Modified Cellulose-Based Waste for Enhanced Adsorption of Selected Heavy Metals from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Material Characterization
2.3. Adsorption Experiments
3. Results and Discussion
3.1. Material Characterization
3.2. Adsorption Experiments
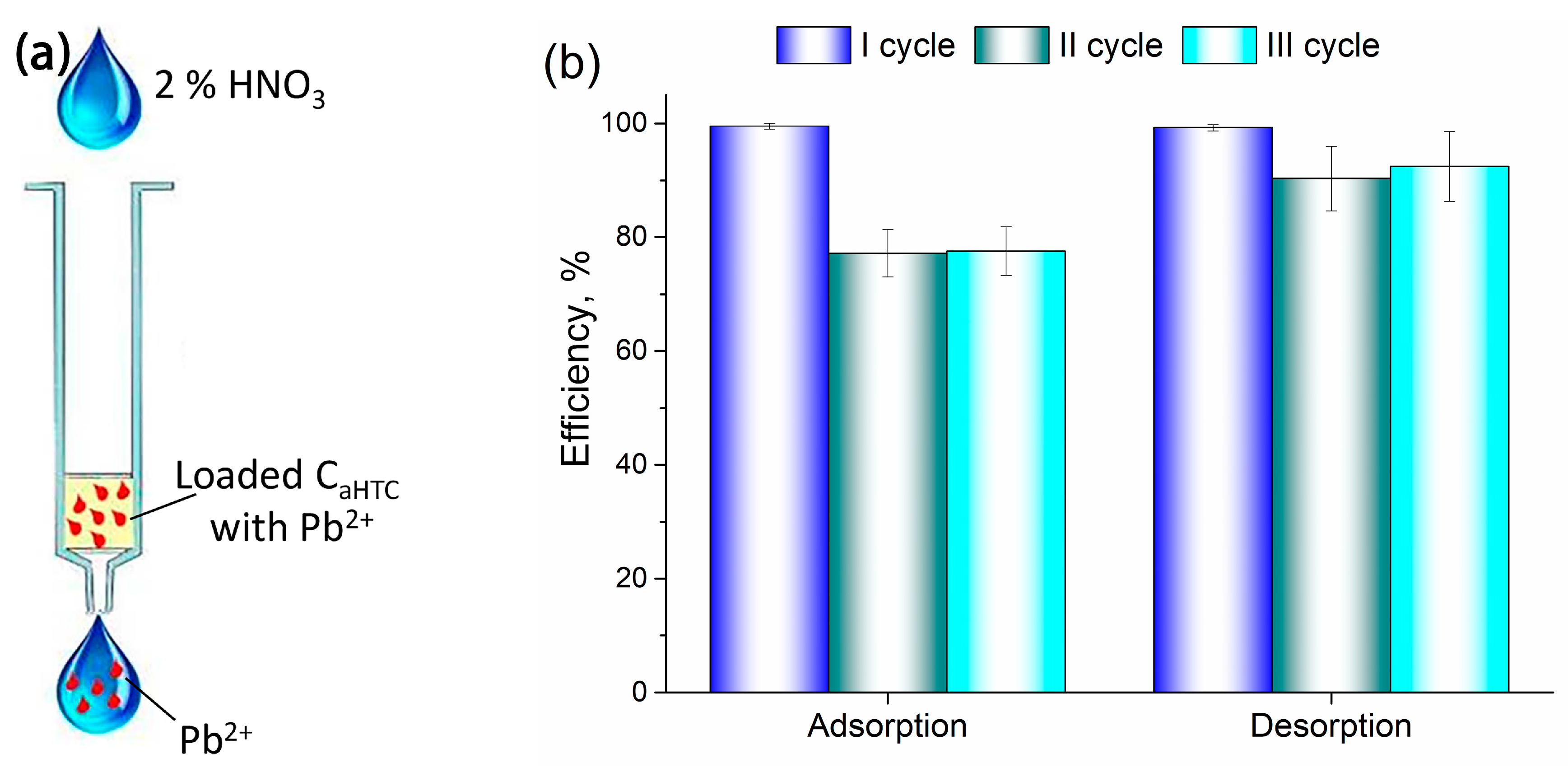
3.3. Adsorption/Desorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Panigrahi, T.; Santhoskumar, A.U. Adsorption process for reducing heavy metals in Textile Industrial Effluent with low cost adsorbents. Prog. Chem. Biochem. Res. 2020, 3, 135–139. [Google Scholar] [CrossRef]
- Al-Asadi, S.T.; Al-Qaim, F.F.; Al-Saedi, H.F.S.; Deyab, I.F.; Kamyab, H.; Chelliapan, S. Adsorption of methylene blue dye from aqueous solution using low-cost adsorbent: Kinetic, isotherm adsorption, and thermodynamic studies. Environ. Monit. Assess. 2023, 195, 676. [Google Scholar] [CrossRef] [PubMed]
- Minelgaitė, A.; Liobikienė, G. Waste problem in European Union and its influence on waste management behaviours. Sci. Total Environ. 2019, 667, 86–93. [Google Scholar] [CrossRef]
- John, M.J.; Anandjiwala, R.D. Recent developments in chemical modification and characterization of natural fiber-reinforced composites. Polym. Composite 2008, 29, 187–207. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classifcation, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; Wagner, J.R. Use of edible films and coatings for functional foods developments: A review. In Functional Foods: Sources, Health Effects and Future Perspectives; Nelson, D.L., Ed.; Nova Science Publishing: Hauppauge, NY, USA, 2016; pp. 1–26. [Google Scholar]
- Textile Exchange. Available online: https://textileexchange.org/app/uploads/2021/08/Textile-Exchange_Preferred-Fiber-and-Materials-Market-Report_2021.pdf (accessed on 5 June 2024).
- Bediako, J.K.; Apalangya, V.; Hodgson, I.O.A.; Anugwom, I.; Repo, E. Adsorbents for water decontamination: A recycling alternative for fiber precursors and textile fiber wastes. Sci. Total Environ. 2024, 919, 171000. [Google Scholar] [CrossRef]
