Butnoi, P.; Intasanta, V. The hybrid structure of nanoflower-like CoxMnyNizO4 nanoparticles embedded biomass-lignin carbon nanofibers as free-standing and binder-free electrodes for high performance supercapacitors. J. Alloys Compd. 2022, 918, 165659. [Google Scholar] [CrossRef]
- Tong, Y.; Yang, J.; Li, J.; Cong, Z.; Wei, L.; Liu, M.; Zhai, S.; Wang, K.; An, Q. Lignin-derived electrode materials for supercapacitor applications: Progress and perspectives. J. Mater. Chem. A 2023, 11, 1061–1082. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Dang, V.D.; Thamilselvan, A.; Doong, R.-a.; Pandit, B. Sustainable high-energy supercapacitors: Metal oxide-agricultural waste biochar composites paving the way for a greener future. J. Energy Storage 2024, 77, 109723. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, T.; Liu, K.; Zhang, M.; Cai, X.-M.; Si, C. Biomass-based materials for advanced supercapacitor: Principles, progress, and perspectives. Aggregate 2024, 5, e428. [Google Scholar] [CrossRef]
- Pontiroli, D.; Scaravonati, S.; Magnani, G.; Fornasini, L.; Bersani, D.; Bertoni, G.; Milanese, C.; Girella, A.; Ridi, F.; Verucchi, R.; et al. Super-activated biochar from poultry litter for high-performance supercapacitors. Microporous Mesoporous Mater. 2019, 285, 161–169. [Google Scholar] [CrossRef]
- Lin, Z.; Li, X.; Zhang, H.; Xu, B.B.; Wasnik, P.; Li, H.; Singh, M.V.; Ma, Y.; Li, T.; Guo, Z. Research progress of MXenes and layered double hydroxides for supercapacitors. Inorg. Chem. Front. 2023, 10, 4358–4392. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, M.A.; Rasool, P.I.; Mohmand, N.Z.K.; Khan, A.J.; Ullah, H.; Feng, X.; Oyama, M. Electrochemical synergy and future prospects: Advancements and challenges in MXene and MOFs composites for hybrid supercapacitors. Sustain. Mater. Technol. 2024, 39, e00814. [Google Scholar] [CrossRef]
- Sheikh, Z.A.; Katkar, P.K.; Kim, H.; Rehman, S.; Khan, K.; Chavan, V.D.; Jose, R.; Khan, M.F.; Kim, D.-k. Transition metal chalcogenides, MXene, and their hybrids: An emerging electrochemical capacitor electrodes. J. Energy Storage 2023, 71, 107997. [Google Scholar] [CrossRef]
- Naffakh, M.; Shuttleworth, P.S.; Ellis, G. Bio-based polymer nanocomposites based on nylon 11 and WS2 inorganic nanotubes. RSC Adv. 2015, 5, 17879–17887. [Google Scholar] [CrossRef]
- Adini, A.R.; Redlich, M.; Tenne, R. Medical applications of inorganic fullerene-like nanoparticles. J. Mater. Chem. 2011, 21, 15121–15131. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Bissett, M.A.; Worrall, S.D.; Kinloch, I.A.; Dryfe, R.A.W. Comparison of Two-Dimensional Transition Metal Dichalcogenides for Electrochemical Supercapacitors. Electrochim. Acta 2016, 201, 30–37. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berge, N.; et al. Recent advances in hydrothermal carbonisation: From tailored carbon materials and biochemicals to applications and bioenergy. Green Chem. 2020, 22, 4747–4800. [Google Scholar] [CrossRef]
- Position of European Bioplastics-Industrial Use of Agricultural Feedstocks; European Bioplastics: Berlin, Germany, 2019.
- Williams, L.; Emerson, R.; Tumuluru, J.S. Biomass Compositional Analysis for Conversion to Renewable Fuels and Chemicals. In Biomass Volume Estimation and Valorization for Energy; Books on Demand: Norderstedt, Germany, 2017. [Google Scholar]
- Eurostat. Food Waste and Food Waste Prevention-Estimates. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 14 September 2024).
