Recent Applications of PLGA in Drug Delivery Systems
Abstract
1. Introduction
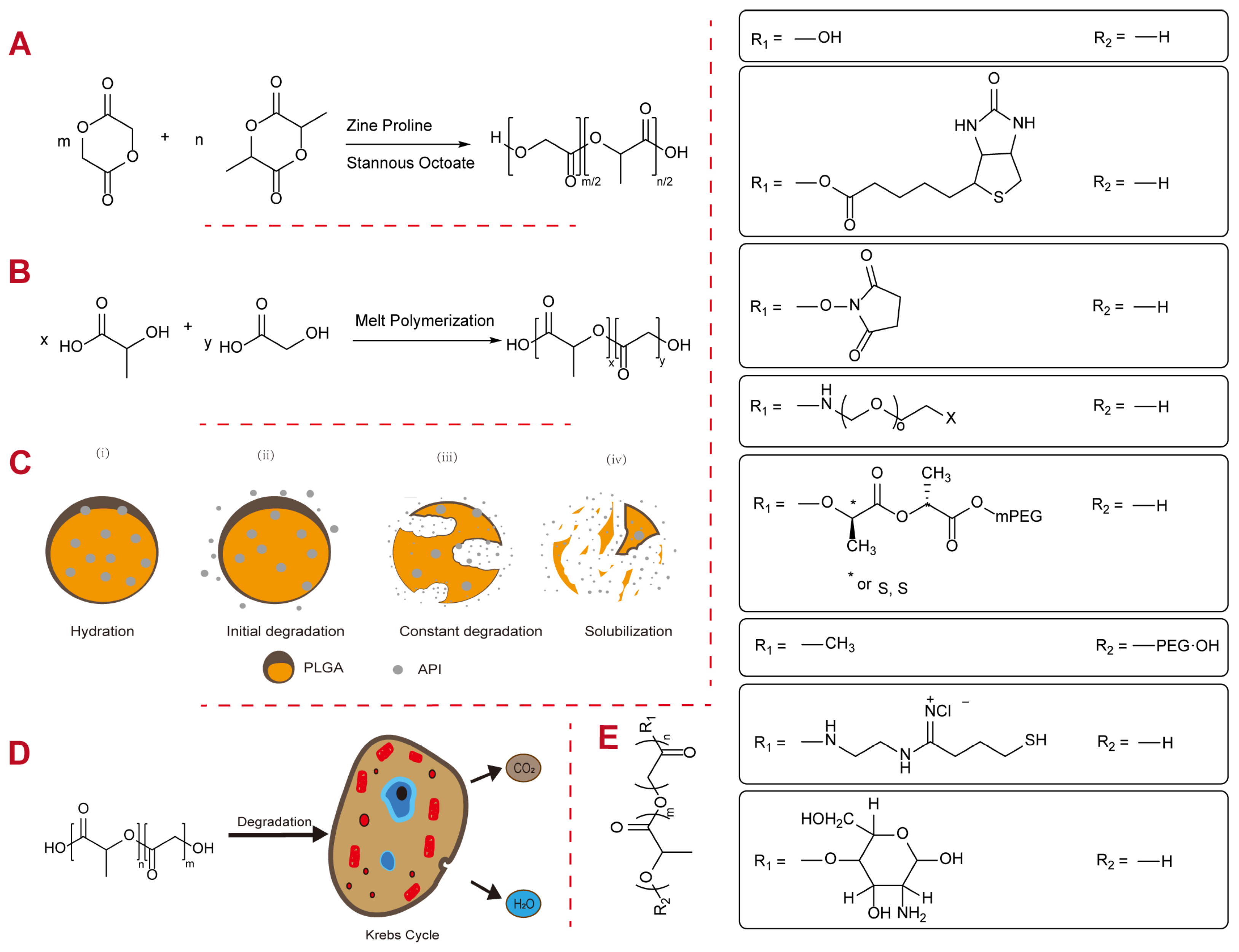
2. Physicochemical Properties of PLGA
2.1. Molecular Weight
2.2. Intrinsic Viscosity
2.3. Monomer Ratio
2.4. Blockiness
2.5. End Caps
3. Modification of PLGA-Based DDSs
4. Load of PLGA-Based DDSs
4.1. Small-Molecule Drugs
4.2. Natural Product
4.3. Protein or Peptide
4.4. Antibiotics or Antiviral Drugs
5. Applications of PLGA-Based DDSs
5.1. Pain
5.2. Cancers
5.3. Neurological Disorders
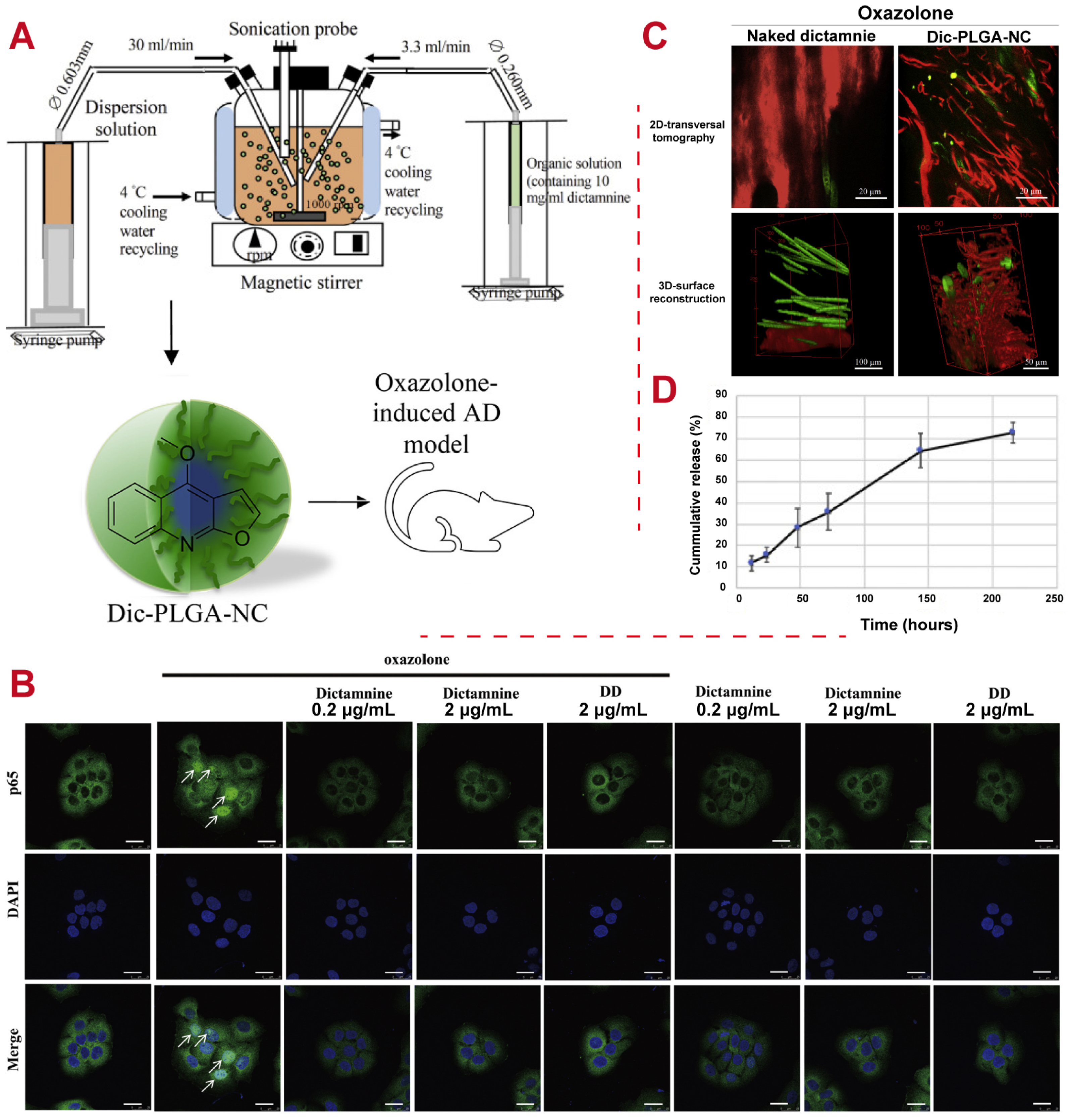
5.4. Inflammation

5.5. Vaccines
5.6. Tissue Regeneration
6. Limitations and Challenges of Using PLGA-Based DDSs
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jia, Y.; Kong, X.; Nie, Y.; Deng, Y.; Liu, Y. Novel drug delivery systems and disease models for pulmonary fibrosis. J. Control. Release 2022, 348, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Kashkooli, F.M.; Soltani, M.; Souri, M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J. Control. Release 2020, 327, 316–349. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef]
- Laracuente, M.L.; Marina, H.Y.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef]
- Shen, X.; Li, T.; Xie, X.; Feng, Y.; Chen, Z.; Yang, H.; Wu, C.; Deng, S.; Liu, Y. PLGA-based drug delivery systems for remotely triggered cancer therapeutic and diagnostic applications. Front. Bioeng. Biotechnol. 2020, 8, 381. [Google Scholar] [CrossRef]
- Rigon, L.; Salvalaio, M.; Pederzoli, F.; Legnini, E.; Duskey, J.T.; D’Avanzo, F.; De Filippis, C.; Ruozi, B.; Marin, O.; Vandelli, M.A.; et al. Targeting brain disease in MPSII: Preclinical evaluation of IDS-loaded PLGA nanoparticles. Int. J. Mol. Sci. 2019, 20, 2014. [Google Scholar] [CrossRef]
- Lee, D.; Nah, H.; Ko, W.K.; Kim, S.J.; Han, G.H.; Jeong, D.; Lee, D.; Han, I.; Sheen, S.H.; Heo, D.N.; et al. Thiolate poly (lactic-co-glycolic acid) nanofibers loaded with dexamethasone and ropivacaine show enhanced sustained release in the treatment of neuropathic pain through a local therapy technique. Chem. Eng. J. 2022, 431, 133356. [Google Scholar] [CrossRef]
- Deng, M.; Tan, J.; Hu, C.; Hou, T.; Peng, W.; Liu, J.; Yu, B.; Dai, Q.; Zhou, J.; Yang, Y.; et al. Modification of PLGA scaffold by MSC-derived extracellular matrix combats macrophage inflammation to initiate bone regeneration via TGF-β-induced protein. Adv. Healthc. Mater. 2020, 9, 2000353. [Google Scholar] [CrossRef]