- Stefan, D.S.; Bosomoiu, M.; Stefan, M. Methods for Natural and Synthetic Polymers Recovery from Textile Waste. Polymers 2022, 1419, 3939. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Sustainable Reuse of Waste Tire Textile Fibers WTTF, as Reinforcements. Polymers 2022, 1419, 3933. [Google Scholar] [CrossRef]
- Srichola, P.; Witthayolankowit, K.; Sukyai, P.; Sampoompuang, C.; Lobyam, K.; Kampakun, P.; Toomtong, R. Recycling of Nanocellulose from Polyester–Cotton Textile Waste for Modification of Film Composites. Polymers 2023, 1515, 3324. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, R.; Zhang, D.; Gao, Y.; Xiong, M.; Chen, W. Co-hydrothermal carbonization of cotton textile waste and polyvinyl chloride waste for the production of solid fuel: Interaction mechanisms and combustion behaviors. J. Clean. Prod. 2021, 316, 128306. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, W.; Liu, F.; Zhao, P. Hydrothermal pretreatment of cotton textile wastes: Biofuel characteristics and biochar electrocatalytic performance. Fuel 2022, 316, 123327. [Google Scholar] [CrossRef]
- Shirvanimoghaddama, K.; Czech, B.; Wiącek, A.E.; Ćwikła-Bundyra, W.; Naebe, M. Sustainable carbon microtube derived from cotton waste for environmental applications. Chem. Eng. J. 2019, 361, 1605–1616. [Google Scholar] [CrossRef]
- Adolfsson, K.H.; Yadav, N.; Hakkarainen, M. Cellulose-derived hydrothermally carbonized materials and their emerging applications. Curr. Opin. Green Sustain. Chem. 2020, 23, 18–24. [Google Scholar] [CrossRef]
- Kawamura, K.; Sako, K.; Ogata, T.; Tanabe, K. Environmentally friendly, hydrothermal treatment of mixed fabric wastes containing polyester, cotton, and wool fibers: Application for HMF production. Bioresour. Technol. Rep. 2020, 11, 100478. [Google Scholar] [CrossRef]
- Sun, K.; Tang, J.; Gong, Y.; Zhang, H. Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ. Sci. Pollut. Res. 2015, 22, 16640. [Google Scholar] [CrossRef]
- Shi, S.; Feng, X.; Gao, L.; Tang, J.; Guo, H.; Wang, S. Hydrolysis and carbonization of reactive dyes/cotton fiber in hydrothermal environment. Waste Manag. 2020, 103, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Soffian, M.S.; Halim, F.Z.A.; Aziz, F.; Rahman, M.A.; Amin, M.A.M.; Chee, D.N.A. Carbon-based material derived from biomass waste for wastewater treatment. Environ. Adv. 2022, 9, 100259. [Google Scholar] [CrossRef]