- Xu, S.; He, H.; Luo, L. Status and Prospects of Municipal Solid Waste to Energy Technologies in China. In Recycling of Solid Waste for Biofuels and Bio-Chemicals; Karthikeyan, O.P., Heimann, K., Muthu, S.S., Eds.; Springer: Singapore, 2016; pp. 31–54. [Google Scholar]
- Peter, Z. Order in cellulosics: Historical review of crystal structure research on cellulose. Carbohydr. Polym. 2021, 254, 117417. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Banwell, M.G.; Pollard, B.; Liu, X.; Connal, L.A. Exploiting Nature’s Most Abundant Polymers: Developing New Pathways for the Conversion of Cellulose, Hemicellulose, Lignin and Chitin into Platform Molecules (and Beyond). Chem.–Asian J. 2021, 16, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Katahira, R.; Elder, T.J.; Beckham, G.T. Chapter 1 A Brief Introduction to Lignin Structure. In Lignin Valorization: Emerging Approaches; The Royal Society of Chemistry: London, UK, 2018; pp. 1–20. [Google Scholar]
- Otromke, M.; Shuttleworth, P.S.; Sauer, J.; White, R.J. Hydrothermal base catalysed treatment of Kraft Lignin for the preparation of a sustainable carbon fibre precursor. Bioresour. Technol. Rep. 2019, 5, 251–260. [Google Scholar] [CrossRef]
- Otromke, M.; Shuttleworth, P.S.; Sauer, J.; White, R.J. Hydrothermal base catalysed treatment of Kraft lignin-time dependent analysis and a techno-economic evaluation for carbon fibre applications. Bioresour. Technol. Rep. 2019, 6, 241–250. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Garcia, I.L.; Leiva-Candia, D.; Dorado, M.P. Valorization of food waste based on its composition through the concept of biorefinery. Curr. Opin. Green Sustain. Chem. 2018, 14, 67–79. [Google Scholar] [CrossRef]
- Tester, R.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Bergius, F. Die Anwendung Hoher Drucke Bei Chemischen Vorgängen Und Eine Nechbildung Des Entstehungsprozesses Der Steinkohle; W. Knapp: Halle, Germany, 1913. [Google Scholar]
- Berl, E.; Schmidt, A. Über die Entstehung der Kohlen. II. Die Inkohlung von Cellulose und Lignin in neutralem Medium. Justus Liebigs Ann. Der Chem. 1932, 493, 97–123. [Google Scholar] [CrossRef]
- Kruse, A.; Dahmen, N. Water–A magic solvent for biomass conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Sun, S.; Zhong, L.; Chen, W.; Wang, S.; Sun, R.-C. Hydrothermal conversion of xylose, glucose, and cellulose under the catalysis of transition metal sulfates. Carbohydr. Polym. 2015, 118, 44–51. [Google Scholar] [CrossRef]
- Aida, T.M.; Shiraishi, N.; Kubo, M.; Watanabe, M.; Smith, R.L. Reaction kinetics of d-xylose in sub- and supercritical water. J. Supercrit. Fluids 2010, 55, 208–216. [Google Scholar] [CrossRef]
- Jung, D.; Körner, P.; Kruse, A. Kinetic study on the impact of acidity and acid concentration on the formation of 5-hydroxymethylfurfural (HMF), humins, and levulinic acid in the hydrothermal conversion of fructose. Biomass Convers. Biorefinery 2021, 11, 1155–1170. [Google Scholar] [CrossRef]
- Titirici, M.M. Hydrothermal Carbonisation: A Sustainable Alternative to Versatile Carbon Materials. Doctoral Thesis, University of Postdam, Potsdam, Germany, 2012. [Google Scholar]
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. Mechanism of Brønsted acid-catalyzed conversion of carbohydrates. J. Catal. 2012, 295, 122–132. [Google Scholar] [CrossRef]
- Horvat, J.; Klaić, B.; Metelko, B.; Šunjić, V. Mechanism of levulinic acid formation. Tetrahedron Lett. 1985, 26, 2111–2114. [Google Scholar] [CrossRef]
- Sumerskii, I.V.; Krutov, S.M.; Zarubin, M.Y. Humin-like substances formed under the conditions of industrial hydrolysis of wood. Russ. J. Appl. Chem. 2010, 83, 320–327. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Nezafat, Z.; Shafiei, N. Chapter 5-Lignin chemistry and valorization. In Biopolymer-Based Metal Nanoparticle Chemistry for Sustainable Applications; Nasrollahzadeh, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–183. [Google Scholar]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Knežević, D.; van Swaaij, W.; Kersten, S. Hydrothermal Conversion Of Biomass. II. Conversion Of Wood, Pyrolysis Oil, And Glucose In Hot Compressed Water. Ind. Eng. Chem. Res. 2010, 49, 104–112. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Sohn, D.; Kim, Y.M.; Park, K.Y. Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel. Energy 2018, 153, 913–920. [Google Scholar] [CrossRef]
- Yang, W.; Wang, H.; Zhang, M.; Zhu, J.; Zhou, J.; Wu, S. Fuel properties and combustion kinetics of hydrochar prepared by hydrothermal carbonization of bamboo. Bioresour. Technol. 2016, 205, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shimanouchi, T.; Kimura, Y. Characterization of the Residue and Liquid Products Produced from Husks of Nuts from Carya cathayensis Sarg by Hydrothermal Carbonization. ACS Sustain. Chem. Eng. 2015, 3, 591–598. [Google Scholar] [CrossRef]