- Gao, J.; Karp, J.M.; Langer, R.; Joshi, N. The future of drug delivery. Chem. Mater. 2023, 35, 359–363. [Google Scholar] [CrossRef]
- Wang, S.; Downing, G.; Olsen, K.F.; Sawyer, T.K.; Cone, R.D.; Schwendeman, S.P. Aqueous remote loading of setmelanotide in poly (lactic-co-glycolic acid) microspheres for long-term obesity treatment. J. Control. Release 2023, 364, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Guarecuco, R.; Lu, J.; McHugh, K.J.; Norman, J.J.; Thapa, L.S.; Lydon, E.; Langer, R.; Jaklenec, A. Immunogenicity of pulsatile-release PLGA microspheres for single-injection vaccination. Vaccine 2018, 36, 3161–3168. [Google Scholar] [CrossRef]
- Li, W.; Tang, J.; Lee, D.; Tice, T.R.; Schwendeman, S.P.; Prausnitz, M.R. Clinical translation of long-acting drug delivery formulations. Nat. Rev. Mater. 2022, 7, 406–420. [Google Scholar] [CrossRef]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Gao, Q.; Lan, P.; Shao, H.; Hu, X. Direct synthesis with melt polycondensation and microstructure analysis of poly (L-lactic acid-co-glycolic acid). Polym. J. 2002, 34, 786–793. [Google Scholar] [CrossRef]
- Giram, P.S.; Wang, J.T.W.; Walters, A.A.; Rade, P.P.; Akhtar, M.; Han, S.; Faruqu, F.N.; Abdel-Bar, H.M.; Garnaik, B.; Al-Jamal, K.T. Green synthesis of methoxy-poly (ethylene glycol)-block-poly (L-lactide-co-glycolide) copolymer using zinc proline as a biocompatible initiator for irinotecan delivery to colon cancer in vivo. Biomater. Sci. 2021, 9, 795–806. [Google Scholar] [CrossRef]
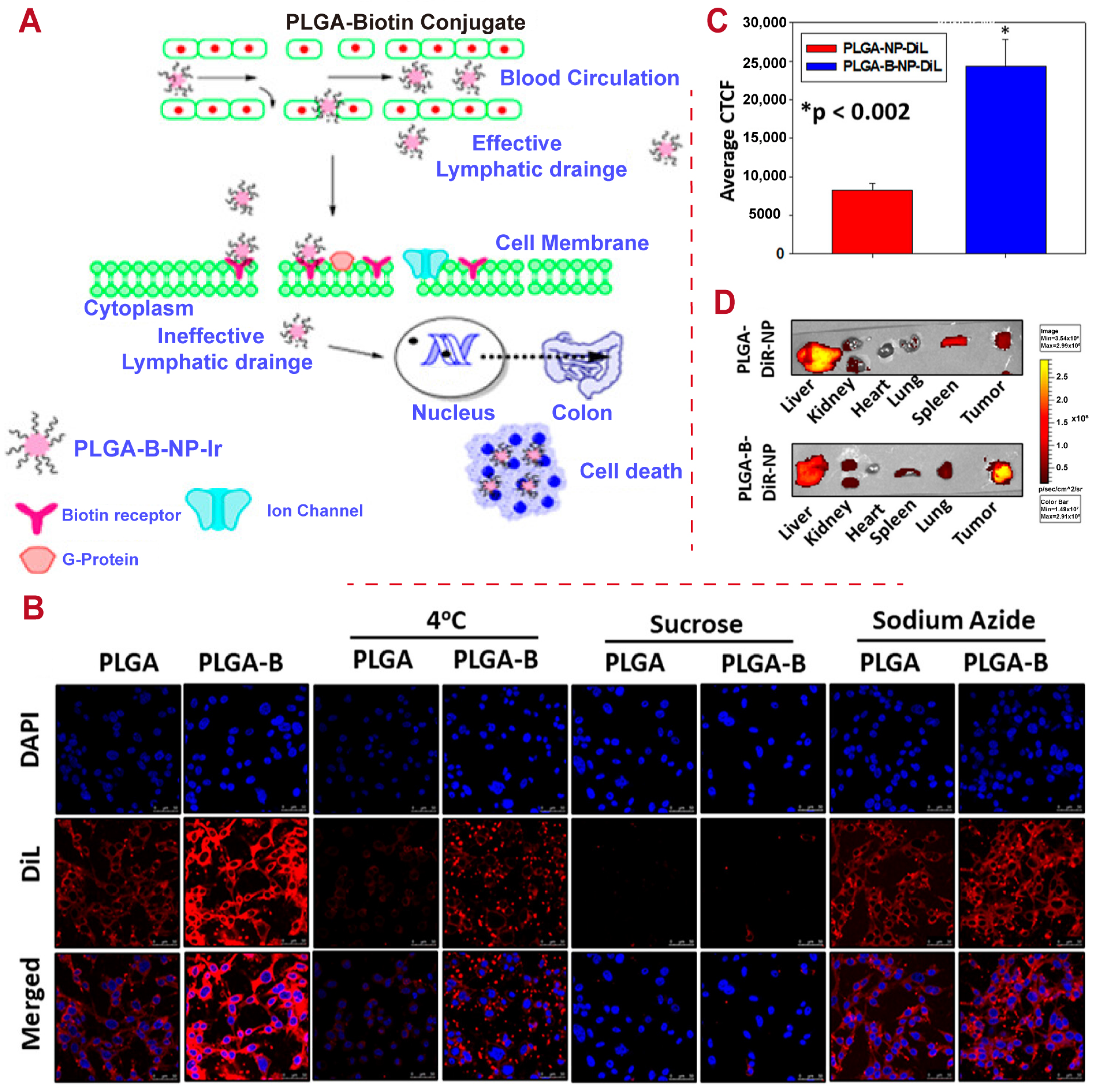
- Giram, P.S.; Nimma, R.; Bulbule, A.; Yadav, A.S.; Gorain, M.; Venkata Radharani, N.N.; Kundu, G.C.; Garnaik, B. Poly (d, l-lactide-co-glycolide) Surface-Anchored Biotin-Loaded Irinotecan Nanoparticles for Active Targeting of Colon Cancer. ACS Omega 2024, 9, 3807–3826. [Google Scholar] [CrossRef]
- Rabanel, J.M.; Piec, P.A.; Landri, S.; Patten, S.A.; Ramassamy, C. Transport of PEGylated-PLA nanoparticles across a blood brain barrier model, entry into neuronal cells and in vivo brain bioavailability. J. Control. Release 2020, 328, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Samadi, N.; Abbadessa, A.; Di Stefano, A.; Van Nostrum, C.; Vermonden, T.; Rahimian, S.; Teunissen, E.; Van Steenbergen, M.; Amidi, M.; Hennink, W. The effect of lauryl capping group on protein release and degradation of poly (d, l-lactic-co-glycolic acid) particles. J. Control. Release 2013, 172, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Sousa, F.; Araujo, F.; Sarmento, B. Functionalizing PLGA and PLGA derivatives for drug delivery and tissue regeneration applications. Adv. Healthc. Mater. 2018, 7, 1701035. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly (lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef]