- Carraro, P.S.; Spessato, L.; Crespo, L.H.S.; Yokoyama, J.T.C.; Fonseca, J.M.; Bedin, K.C.; Ronix, A.; Cazetta, A.L.; Silva, T.L.; Almeida, V.C. Activated carbon fibers prepared from cellulose and polyester–derived residues and their application on removal of Pb2+ ions from aqueous solution. J. Mol. Liq. 2019, 289, 111150. [Google Scholar] [CrossRef]
- Parmakoğlu, E.Ü.; Cxay, A.; Yanik, J. Valorization of Solid Wastes from Textile Industry as an Adsorbent Through Activated Carbon Production. AATCC J. Res. 2023, 10, 133–143. [Google Scholar] [CrossRef]
- Bediako, J.K.; Wei, W.; Yun, Y.-S. Conversion of waste textile cellulose fibers into heavy metal adsorbents. J. Ind. Eng. Chem. 2016, 43, 61–68. [Google Scholar] [CrossRef]
- Racho, P.; Waiwong, W. Modified textile waste for heavy metals removal. Energy Rep. 2020, 6, 927–932. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Gao, L.; Li, Z.; Yi, W.; Li, Y.; Zhang, P.; Zhang, A.; Wang, L. Impacts of pyrolysis temperature on lead adsorption by cotton stalk-derived biochar and related mechanisms. J. Environ. Chem. Eng. 2021, 9, 105602. [Google Scholar] [CrossRef]
- Cui, L.-P.; Shi, S.; Hou, W.-S.; Yan, Z.-F.; Dan, J.-M. Hydrolysis and carbonization mechanism of cotton fibers in subcritical water. New Carbon Mater. 2018, 33, 245–251. [Google Scholar] [CrossRef]
- Luo, M.; Chen, J.; Li, Q.; Wang, J. Cotton-Based Activated Carbon Fiber with High Specific Surface Area Prepared by Low-Temperature Hydrothermal Carbonization with Urea Enhancement. Ind. Eng. Chem. Res. 2023, 62, 8744–8753. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Singh, V.; Ahmed, G.; Vedika, S.; Kumar, P.; Chaturvedi, S.K.; Rai, S.N.; Vamanu, E.; Kumar, A. Toxic heavy metal ions contamination in water and their sustainable reduction by eco-friendly methods: Isotherms, thermodynamics and kinetics study. Sci. Rep. 2024, 14, 7595. [Google Scholar] [CrossRef] [PubMed]