- Yang, W.; Shimanouchi, T.; Iwamura, M.; Takahashi, Y.; Mano, R.; Takashima, K.; Tanifuji, T.; Kimura, Y. Elevating the fuel properties of Humulus lupulus, Plumeria alba and Calophyllum inophyllum L. through wet torrefaction. Fuel 2015, 146, 88–94. [Google Scholar] [CrossRef]
- Cai, J.; Li, B.; Chen, C.; Wang, J.; Zhao, M.; Zhang, K. Hydrothermal carbonization of tobacco stalk for fuel application. Bioresour. Technol. 2016, 220, 305–311. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.-H.; Yang, H.-P.; Chen, H.-P. Characterization of products from hydrothermal treatments of cellulose. Energy 2012, 42, 457–465. [Google Scholar] [CrossRef]
- Yang, W.; Shimizu, I.; Ono, T.; Kimura, Y. Preparation of Biodegradable Foam from Walnut Shells Treated by Subcritical Water. J. Chem. Technol. Biotechnol. 2015, 90, 44–49. [Google Scholar] [CrossRef]
- Krysanova, K.; Krylova, A.; Zaichenko, V.; Lavrenov, V.; Khaskhachikh, V. Influence of the parameters of the hydrothermal carbonization of the biomass on the biocoal obtained from peat. E3S Web Conf. 2019, 114, 07003. [Google Scholar] [CrossRef]
- Kim, H.; Han, S.; Song, E.; Park, S. Estimation of the characteristics with hydrothermal carbonisation temperature on poultry slaughterhouse wastes. Waste Manag. Res. 2018, 36, 0734242X1877208. [Google Scholar] [CrossRef]
- Park, K.Y.; Lee, K.; Kim, D. Characterized hydrochar of algal biomass for producing solid fuel through hydrothermal carbonization. Bioresour. Technol. 2018, 258, 119–124. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Wang, J.; Li, X.; Cheng, J.; Haiping, Y.; Chen, H. Effect of residence time on chemical and structural properties of hydrochar obtained by hydrothermal carbonization of water hyacinth. Energy 2013, 58, 376–383. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Wang, B.; Wang, Q.; Yang, G.; Chen, J. Effect of Residence Time on Hydrothermal Carbonization of Corn Cob Residual. BioResources 2015, 10, 3979–3986. [Google Scholar] [CrossRef]
- Al-Kaabi, Z.; Pradhan, R.; Thevathasan, N.; Gordon, A.; Chiang, Y.W.; Dutta, A. Bio-carbon production by oxidation and hydrothermal carbonization of paper recycling black liquor. J. Clean. Prod. 2019, 213, 332–341. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.; Mumme, J. Review Article: Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Siskin, M.; Katritzky, A.R. Reactivity of Organic Compounds in Superheated Water: General Background. Chem. Rev. 2001, 101, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, Y.; Li, S.; Wang, B.; Wang, T.; Liu, Y.; Qiu, Z.; Li, C. Hydrothermal carbonization of sewage sludge: Effect of feed-water pH on hydrochar’s physicochemical properties, organic component and thermal behavior. J. Hazard. Mater. 2020, 388, 122084. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Wang, T.; Xu, B.; Li, C.; Zeng, G. Feedwater pH affects phosphorus transformation during hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2017, 245, 182–187. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Acetic Acid and Sodium Hydroxide-Aided Hydrothermal Carbonization of Woody Biomass for Enhanced Pelletization and Fuel Properties. Energy Fuels 2017, 31, 12200–12208. [Google Scholar] [CrossRef]
- Liang, X.; Yang, J. Synthesis of a Novel Carbon Based Strong Acid Catalyst Through Hydrothermal Carbonization. Catal. Lett. 2009, 132, 460. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Catalytic hydrothermal treatment of pine wood biomass: Effect of RbOH and CsOH on product distribution. J. Chem. Technol. Biotechnol. 2005, 80, 1097–1102. [Google Scholar] [CrossRef]
- Zhong, C.; Wei, X. A comparative experimental study on the liquefaction of wood. Energy 2004, 29, 1731–1741. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Thygesen, A.; Thomsen, A.B. Hydrothermal treatment of wheat straw at pilot plant scale using a three-step reactor system aiming at high hemicellulose recovery, high cellulose digestibility and low lignin hydrolysis. Bioresour. Technol. 2008, 99, 4221–4228. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Tayyab, M. Bioethanol Production From Lignocellulosic Biomass By Environment-Friendly Pretreatment Methods: A Review. Appl. Ecol. Environ. Res. 2018, 16, 225–249. [Google Scholar] [CrossRef]
- Cui, X.; Lee, J.J.L.; Chen, W.N. Eco-friendly and biodegradable cellulose hydrogels produced from low cost okara: Towards non-toxic flexible electronics. Sci. Rep. 2019, 9, 18166. [Google Scholar] [CrossRef]
- Liu, M.; Tong, S.; Tong, Z.; Guan, Y.; Sun, Y. A strong, biodegradable and transparent cellulose-based bioplastic stemmed from waste paper. J. Appl. Polym. Sci. 2023, 140, e53671. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Volf, I.; Belosinschi, D.; Tofanica, B.-M.; Ungureanu, E. Cellulose-Based Metallogels—Part 1: Raw Materials and Preparation. Gels 2023, 9, 390. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Effect of heating rate on the pyrolysis yields of rapeseed. Renew. Energy 2006, 31, 803–810. [Google Scholar] [CrossRef]