- Li, Q.; Chang, B.; Dong, H.; Liu, X. Functional microspheres for tissue regeneration. Bioact. Mater. 2023, 25, 485–499. [Google Scholar] [CrossRef]
- Behera, A. Nanomaterials. In Advanced Materials: An Introduction to Modern Materials Science; Springer International Publishing: Cham, Switzerland, 2022; pp. 77–125. [Google Scholar] [CrossRef]
- Mei, H.; Cai, S.; Huang, D.; Gao, H.; Cao, J.; He, B. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: From intrinsic physicochemical properties to external modification. Bioact. Mater. 2022, 8, 220–240. [Google Scholar] [CrossRef]
- Magill, E.; Demartis, S.; Gavini, E.; Permana, A.D.; Thakur, R.R.S.; Adrianto, M.F.; Waite, D.; Glover, K.; Picco, C.J.; Korelidou, A.; et al. Solid implantable devices for sustained drug delivery. Adv. Drug Deliv. Rev. 2023, 199, 114950. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Oz, Y.; Gu, Y.; Ahamad, N.; Shariati, K.; Chevalier, J.; Kapur, D.; Annabi, N. Rational design of polymeric micelles for targeted therapeutic delivery. Nano Today 2024, 55, 102147. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Pereira, M.C. PLGA based drug carrier and pharmaceutical applications: The most recent advances. Pharmaceutics 2020, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, W.; Choi, S. FDA’s regulatory science program for generic PLA/PLGA-based drug products. Am. Pharm. Rev. 2016, 20. [Google Scholar]
- Wan, B.; Bao, Q.; Burgess, D. Long-acting PLGA microspheres: Advances in excipient and product analysis toward improved product understanding. Adv. Drug Deliv. Rev. 2023, 198, 114857. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Qi, J.; Lu, Y.; He, H.; Wu, W. PLGA-based implants for sustained delivery of peptides/proteins: Current status, challenge and perspectives. Chin. Chem. Lett. 2023, 34, 108250. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-based nanomedicine: History of advancement and development in clinical applications of multiple diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Available online: https://www.clinicaltrials.gov/ (accessed on 8 June 2024).