- Bayuo, J.; Rwiza, M.J.; Choi, J.W.; Mtei, K.M.; Bandegharaei, A.H.; Sillanpa, M. Adsorption and desorption processes of toxic heavy metals, regeneration and reusability of spent adsorbents: Economic and environmental sustainability approach. Adv. Colloid. Interface Sci. 2024, 329, 103196. [Google Scholar] [CrossRef] [PubMed]
- Husien, S.; El-taweel, R.M.; Salim, A.I.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Curr. Res. Green Sustain. Chem. 2022, 5, 100325. [Google Scholar] [CrossRef]
- Shukla, S.K.; Al Mushaiqri, N.R.S.; Al Subhi, H.M.; Yoo, K.; Al Sadeq, H. Low-cost activated carbon production from organic waste and its utilization for wastewater treatment. Appl. Water Sci. 2020, 10, 62. [Google Scholar] [CrossRef]
- Azharul Islam, M.; Benhouria, A.; Asif, M.; Hameed, B.H. Methylene blue adsorption on factory-rejected tea activated carbon prepared by conjunction of hydrothermal carbonization and sodium hydroxide activation processes. J. Taiwan Inst. Chem. Eng. 2015, 52, 57–64. [Google Scholar] [CrossRef]
- Babeker, T.M.A.; Chen, Q. Heavy Metal Removal from Wastewater by Adsorption with Hydrochar Derived from Biomass: Current Applications and Research Trends. Curr. Pollut. Rep. 2021, 7, 54–71. [Google Scholar] [CrossRef]
- Pap, S.; Radonić, J.; Trifunović, S.; Adamović, D.; Mihajlović, I.; Vojinović Miloradov, M.; Turk Sekulić, M. Evaluation of the adsorption potential of eco-friendly activated carbon prepared from cherry kernels for the removal of Pb2+, Cd2+ and Ni2+ from aqueous wastes. J. Environ. Manag. 2016, 184, 297–306. [Google Scholar] [CrossRef]
- Saini, R.; Pandey, M.; Mishra, R.K.; Kumar, P. Adsorption potential of hydrochar derived from hydrothermal carbonization of waste biomass towards the removal of methylene blue dye from wastewater. Biomass. Convers. Biorefin. 2024. [CrossRef]
- Lagergren, S. Zur the oriedersogennanten adsorption geloesterstoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Bio-Chem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Adsorption in solutions. J. Phys. Chem. 1906, 57, 384–410. [Google Scholar]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, Z.; Jiang, W.; Zhang, G.; Tan, Y.; Guan, Y.; Zhou, L.; Cui, L.; Choi, S.W.; Li, M.-X. Preparation of Y2O3/TiO2-Loaded Polyester Fabric and Its Photocatalytic Properties under Visible Light Irradiation. Polymers 2022, 14, 2760. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796. [Google Scholar] [CrossRef]