- Zhang, B.; von Keitz, M.; Valentas, K. Thermal Effects on Hydrothermal Biomass Liquefaction. Appl. Biochem. Biotechnol. 2008, 147, 143–150. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Knox, J.H.; Kaur, B.; Millward, G.R. Structure and performance of porous graphitic carbon in liquid chromatography. J. Chromatogr. A 1986, 352, 3–25. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, L.; Liu, H.; Zeng, J.; Zhou, J.; Li, H.; Xia, H. Soft-template assisted hydrothermal synthesis of size-tunable, N-doped porous carbon spheres for supercapacitor electrodes. Results Phys. 2019, 12, 1984–1990. [Google Scholar] [CrossRef]
- Su, J.; Fang, C.; Yang, M.; Cheng, Y.; Wang, Z.; Huang, Z.; You, C. A controllable soft-templating approach to synthesize mesoporous carbon microspheres derived from d-xylose via hydrothermal method. J. Mater. Sci. Technol. 2020, 38, 183–188. [Google Scholar] [CrossRef]
- Kubo, S.; White, R.J.; Yoshizawa, N.; Antonietti, M.; Titirici, M.-M. Ordered Carbohydrate-Derived Porous Carbons. Chem. Mater. 2011, 23, 4882–4885. [Google Scholar] [CrossRef]
- Xiao, P.-W.; Guo, D.; Zhao, L.; Han, B.-H. Soft templating synthesis of nitrogen-doped porous hydrothermal carbons and their applications in carbon dioxide and hydrogen adsorption. Microporous Mesoporous Mater. 2016, 220, 129–135. [Google Scholar] [CrossRef]
- Román, S.; González, J.F.; González-García, C.M.; Zamora, F. Control of pore development during CO2 and steam activation of olive stones. Fuel Process. Technol. 2008, 89, 715–720. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Molina-Sabio, M. Activated carbons from lignocellulosic materials by chemical and/or physical activation: An overview. Carbon 1992, 30, 1111–1118. [Google Scholar] [CrossRef]
- Downie, A.; Crosky, A.; Munroe, P. Physical Properties of Biochar. In Biochar for Environmental Management; Earthscan: Oxford, UK, 2009; pp. 13–32. [Google Scholar]
- Antero, R.V.P.; Alves, A.C.F.; Ferreira Sales, P.d.T.; de Oliveira, S.B.; Ojala, S.A.; Brum, S.S. A new approach to obtain mesoporous-activated carbon via hydrothermal carbonization of Brazilian Cerrado biomass combined with physical activation for bisphenol-A removal. Chem. Eng. Commun. 2019, 206, 1498–1514. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Removal of copper (II) and phenol from aqueous solution using porous carbons derived from hydrothermal chars. Desalination 2011, 267, 101–106. [Google Scholar] [CrossRef]
- Thommes, M. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Chem. Int. 2016, 38, 25. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.T.; Fan, M.; Daugaard, D.; Brown, R.C. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Kohandehghan, A.; Li, Z.; Cui, K.; Tan, X.; Stephenson, T.J.; King’ondu, C.K.; Holt, C.M.B.; Olsen, B.C.; et al. Interconnected Carbon Nanosheets Derived from Hemp for Ultrafast Supercapacitors with High Energy. ACS Nano 2013, 7, 5131–5141. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal Carbonization of Abundant Renewable Natural Organic Chemicals for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef]
- Sevilla, M.; Gu, W.; Falco, C.; Titirici, M.M.; Fuertes, A.B.; Yushin, G. Hydrothermal synthesis of microalgae-derived microporous carbons for electrochemical capacitors. J. Power Sources 2014, 267, 26–32. [Google Scholar] [CrossRef]
- Jain, A.; Xu, C.; Jayaraman, S.; Balasubramanian, R.; Lee, J.Y.; Srinivasan, M.P. Mesoporous activated carbons with enhanced porosity by optimal hydrothermal pre-treatment of biomass for supercapacitor applications. Microporous Mesoporous Mater. 2015, 218, 55–61. [Google Scholar] [CrossRef]
- Falco, C.; Sieben, J.M.; Brun, N.; Sevilla, M.; Van Der Mauelen, T.; Morallón, E.; Cazorla-Amorós, D.; Titirici, M.M. Hydrothermal carbons from hemicellulose-derived aqueous hydrolysis products as electrode materials for supercapacitors. ChemSusChem 2013, 6, 374–382. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Z.; Wang, X.; Liu, Z.; Liu, X. A simple route for hierarchically porous carbon derived from corn straw for supercapacitor application. J. Renew. Sustain. Energy 2019, 11, 024102. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef]
- Miliotti, E.; Bettucci, L.; Lotti, G.; Dell’Orco, S.; Rizzo, A.M.; Chiaramonti, D. Hydrothermal Carbonization and Activation of Lignin-Rich Ethanol Co-Product. In Proceedings of the 26th European Biomass Conference and Exhibition, Copenhagen, Denmark, 14–18 May 2018. [Google Scholar]
- Le Van, K.; Luong Thi Thu, T. Preparation of Pore-Size Controllable Activated Carbon from Rice Husk Using Dual Activating Agent and Its Application in Supercapacitor. J. Chem. 2019, 2019, 4329609. [Google Scholar] [CrossRef]