- Hua, Y.; Wang, Z.; Wang, D.; Lin, X.; Liu, B.; Zhang, H.; Gao, J.; Zheng, A. Key factor study for generic long-acting PLGA microspheres based on a reverse engineering of Vivitrol®. Molecules 2021, 26, 1247. [Google Scholar] [CrossRef]
- Schutzman, R.; Shi, N.Q.; Olsen, K.F.; Ackermann, R.; Tang, J.; Liu, Y.Y.; Hong, J.K.; Wang, Y.; Qin, B.; Schwendeman, A.; et al. Mechanistic evaluation of the initial burst release of leuprolide from spray-dried PLGA microspheres. J. Control. Release 2023, 361, 297–313. [Google Scholar] [CrossRef]
- He, Y.; Chen, Q.W.; Yu, J.X.; Qin, S.Y.; Liu, W.L.; Ma, Y.H.; Chen, X.S.; Zhang, A.Q.; Zhang, X.Z.; Cheng, Y.J. Yeast cell membrane-camouflaged PLGA nanoparticle platform for enhanced cancer therapy. J. Control. Release 2023, 359, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.M.; Shrestha, N.; Kipping, T.; Banga, A.K. Formulation development of tazarotene-loaded PLGA nanoparticles for follicular delivery in the treatment of inflammatory skin diseases. Eur. J. Pharm. Biopharm. 2024, 200, 114346. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ain, Q.U.; Weigmann, B.; Story, D.; Smith, B.R.; Ali, H. Dual pH and microbial-sensitive galactosylated polymeric nanocargoes for multi-level targeting to combat ulcerative colitis. Asian J. Pharm. Sci. 2023, 18, 100831. [Google Scholar] [CrossRef]
- Wei, D.; Sun, Y.; Zhu, H.; Fu, Q. Stimuli-responsive polymer-based nanosystems for cancer theranostics. ACS Nano 2023, 17, 23223–23261. [Google Scholar] [CrossRef]
- Braet, H.; Fransen, P.P.; Chen, Y.; Van Herck, S.; Mariën, R.; Vanhoorne, V.; Ceelen, W.; Madder, A.; Ballet, S.; Hoogenboom, R.; et al. Smart hydrogels delivered by high pressure aerosolization can prevent peritoneal adhesions. J. Control. Release 2023, 362, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, D.S.; Hwang, G.Y.; Lee, J.K.; Lee, H.L.; Jung, J.W.; Hwang, S.Y.; Baek, S.W.; lip Yoon, S.; Ha, Y.; et al. Multi-modulation of immune-inflammatory response using bioactive molecule-integrated PLGA composite for spinal fusion. Mater. Today Bio 2023, 19, 100611. [Google Scholar] [CrossRef]
- Watcharadulyarat, N.; Rattanatayarom, M.; Ruangsawasdi, N.; Patikarnmonthon, N. PEG–PLGA nanoparticles for encapsulating ciprofloxacin. Sci. Rep. 2023, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Giacon, N.; Lo Cascio, E.; Pennacchietti, V.; De Maio, F.; Santarelli, G.; Sibilia, D.; Tiberio, F.; Sanguinetti, M.; Lattanzi, W.; Toto, A.; et al. PDZ2-conjugated-PLGA nanoparticles are tiny heroes in the battle against SARS-CoV-2. Sci. Rep. 2024, 14, 13059. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Shekhar, S.; Yadav, B.; Garg, V.; Dutt, R.; Mehata, A.K.; Goswami, P.; Koch, B.; Muthu, M.S.; Singh, R.P.; et al. AS1411 aptamer/RGD dual functionalized theranostic chitosan-PLGA nanoparticles for brain cancer treatment and imaging. Biomater. Adv. 2024, 160, 213833. [Google Scholar] [CrossRef]
- Hua, Y.; Su, Y.; Zhang, H.; Liu, N.; Wang, Z.; Gao, X.; Gao, J.; Zheng, A. Poly (lactic-co-glycolic acid) microsphere production based on quality by design: A review. Drug Deliv. 2021, 28, 1342–1355. [Google Scholar] [CrossRef]
- Pardeshi, S.R.; Nikam, A.; Chandak, P.; Mandale, V.; Naik, J.B.; Giram, P.S. Recent advances in PLGA based nanocarriers for drug delivery system: A state of the art review. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 49–78. [Google Scholar] [CrossRef]
- Wan, B.; Bao, Q.; Burgess, D.J. In vitro-in vivo correlation of PLGA microspheres: Effect of polymer source variation and temperature. J. Control. Release 2022, 347, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.R.S.; McMillan, H.L.; Jones, D.S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 2014, 176, 8–23. [Google Scholar] [CrossRef]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef]
- Wan, B.; Bao, Q.; Zou, Y.; Wang, Y.; Burgess, D.J. Effect of polymer source variation on the properties and performance of risperidone microspheres. Int. J. Pharm. 2021, 610, 121265. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Burgess, D.J. Drug release from in situ forming implants and advances in release testing. Adv. Drug Deliv. Rev. 2021, 178, 113912. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bao, Q.; Wang, R.; Wan, B.; Wang, Y.; Qin, B.; Burgess, D.J. Reverse engineering of Perseris and development of compositionally equivalent formulations. Int. J. Pharm. 2023, 639, 122948. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, C.; Wu, G.; Ye, Q.; Wang, C. Poly (lactic-co-glycolic acid): Applications and future prospects for periodontal tissue regeneration. Polymers 2017, 9, 189. [Google Scholar] [CrossRef]
- Park, K.; Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Otte, A.; Soh, B.K.; Yoon, G.; Yu, D.; Yun, Y.; et al. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation. J. Control. Release 2019, 304, 125–134. [Google Scholar] [CrossRef]
- Hadar, J.; Garner, J.; Skidmore, S.; Park, H.; Park, K.; Jhon, Y.; Wang, Y. Correlation analysis of refractive index (dn/dc) for PLGAs with different ratios of lactide to glycolide. In Proceedings of the 2018 Controlled Release Society (CRS) Annual Meeting, New York, NY, USA, 22–24 July 2018. [Google Scholar]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef]
- Smith, A.N.; Ulsh, J.B.; Gupta, R.; Tang, M.M.; Peredo, A.P.; Teinturier, T.D.; Mauck, R.L.; Gullbrand, S.; Hast, M.W. Characterization of degradation kinetics of additively manufactured PLGA under variable mechanical loading paradigms. J. Mech. Behav. Biomed. Mater. 2024, 153, 106457. [Google Scholar] [CrossRef]
- Molavi, F.; Barzegar-Jalali, M.; Hamishehkar, H. Polyester based polymeric nano and microparticles for pharmaceutical purposes: A review on formulation approaches. J. Control. Release 2020, 320, 265–282. [Google Scholar] [CrossRef]