- Amen, R.; Bashir, H.; Bibi, I.; Shaheen, S.M.; Niazi, N.K.; Shahid, M.; Hussain, M.M.; Antoniadis, V.; Shakoor, M.B.; Al-Solaimani, S.G.; et al. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem. Eng. J. 2020, 396, 125195. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Jin, C.E.S.; Sheng, K.; Zhang, X. Effect of swelling pretreatment on properties of cellulose-based hydrochar. ACS Sustain. Chem. Eng. 2019, 7, 10821–10829. [Google Scholar] [CrossRef]
- Chen, J.; Li, S. Characterization of biofuel production from hydrothermal treatment of hyperaccumulator waste (Pteris vittata L.) in sub- and supercritical water. RSC Adv. 2020, 10, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Kharrazi, S.M.; Mirghaffari, N.; Dastgerdi, M.M.; Soleimani, M. A novel post-modification of powdered activated carbon prepared from lignocellulosic waste through thermal tension treatment to enhance the porosity and heavy metals adsorption. Powder Technol. 2020, 366, 358–368. [Google Scholar] [CrossRef]
- Kalijadis, A.; Đorđević, J.; Trtić-Petrović, T.; Vukčević, M.; Popović, M.; Maksimović, V.; Rakočević, Z.; Laušević, Z. Preparation of boron-doped hydrothermal carbon from glucose for carbon paste electrode. Carbon 2015, 95, 42–50. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef]
- Tyagi, U. Enhanced adsorption of metal ions onto Vetiveriazizanioides biochar via batch and fixed bed studies. Bioresour. Technol. 2022, 345, 126475. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Zhu, N.; Li, D.; Wang, Z.; Lang, Q.; Liu, Z.; Jiao, W. Enhanced adsorption of Pb(II) onto modified hydrochar: Modeling and mechanism analysis. Bioresour. Technol. 2019, 288, 1–8. [Google Scholar] [CrossRef]