- Brun, N.; Yu, S.-H.; White, R.J. Chapter 6 Porous Hydrothermal Carbon Materials, Nanoparticles, Hybrids and Composites. In Porous Carbon Materials from Sustainable Precursors; The Royal Society of Chemistry: London, UK, 2015; pp. 156–190. [Google Scholar]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Zein, S.H.S.; Najafpour, G.; Sata, S.A.; Buniran, S. Multiwalled carbon nanotubes based nanocomposites for supercapacitors: A review of electrode materials. Nano 2012, 7, 1230002. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Feng, Y.P. Carbon nanotubes for supercapacitor. Nanoscale Res. Lett. 2010, 5, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Liu, M.; Li, F.; Cheng, H.M. Frequency response characteristic of single-walled carbon nanotubes as supercapacitor electrode material. Appl. Phys. Lett. 2008, 92, 143108. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Fan, L.Z.; Hu, Y.S.; Maier, J.; Adelhelm, P.; Smarsly, B.; Antonietti, M. High Electroactivity of Polyaniline in Supercapacitors by Using a Hierarchically Porous Carbon Monolith as a Support. Adv. Funct. Mater. 2007, 17, 3083–3087. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Lai-Peng, M.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, Z.; Li, J. Carbon nanotubes/TiO2 nanotubes hybrid supercapacitor. J. Nanosci. Nanotechnol. 2007, 7, 3328–3331. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene Oxide-MnO2 nanocomposites for supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef]
- Garche, J.; Moseley, P.T. Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Park, B.O.K.; Lokhande, C.D.; Park, H.S.; Jung, K.D.; Joo, O.H.S. Electrodeposited ruthenium oxide (RuO2) films for electrochemical supercapacitors. J. Mater. Sci. 2004, 39, 4313–4317. [Google Scholar] [CrossRef]
- Boota, M.; Gogotsi, Y. MXene—Conducting Polymer Asymmetric Pseudocapacitors. Adv. Energy Mater. 2019, 9, 1802917. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Huang, P.; Wang, H.; Cao, C.; Song, W. Carbonaceous aerogel and CoNiAl-LDH@CA nanocomposites derived from biomass for high performance pseudo-supercapacitor. Sci. Bull. 2017, 62, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Chai, H.; Seong, K.D.; Lee, C.; Kang, J.; Zhang, W.; Piao, Y. Yeast-derived N-doped carbon microsphere/polyaniline composites as high performance pseudocapacitive electrodes. Electrochim. Acta 2018, 291, 256–266. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef]
- Herou, S.; Schlee, P.; Jorge, A.B.; Titirici, M. Biomass-derived electrodes for flexible supercapacitors. Curr. Opin. Green Sustain. Chem. 2018, 9, 18–24. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Liu, C.; Yan, X.; Hu, F.; Gao, G.; Wu, G.; Yang, X. Toward Superior Capacitive Energy Storage: Recent Advances in Pore Engineering for Dense Electrodes. Adv. Mater. 2018, 30, 1705713. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.-M. 3D Aperiodic Hierarchical Porous Graphitic Carbon Material for High-Rate Electrochemical Capacitive Energy Storage. Angew. Chem. Int. Ed. 2008, 47, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Tailoring porosity in carbon materials for supercapacitor applications. Mater. Horiz. 2014, 1, 157–168. [Google Scholar] [CrossRef]
- Tamai, H.; Kunihiro, M.; Morita, M.; Yasuda, H. Mesoporous activated carbon as electrode for electric double layer capacitor. J. Mater. Sci. 2005, 40, 3703–3707. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zhou, J.; Liang, T. Biomass-Derived Porous Carbon Materials for Supercapacitor. Front. Chem. 2019, 7, 274. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Replication and Coating of Silica Templates by Hydrothermal Carbonization. Adv. Funct. Mater. 2007, 17, 1010–1018. [Google Scholar] [CrossRef]
- Yuan, K.; Hu, T.; Xu, Y.; Graf, R.; Brunklaus, G.; Forster, M.; Chen, Y.; Scherf, U. Engineering the Morphology of Carbon Materials: 2D Porous Carbon Nanosheets for High-Performance Supercapacitors. ChemElectroChem 2016, 3, 822–828. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, H.; Lin, Z.; Yin, J.; Lu, H.; Liu, D.; Zhao, M. 3D Hierarchical Porous Carbon for Supercapacitors Prepared from Lignin through a Facile Template-Free Method. ChemSusChem 2015, 8, 2114–2122. [Google Scholar] [CrossRef]
- Lv, Y.; Gan, L.; Liu, M.; Xiong, W.; Xu, Z.; Zhu, D.; Wright, D.S. A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes. J. Power Sources 2012, 209, 152–157. [Google Scholar] [CrossRef]
- Gibson, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Xiao, K.; Ding, L.-X.; Liu, G.; Chen, H.; Wang, S.; Wang, H. Freestanding, Hydrophilic Nitrogen-Doped Carbon Foams for Highly Compressible All Solid-State Supercapacitors. Adv. Mater. 2016, 28, 5997–6002. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Myung, Y.; Kim, B.N.; Kim, I.G.; You, I.K.; Kim, T. Activated Biomass-derived Graphene-based Carbons for Supercapacitors with High Energy and Power Density. Sci. Rep. 2018, 8, 1915. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Preparation of Ginger Straw based Porous Carbon using One-step Pyrolysis Process as Electrode Material for Supercapacitor. Int. J. Electrochem. Sci. 2019, 14, 10289–10305. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Idrees, F.; Ma, X. Hierarchical Porous Nitrogen-Doped Carbon Nanosheets Derived from Silk for Ultrahigh-Capacity Battery Anodes and Supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Gao, Z.; Wang, X.; Wu, D.; Xu, F.; Wang, X.; Guo, Y.; Jiang, K. Activated porous carbon prepared from paulownia flower for high performance supercapacitor electrodes. Electrochim. Acta 2015, 157, 290–298. [Google Scholar] [CrossRef]