- Butreddy, A.; Gaddam, R.P.; Kommineni, N.; Dudhipala, N.; Voshavar, C. PLGA/PLA-based long-acting injectable depot microspheres in clinical use: Production and characterization overview for protein/peptide delivery. Int. J. Mol. Sci. 2021, 22, 8884. [Google Scholar] [CrossRef]
- Li, S.; Niu, D.; Shi, T.; Yun, W.; Yan, S.; Xu, G.; Yin, J. Injectable, in situ self-cross-linking, self-healing poly (l-glutamic acid)/polyethylene glycol hydrogels for cartilage tissue engineering. ACS Biomater. Sci. Eng. 2023, 9, 2625–2635. [Google Scholar] [CrossRef]
- Garner, J.; Skidmore, S.; Hadar, J.; Park, H.; Park, K.; Jhon, Y.K.; Qin, B.; Wang, Y. Analysis of semi-solvent effects for PLGA polymers. Int. J. Pharm. 2021, 602, 120627. [Google Scholar] [CrossRef] [PubMed]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Wang, X.; Bao, Q.; Suh, M.S.; Kastellorizios, M.; Wang, R.; Burgess, D.J. Novel adapter method for in vitro release testing of in situ forming implants. Int. J. Pharm. 2022, 621, 121777. [Google Scholar] [CrossRef]
- Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the application of micellar drug delivery systems in cancer nanomedicine. Pharmaceuticals 2023, 16, 433. [Google Scholar] [CrossRef]
- Kim, G.; Gavande, V.; Shaikh, V.; Lee, W.K. Degradation behavior of poly (lactide-co-glycolide) monolayers investigated by Langmuir technique: Accelerating Effect. Molecules 2023, 28, 4810. [Google Scholar] [CrossRef]
- Murcia Valderrama, M.A.; van Putten, R.J.; Gruter, G.J.M. PLGA barrier materials from CO2. The influence of lactide co-monomer on glycolic acid polyesters. ACS Appl. Polym. Mater. 2020, 2, 2706–2718. [Google Scholar] [CrossRef] [PubMed]
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Avgoustakis, K. Polylactic-co-glycolic acid (PLGA). Encycl. Biomater. Biomed. Eng. 2005, 1, 1–11. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Patil, S.M.; Won, Y.Y. Effect of Monomer Sequence Distribution on the Glass Transition Temperature of Poly (d, l-lactic-co-glycolic acid) (PLGA). Macromolecules 2024, 57, 4947–4962. [Google Scholar] [CrossRef]
- De, R.; Mahata, M.K.; Kim, K.T. Structure-based varieties of polymeric nanocarriers and influences of their physicochemical properties on drug delivery profiles. Adv. Sci. 2022, 9, 2105373. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.; Skidmore, S.; Park, H.; Park, K.; Choi, S.; Wang, Y. A protocol for assay of poly (lactide-co-glycolide) in clinical products. Int. J. Pharm. 2015, 495, 87–92. [Google Scholar] [CrossRef]
- Wang, R.; Bao, Q.; Clark, A.G.; Wang, Y.; Zhang, S.; Burgess, D.J. Characterization and in vitro release of minocycline hydrochloride microspheres prepared via coacervation. Int. J. Pharm. 2022, 628, 122292. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Kumar, S.; Tan, J.J.; Boey, F.Y.; Venkatraman, S.S.; Steele, T.W.; Loo, J.S. Modulating drug release from poly (lactic-co-glycolic acid) thin films through terminal end-groups and molecular weight. Polym. Degrad. Stab. 2013, 98, 619–626. [Google Scholar] [CrossRef]
- Lanao, R.P.F.; Jonker, A.M.; Wolke, J.G.; Jansen, J.A.; van Hest, J.C.; Leeuwenburgh, S.C. Physicochemical properties and applications of poly (lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng. Part B Rev. 2013, 19, 380–390. [Google Scholar] [CrossRef]
- Zare, E.N.; Jamaledin, R.; Naserzadeh, P.; Afjeh-Dana, E.; Ashtari, B.; Hosseinzadeh, M.; Vecchione, R.; Wu, A.; Tay, F.R.; Borzacchiello, A.; et al. Metal-based nanostructures/PLGA nanocomposites: Antimicrobial activity, cytotoxicity, and their biomedical applications. ACS Appl. Mater. Interfaces 2019, 12, 3279–3300. [Google Scholar] [CrossRef]
- Locatelli, E.; Comes Franchini, M. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J. Nanopart. Res. 2012, 14, 1316. [Google Scholar] [CrossRef]
- Yu, B.; Pu, Y.; Liu, J.; Liao, J.; Chen, K.; Zhang, J.; Zhong, W.; Hu, Y.; Wang, X.Q.; Liu, B.; et al. Targeted delivery of emodin to adipocytes by aptamer-functionalized PEG-PLGA nanoparticles in vitro. J. Drug Deliv. Sci. Technol. 2020, 57, 101739. [Google Scholar] [CrossRef]
- Sheffey, V.V.; Siew, E.B.; Tanner, E.E.; Eniola-Adefeso, O. PLGA’s plight and the role of stealth surface modification strategies in its use for intravenous particulate drug delivery. Adv. Healthc. Mater. 2022, 11, 2101536. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Serra, É.; Lima, J.; Loureiro, J.A.; Pereira, M.C. Chitosan-PLGA mucoadhesive nanoparticles for gemcitabine repurposing for glioblastoma therapy. Eur. J. Pharm. Biopharm. 2024, 200, 114326. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, T.; Zhang, Z.; Yu, D.G.; Liu, P.; Wang, L.; Zhu, Y. Electrospun Janus core (ethyl cellulose//polyethylene oxide)@ shell (hydroxypropyl methyl cellulose acetate succinate) hybrids for an enhanced colon-targeted prolonged drug absorbance. Adv. Compos. Hybrid Mater. 2023, 6, 189. [Google Scholar] [CrossRef]
- Soares, D.C.F.; Domingues, S.C.; Viana, D.B.; Tebaldi, M.L. Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed. Pharmacother. 2020, 131, 110695. [Google Scholar]
- Malcor, J.D.; Mallein-Gerin, F. Biomaterial functionalization with triple-helical peptides for tissue engineering. Acta Biomater. 2022, 148, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, W.; Xia, Y.; Zhang, W.; Zhao, Q.; Li, X.; Gao, H.; Liang, Z.; Ma, G.; Yang, K.; et al. Targeted cross-linker delivery for the in situ mapping of protein conformations and interactions in mitochondria. Nat. Commun. 2023, 14, 3882. [Google Scholar] [CrossRef]
- Barros, C.H.; Hiebner, D.W.; Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Synthesis and self-assembly of curcumin-modified amphiphilic polymeric micelles with antibacterial activity. J. Nanobiotechnol. 2021, 19, 104. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Z.; Zhu, X.; Jiang, L.; Shi, W.; Wang, Y.; Xu, N.; Gang, F.; Wang, X.; Zhao, L.; et al. Dual directions to address the problem of aseptic loosening via electrospun PLGA@ aspirin nanofiber coatings on titanium. Biomaterials 2020, 257, 120237. [Google Scholar] [CrossRef]