- Khoshbouy, R.; Lejiu, R.; Takahashi, F.; Yoshikawa, K. Effect of acid-assisted hydrothermal carbonization (HTC) process of tree branches using nitric acid on cadmium adsorption. E3S Web Conf. 2018, 67, 03033. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, Z.; Feng, Q.; Yao, D.; Yu, J.; Wang, D.; Lv, S.; Liu, Y.; Zhou, N.; Zhong, M.E. Effect of pyrolysis condition on the adsorption mechanism of lead, cadmium and copper on tobacco stem biochar. J. Clean. Prod. 2018, 187, 996–1005. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-Bandegharaei, A.; Giannakoudakis, D.A.; Robalds, A.; Usman, M.; Escudero, L.B.; Zhou, Y.; Colmenares, J.C.; Núńez-Delgado, A.; et al. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019, 295, 111684. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Salehi, E. Adsorption of cations on nanofiltration membrane: Separation mechanism, isotherm confirmation and thermodynamic analysis. Chem. Eng. J. 2009, 150, 114–121. [Google Scholar] [CrossRef]
- Yin, W.; Zhao, C.; Xu, J. Enhanced adsorption of Cd (II) from aqueous solution by a shrimp bran modified Typha orientalis biochar. Environ. Sci. Pollut. Res. 2019, 26, 37092–37100. [Google Scholar] [CrossRef]
- Bhakta, J.N.; Majumdar, P.B.; Munekage, Y. Development of Activated Carbon from Cotton Fibre Waste as Potential Mercury Adsorbent: Kinetic and Equilibrium Studies. Int. J. Chem. Eng. 2014, 1, 176483. [Google Scholar] [CrossRef]
- Mosa, A.A.; El-Ghamry, A.; Al-Zahrani, H.; Selim, E.M.; El-Khateeb, A. Chemically Modified Biochar Derived from Cotton Stalks: Characterization And Assessing Its Potential for Heavy Metals Removal from Wastewater. Environ. Biodivers. Soil Secur. 2017, 1, 33–45. [Google Scholar] [CrossRef]


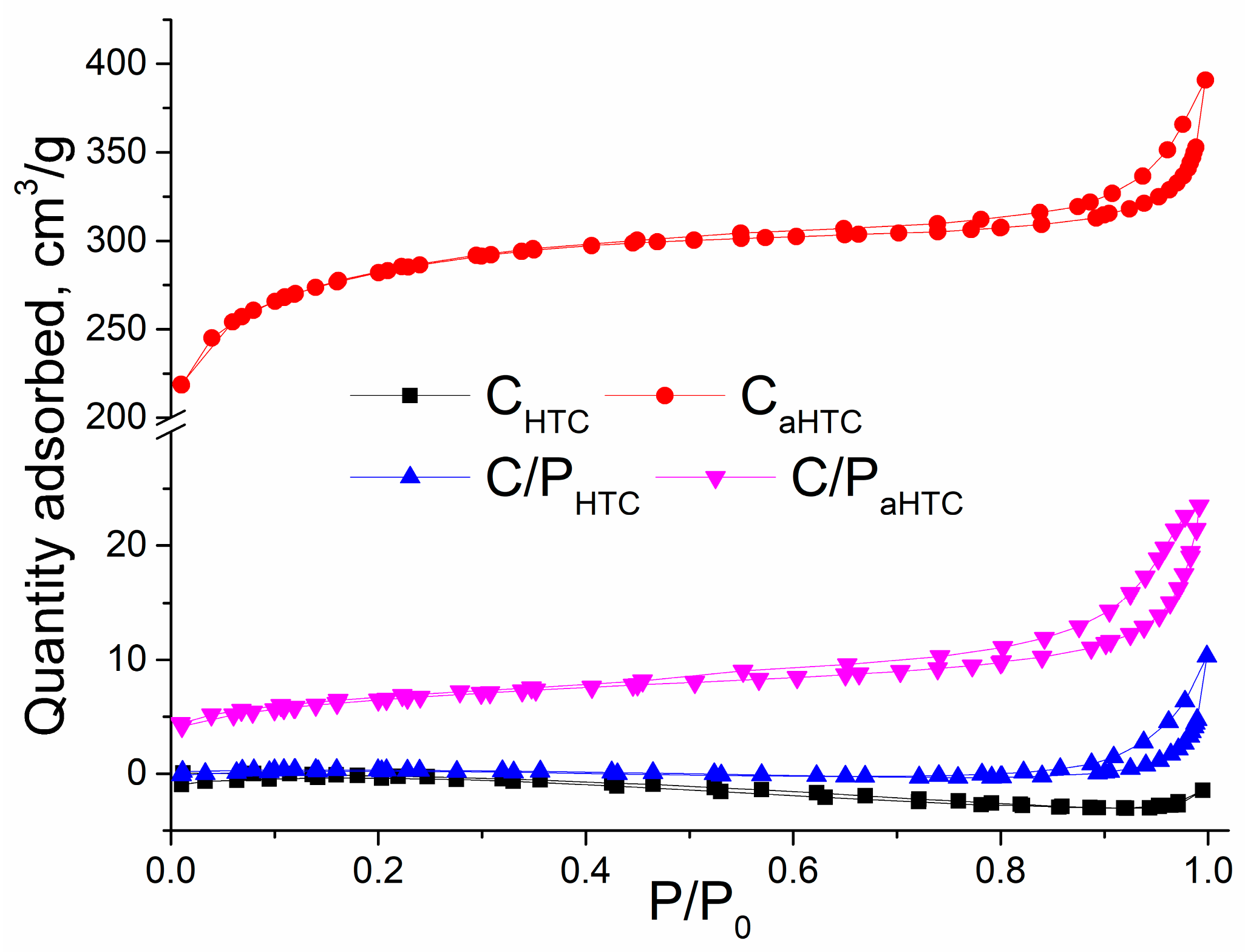


| Sample | SBET, m2/g | Sext, m2/g | Smicro, m2/g | Vtotal, cm3/g | Vmicro, cm3/g | Dm, nm | Amount of Surface Oxygen Groups, mmol/g | ||
|---|---|---|---|---|---|---|---|---|---|
| Basic | Acidic | Total | |||||||
| CHTC | 0.003 | - | - | - | - | - | 0.983 ± 0.020 | 3.782 ± 0.017 | 4.765 ± 0.037 |
| C/PHTC | 0.64 | 0.122 | 0.516 | 0.01 | 4 × 10−4 | 28.75 | 0.345 ± 0.015 | 4.866 ± 0.012 | 5.211 ± 0.027 |
| CaHTC | 872.2 | 386.1 | 486 | 0.532 | 0.269 | 4.82 | 2.134 ± 0.008 | 3.940 ± 0.025 | 6.074 ± 0.033 |
| C/PaHTC | 21.75 | 19.03 | 2.72 | 0.035 | 0.002 | 8.93 | 2.333 ± 0.010 | 4.811 ± 0.015 | 7.144 ± 0.025 |