- Du, W.; Zhang, Z.; Du, L.; Fan, X.; Shen, Z.; Ren, X.; Zhao, Y.; Wei, C.; Wei, S. Designing synthesis of porous biomass carbon from wheat straw and the functionalizing application in flexible, all-solid-state supercapacitors. J. Alloys Compd. 2019, 797, 1031–1040. [Google Scholar] [CrossRef]
- He, X.; Ling, P.; Qiu, J.; Yu, M.; Zhang, X.; Yu, C.; Zheng, M. Efficient preparation of biomass-based mesoporous carbons for supercapacitors with both high energy density and high power density. J. Power Sources 2013, 240, 109–113. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, C.; Zhang, S.-W.; Wei, J.; Lv, W.; Yang, Q.-H. Porous carbons derived from carbonization of tissue papers for supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 11250–11256. [Google Scholar] [CrossRef]
- Jia, H.; Wang, S.; Sun, J.; Yin, K.; Xie, X.; Sun, L. Nitrogen-doped microporous carbon derived from a biomass waste-metasequoia cone for electrochemical capacitors. J. Alloys Compd. 2019, 794, 163–170. [Google Scholar] [CrossRef]
- Wang, X.; Kong, D.; Wang, B.; Song, Y.; Zhi, L. Activated pyrolysed bacterial cellulose as electrodes for supercapacitors. Sci. China Chem. 2016, 59, 713–718. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Xu, F.; Li, M.; Han, C.; Gong, Y.; Wang, H.; Wang, Y. Inspired by bread leavening: One-pot synthesis of hierarchically porous carbon for supercapacitors. Green Chem. 2015, 17, 4053–4060. [Google Scholar] [CrossRef]
- Cai, N.; Cheng, H.; Jin, H.; Liu, H.; Zhang, P.; Wang, M. Porous carbon derived from cashew nut husk biomass waste for high-performance supercapacitors. J. Electroanal. Chem. 2020, 861, 113933. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, G.; Yang, Y. Carbon nanotube arrays and their composites for electrochemical capacitors and lithium-ion batteries. Energy Environ. Sci. 2009, 2, 932–943. [Google Scholar] [CrossRef]
- Roberts, M.E.; Wheeler, D.R.; McKenzie, B.B.; Bunker, B.C. High specific capacitance conducting polymer supercapacitor electrodes based on poly(tris(thiophenylphenyl)amine). J. Mater. Chem. 2009, 19, 6977–6979. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Tong, X.; Zhong, L.; Peng, X.; Sun, R. Sustainable hierarchical porous carbon aerogel from cellulose for high-performance supercapacitor and CO2 capture. Ind. Crops Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, C.; Zhao, Y.; Li, Y.; Yu, S.; Huang, L. Cellulose nanofiber-based hybrid hydrogel electrode with superhydrophilicity enabling flexible high energy density supercapacitor and multifunctional sensors. Int. J. Biol. Macromol. 2024, 276, 134003. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Du, Y.; Shan, Y.; Duan, P.; Ramzan, N. Hydrothermal Carbonization of Cellulose with Ammonium Sulfate and Thiourea for the Production of Supercapacitor Carbon. Polymers 2023, 15, 4478. [Google Scholar] [CrossRef]
- Gamry. Two, Three and Four Electrode Experiments. Available online: https://www.gamry.com/application-notes/instrumentation/two-three-four-electrode-experiments/#:~:text=Three%2Delectrode%20setups%20have%20a,occur%20at%20the%20counter%20electrode (accessed on 14 September 2024).
- Lu, X.; Jiang, C.; Hu, Y.; Zhong, H.; Zhao, Y.; Xu, X.; Liu, H. Preparation of hierarchically porous carbon spheres by hydrothermal carbonization process for high-performance electrochemical capacitors. J. Appl. Electrochem. 2018, 48, 233–241. [Google Scholar] [CrossRef]
- Tong, X.; Chen, Z.; Zhuo, H.; Hu, Y.; Jing, S.; Liu, J.; Zhong, L. Tailoring the physicochemical properties of chitosan-derived N-doped carbon by controlling hydrothermal carbonization time for high-performance supercapacitor application. Carbohydr. Polym. 2019, 207, 764–774. [Google Scholar] [CrossRef]
- Li, H.; Shi, F.; An, Q.; Zhai, S.; Wang, K.; Tong, Y. Three-dimensional hierarchical porous carbon derived from lignin for supercapacitors: Insight into the hydrothermal carbonization and activation. Int. J. Biol. Macromol. 2021, 166, 923–933. [Google Scholar] [CrossRef]
- Yang, L.; Feng, Y.; Cao, M.; Yao, J. Two-step preparation of hierarchical porous carbon from KOH-activated wood sawdust for supercapacitor. Mater. Chem. Phys. 2019, 238, 121956. [Google Scholar] [CrossRef]
- Ren, M.; Jia, Z.; Tian, Z.; Lopez, D.; Cai, J.; Titirici, M.-M.; Jorge, A.B. High Performance N-Doped Carbon Electrodes Obtained via Hydrothermal Carbonization of Macroalgae for Supercapacitor Applications. ChemElectroChem 2018, 5, 2686–2693. [Google Scholar] [CrossRef]
- Siva Sankari, M.; Vivekanandhan, S. Jatropha Oil Cake Based Activated Carbon for Symmetric Supercapacitor Application: A Comparative Study on Conventional and Hydrothermal Carbonization Processes. ChemistrySelect 2020, 5, 1375–1384. [Google Scholar] [CrossRef]