- Monteleone, P.; Cascino, G.; Rossi, A.; Rocca, P.; Bertolino, A.; Aguglia, E.; Amore, M.; Andriola, I.; Bellomo, A.; Biondi, M.; et al. Evolution of antipsychotic-induced extrapyramidal symptoms in patients with schizophrenia in the real-life: A 4-year follow-up naturalistic study. Schizophr. Res. 2022, 248, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bao, Q.; Wang, R.; Kwok, O.; Maurus, K.; Wang, Y.; Qin, B.; Burgess, D.J. In situ forming risperidone implants: Effect of PLGA attributes on product performance. J. Control. Release 2023, 361, 777–791. [Google Scholar] [CrossRef]
- Shen, J.; Choi, S.; Qu, W.; Wang, Y.; Burgess, D.J. In vitro-in vivo correlation of parenteral risperidone polymeric microspheres. J. Control. Release 2015, 218, 2–12. [Google Scholar] [CrossRef]
- Andhariya, J.V.; Shen, J.; Choi, S.; Wang, Y.; Zou, Y.; Burgess, D.J. Development of in vitro-in vivo correlation of parenteral naltrexone loaded polymeric microspheres. J. Control. Release 2017, 255, 27–35. [Google Scholar] [CrossRef]
- Liu, J.C.T.; De La Peña, R.; Tocol, C.; Sattely, E.S. Reconstitution of early paclitaxel biosynthetic network. Nat. Commun. 2024, 15, 1419. [Google Scholar] [CrossRef] [PubMed]
- Luiz, M.T.; Abriata, J.P.; Raspantini, G.L.; Tofani, L.B.; Fumagalli, F.; de Melo, S.M.G.; da Silva Emery, F.; Swiech, K.; Marcato, P.D.; Lee, R.; et al. In vitro evaluation of folate-modified PLGA nanoparticles containing paclitaxel for ovarian cancer therapy. Mater. Sci. Eng. C 2019, 105, 110038. [Google Scholar] [CrossRef]
- Khaled, S.S.; Soliman, H.A.; Abdel-Gabbar, M.; Ahmed, N.A.; Attia, K.A.H.A.; Mahran, H.A.; El-Nahass, E.S.; Ahmed, O.M. The preventive effects of naringin and naringenin against paclitaxel-induced nephrotoxicity and cardiotoxicity in male Wistar rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 8739815. [Google Scholar] [CrossRef]
- Xu, D.; Song, X.J.; Chen, X.; Wang, J.W.; Cui, Y.L. Advances and future perspectives of intranasal drug delivery: A scientometric review. J. Control. Release 2024, 367, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, J.; Fan, Y.; Sun, J.; Cheng, J.X.; Zhang, X.F.; Zhai, B.T.; Guo, D.Y. Development of curcumin-loaded galactosylated chitosan-coated nanoparticles for targeted delivery of hepatocellular carcinoma. Int. J. Biol. Macromol. 2023, 253, 127219. [Google Scholar] [CrossRef]
- Rawal, S.; Patel, M.M. Threatening cancer with nanoparticle aided combination oncotherapy. J. Control. Release 2019, 301, 76–109. [Google Scholar] [CrossRef]
- Hu, H.; Liao, Z.; Xu, M.; Wan, S.; Wu, Y.; Zou, W.; Wu, J.; Fan, Q. Fabrication, optimization, and evaluation of paclitaxel and curcumin coloaded PLGA nanoparticles for improved antitumor activity. ACS Omega 2022, 8, 976–986. [Google Scholar] [CrossRef]
- Shi, M.; McHugh, K.J. Strategies for overcoming protein and peptide instability in biodegradable drug delivery systems. Adv. Drug Deliv. Rev. 2023, 199, 114904. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, C.M.; Dasanayake, G.S.; Gorniak, M.E.; Pride, M.C.; Monroe, W.; Chism, C.M.; Heintz, R.; Jarrett, E.; Singh, G.; Edgecomb, S.X.; et al. Development of ionic liquid-coated PLGA nanoparticles for applications in intravenous drug delivery. Nat. Protoc. 2023, 18, 2509–2557. [Google Scholar] [CrossRef]
- Müller, M.; Vörös, J.; Csucs, G.; Walter, E.; Danuser, G.; Merkle, H.; Spencer, N.; Textor, M. Surface modification of PLGA microspheres. J. Biomed. Mater. Res. Part A 2003, 66, 55–61. [Google Scholar] [CrossRef]
- Ochi, M.; Wan, B.; Bao, Q.; Burgess, D.J. Influence of PLGA molecular weight distribution on leuprolide release from microspheres. Int. J. Pharm. 2021, 599, 120450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qin, F.; Ji, Z.; Fei, W.; Tan, Z.; Hu, Y.; Zheng, C. Mannose-modified PLGA nanoparticles for sustained and targeted delivery in hepatitis B virus immunoprophylaxis. AAPS PharmSciTech 2020, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Islan, G.A.; Rodenak-Kladniew, B.; Noacco, N.; Duran, N.; Castro, G.R. Prodigiosin: A promising biomolecule with many potential biomedical applications. Bioengineered 2022, 13, 14227–14258. [Google Scholar] [CrossRef] [PubMed]
- Obayemi, J.; Danyuo, Y.; Dozie-Nwachukwu, S.; Odusanya, O.; Anuku, N.; Malatesta, K.; Yu, W.; Uhrich, K.; Soboyejo, W. PLGA-based microparticles loaded with bacterial-synthesized prodigiosin for anticancer drug release: Effects of particle size on drug release kinetics and cell viability. Mater. Sci. Eng. C 2016, 66, 51–65. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, H.; Du, S.; Zhang, X.Q.; Lin, F.; Ji, F.; Tsou, Y.H.; Li, Z.; Feng, Y.; Ticehurst, K.; et al. Injectable PLGA-coated ropivacaine produces a long-lasting analgesic effect on incisional pain and neuropathic pain. J. Pain 2021, 22, 180–195. [Google Scholar] [CrossRef]
- Gao, G.; Sun, X.; Liang, G. Nanoagent-promoted mild-temperature photothermal therapy for cancer treatment. Adv. Funct. Mater. 2021, 31, 2100738. [Google Scholar] [CrossRef]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.F.; Zhao, Y.; Zhang, H.; et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef]