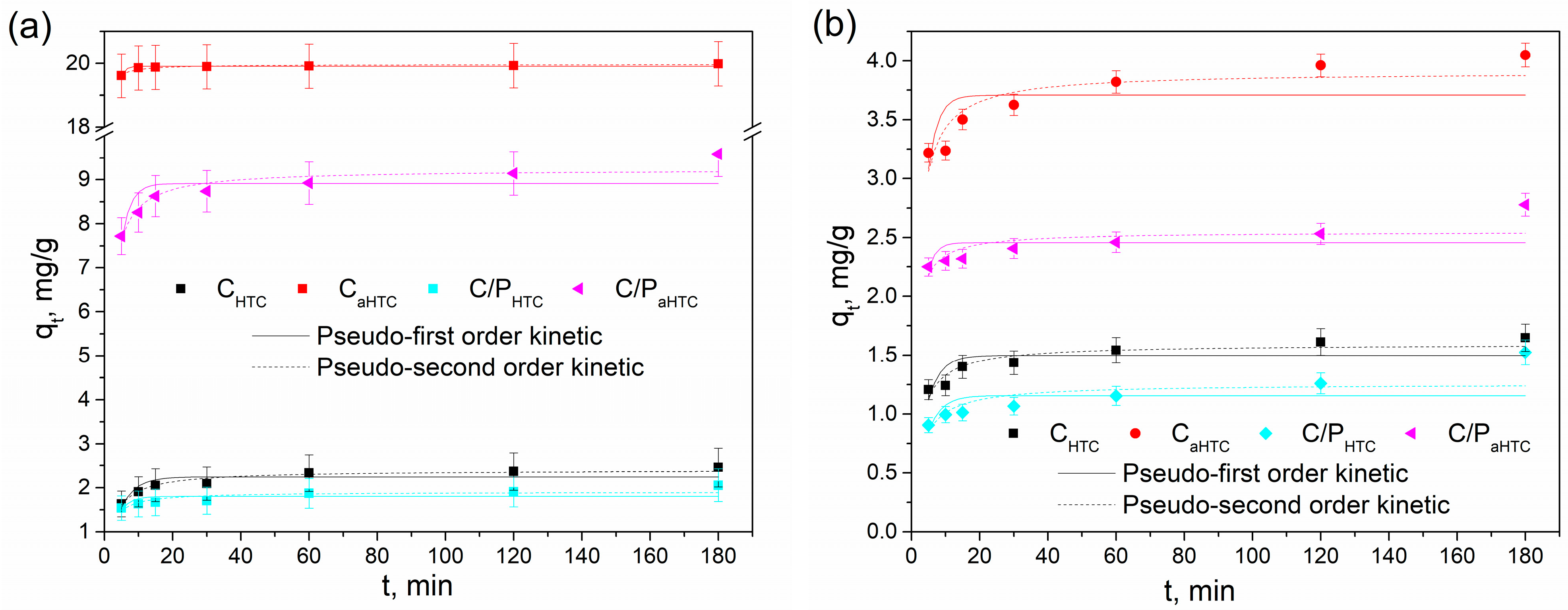
| Metal Ion | Sample | Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | k1 (1/min) | qe,mod (mg/g) | R2 | k2 (g/(mg min)) | qe,mod (mg/g) | qe,exp (mg/g) | ||
| Pb(II) | CHTC | 0.71124 | 0.216 | 2.28 | 0.94000 | 0.159 | 2.42 | 2.46 |
| C/PHTC | 0.28953 | 0.325 | 1.83 | 0.70164 | 0.325 | 1.93 | 2.06 | |
| CaHTC | 0.85576 | 0.842 | 19.91 | 0.91098 | 0.607 | 19.96 | 19.98 | |
| C/PaHTC | 0.54732 | 0.372 | 8.95 | 0.86901 | 0.100 | 9.26 | 9.59 | |
| Cd(II) | CHTC | 0.47925 | 0.255 | 1.52 | 0.85098 | 0.295 | 1.61 | 1.65 |
| C/PHTC | 0.23811 | 0.212 | 1.22 | 0.56231 | 0.243 | 1.31 | 1.52 | |
| CaHTC | 0.31121 | 0.337 | 3.75 | 0.78406 | 0.175 | 3.93 | 4.05 | |
| C/PaHTC | 0.10068 | 0.446 | 2.48 | 0.50107 | 0.417 | 2.57 | 2.78 | |
| Metal Ion | Sample | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|---|
| qmax (mg/g) | b (dm3/mg) | R2 | Kf ((mg/g)/(mg/dm3)1/n) | 1/n | R2 | ||
| Pb(II) | CHTC | 260.8 | 0.006 | 0.99999 | 1.683 | 0.372 | 0.98237 |
| C/PHTC | 81.6 | 0.004 | 0.94043 | 0.361 | 0.694 | 0.92571 | |
| CaHTC | 3345.0 | 0.012 | 0.99999 | 40.596 | 0.448 | 0.95892 | |
| C/PaHTC | 49.7 | 0.046 | 0.98189 | 3.634 | 0.501 | 0.97498 | |
| Cd(II) | CHTC | 1357.0 | 4.62 × 10−5 | 0.93807 | 0.063 | 1.906 | 0.96911 |
| C/PHTC | 2.2 | 1.01 × 10−6 | 0.92372 | 0.086 | 1.275 | 0.71281 | |
| CaHTC | 843.4 | 0.00414 | 0.97853 | 3.480 | 0.573 | 0.98945 | |
| C/PaHTC | 2.4 | 4.81 × 10−13 | 0.94990 | 0.026 | 1.807 | 0.76007 | |
| Material | Modification | Initial Concentration, mg/dm3 | Heavy Metal | Adsorption Capacity qmax, mg/g | Reference |
|---|---|---|---|---|---|
| Cotton fabric | Pyrolysis + Activation H3PO4 | 80–500 | Pb(II) | 361.54 | [20] |
| Cotton/polyester fabric 75:25 | 80–500 | 385.77 | |||
| Cotton fibre | Microwave-assisted carbonization | 0.05–0.4 | Hg(II) | 169.2 | [58] |
| Cotton stalk | Pyrolysis | 100–500 | Pb(II) | 146.78 | [25] |
| Cotton stalk | Hydrothermal carbonization + Activation KOH | 5–300 | Cd(II) | 30.40 | [17] |
| Cotton stalk | Pyrolysis | 10–80 | Pb(II) | 42.55 | [59] |
| 0.1–1.0 | Cd(II) | 0.53 | |||