- Lim, Y.S.; Lai, C.W.; Abd Hamid, S.B. Porous 3D carbon decorated Fe3O4 nanocomposite electrode for highly symmetrical supercapacitor performance. RSC Adv. 2017, 7, 23030–23040. [Google Scholar] [CrossRef]
- Siddiqa, A.; Nagaraju, D.H.; Padaki, M. High-Energy-Density Asymmetric Supercapacitor Based on Layered-Double-Hydroxide-Derived CoNi2S4 and Eco-friendly Biomass-Derived Activated Carbon. Energy Fuels 2022, 36, 13286–13295. [Google Scholar] [CrossRef]
- Reddygunta, K.K.R.; Šiller, L.; Ivaturi, A. Sheet-like ZnCo2O4 microspheres and pomelo peel waste-derived activated carbon for high performance solid state asymmetric supercapacitors. Sustain. Energy Fuels 2024, 8, 2751–2761. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, J.; Zhou, Y.; Xie, J.; Zhang, X.; Guan, M.; Pan, B.; Xie, Y. High-performance flexible electrochromic device based on facile semiconductor-To-metal transition realized by WO3∙2H2O ultrathin nanosheets. Sci. Rep. 2013, 3, 1936. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, Y.; Wang, H.; Wang, L.; Kong, T.; Zhang, H.; Meng, S. Recent advances on TMDCs for medical diagnosis. Biomaterials 2021, 269, 120471. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, S.Y.; Loy, A.; Shen, H.; Leong, W.; Tao, X. Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review. Nanomaterials 2020, 10, 1012. [Google Scholar] [CrossRef]
- Xiang, J.; Dong, D.; Wen, F.; Zhao, J.; Zhang, X.; Wang, L.; Liu, Z. Microwave synthesized self-standing electrode of MoS2 nanosheets assembled on graphene foam for high-performance Li-Ion and Na-Ion batteries. J. Alloys Compd. 2016, 660, 11–16. [Google Scholar] [CrossRef]
- Zhao, G.; Cheng, Y.; Sun, P.; Ma, W.; Hao, S.; Wang, X.; Xu, X.; Xu, Q.; Liu, M. Biocarbon based template synthesis of uniform lamellar MoS2 nanoflowers with excellent energy storage performance in lithium-ion battery and supercapacitors. Electrochim. Acta 2020, 331, 135262. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, X.; Xu, L.; Xu, X.; Zhang, L.; Chen, W. Chitosan-assisted fabrication of ultrathin MoS2/graphene heterostructures for Li-ion battery with excellent electrochemical performance. Electrochim. Acta 2015, 167, 39–47. [Google Scholar] [CrossRef]
- Bhaskar, A.; Deepa, M.; Narasinga Rao, T. MoO2/Multiwalled Carbon Nanotubes (MWCNT) Hybrid for Use as a Li-Ion Battery Anode. ACS Appl. Mater. Interfaces 2013, 5, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Simsir, H.; Eltugral, N.; Frohnhoven, R.; Ludwig, T.; Gönüllü, Y.; Karagoz, S.; Mathur, S. Anode performance of hydrothermally grown carbon nanostructures and their molybdenum chalcogenides for Li-ion batteries. MRS Commun. 2018, 8, 610–616. [Google Scholar] [CrossRef]
- Ratha, S.; Rout, C.S. Supercapacitor Electrodes Based on Layered Tungsten Disulfide-Reduced Graphene Oxide Hybrids Synthesized by a Facile Hydrothermal Method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, L.; Fan, L.-Z. Immobilization of tungsten disulfide nanosheets on active carbon fibers as electrode materials for high performance quasi-solid-state asymmetric supercapacitors. J. Mater. Chem. A 2018, 6, 7835–7841. [Google Scholar] [CrossRef]
- Xing, L.-L.; Huang, K.-J.; Fang, L.-X. Preparation of layered graphene and tungsten oxide hybrids for enhanced performance supercapacitors. Dalton Trans. 2016, 45, 17439–17446. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Zeng, D.; Liu, Q.; Yi, F.; Shu, D.; Cheng, H.; Zhou, X.; Li, S.; Zhang, F. Molecular self-assembly assisted synthesis of carbon nanoparticle-anchored MoS2 nanosheets for high-performance supercapacitors. Electrochim. Acta 2019, 295, 187–194. [Google Scholar] [CrossRef]
- Huang, K.-J.; Wang, L.; Liu, Y.-J.; Liu, Y.-M.; Wang, H.-B.; Gan, T.; Wang, L.-L. Layered MoS2–graphene composites for supercapacitor applications with enhanced capacitive performance. Int. J. Hydrog. Energy 2013, 38, 14027–14034. [Google Scholar] [CrossRef]
- Sangeetha, D.N.; Selvakumar, M. Active-defective activated carbon/MoS2 composites for supercapacitor and hydrogen evolution reactions. Appl. Surf. Sci. 2018, 453, 132–140. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Liu, G.-J.; Zhang, C.-Y.; Wu, J.-H.; Wei, Y.-L. Facile one-step hydrothermal preparation of molybdenum disulfide/carbon composite for use in supercapacitor. Int. J. Hydrog. Energy 2015, 40, 10150–10157. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; Zhou, K.; Li, X. MoS2/corncob-derived activated carbon for supercapacitor application. Mater. Chem. Phys. 2020, 244, 122215. [Google Scholar] [CrossRef]
- Lin, Q.J.; Wang, J.M.; Chen, J.H.; Yang, Q.; Fang, L.J.; Huang, Y.D. Collaborative Improvement Electrochemical Properties of Supercapacitor Electrodes by Loading MoS2 Nanosheets on Biomass Hierarchical Porous Carbon. J. Electrochem. Soc. 2022, 169, 020502. [Google Scholar] [CrossRef]