- Yan, J.; Shan, C.; Zhang, Z.; Li, F.; Sun, Y.; Wang, Q.; He, B.; Luo, K.; Chang, J.; Liang, Y. Autophagy-induced intracellular signaling fractional nano-drug system for synergistic anti-tumor therapy. J. Colloid Interface Sci. 2023, 645, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, X.; Song, H.; Zhang, C.; Wang, X.; Huang, P.; Dong, A.; Zhang, Y.; Kong, D.; Wang, W. Polymer-lipid hybrid nanovesicle-enabled combination of immunogenic chemotherapy and RNAi-mediated PD-L1 knockdown elicits antitumor immunity against melanoma. Biomaterials 2021, 268, 120579. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Zeng, X.; Liang, X.; Li, X.; Tao, W.; Chen, H.; Jiang, Y.; Mei, L.; Feng, S.S. The effect of autophagy inhibitors on drug delivery using biodegradable polymer nanoparticles in cancer treatment. Biomaterials 2014, 35, 1932–1943. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- Koerner, J.; Horvath, D.; Herrmann, V.L.; MacKerracher, A.; Gander, B.; Yagita, H.; Rohayem, J.; Groettrup, M. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy. Nat. Commun. 2021, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Biswaro, L.S.; Garcia, M.P.; da Silva, J.R.; Neira Fuentes, L.F.; Vera, A.; Escobar, P.; Azevedo, R.B. Itraconazole encapsulated PLGA-nanoparticles covered with mannose as potential candidates against leishmaniasis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Handa, M.; Sanap, S.N.; Bhatta, R.S.; Patil, G.P.; Ghose, S.; Singh, D.P.; Shukla, R. Combining donepezil and memantine via mannosylated PLGA nanoparticles for intranasal delivery: Characterization and preclinical studies. Biomater. Adv. 2023, 154, 213663. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jang, J.H.; Lee, Y.B. Drug delivery to the brain via the nasal route of administration: Exploration of key targets and major consideration factors. J. Pharm. Investig. 2023, 53, 119–152. [Google Scholar] [CrossRef] [PubMed]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef]
- Maiti, P.; Dunbar, G.L. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Zhang, J.; Zheng, Z.; Kaplan, D.L.; Li, G.; Wang, X. Oral delivery of curcumin using silk nano-and microparticles. ACS Biomater. Sci. Eng. 2018, 4, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, W.; Zhou, H.; Wang, H.; Kong, B.; Bai, F. Ginkgo biloba extract-loaded PLGA microcapsules generated from microfluidics for Alzheimer’s disease treatment. Mater. Des. 2024, 238, 112735. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Y.; Zhang, Z.; Yang, Z.; Huang, G. Intra-articular delivery of tetramethylpyrazine microspheres with enhanced articular cavity retention for treating osteoarthritis. Asian J. Pharm. Sci. 2018, 13, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hsieh, Y.T.; Chan, L.Y.; Yang, T.Y.; Maeda, T.; Chang, T.M.; Huang, H.C. Dictamnine delivered by PLGA nanocarriers ameliorated inflammation in an oxazolone-induced dermatitis mouse model. J. Control. Release 2021, 329, 731–742. [Google Scholar] [CrossRef]
- Karami, Z.; Mehrzad, J.; Akrami, M.; Hosseinkhani, S. Anti-inflammation-based treatment of atherosclerosis using Gliclazide-loaded biomimetic nanoghosts. Sci. Rep. 2023, 13, 13880. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- McHugh, K.J.; Guarecuco, R.; Langer, R.; Jaklenec, A. Single-injection vaccines: Progress, challenges, and opportunities. J. Control. Release 2015, 219, 596–609. [Google Scholar] [CrossRef]
- Watkins, H.C.; Pagan, C.L.; Childs, H.R.; Posada, S.; Chau, A.; Rios, J.; Guarino, C.; DeLisa, M.P.; Whittaker, G.R.; Putnam, D. A single dose and long lasting vaccine against pandemic influenza through the controlled release of a heterospecies tandem M2 sequence embedded within detoxified bacterial outer membrane vesicles. Vaccine 2017, 35, 5373–5380. [Google Scholar] [CrossRef]
- Danaeifar, M.; Negahdari, B.; Eslam, H.M.; Zare, H.; Ghanaat, M.; Koushali, S.S.; Malekshahi, Z.V. Polymeric nanoparticles for DNA vaccine-based cancer immunotherapy: A review. Biotechnol. Lett. 2023, 45, 1053–1072. [Google Scholar] [CrossRef]
- Rubsamen, R.; Herst, C.; Lloyd, P.; Heckerman, D. Eliciting cytotoxic T-lymphocyte responses from synthetic vectors containing one or two epitopes in a C57BL/6 mouse model using peptide-containing biodegradable microspheres and adjuvants. Vaccine 2014, 32, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- Rosalia, R.A.; Cruz, L.J.; van Duikeren, S.; Tromp, A.T.; Silva, A.L.; Jiskoot, W.; de Gruijl, T.; Löwik, C.; Oostendorp, J.; van der Burg, S.H.; et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 2015, 40, 88–97. [Google Scholar] [CrossRef]
- Silva, A.; Soema, P.; Slütter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccines Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
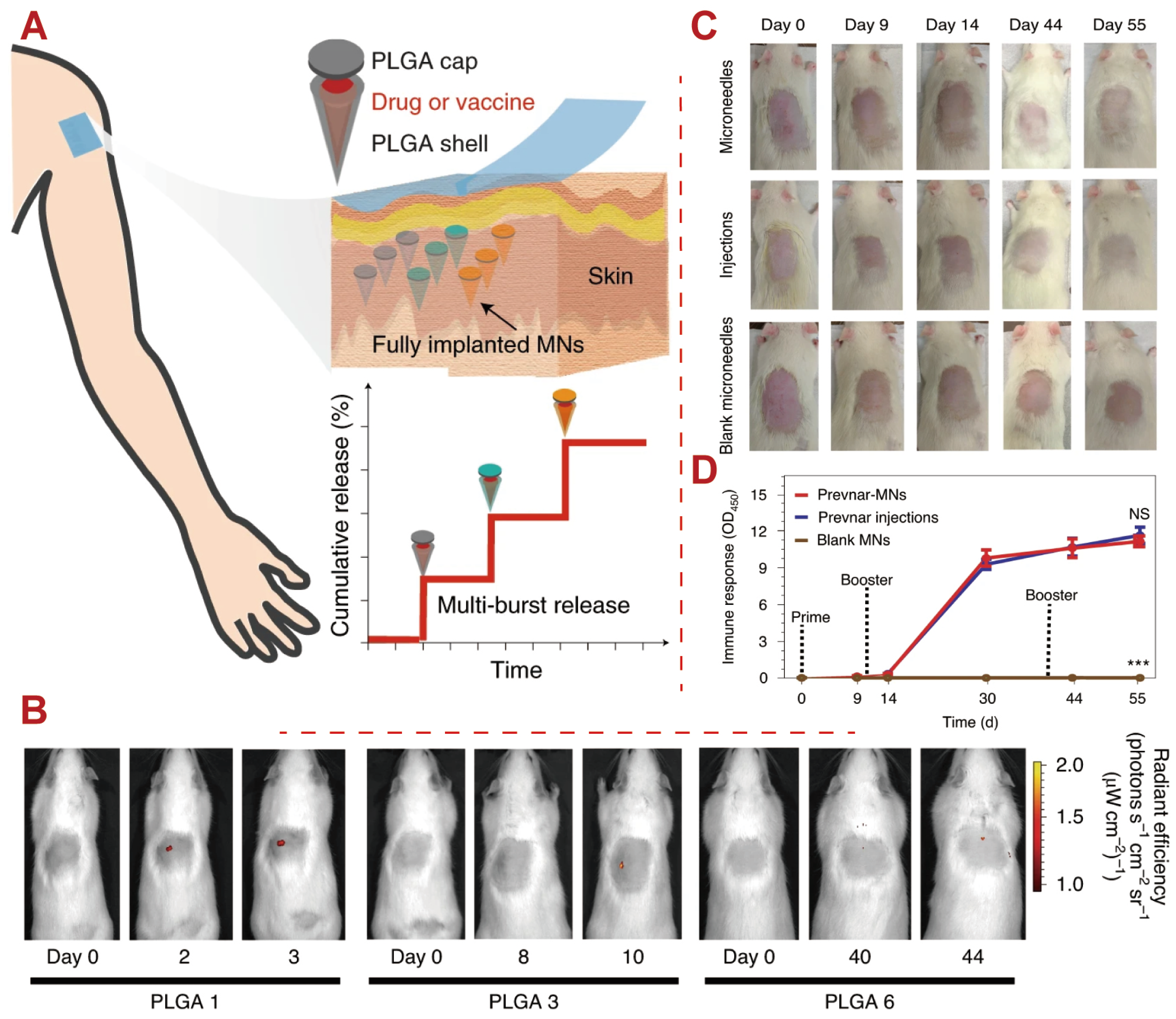
- Tran, K.T.; Gavitt, T.D.; Farrell, N.J.; Curry, E.J.; Mara, A.B.; Patel, A.; Brown, L.; Kilpatrick, S.; Piotrowska, R.; Mishra, N.; et al. Transdermal microneedles for the programmable burst release of multiple vaccine payloads. Nat. Biomed. Eng. 2021, 5, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, M.A.; Shin, J.Y.; Jeon, J.H.; Lee, S.J.; Yoon, M.Y.; Kim, H.J.; Choi, E.J.; Do, S.H.; Yang, V.C.; et al. Intra-articular delivery of synovium-resident mesenchymal stem cells via BMP-7-loaded fibrous PLGA scaffolds for cartilage repair. J. Control. Release 2019, 302, 169–180. [Google Scholar] [CrossRef]
- Xu, T.M.; Chu, H.Y.; Li, M.; Talifu, Z.; Ke, H.; Pan, Y.Z.; Xu, X.; Wang, Y.H.; Guo, W.; Wang, C.L.; et al. Establishment of FK506-Enriched PLGA Nanomaterial Neural Conduit Produced by Electrospinning for the Repair of Long-Distance Peripheral Nerve Injury. J. Nanomater. 2022, 2022, 3530620. [Google Scholar] [CrossRef]
- Wang, Y.; Kankala, R.K.; Cai, Y.Y.; Tang, H.X.; Zhu, K.; Zhang, J.T.; Yang, D.Y.; Wang, S.B.; Zhang, Y.S.; Chen, A.Z. Minimally invasive co-injection of modular micro-muscular and micro-vascular tissues improves in situ skeletal muscle regeneration. Biomaterials 2021, 277, 121072. [Google Scholar] [CrossRef]
- Lü, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef]