| 1.0–10 | Ni(II) | 5.25 | |||
| 0.1–1.0 | Co(II) | 0.54 | |||
| Pyrolysis + H2SO4 | 10–80 | Pb(II) | 38.76 | ||
| 0.1–1.0 | Cd(II) | 0.53 | |||
| 1.0–10 | Ni(II) | 2.21 | |||
| 0.1–1.0 | Co(II) | 0.52 | |||
| Pyrolysis + NaOH | 10–80 | Pb(II) | 37.59 | ||
| 0.1–1.0 | Cd(II) | 0.51 | |||
| 1.0–10 | Ni(II) | 2.05 | |||
| 0.1–1.0 | Co(II) | 0.50 | |||
| Pyrolysis + H2C2O4 | 10–80 | Pb(II) | 44.64 | ||
| 0.1–1.0 | Cd(II) | 0.65 | |||
| 1.0–10 | Ni(II) | 6.20 | |||
| 0.1–1.0 | Co(II) | 0.52 | |||
| Waste cotton yarns | Hydrothermal carbonization | 5–100 | Pb(II) | 260.8 | This study |
| Waste cotton/polyester yarns | 5–100 | 81.6 | |||
| Waste cotton yarns | 5–15 | Cd(II) | 3345.0 | ||
| Waste cotton/polyester yarns | 5–15 | 49.7 | |||
| Waste cotton yarns | Hydrothermal carbonization + Activation KOH | 5–100 | Pb(II) | 1357.0 | |
| Waste cotton/polyester yarns 50:50 | 5–100 | 2.2 | |||
| Waste cotton yarns | 5–15 | Cd(II) | 843.4 | ||
| Waste cotton/polyester yarns 50:50 | 5–15 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivunac, K.; Mihajlović, S.; Vukčević, M.; Maletić, M.; Pejić, B.; Kalijadis, A.; Perić Grujić, A. Modified Cellulose-Based Waste for Enhanced Adsorption of Selected Heavy Metals from Wastewater. Polymers 2024, 16, 2610. https://doi.org/10.3390/polym16182610
Trivunac K, Mihajlović S, Vukčević M, Maletić M, Pejić B, Kalijadis A, Perić Grujić A. Modified Cellulose-Based Waste for Enhanced Adsorption of Selected Heavy Metals from Wastewater. Polymers. 2024; 16(18):2610. https://doi.org/10.3390/polym16182610
Chicago/Turabian StyleTrivunac, Katarina, Snežana Mihajlović, Marija Vukčević, Marina Maletić, Biljana Pejić, Ana Kalijadis, and Aleksandra Perić Grujić. 2024. "Modified Cellulose-Based Waste for Enhanced Adsorption of Selected Heavy Metals from Wastewater" Polymers 16, no. 18: 2610. https://doi.org/10.3390/polym16182610
APA StyleTrivunac, K., Mihajlović, S., Vukčević, M., Maletić, M., Pejić, B., Kalijadis, A., & Perić Grujić, A. (2024). Modified Cellulose-Based Waste for Enhanced Adsorption of Selected Heavy Metals from Wastewater. Polymers, 16(18), 2610. https://doi.org/10.3390/polym16182610