- Hu, B.; Qin, X.; Asiri, A.M.; Alamry, K.A.; Al-Youbi, A.O.; Sun, X. Synthesis of porous tubular C/MoS2 nanocomposites and their application as a novel electrode material for supercapacitors with excellent cycling stability. Electrochim. Acta 2013, 100, 24–28. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Jiang, H.-W.; Zou, B.-X. WO3 Nanowires on Graphene Sheets as Negative Electrode for Supercapacitors. J. Nanomater. 2017, 2017, 2494109. [Google Scholar] [CrossRef]



| Li-Ion Batteries | |||||
| Electrode Material | Synthesis Method | Electrolyte 1 | Stability | Capacity (mA·h/g) | |
| MoS2/Graphene [203] | HTC (180 °C,12 h) of MoS2 precursors and graphene | 1 M LiPF6 solution in an EC/DEC mixture | 1127 mA·h/g after 200 cycles | >1300 | |
| Cornstalk-derived C/MoS2 [204] | HTC (200 °C, 1 h) of precursors and corn; pyrolysis at 1000 °C | 1 M LiPF6 solution in a mixture of EC/DEC/DMC | 1129 mA·h/g after 200 cycles | > 1300 | |
| AC from chitosan/graphene oxide/MoS2 [205] | HTC (240 °C, 24 h) of all materials and annealing at 800 °C | 1 M LiPF6 solution in an EC/DMC mixture | Stable over 100 cycles | >1000 | |
| MoO2/Multiwalled carbon nanotubes [206] | HTC (200 °C, 36 h) of CNT and MoO2 precursors. | 1 M LiPF6 solution in an EC/DMC mixture | 1143 mA·h/g after 200 cycles | >1200 | |
| AC from glucose and MoS2 [207] | HTC (200 °C, 48 h) of glucose; HTC (200 °C, 18 h) of MoS2 precursor and hydrochar. Pyrolysis at 600 °C | 1 M LiPF6 solution in an EC/EMC/DEC mixture | 98% retention after 50 cycles | 484 | |
| Supercapacitors | |||||
| Electrode material | Synthesis method | Electrolyte | BET area (m2/g) | Stability | Capacitance 2 (F/g) |
| Graphene oxide/WS2 [208] | HTC (265 °C, 24 h) of GO and WS2 precursors | 1 M Na2SO4 | - | 94% after 1000 cycles | 274 * |
| AC fiber/WS2 [209] | Fiber activ. *** (800 °C) with KOH (3:1). HTC (180 °C, 24 h) of AC and WS2 precursors | 1 M KOH | 11 | 93% after 1000 cycles | 255 */600 ** |
| Graphene oxide/WO3 [210] | Hydrothermal heating (90 °C, 3 h) of precursor; heating at 500 °C. HTC (180 °C, 12 h) of WO3/GO | 2 M KOH | 17 | >320 F/g after 1000 cycles | 580 ** |
| Carbon/MoS2 [211] | HTC (200 °C, 12 h) of all precursors | 1 M Na2SO4 | 16 | 60% after 2000 cycles | 394 ** at 5 mV/s |
| Graphene/MoS2 [212] | HTC (180 °C, 36 h) of GO and MoS2 precursor. | 1 M Na2SO4 | 103 | 92% after 1000 cycles | 243 ** |
| Biomass-derived electrodes for supercapacitors | |||||
| Tendu leaf-derived AC/MoS2 [213] | Heating (450 °C) and KOH (3:1) activ. (650 °C) of leaves. HTC (180 °C, 20 h) of MoS2 precursor; HTC (180 °C,12 h) and heating (800 °C) of AC | 1 M Na2SO4 | 1509 | 89% after 5000 cycles | 261 * at 2 mV/s |
| Glucose/PEG/Thiourea/MoS2 [214] | HTC (200 °C, 24 h) of MoS2 precursor and rest of materials. | 1 M Na2SO4 | 69 | 95% after 1000 cycles | 186 * |
| Corncob-derived carbon/MoS2 [215] | Pyrolysis (750 °C) of corncob. HTC (200 °C, 16 h) of MoS2 precursors and carbon. Mix with KOH and drying. | 1 M Na2SO4 | 101 | 82% after 7000 cycles | 38 */333 ** |
| Pomelo peel-derived AC/MoS2 [216] | KOH (5 mol/L) activ. (700 °C) of biomass. HTC (220 °C, 24 h) of carbon and MoS2 precursor | 3 M KOH | 320 | 94% after 2000 cycles | 361 ** |
| Cornstalk-derived C/MoS2 [204] | HTC (200 °C, 1 h) of precursors and corn; pyrolysis at 1000 °C | 1 M Na2SO4 in a mixture of EC/DEC/DMC | 326 | 79% after 5000 cycles | 338 ** |
| Glucose/Al2O3/MoS2 [217]. | HTC (200 °C, 24 h) of glucose and MoS2 precursor. Annealing at 500 °C | 3 M KOH | - | Increase 5% after 1000 cycles | 210 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto, M.; Yue, H.; Brun, N.; Ellis, G.J.; Naffakh, M.; Shuttleworth, P.S. Hydrothermal Carbonization of Biomass for Electrochemical Energy Storage: Parameters, Mechanisms, Electrochemical Performance, and the Incorporation of Transition Metal Dichalcogenide Nanoparticles. Polymers 2024, 16, 2633. https://doi.org/10.3390/polym16182633
Prieto M, Yue H, Brun N, Ellis GJ, Naffakh M, Shuttleworth PS. Hydrothermal Carbonization of Biomass for Electrochemical Energy Storage: Parameters, Mechanisms, Electrochemical Performance, and the Incorporation of Transition Metal Dichalcogenide Nanoparticles. Polymers. 2024; 16(18):2633. https://doi.org/10.3390/polym16182633
Chicago/Turabian StylePrieto, Manuel, Hangbo Yue, Nicolas Brun, Gary J. Ellis, Mohammed Naffakh, and Peter S. Shuttleworth. 2024. "Hydrothermal Carbonization of Biomass for Electrochemical Energy Storage: Parameters, Mechanisms, Electrochemical Performance, and the Incorporation of Transition Metal Dichalcogenide Nanoparticles" Polymers 16, no. 18: 2633. https://doi.org/10.3390/polym16182633
APA StylePrieto, M., Yue, H., Brun, N., Ellis, G. J., Naffakh, M., & Shuttleworth, P. S. (2024). Hydrothermal Carbonization of Biomass for Electrochemical Energy Storage: Parameters, Mechanisms, Electrochemical Performance, and the Incorporation of Transition Metal Dichalcogenide Nanoparticles. Polymers, 16(18), 2633. https://doi.org/10.3390/polym16182633