- Ramezanpour, S.; Tavatoni, P.; Akrami, M.; Navaei-Nigjeh, M.; Shiri, P. Potential wound healing of PLGA nanoparticles containing a novel L-Carnitine–GHK peptide conjugate. J. Nanomater. 2022, 2022, 6165759. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Castano, O.; Mateos-Timoneda, M.Á.; Engel, E.; Pérez-Amodio, S. Nanotechnology approaches in chronic wound healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, Z.R.; Jacobsen, D.E.; Kocheril, P.A.; Kubicek-Sutherland, J.Z. Chapter 17 - Biological toxicity and environmental hazards associated with PLGA nanoparticles. In Poly(lactic-co-glycolic acid) (PLGA) Nanoparticles for Drug Delivery; Kesharwani, P., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 457–475. [Google Scholar] [CrossRef]
- Yoo, J.; Won, Y.Y. Phenomenology of the initial burst release of drugs from PLGA microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Hamdallah, S.I.; Zoqlam, R.; Yang, B.; Campbell, A.; Booth, R.; Booth, J.; Belton, P.; Qi, S. Using a systematic and quantitative approach to generate new insights into drug loading of PLGA nanoparticles using nanoprecipitation. Nanoscale Adv. 2024, 6, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, W.; Zhou, J.; Yu, Z.; An, M.; He, W.; Xue, Y.; Chen, F. Transdermal delivery of IBU-PLGA nanoparticles with dissolving microneedle array patch. J. Drug Deliv. Sci. Technol. 2024, 95, 105528. [Google Scholar] [CrossRef]
- Bausart, M.; Rodella, G.; Dumont, M.; Ucakar, B.; Vanvarenberg, K.; Malfanti, A.; Préat, V. Combination of local immunogenic cell death-inducing chemotherapy and DNA vaccine increases the survival of glioblastoma-bearing mice. Nanomed. Nanotechnol. Biol. Med. 2023, 50, 102681. [Google Scholar] [CrossRef]
- Casanova, E.A.; Rodriguez-Palomo, A.; Stähli, L.; Arnke, K.; Gröninger, O.; Generali, M.; Neldner, Y.; Tiziani, S.; Dominguez, A.P.; Guizar-Sicairos, M.; et al. SAXS imaging reveals optimized osseointegration properties of bioengineered oriented 3D-PLGA/aCaP scaffolds in a critical size bone defect model. Biomaterials 2023, 294, 121989. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, K.; He, A.; Wan, S.; Wu, M.; Hu, D.; Xu, K.; Wei, P.; Yin, J. Dual-layer conduit containing VEGF-A–Transfected Schwann cells promotes peripheral nerve regeneration via angiogenesis. Acta Biomater. 2024, 180, 323–336. [Google Scholar] [CrossRef]




| Product name, Manufacturer | API | Type | Approbsl Date(s), Indication(s) | Dose |
|---|---|---|---|---|
| Vivitrol® Alkermes, Inc., Dublin, Ireland | Naltrexone | Microparticle | Alcohol dependence, relapse in opioid dependence | 380 mg per month |
| Zoladex® Depot AstraZeneca, Cambridge, UK | Goserelin acetate | Implant | Breast cancer, prostate cancer, endometriosis | 3.6 mg/10.8 per month |
| Lupron Depot® AbbVie Inc., North Chicago, IL, USA | Leuprolide acetate | Microsphere | Endometriosis, advanced prostate cancer | 7.5 mg per month |
| Lupron® AbbVie Inc., North Chicago, IL, USA | Leuprolide acetate | Microsphere | Endometriosis | 3.75 mg per month |
| Eligard® Tolmar, Inc., Fort Collins, CO, USA | Leuprolide acetate | In situ gel | Prostate cancer symptoms | 7.5 mg a month 22.5 mg per 3 months 30 mg for 4 months 45 mg per 6 months |
| Sandostatin® LAR Novartis, Basel, Switzerland | Octreotide acetate | Microsphere | Acromegaly, flushing episodes and watery diarrhea (caused by vasoactive intestinal peptide tumors) | 10/20/30 mg per month |
| Atridox® Atrix, Inc., Fort Collins, CO, USA | Doxycycline hyclate | In situ gel | Chronic periodontitis. | 42.5 mg per week |
| Nutropin Depot® Genentech, Inc., South San Francisco, CA, USA | Somatotropin | Microparticle | Growth failure, growth hormone deficiency | 13.5/18/22.5 mg per month |
| Trelstar® Tolmar, Inc., Fort Collins, CO, USA | Triptorelin pamoate | Microparticle | Advanced prostate cancer relief treatment | 3.75 mg a month 11.25 mg per 3 months 22.5 mg for 6 months |
| Somatuline® Depot Ipsen, Inc., Basking Ridge, NJ, USA | Lanreotide | Microparticle | Acromegaly, symptoms caused by neuroendocrine tumors | 60 mg per month |
| Arestin® OraPharma, Inc., Warminster, PA, USA | Minocycline HCl | Microparticle | Periodontitis | 1 mg per 2 weeks |
| Risperidal® Consta Janssen, Inc., Titusville, NJ, USA | Risperidone | Microparticle | Schizophrenia | 12.5/25/37.5/50 mg per 2 weeks |
| Perseris™ Indivior Inc., Richmond, VA, USA | Risperidone | In situ gel | Schizophrenia | 90/120 mg per month |
| Ozurdex® Allergan, Dublin, Ireland | Dexamethasone | Implant | Macular edema, diabetic macular edema, non-infectious uveitis | 0.7 mg variable dosing frequency |
| Propel® Intersect ENT, Inc., Menlo Park, CA, USA | Mometasone furoate | Implant | Chronic sinusitis | 0.37 mg per month |
| Bydureon® AstraZeneca, Cambridge, UK | Exenatide | Microparticle | Type 2 diabetes mellitus | 2.0 mg per week |
| Signifor® LAR Novartis, Basel, Switzerland | Pasireotide | Microparticle | Alcohol dependence, relapse in opioid dependence | 380 mg per month |
| Zilretta® Flexion, Inc., Burlington, MA, USA | Triamcinolone acetoamide | Microparticle | Osteoarthritis | 32 mg per 3 months |
| Sublocade™ Indivior Inc., Berkshire, UK | Buprenorphine | in situ gel | Moderate-to-severe opioid use disorder | 100/300 mg per month |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in Drug Delivery Systems. Polymers 2024, 16, 2606. https://doi.org/10.3390/polym16182606
Yang J, Zeng H, Luo Y, Chen Y, Wang M, Wu C, Hu P. Recent Applications of PLGA in Drug Delivery Systems. Polymers. 2024; 16(18):2606. https://doi.org/10.3390/polym16182606
Chicago/Turabian StyleYang, Jie, Huiying Zeng, Yusheng Luo, Ying Chen, Miao Wang, Chuanbin Wu, and Ping Hu. 2024. "Recent Applications of PLGA in Drug Delivery Systems" Polymers 16, no. 18: 2606. https://doi.org/10.3390/polym16182606
APA StyleYang, J., Zeng, H., Luo, Y., Chen, Y., Wang, M., Wu, C., & Hu, P. (2024). Recent Applications of PLGA in Drug Delivery Systems. Polymers, 16(18), 2606. https://doi.org/10.3390/polym16182606










